Glycogen synthase kinase 3 (GSK-3), initially identified in the late 1970s as a regulator of glycogen metabolism,[1, 2] is now well known to be involved in a variety of intracellular signaling events.[3] GSK-3 is a serine/threonine protein kinase, which is encoded in mammals by two highly homologous isoforms, GSK-3α and GSK-3β.[4] Since mood stabilizers, such as lithium, likely owe their effects to the inhibition of GSK-3β, considerable effort has gone into the discovery and design of small, organic molecules that serve as GSK-3-selective lithium mimetics. Properly designed inhibitors could possibly overcome the shortcomings of this drug with considerable side effects,[5] thereby finding applications in the treatment of central nervous system (CNS) disorders including Alzheimer’s disease (AD),[6] Parkinson’s disease, stroke, traumatic brain injury, and bipolar disorders.[7, 8]
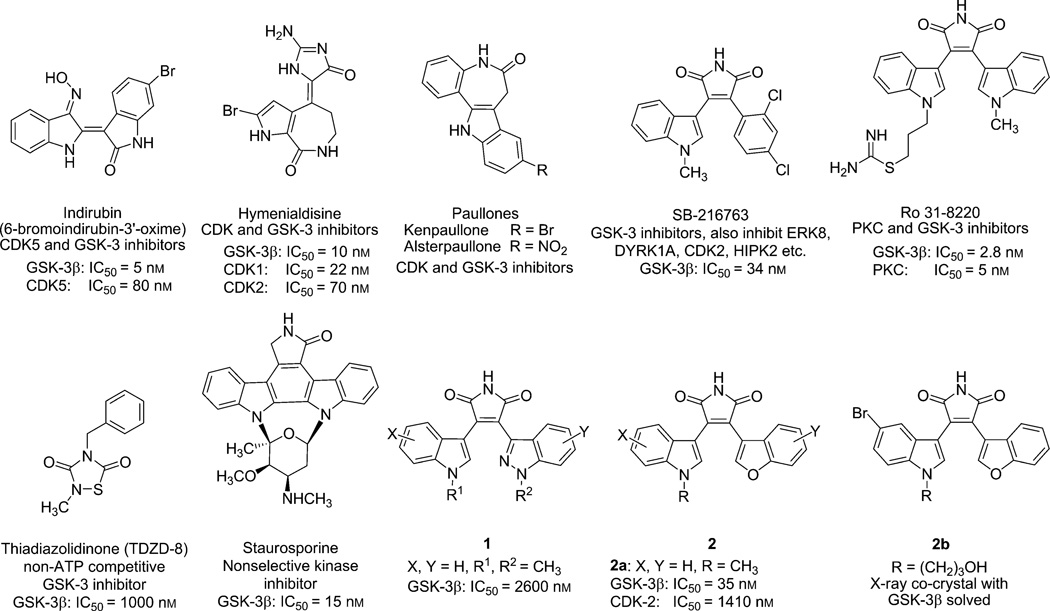
A host of both natural and unnatural products have been investigated as ATP-competitive GSK-3 inhibitors over the past few decades. Natural products, such as staurosporine,[9] indirubins,[10] hymenialdisines,[11] and paullones,[12] have been extensively studied in this connection (Figure 1). In addition, a number of synthetic, small-molecule GSK-3 inhibitors have been reported.[13] All of these compounds are ATP-competitive GSK-3β inhibitors, most of which show nonselective protein kinase inhibition profiles including activity against the cyclin-dependent kinases (CDKs) and PKCs (protein kinase C) as shown in Figure 1. Poor kinase selectivity is a common problem for most ATP-competitive GSK-3β inhibitors. Thiadiazolidinones (TDZDs) are the first reported non-ATP-competitive compounds.[14] The lead TDZD, NP-12 (also named tideglusib), is now in phase II clinical trials for the treatment of AD and progressive supranuclear palsy (PSP). NP-12 is the only GSK-3 inhibitor under clinical development for both PSP and AD. However, none of the ATP-competitive compounds have made it into clinical trials. Improved compounds demonstrating higher selectivity for GSK-3β are likely needed in order to properly validate the ability of this compound class to function as lithium mimetics.
Figure 1.
Selected examples of GSK-3β inhibitors from the literature.[9–14]
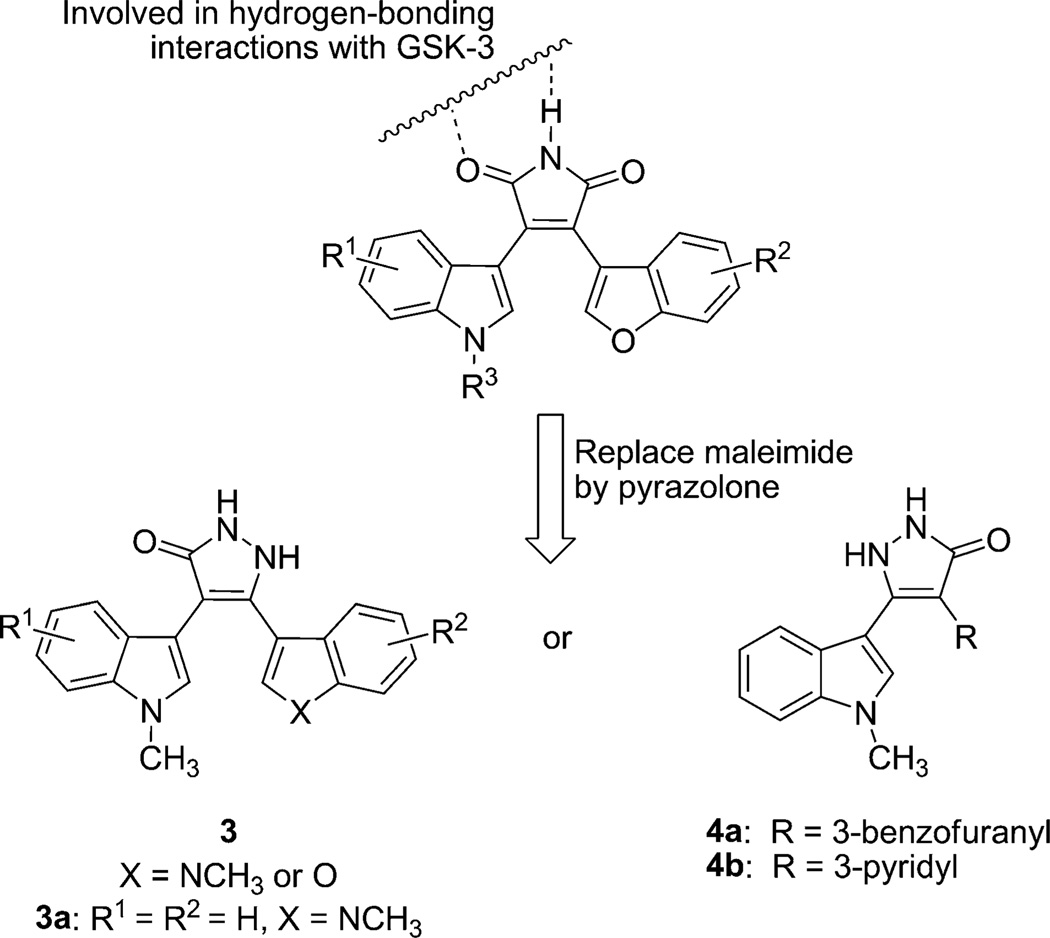
Previously, we described the identification of 3-indolyl-4-indazolylmaleimides (1) and benzofuran-3-yl-(indol-3-yl)maleimides (2) as reasonable GSK-3β inhibitor candidates showing some elements of kinase selectivity and behavioral activity in the mouse mania model.[15, 16] Both of these compounds are derived from the natural product staurosporine (Figure 1). In further investigations aimed at improving largely target selectivity, we decided to explore the possibility of replacing the 5-membered maleimide core with other heterocyclic systems capable of maintaining the required hydrogen-bonding patterns between the small molecule and enzyme. Pyrazolones appeared to dock reasonably well in the X-ray co-crystal structure complexes [minus the original ligand] obtained previously for some of our GSK-3β inhibitors. Herein, we present the synthesis and activity of members of a pyrazolone family. In particular, one of the lead pyrazolones showed excellent selectivity for GSK-3β when tested against a panel of 40 other kinases. Moreover, this compound was found to be superior as a neuroprotective agent when tested in the homocysteic acid (HCA) model of oxidative stress.
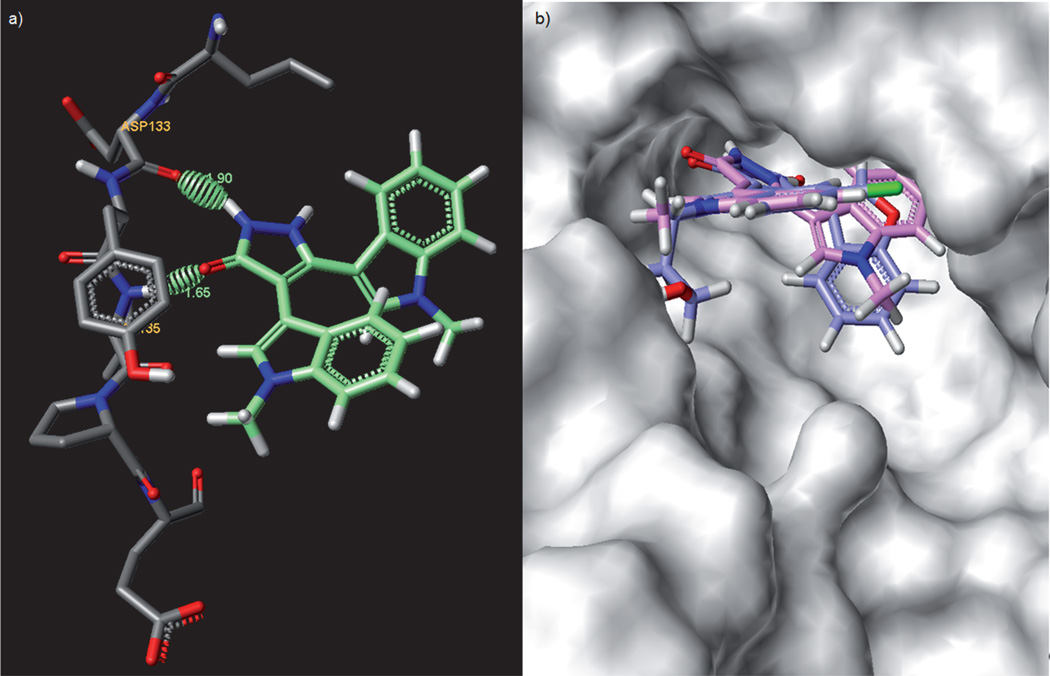
In order to better understand the interactions between human GSK-3β and our maleimide-based inhibitors, we obtained a co-crystal structure of our previously identified lead compound 2b in complex with human GSK-3β.[17] Since only the NH group and one of the two carbonyl groups are involved in the tight binding to GSK-3β with a water molecule acting as a bridge between the other carbonyl group of maleimide and the backbone, we assumed that it may be possible to further enhance the kinase selectivity of the inhibitors by removing one of the maleimide carbonyl groups. By altering one point of interaction that is not required for complexation with GSK-3, we believed that we might reduce some of the undesirable off-target interactions with other kinases. Taking into consideration both the drug-like properties of new analogues, as well as their synthetic accessibility, we now sought to replace the GSK-3β noninteracting carbonyl group of the maleimide group by a potential hydrogen-bond donor NH group. Following this logic, a series of compounds, the 5-membered-ring pyrazolones, was considered as displayed in Figure 2. Employing the original X-ray co-crystal structures, we docked[16] the new pyrazolones to the active site of GSK-3β and observed that the pyrazolone portion of compound 3a provides key hydrogen-bonding interactions with the backbone carbonyl group of Asp 133 (1.90 Å) and the backbone amide of Val 135 (1.65 Å) as shown in Figure 3. The characteristic hydrogen-bonding interaction patterns were maintained for these new compounds. Compound 3a was previously reported as a PKC inhibitor,[18] however no GSK-3 data has previously been shown.
Figure 2.
Alternative GSK-3 inhibitor scaffolds with a pyrazolone (5-membered heterocycle) as a replacement for the maleimide core.
Figure 3.
Docking of the pyrazolone to the X-ray structure of GSK-3β. a) Binding orientation of 3a in the active site showing key hydrogen-bonding interactions. b) Solvent-exposed surface map of GSK-3β (grey) showing the binding pocket for 2b (blue) and 3a (pink).
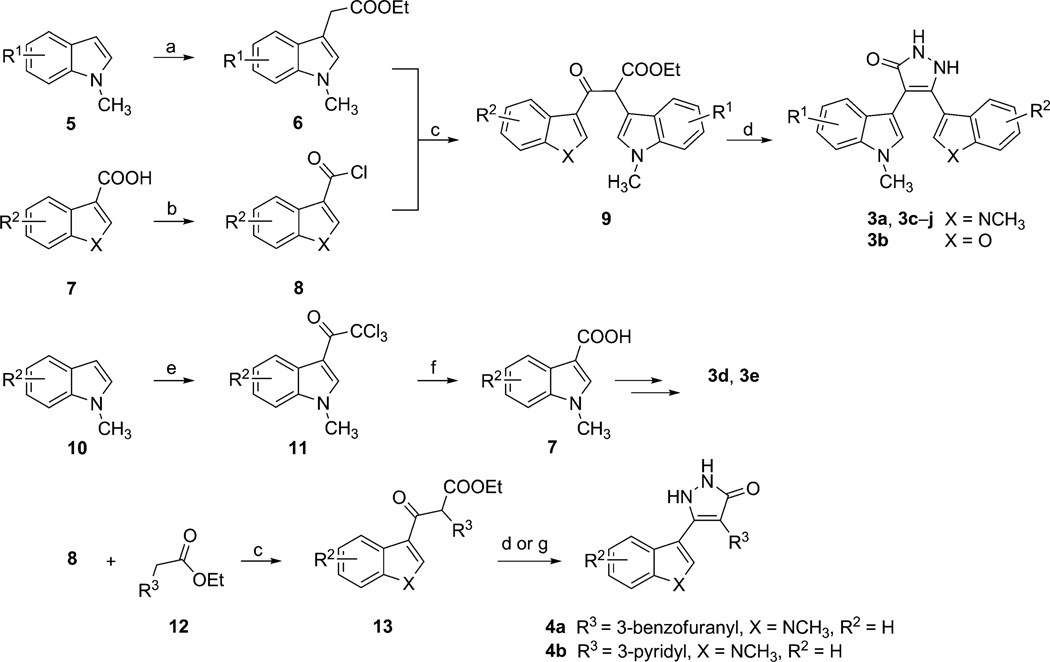
With the aforementioned hypothesis in hand, a series of pyrazolones were synthesized by the general method shown in Scheme 1. The synthesis of pyrazolone 3 is straightforward and based on the cyclization of the corresponding β-keto esters 9 and hydrazine. Compound 9 was prepared by the addition of the appropriately substituted ethyl-3-indoleacetates and indole-3-carbonyl chlorides or benzofuran-3-carbonyl chlorides (Scheme 1).[18] Preparation of indolyl-based acetates commences with alkylation of the indole 5 to afford compound 6. The other required heterocycle-3-carbonyl chlorides 8 were prepared from indole or benzofuran-based carboxylic acid 7. Some of the indole-3-carboxylic acids were prepared by acylation of indole 10 with trichloroacetyl chloride to form intermediate 11, followed by basic hydrolysis. The enolates generated from indole-3-acetic acid ester 6 and lithium diisopropylamide (LDA) reacted with acyl chloride 8 to form β-ketoester 9. Treatment of compound 9 with hydrazine in the presence of p-toluenesulfonic acid (p-TsOH) yielded the pyrazolones 3. For the synthesis of pyrazolones 4, heterocyclic-3-acetic acid esters 12 were used in place of the indole-3-acetic acid esters to form intermediate 13.
Scheme 1.
Reagents and conditions: a) 5% Cu(OTf)2, ethyl diazoacetate, CH2Cl2, 12 h, 45–55 %; b) SOCl2, cat. DMF, RT, 16 h; c) LDA, THF, −78°C, 16 h, 28–66 %; d) NH2NH2·XH2O, EtOH, p-TsOH, MW, 5 h, 15–40 %; e) Trichloroacetyl chloride, pyridine, THF, 0 °C→RT, 3 h, 80–90 %; f) 2m NaOH, 1,4-dioxane, H2O, reflux, 16 h, then H+, 60–70 %; g) NH2NH2·XH2O, 1,1-dioxane, AcOH, reflux, 16 h, 58%.
The newly synthesized pyrazolones were then examined for their inhibitory activity against GSK-3β (Table 1). Pyrazolones containing both an indole and a benzofuran group displayed micromolar potency against GSK-3β (IC50=2270 nm for 4a; IC50=2950 nm for 3b). To our delight, the bisindolyl-pyrazolone 3a displayed very good GSK-3β inhibitory activity in the in vitro assay (IC50=34 nm), as was anticipated from the modeling studies. To ascertain the importance of substitution on either of the indole rings of this pyrazolone series, a 5-bromo substituent was introduced as in compounds 3c and 3d. The kinase assays revealed that one isomer was about six-fold more active than the other, with compound 3c exhibiting an IC50 value of 535 nm. Further variations of the substituent located at the 5-position of compound 3c, as in the 5-fluoro compound 3f and 5-chloro compound 3g, led to further decreases in potency (IC50=574 nm for 3f; IC50=2470 nm for 3g), while the 5-benzyloxy compound 3j was inactive against GSK-3β at the maximum test concentration of 10 µm. In addition, we found that the introduction of a halogen in the 6-position of the indole ring as in compound 3e was also unfavorable. Replacement of one of the indoles by a pyridine ring as in 4b also led to poorer activity (IC50=4280 nm) in comparison with 3a. From these results, we chose compound 3a as the lead compound for further biological studies.
Table 1.
GSK-3β inhibition by pyrazolones and staurosporine.
| Compd | R1 | R2 | IC50[a] [nm] |
|---|---|---|---|
| Staurosporine | – | – | 11.2 |
| 3a | H | H | 34.0 |
| 4a | – | H | 2270 |
| 3b | H | H | 2950 |
| 3c | 5-Br | H | 535 |
| 3d | H | 5-Br | 3090 |
| 3e | H | 6-F | 2060 |
| 3f | 5-F | H | 574 |
| 3g | 5-Cl | H | 2470 |
| 3h | 4-F | H | 1490 |
| 3i | 7-F | H | 2070 |
| 3j | 5-OBn | H | NA[b] |
| 4b | – | H | 4280 |
The synthesized pyrazolones were evaluated for their ability to inhibit phosphorylation of primed substrate (YRRAAVPPSPSLSRHSSPHQ(pS)E DEEE; 20 µm) by human GSK-3β in the presence of 10 µm ATP. These compounds were tested by Reaction Biology, Inc. (http://www.reactionbiology.com).
Not active (maximum test concentration = 10 µm).
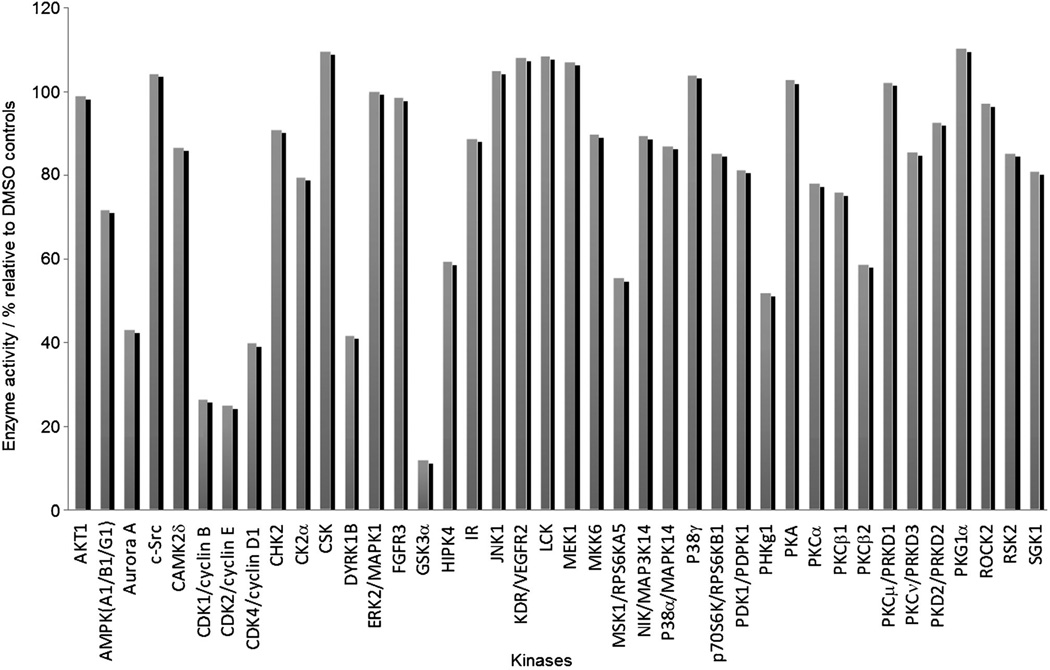
A number of GSK-3β inhibitors have been reported, however, many of these are poorly selective drugs, as they show cross-reactivity to PKC and CDK, a consequence of high homology among the catalytic domains of these enzymes. To determine whether the selectivity of pyazolone 3a is, in fact, better than the previously reported compounds, it was tested for inhibition at a single concentration (10 µm) against a panel of 40 related kinases. In the presence of ATP (10 µm), compound 3a exhibited low inhibitory activity against 37 of the other kinases, with the exception of showing moderate inhibitory activity against the CDKs and GSK-3α (Figure 4). A second round of kinase screening in which IC50 values were determined, revealed that compound 3a is, in fact, spectacularly selective for GSK-3 (Table 2). Given the nearly identical sequences of the α and β isoforms of GSK-3, we did not anticipate any selectivity for these two kinases.
Figure 4.
First-pass kinase screening of 3a at 10 µm concentration against a panel of 40 kinases.
Table 2.
Further in vitro kinase selectivity summary of lead compound 3a.[a]
| Kinases | IC50[nm] | SI[b] | |
|---|---|---|---|
| 3a | Staurosporine | ||
| ALK5/TGFBR1 | >100 000 | 10 100 | >2941 |
| Aurora A | 7900 | <1.0 | 232 |
| CDK1 cyclin B | 3250 | <1.0 | 96 |
| CDK2 cyclin E | 1800 | 1.3 | 53 |
| CDK3 cyclin E | 7090 | 5.4 | 209 |
| CDK4 cyclin D1 | 2970 | 6.1 | 87 |
| CDK5 p25 | 4220 | 2.4 | 124 |
| CDK6 cyclin D3 | 8180 | 17.2 | 241 |
| CDK7 cyclin H | – | 155 | – |
| CDK9 cyclin T1 | 8800 | 7.4 | 259 |
| DYRK1 B | 5970 | 6.3 | 176 |
| GSK3α | 37.6 | 2.8 | 1 |
| HIPK4 | 10 000 | 408 | 294 |
| MSK1 | 14 000 | <1.0 | 412 |
| PHKγ1 | 14 100 | 1.2 | 415 |
| PKCα | 45 700 | 1.6 | 1344 |
| PKCβ2 | 23 000 | <1.0 | 677 |
The compound was tested in ten-dose IC50 mode with three-fold serial dilution starting at 100 µm. Control compound staurosporine was tested in ten-dose IC50 mode with three-fold serial dilution starting at 20 µm. Reactions were carried out at 10 µm ATP. These compounds were tested by Reaction Biology, Inc. (http://www.reactionbiology.com).
Kinase selectivity index (SI) of compound 3a for GSK-3β over other kinases tested.
Compound 3a was also tested in a panel of 44 cloned human and rodent CNS receptors, channels and transporters through the US National Institute of Mental Health’s (NIMH) Psychoactive Drug Screening Program (PDSP). No significant activity was found in these studies, with the exception of some activity at the 5-HT1a receptor (IC50=704 nm; further data can be found in the Supporting Information).
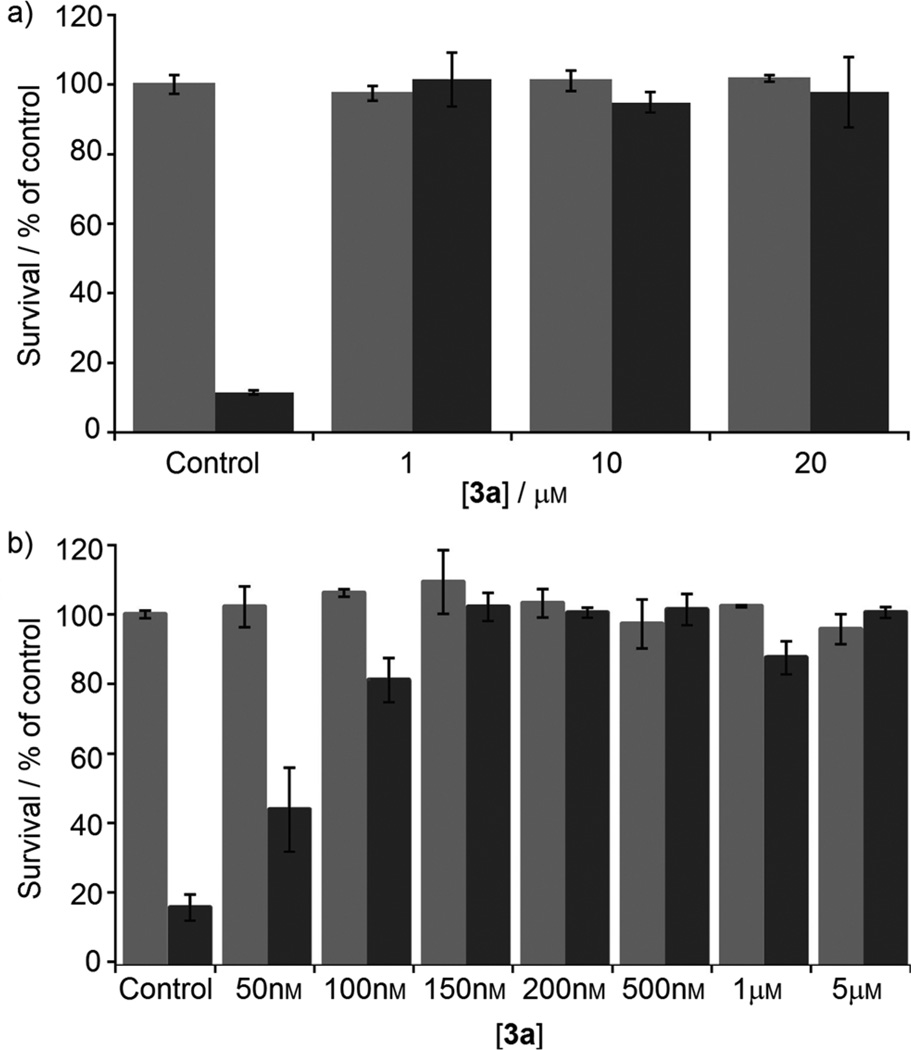
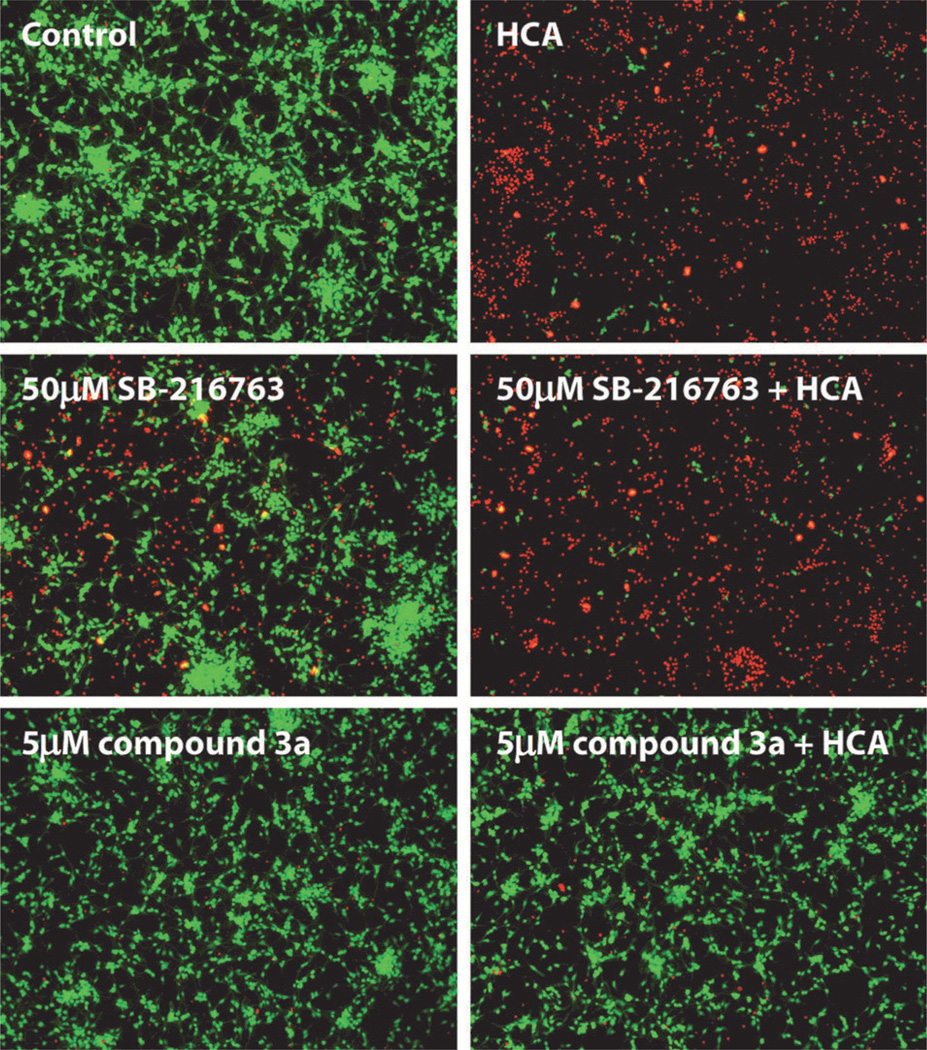
Next, lead pyrazolone 3a was examined in a model of oxidative stress induced by homocysteic acid (HCA), where neurons were treated with the test compound alone or in the presence of HCA. Cell viability was assessed using the MTT assay, and the results are shown in Figure 5. In the preliminary assays, pyrazolone 3a was tested at concentrations ranging from 1 µm to 20 µm. Full neuroprotective activity was seen at 1 µm and up to 20 µm without any observable toxicity at the higher doses. In a follow-up expanded dose-finding study starting at 50 nm, 3a was found to afford nearly complete protection at 150 nm. The cell micrographs provide a more exact depiction of the live versus dead cells (Figure 6). In comparison to the known GSK-3 inhibitor SB-216763, pyrazolone 3a showed superior neuroprotective activity.
Figure 5.
HCA oxidative stress assay: neurons were treated with pyrazolone 3a, with ( ) or without (
) or without ( ) addition of HCA. Viability was assessed after 48 h by an MTT assay.
) addition of HCA. Viability was assessed after 48 h by an MTT assay.
Figure 6.
Oxidative stress model: neuronal cell micrographs of live ( ) and dead (
) and dead ( ) cells in the presence of the GSK-3 inhibitors alone or in combination with HCA.
) cells in the presence of the GSK-3 inhibitors alone or in combination with HCA.
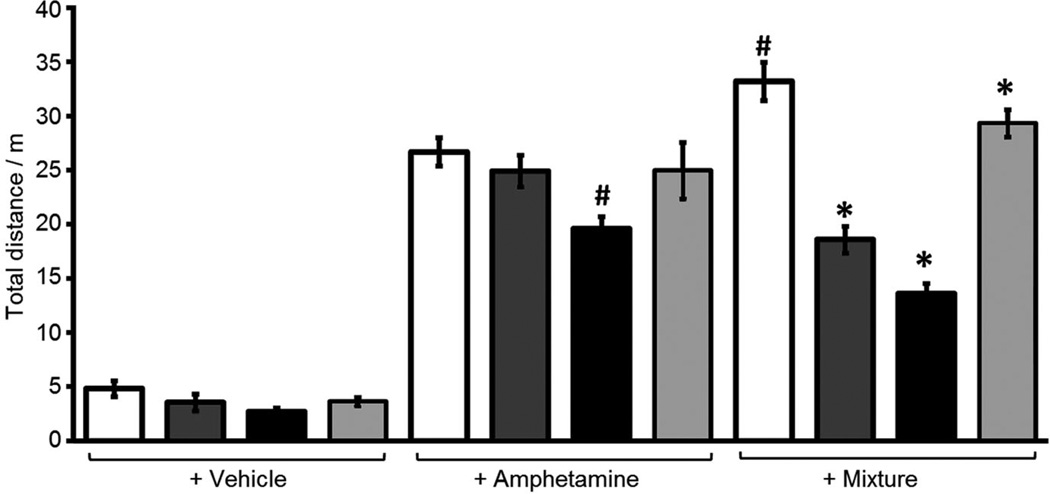
As our interest in developing GSK-3 inhibitors relates largely to their possible use in treating bipolar disorders, we next chose to examine 3a in an animal model of mania/hyperactivity induced by the combination of the psychostimulant amphetamine (Amph) and the anxiolytic agent chlordiazepoxide (CDP). In this model, the combination produces an increase in locomotor activity, which is blocked by known mood stabilizers, such as lithium and valproate.[16, 19] Following pretreatment with vehicle, valproate or compound 3a (150 mg kg−1), eight-week old C57BL/6J male mice were injected with water, Amph (4 mg kg−1) or a mixture of Amph (4 mg kg−1) and CDP (2.5 mg kg−1) and placed in open field chambers where locomotor activity was monitored for 60 min. Data were analyzed with analysis of variance followed by Fisher’s PLSD post-hoc tests. Pretreatment with compound 3a (5 min) or valproate (30 min) attenuated the locomotor activity induced by the mixture of Amph + CDP, without affecting the activity induced by Amph alone. Decreasing the pretreatment time of valproate (5 min) to match that of compound 3a, resulted in a decrease in both Amph and mixture-induced activity (Figure 7). The results demonstrate that inhibitor 3a selectively reduces locomotor activity induced by a combination of amphetamine and CDP, similar to known mood stabilizers such as valproate.
Figure 7.
Compound 3a or valproate reduced the locomotor hyperactivity induced by chlordiazepoxide (CDP) + amphetamine (Amph) mixture in C57BL/6J mice. Locomotor activity was quantified for a period of 1 h. Each bar represents the mean (±SEM) of the total distance traveled (m) for at least eleven mice. The mixture of Amph + CDP produced greater locomotor activity than Amph alone (#, p <0.05 Veh–Mix vs Veh–Amph; Fisher’s PLSD). Pretreatment with 3a or valproate reduced the locomotor activity produced by the Amph + CDP mixture (*, p <0.05 3a–Mix or Val–Mix vs Veh–Mix; Fisher’s PLSD). Vehicle control:  ; valproate (400 mg kg−1>; 30 min):
; valproate (400 mg kg−1>; 30 min):  ; valproate (400 mg kg−1>; 5 min):
; valproate (400 mg kg−1>; 5 min):  ; 3a (150 mg kg−1>; 5 min):
; 3a (150 mg kg−1>; 5 min):  .
.
In summary, with the aid of molecular modeling and X-ray studies, we have examined a new chemical scaffold to replace the heterocyclic core of the nonselective kinase inhibitor staurosporine. We designed, synthesized, and tested a small series of pyrazolones specifically for their ability to function as GSK-3β inhibitors. The best of these analogues was found to be selective for GSK-3 over 40 other homologous kinases. In particular, compound 3a gave an impressive dose–response curve for neuroprotection in the homocysteic acid model of oxidative stress. Additionally, compound 3a was able to attenuate locomotor activity in the chlordiazepoxide/amphetamine-induced hyperactivity model in vivo. This new lead compound represents one of the best all-around GSK-3 inhibitors we have identified to date.
Experimental Section
Full experimental protocols can be found in the Supporting Information. All experiments were conducted in accordance with the US National Institutes of Health guidelines and using an approved protocol from the Institutional Animal Care and Use Committee of PsychoGenics, Inc. (Tarrytown, USA).
Supplementary Material
Acknowledgements
This work was supported by the US National Institutes of Health (grant: 1R01 MH072940–01 to A.P.K.). The authors thank Prof. Brett Langley (Burke–Cornell Medical Research Institute, White Plains, USA) for performing the neuroprotection tests, Prof. Bryan Roth (University of North Carolina at Chapel Hill, USA) for PDSP screening, and Dr. Daniela Brunner (PsychoGenics Inc., Tarrytown, USA) for the mania model test and critical reading of the manuscript. The authors also thank Dr. Po-Chih Chen and Mr. Jay Hans Kalin (University of Illinois at Chicago, USA) for their help with the modeling studies.
References
- 1.Embi N, Rylatt DB, Cohen P. Eur. J. Biochem. 1980;107:519–527. [PubMed] [Google Scholar]
- 2.Welsh GI, Proud CG. Biochem. J. 1993;294(Pt. 3):625–629. doi: 10.1042/bj2940625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen P, Frame S. Nat. Rev. Mol. Cell Biol. 2001;2:769–776. doi: 10.1038/35096075. [DOI] [PubMed] [Google Scholar]
- 4.Woodgett JR. EMBO. J. 1990;9:2431–2438. doi: 10.1002/j.1460-2075.1990.tb07419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Persson G. Acta Psychiatr. Scand. 1977;55:208–213. doi: 10.1111/j.1600-0447.1977.tb00163.x. [DOI] [PubMed] [Google Scholar]
- 6.Phiel CJ, Wilson CA, Lee VM, Klein PS. Nature. 2003;423:435–439. doi: 10.1038/nature01640. [DOI] [PubMed] [Google Scholar]
- 7.Hall AC, Brennan A, Goold RG, Cleverley K, Lucas FR, Gordon-Weeks PR, Salinas PC. Mol. Cell. Neurosci. 2002;20:257–270. doi: 10.1006/mcne.2002.1117. [DOI] [PubMed] [Google Scholar]
- 8.Klein PS, Melton DA. Proc. Natl. Acad. Sci. USA. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leclerc S, Garnier M, Hoessel R, Marko D, Bibb JA, Snyder GL, Greengard P, Biernat J, Wu YZ, Mandelkow EM, Eisenbrand G, Meijer L. J. Biol. Chem. 2001;276:251–260. doi: 10.1074/jbc.M002466200. [DOI] [PubMed] [Google Scholar]
- 10.Meijer L, Skaltsounis AL, Magiatis P, Polychronopoulos P, Knockaert M, Leost M, Ryan XP, Vonica CA, Brivanlou A, Dajani R, Crovace C, Tarricone C, Musacchio A, Roe SM, Pearl L, Greengard P. Chem. Biol. 2003;10:1255–1266. doi: 10.1016/j.chembiol.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Meijer L, Thunnissen AM, White AW, Garnier M, Nikolic M, Tsai LH, Walter J, Cleverley KE, Salinas PC, Wu YZ, Biernat J, Mandelkow EM, Kim SH, Pettit GR. Chem. Biol. 2000;7:51–63. doi: 10.1016/s1074-5521(00)00063-6. [DOI] [PubMed] [Google Scholar]
- 12.Leost M, Schultz C, Link A, Wu YZ, Biernat J, Mandelkow EM, Bibb JA, Snyder GL, Greengard P, Zaharevitz DW, Gussio R, Senderowicz AM, Sausville EA, Kunick C, Meijer L. Eur. J. Biochem. 2000;267:5983–5994. doi: 10.1046/j.1432-1327.2000.01673.x. [DOI] [PubMed] [Google Scholar]
- 13.a) Phukan S, Babu VS, Kannoji A, Hariharan R, Balaji VN. Br. J. Pharmacol. 2010;160:1–19. doi: 10.1111/j.1476-5381.2010.00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Martinez A, Perez DI. J. Alzheimer’s Dis. 2008;15:181–191. doi: 10.3233/jad-2008-15204. and references therein. [DOI] [PubMed] [Google Scholar]
- 14.a) Martinez A, Alonso M, Castro A, Dorronsoro I, Gelpi JL, Luque FJ, Perez C, Moreno FJ. J. Med. Chem. 2005;48:7103–7112. doi: 10.1021/jm040895g. [DOI] [PubMed] [Google Scholar]; b) Castro A, Alonso M, Martinez A. Glycogen Synthase Kinase-3 (GSK-3) and Its Inhibitors: Drug Discovery and Development. New York: Jonh Wiley& Sons; 2006. pp. 257–280. [Google Scholar]
- 15.Kozikowski AP, Gaisina IN, Petukhov PA, Sridhar J, King LT, Blond SY, Duka T, Rusnak M, Sidhu A. ChemMedChem. 2006;1:256–266. doi: 10.1002/cmdc.200500039. [DOI] [PubMed] [Google Scholar]
- 16.a) Kozikowski AP, Gaisina IN, Yuan H, Petukhov PA, Blond SY, Fedolak A, Caldarone B, McGonigle P. J. Am. Chem. Soc. 2007;129:8328–8332. doi: 10.1021/ja068969w. [DOI] [PubMed] [Google Scholar]; b) Kim KH, Gaisina I, Gallier F, Holzle D, Blond SY, Mesecar A, Kozikowski AP. J. Mol. Model. 2009;15:1463–1479. doi: 10.1007/s00894-009-0498-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kozikowski AP, Gunosewoyo H, Guo S, Gaisina IN, Walter RL, Ketcherside KA, McClung CA, Mesecar AD, Caldarone B. ChemMed-Chem. 6 doi: 10.1002/cmdc.201100188. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brana MF, Gradillas A, Ovalles AG, Lopez B, Acero N, Llinares F, Munoz Mingarro D. Bioorg. Med. Chem. 2006;14:9–16. doi: 10.1016/j.bmc.2005.09.059. [DOI] [PubMed] [Google Scholar]
- 19.Vale AL, Ratcliffe F. Psychopharmacology. 1987;91:352–355. doi: 10.1007/BF00518190. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.