Abstract
Sarcomas of soft tissue and bone are a rare group of cancers hallmarked by relative insensitivity to cytotoxic chemotherapy. The development of targeted therapies in the treatment of sarcoma has been difficult due to the significant heterogeneity and rarity of these diseases. Inhibition of the mammalian target of rapamycin (mTOR) has emerged as an exciting treatment approach and is being studied extensively in sarcoma patients. Ridaforolimus is a second generation mTOR inhibitor that has shown potential benefit in the treatment of sarcoma. Recently a Phase III study demonstrated an improvement in progression-free survival when patients with at least stable disease after treatment with standard chemotherapy received maintenance ridaforolimus compared to placebo. The results of this study show that mTOR is an important pathway in soft tissue and bone sarcomas and represents an exciting opportunity for the improvement in the treatment of our patients.
Keywords: sarcoma, mTOR, ridaforolimus
Introduction
Sarcomas are a heterogeneous group of tumors that arise from mesenchymal cells and represent approximately 1% of all adult cancers and 15% of cancers in children and adolescents.1,2 For those patients with metastatic disease, median survival remains around 1 year. Those sarcomas primarily thought of as pediatric sarcomas, namely rhabdomyosarcoma, Ewing sarcoma, and osteosarcoma, are relatively chemotherapy-sensitive; however, survival after the development of recurrent disease remains poor. Unfortunately, for most adult soft tissue sarcomas response rates to chemotherapy are only 13%–34%, and this has not led to a significant improvement in overall survival.3
Doxorubicin has been used in soft tissue sarcomas for over 30 years, and response rates with single agent doxorubicin range from 16%–27%.4,5 Attempts to improve overall survival using combinations and/or higher doses of chemotherapy have not been successful.6–9 Other agents found to have activity when used alone include decarbonize and ifosfamide, although response rates remain low at 18%–36%.10 Trabectedin is approved by the European Medicines Agency, and is currently being evaluated further in ongoing Phase III trials. More recently, the combination of gemcitabine and docetaxel showed promising progression-free survival (PFS) rates in the second line setting; however, response rates and overall survival (OS) remained disappointing, 16% and 6.2 months (95% CI: 3.6–8.8), respectively.11
Bone sarcomas include Ewing sarcoma and osteosarcoma. Ewing sarcoma and osteosarcoma are typically treated with multiagent chemotherapy with relatively high response rates to primary therapy of approximately 70% and 50%, respectively.12–15 Unfortunately, most patients who go on to develop recurrences often have chemotherapy refractory disease and outcomes are poor.16,17 Ewing sarcoma is characterized by translocations involving the EWS gene; however, the search for targeted therapies directed to these driver mutations has been disappointing.
Few advancements have been made in this disease with chemotherapy, thus there is an urgent need for the evaluation of alternative treatment strategies. Because of the heterogeneity and complexity of these tumors, with a few exceptions, effective targeted therapies have remained elusive. The mammalian target of rapamycin (mTOR) represents a point of convergence of many cellular signaling pathways, and the rationale for its inhibition will be described.
Mammalian target of rapamycin
The macrolide antifungal rapamycin, produced by Streptomyces hygroscopicus, and its immunosuppressive effects were first identified in the 1970s, though its benefit as an antirejection immunosuppressant was not fully appreciated until the 1990s.18–20 The target of rapamycin (TOR) was identified in yeast possessing mutations that rendered them resistant to rapamycin, and Sabers et al first identified the mammalian homolog (mTOR) in 1995.21–24 Rapamycin inhibits T-cell proliferation by preventing cell cycle progression from G1 to S phase though its interaction with mTOR.25,26
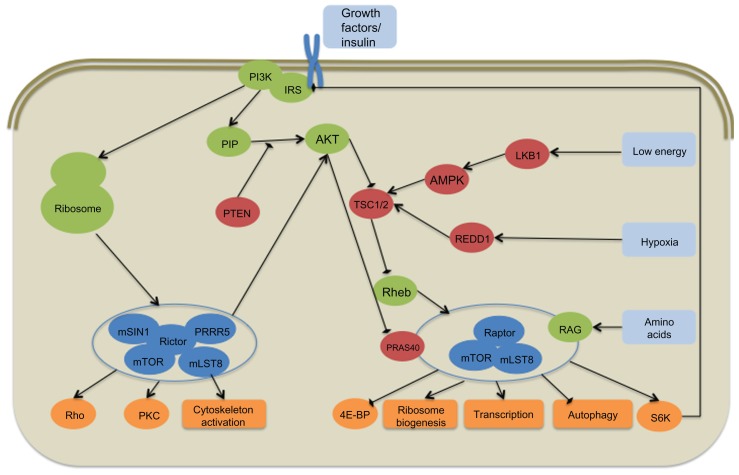
mTOR is a member of the serine-threonine protein PI3K-related kinases and is part of two multiprotein complexes, mTORC1 and mTORC2 (Figure 1).27 mTORC1 consists of several proteins including regulatory-associated protein of mTOR, mLST8, and proline-rich AKT substrate (PRAS40). Upstream regulators of mTORC1 include growth factors through their receptors via the PI3K/AKT pathway, amino acids through the RAG guanosine triphosphate (GTP)-ase pathway, cellular energy through LKB1 and AMP-activated protein kinase, and cellular stress including hypoxia through REDD1.28,29 mTOR is negatively regulated by PRAS40 as well as the tuberous sclerosis complex proteins TSC1 and TSC2, which inhibit the small GTP-binding protein Rheb from activating the complex.30,31–34 mTORC2 includes the scaffolding protein rapamycin-insensitive companion of mTOR, mSIN-1, proline-rich protein 5, and mLST-8. mTORC2 is primarily resistant to rapamycin, although chronic exposure does lead to mTORC2 disruption in some cell lines.35,36 Upstream regulators of mTORC2 are less well defined, but it appears to be activated by growth factors and amino acids, including insulin, through PI3K.37,38 PI3K signaling promotes mTORC2 binding to ribosomes leading to its activation.39
Figure 1.
Simplified schematic representation of the mammalian target of rapamycin (mTOR) signaling pathway.
Notes: Red, pathway inhibitor; Green, pathway activator; Blue, mTOR complex protein.
Abbreviation: NOS, not otherwise specified.
mTOR acts as a central mediator of the cell’s translational control in response to nutritional, growth factor, and stress-induced signals. Downstream targets of mTORC1 include 4E-binding protein (4EBP) and S6K, which are integral components of translational initiation. When activated, mTORC1 hyperphosphorylates the 4E-BP1 and leads to its dissociation from the initiation factor complex 4e (eIF4E). This allows recruitment of eIF4G and its binding to the 5′ cap.40 mTORC1 also binds and phosphorylates S6K1, which is involved in several areas of translational control.40,41 Downstream effects of mTORC1 activation include protein, ribosome, and lipid synthesis, and nutrient transport, leading to increased cell mass and autophagy.34,42 Similar to the upstream regulation of mTORC2, less is known about its downstream effects. It is accepted that mTORC2 is important in the activation of AKT and members of protein kinase C (PKC) family.36,43 Additionally, it appears to be involved in regulation of cytoskeleton organization through its interaction with Rho GTP-ases.44,45 mTORC2 is upstream to mTORC1 as it phosphorylates AKT and is required for its activation, and thus regulates mTORC1.36 Alternatively, mTORC1 appears to inhibit mTORC2 through its interaction with insulin receptor substrate 1 (IRS1).46
mTOR in neoplasia
As mTOR appears to be a major regulator of translational control in response to environmental signals, it is not difficult to see how its dysregulation could lead to the development of several disease processes. In vitro and in vivo models have shown that manipulation of the mTORC complexes can lead to impaired development and alterations in cellular function.47 Mutations in tsc1 and tsc2 lead to the tuberous sclerosis complex, which is characterized by the formation of benign tumors suggesting a link between the mTOR pathway and neoplasia. The discovery of the strong interaction between mTOR and AKT also suggests its relevance to neoplasia. The AKT pathway and AKT itself have been shown to be frequently upregulated in most cancers, with both amplification and activation of AKT being described.48
Both germline and spontaneous mutations in other components of the mTOR pathway also point to its strong connection with neoplasia. Patients with Cowden’s disease, a syndrome characterized by benign hamartomas, have loss of the tumor-suppressor phosphatase related to tensin (PTEN) gene which leads to increased PI3K/AKT/mTOR signaling by loss of inhibition of PI3K.49,50 Other mTOR-related neoplastic syndromes include, Peutz–Jehgers syndrome, lymphangioleiomyomatosis and other PTEN-related harmatomatous tumor syndromes.49,51 LKB1 germline mutations lead to Peutz–Jehgers syndrome characterized by mucocutaneous pigmentation and hamartomas of the gastrointestinal tract.29 Subsequently, mutations of the mTOR pathway and altered regulation have been found in many cancers.52 LKB1 is mutated in non-small cell lung cancer (NSCLC).53 Somatic PTEN mutations and inactivation have been identified in several cancers including hematologic malignancies, melanoma, glioblastoma, prostate, endometrial, breast, lung, pancreas, liver, and adrenal gland cancers.54 In addition, somatic mutations or abnormal activation of several of the components of the PI3K-AKT-mTOR signaling pathway are frequently found in nearly all malignancies, underscoring the importance of this pathway in neoplasia.
Rapamycin was first recognized as an antifungal but was primarily marketed and first approved as an immunosuppressant agent. Its antineoplastic effects were first recognized well before its target was identified in the 1980s by Eng et al, who acknowledged its antiproliferative properties and studied it in hematologic and solid tumor xenograft models.55 Subsequent preclinical studies confirmed the antineoplastic properties in lymphoma and thymoma models.56 Some small cell lung cancer cell lines possess constitutively phosphorylated S6K which are effectively suppressed by rapamycin.57
Because the differential activities of mTORC1 and mTORC2 are complex and mTOR is vital to so many cellular functions, determining the overall effect of inhibiting these pathways has been difficult. Similarly, as our understanding of the mTORC2 complex is less well defined, we are only now recognizing how inhibiting either complex or both will impact tumor growth.
mTOR in sarcoma
The mTOR pathway was first implicated in the development of mesenchymal cells by the observation that protein kinase C-a (PKC-a) and p-38 mitogen-activated protein kinase (MAPK) inhibition by rapamycin inhibits chondrogenesis.58 Both PKC-a and MAPK are activated in malignant mesenchymal chondroblasts. The mTOR pathway is also involved in adipocyte differentiation, possibly through its interaction with the peroxisome proliferator-activated receptor-γ.59
Several studies have demonstrated that inhibition of mTOR by rapamycin induces apoptosis or delays growth in several sarcoma models including rhabdomyosarcoma, Ewing sarcoma, and Kaposi sarcoma.60–63 Hu et al discovered loss of PTEN through 10q deletions in over half of human leiomyosarcomas, and it was subsequently discovered that PTEN deletion leads to smooth muscle cell hyperplasia and initiation of leiomyosarcoma.64,65 The ewing family of tumors (EFT) includes Ewing sarcoma and primitive neuroectodermal tumors, and is characterized by fusion translocations involving the EWS gene and several genes encoding transcription factors. Inhibition of mTOR by rapamycin in EFT cell lines leads to G1 cell cycle arrest and decreased levels of EWS translocated proteins.62
Alterations of the growth factor receptors and their signaling through PI3K are frequently found in many sarcoma subtypes. The insulin-like growth factor receptor (IGFR) family is frequently studied in sarcomas, particularly because of its importance in the development of normal bone in the postnatal period.66 IGFR1 transcription is directly inhibited by the tumor suppressor Wilms’ tumor protein 1 (WTI). In desmoplastic small cell tumors, the classic EWS-WT1 fusion on coprotein leads to IGFR1 over expression. In Ewing sarcoma, the EWS-FLI1 fusion protein both requires IGFR1 signaling for transformation and regulates IGFR1 signaling.67,68 There is a similar interaction between the IGFR pathway and the SYT-SSX fusion protein found in synovial cell sarcoma.69
Sarcoma clinical trials with mTOR inhibitors
Rapamycin acts at mTORC1 by binding to the intracellular FK506-binding protein, FKBP12, which then binds to mTORC1.21,70 Rapamycin has significant activity against several in vivo cancer models; however, its poor stability and solubility limits its use as an antineoplastic.62,71 With the more recent understanding of the involvement of the mTOR pathway in neoplasia, renewed interest in inhibiting it has led to the development of several rapamycin analogues, including temsirolimus (Torisel; Wyeth, Madison, NJ), everolimus (Afinitor; Novartis, Basel, Switzerland) and ridaforolimus (Merck/Ariad, Cambridge, MA). These rapalogues possess properties that improve aqueous solubility and allow for oral dosing. Currently, everolimus (renal cell and pancreatic neuroendocrine cancers) and temsirolimus (kidney cancer and mantle cell lymphoma) have approved indications in oncology.
Thus far, results of clinical trials using everolimus and temsirolimus in sarcoma have been disappointing. A small Phase II study of everolimus in patients with Kaposi’s sarcoma resulted in only one partial response, and raising human herpesvirus 8 (HHV8) viral loads highlighting the immunosuppressive effects of mTOR inhibitors.72 Another Phase II study in patients with soft tissue sarcomas and GIST demonstrated 16 week progression-free rates of only 13% and 27%, respectively.73
Several Phase II studies of everolimus and temsirolimus, both as monotherapy and in combination, are ongoing in various sarcoma populations and will further define the potential of these drugs in patients with sarcoma.
Ridaforolimus (AP23573, M-8669, formerly deforolimus) is a non prodrug rapalogue which may have improved in vivo stability and is available in both intravenous and oral formulations. 74 Early phase I studies suggested clinical benefit in patients with sarcoma.75,76 Subsequently, a large Phase II study of patients with advanced bone and soft tissue sarcomas using ridaforolimus 12.5 mg orally daily for five consecutive days every 2 weeks has been reported. Patients aged ≥15 years were included with no restrictions on the number of prior therapies. Four cohorts were evaluated: primary bone sarcomas, leiomyosarcomas, liposarcomas, and other soft tissue sarcomas. The primary end point was clinical benefit rate (CBR) defined as complete response (CR), partial response (PR), or stable disease (SD) of ≥16 weeks, using a Simon’s optimal two-stage design.77 A total of 216 patients were enrolled, and the overall CBR was 28.8% with a median PFS of 15.3 weeks (95% confidence interval [CI]: 14.29–16.29). Patients in the bone sarcoma, leiomyosarcoma, and liposarcoma cohorts had slightly higher clinical benefit rates than those patients in the ‘other’ cohort (31.5%, 33.3%, 29.5%, and 21.1%, respectively). This study included pretreatment biomarker analysis of possible predictors of response. No biomarker was predicted for efficacy. The most common adverse events were stomatitis, fatigue, hyperlipidemia, rash, nausea, anemia, and thrombocytopenia, and five patients developed pneumonitis.
Based on the prolonged PFS in patients with advanced sarcomas, it was hypothesized that ridaforolimus may be most beneficial as a maintenance regimen. A randomized, placebo-controlled Phase III study of ridaforolimus as maintenance therapy in 711 patients with advanced bone and soft tissue sarcomas who had at least stable disease following prior chemotherapy has been reported.77 Patients received 40 mg orally daily for 5 days a week. The primary endpoint of PFS was met, with a hazard ratio (HR) of 0.72 (P < 0.001). Median PFS and 6-month PFS rates were 17.7 weeks and 34% in the ridaforolimus group and 14.6 weeks and 23% in the placebo group. Although not the primary endpoint, and thus far only an early analysis has been reported, there was no statistical improvement in overall survival, HR of 0.88 (P = 0.23). The most common adverse events were stomatitis, anemia, hyperlipidemia, hyperglycemia, renal disorders, and pneumonitis. Six deaths due to pulmonary disorders were reported, including pneumonitis, pleural effusions, pulmonary embolism and respiratory distress, compared to none in the placebo arm. Ridaforolimus received fast track and orphan drug status from the US Food and Drug Administration (FDA) and orphan status from the European Medicines Agency. The FDA is currently reviewing its registration for an indication for maintenance therapy in patients with sarcoma based on the results of the Phase III study.
Future directions
Response to mTOR inhibitors as monotherapy has been disappointing, but not unexpected. The pathway is complex and there is still much to discover. Given its importance in maintenance of tumor populations in times of stress, it is not surprising that alternative pathways would be activated. Alternatively, activation of the pathway is involved in resistance to cytotoxic chemotherapies. Upregulation of PI3K and AKT is frequently seen in tumors both when treated with chemotherapy and when mTOR has been inhibited.78,79 Combination therapy will likely be needed to derive significant benefit from the mTOR inhibitors. Several Phase I and Phase II studies are ongoing to evaluate the mTOR inhibitors in combination with chemotherapy in other solid tumors, and the Phase I/II studies of temsirolimus and liposomal doxorubicin in patients with soft tissue and bone sarcomas is ongoing.
As described previously, the IGFR pathway appears to play a significant role in bone development. This has led to significant interest in the use of IGF1R inhibitors in sarcoma patients. IGF1R and members of its pathway are frequently dysregulated in several sarcoma subtypes.66 Because IGF1R is intimately involved with the mTOR pathway and likely involved in resistance to mTOR inhibitors, the combination of IGF1R and mTOR inhibition is actively being studied. A Phase I study of everolimus and CP-751871, a fully human IgG2 anti-IGF1R monoclonal antibody, in patients with sarcoma and other solid tumors demonstrated SD in most patients, with one patient with a solitary fibrous tumor having a PR.80 In a small trial of temsirolimus with cixutumumab, a fully humanized IgG1 monoclonal antibody directed at IGF1R, in heavily pretreated Ewing sarcoma patients, one patient had a prolonged CR and four of 27 patients had a PR.81
Because the kinase domains of mTOR and PI3K are very similar, several drugs designed as PI3K inhibitors also demonstrate both mTORC1 and mTORC2 inhibition. Given the significant interaction and regulation between the mTORC1 and mTORC2 complexes, and that mTORC2 is relatively insensitive to inhibition by the rapalogues, combined inhibition will likely provide better efficacy, and several Phase I studies of dual PI3K/mTOR inhibitors are ongoing.82 To improve mTOR inhibition, companies are now actively developing pan-mTOR inhibitors and we hope they will soon be evaluated in sarcoma patients.
Conclusion
Sarcomas are a diffuse group of diseases with remarkable diversity in their pathophysiology, making drug discovery difficult and improvements in the treatment of our patients disappointingly slow. It is likely that we have reached a therapeutic plateau with the use of cytotoxic chemotherapy, even in those patients with relatively chemotherapy-sensitive tumors. The mTOR pathway is frequently dysregulated and is implicated in the development of mesenchymal neoplasms, making it an interesting target.
Although results with monotherapy treatment with rapalogues have been modest, interesting improvements in PFS have been demonstrated with ridaforolimus. The results of the Phase III study of ridaforolimus as maintenance therapy in patients with advanced soft tissue and bone sarcomas who had at least SD after standard of care chemotherapy is one of the first Phase III studies in many years to demonstrate any improvement in this population. We await the FDA’s decision regarding approval for this indication; however, the benefit in PFS must be balanced with the added toxicity of taking a maintenance drug when no improvement in overall survival is demonstrated. Progression of sarcoma is frequently associated with significant symptoms and decline in quality of life; thus, such an approach may be indicated. Nevertheless, further investigation of mTOR inhibitors using alternative approaches is greatly needed to fully evaluate the impact of these drugs on the treatment of sarcoma patients.
Footnotes
Disclosure
The author declares no conflicts of interest in this work.
References
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61(4):212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Darling J. A different view of sarcoma statistics. The Electronic Sarcoma Update Newsletter. 2007. [Accessed January 2011]. Available from: http://sarcomahelp.org/sarcoma_statistics.html.
- 3.Van Glabbeke M, van Oosterom AT, Oosterhis JW, et al. Prognostic factors for the outcome of chemotherapy in advanced soft tissue sarcoma: An analysis of 2,185 patients treated with anthracycline-containing first-line regimens – A European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol. 1999;17(1):150–157. doi: 10.1200/JCO.1999.17.1.150. [DOI] [PubMed] [Google Scholar]
- 4.Benjamin RS, Wiernik PH, Bachur NR. Adriamycin: a new effective agent in the therapy of disseminated sarcomas. Med Pediatr Oncol. 1975;1(1):63–76. doi: 10.1002/mpo.2950010109. [DOI] [PubMed] [Google Scholar]
- 5.Schoenfeld DA, Rosenbaum C, Horton J, Wolter JM, Falkson G, DeConti RC. A comparison of adriamycin versus vincristine and adriamycin, and cyclophosphamide versus vincristine, actinomycin-D, and cyclophosphamide for advanced sarcoma. Cancer. 1982;50(12):2757–2762. doi: 10.1002/1097-0142(19821215)50:12<2757::aid-cncr2820501211>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 6.Santoro A, Tursz T, Mouridsen H, et al. Doxorubicin versus CYVADIC versus doxorubicin plus ifosfamide in first-line treatment of advanced soft tissue sarcomas: a randomized study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. J Clin Oncol. 1995;13(7):1537–1545. doi: 10.1200/JCO.1995.13.7.1537. [DOI] [PubMed] [Google Scholar]
- 7.Antman K, Crowley J, Balcerzak SP, et al. An intergroup phase III randomized study of doxorubicin and dacarbazine with or without ifosfamide and mesna in advanced soft tissue and bone sarcomas. J Clin Oncol. 1993;11(7):1276–1285. doi: 10.1200/JCO.1993.11.7.1276. [DOI] [PubMed] [Google Scholar]
- 8.Le Cesne A, Judson I, Crowther D, et al. Randomized Phase III study comparing conventional-dose doxorubicin plus ifosfamide versus high-dose doxorubicin plus ifosfamide plus recombinant human granulocyte-macrophage colony-stimulating factor in advanced soft tissue sarcomas: A trial of the European Organization for Research and Treatment of Cancer/Soft Tissue and Bone Sarcoma Group. J Clin Oncol. 2000;18(14):2676–2684. doi: 10.1200/JCO.2000.18.14.2676. [DOI] [PubMed] [Google Scholar]
- 9.Worden FP, Taylor JM, Biermann JS, et al. Randomized phase II evaluation of 6 mg/m2 of ifosfamide plus doxorubicin and granulocyte colony-stimulating factor (G-CSF) compared with 12 g/m2 of ifosfamide plus doxorubicin and G-CSF in the treatment of poor-prognosis soft tissue sarcoma. J Clin Oncol. 2005;23(1):105–112. doi: 10.1200/JCO.2005.05.108. [DOI] [PubMed] [Google Scholar]
- 10.Bramwell VH, Anderson D, Charette ML The Sarcoma Disease Site Group. Doxorubicin-based chemotherapy for the palliative treatment of adult patients with locally advanced or metastatic soft tissue sarcoma. Cochrane Database Syst Rev. 2003;(3):CD003293. doi: 10.1002/14651858.CD003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maki RG, Wathen JK, Patel SR, et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002. J Clin Oncol. 2007;25(19):2755–2763. doi: 10.1200/JCO.2006.10.4117. [DOI] [PubMed] [Google Scholar]
- 12.Grier HE, Krailo MD, Tarbell NJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing’s sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med. 2003;348(8):694–701. doi: 10.1056/NEJMoa020890. [DOI] [PubMed] [Google Scholar]
- 13.Antman K, Crowley J, Balcerzak SP, et al. A southwest oncology group and cancer and leukemia group B phase II study of doxorubicin, dacarbazine, ifosfamide, and mesna in Adults with advanced osteosarcoma, Ewing’s sarcoma, and rhabdomyosarcoma. Cancer. 1998;82(7):1288–1295. [PubMed] [Google Scholar]
- 14.Bramwell VH, Burgers MV, Souhami RL, et al. A randomized comparison of two short intensive chemotherapy regimens in children and young adults with osteosarcoma: results in patients with metastases: A Study of the European Osteosarcoma Intergroup. Sarcoma. 1997;1(3–4):155–160. doi: 10.1080/13577149778245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewis IJ, Nooij MA, Whelan J, et al. Improvement in histologic response but not survival in osteosarcoma patients treated with intensified chemotherapy: a randomized phase III trial of the European Osteosarcoma Intergroup. J Natl Cancer Inst. 2007;99(2):112–128. doi: 10.1093/jnci/djk015. [DOI] [PubMed] [Google Scholar]
- 16.Hunold A, Weddeling N, Paulussen M, Ranft A, Liebscher C, Jürgens H. Topotecan and cyclophosphamide in patients with refractory or relapsed Ewing tumors. Pediatr Blood Cancer. 2006;47(6):795–800. doi: 10.1002/pbc.20719. [DOI] [PubMed] [Google Scholar]
- 17.Kempf-Bielack B, Bielack SS, Jürgens H, et al. Osteosarcoma relapse after combined modality therapy: an analysis of unselected patients in the Cooperative Osteosarcoma Study Group (COSS) J Clin Oncol. 2005;23(3):559–568. doi: 10.1200/JCO.2005.04.063. [DOI] [PubMed] [Google Scholar]
- 18.Vézina C, Kudelski A, Sehgal SN. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J Antibiot (Tokyo) 1975;28(10):721–726. doi: 10.7164/antibiotics.28.721. [DOI] [PubMed] [Google Scholar]
- 19.Blazar BR, Taylor PA, Sehgal SN, Vallera DA. Rapamycin prolongs survival of murine recipients of fully allogeneic donor grafts when administered during the graft-versus-host disease process. Ann N Y Acad Sci. 1993;685:73–85. doi: 10.1111/j.1749-6632.1993.tb35854.x. [DOI] [PubMed] [Google Scholar]
- 20.Almond PS, Moss A, Nakhleh R, et al. Rapamycin in a porcine renal transplant model. Ann N Y Acad Sci. 1993;685:121–122. doi: 10.1111/j.1749-6632.1993.tb35858.x. [DOI] [PubMed] [Google Scholar]
- 21.Sabers CJ, Martin MM, Brunn GJ, et al. Isolation of a protein target of the FKBP12-rapamycin complex in mammalian cells. J Biol Chem. 1995;270(2):815–822. doi: 10.1074/jbc.270.2.815. [DOI] [PubMed] [Google Scholar]
- 22.Heitman J, Movva NR, Hall MN. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991;253(5022):905–909. doi: 10.1126/science.1715094. [DOI] [PubMed] [Google Scholar]
- 23.Zheng XF, Fiorentino D, Chen J, Crabtree GR, Schreiber SL. TOR kinase domains are required for two distinct functions, only one of which is inhibited by rapamycin. Cell. 1995;82(1):121–130. doi: 10.1016/0092-8674(95)90058-6. [DOI] [PubMed] [Google Scholar]
- 24.Kunz J, Henriquez R, Schneider U, Deuter-Reinhard M, Movva NR, Hall MN. Target of rapamycin in yeast, TOR2, is an essential phosphatidylinositol kinase homolog required for G1 progression. Cell. 1993;73(3):585–596. doi: 10.1016/0092-8674(93)90144-f. [DOI] [PubMed] [Google Scholar]
- 25.Dumont FJ, Staruch MJ, Koprak SL, Melino MR, Sigal NH. Distinct mechanisms of suppression of murine T cell activation by the related macrolides FK-506 and rapamycin. J Immunol. 1990;144(1):251–258. [PubMed] [Google Scholar]
- 26.Dumont FJ, Su Q. Mechanism of action of the immunosuppressant rapamycin. Life Sci. 1995;58(5):373–395. doi: 10.1016/0024-3205(95)02233-3. [DOI] [PubMed] [Google Scholar]
- 27.Hara K, Maruki Y, Long X, et al. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002;110(2):177–189. doi: 10.1016/s0092-8674(02)00833-4. [DOI] [PubMed] [Google Scholar]
- 28.Brugarolas J, Lei K, Hurley RL, et al. Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 2004;18(23):2893–2904. doi: 10.1101/gad.1256804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaw RJ, Bardeesy N, Manning BD, et al. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell. 2004;6(1):91–99. doi: 10.1016/j.ccr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 30.Vander Haar E, Lee SI, Bandhakavi S, Griffin TJ, Kim DH. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol. 2007;9(3):316–323. doi: 10.1038/ncb1547. [DOI] [PubMed] [Google Scholar]
- 31.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4(9):648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 32.Manning BD, Cantley LC. United at last: the tuberous sclerosis complex gene products connect the phosphoinositide 3-kinase/Akt pathway to mammalian target of rapamycin (mTOR) signalling. Biochem Soc Trans. 2003;31(Pt 3):573–578. doi: 10.1042/bst0310573. [DOI] [PubMed] [Google Scholar]
- 33.Li Y, Corradetti MN, Inoki K, Guan KL. TSC2: filling the GAP in the mTOR signaling pathway. Trends Biochem Sci. 2004;29(1):32–38. doi: 10.1016/j.tibs.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 34.Fingar DC, Blenis J. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene. 2004;23(18):3151–3171. doi: 10.1038/sj.onc.1207542. [DOI] [PubMed] [Google Scholar]
- 35.Loewith R, Jacinto E, Wullschleger S, et al. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell. 2002;10(3):457–468. doi: 10.1016/s1097-2765(02)00636-6. [DOI] [PubMed] [Google Scholar]
- 36.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307(5712):1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 37.Huang J, Dibble CC, Matsuzaki M, Manning BD. The TSC1-TSC2 complex is required for proper activation of mTOR complex 2. Mol Cell Biol. 2008;28(12):4104–4115. doi: 10.1128/MCB.00289-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tato I, Bartrons R, Ventura F, Rosa JL. Amino acids activate mammalian target of rapamycin complex 2 (mTORC2) via PI3K/Akt signaling. J Biol Chem. 2011;286(8):6128–6142. doi: 10.1074/jbc.M110.166991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zinzalla V, Stracka D, Oppliger W, Hall MN. Activation of mTORC2 by association with the ribosome. Cell. 2011;144(5):757–768. doi: 10.1016/j.cell.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 40.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10(5):307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- 41.Sabine VS, Sims AH, Macaskill EJ, et al. Gene expression profiling of response to mTOR inhibitor everolimus in pre-operatively treated post-menopausal women with oestrogen receptor-positive breast cancer. Breast Cancer Res Treat. 2010;122(2):419–428. doi: 10.1007/s10549-010-0928-6. [DOI] [PubMed] [Google Scholar]
- 42.Neufeld TP. TOR-dependent control of autophagy: biting the hand that feeds. Curr Opin Cell Biol. 2010;22(2):157–168. doi: 10.1016/j.ceb.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ikenoue T, Inoki K, Yang Q, Zhou X, Guan KL. Essential function of TORC2 in PKC and Akt turn motif phosphorylation, maturation and signalling. EMBO J. 2008;27(14):1919–1931. doi: 10.1038/emboj.2008.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jacinto E, Facchinetti V, Liu D, et al. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127(1):125–137. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 45.Sarbassov DD, Ali SM, Kim DH, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14(14):1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 46.Um SH, Frigerio F, Watanabe M, et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431(7005):200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- 47.Russell RC, Fang C, Guan KL. An emerging role for TOR signaling in mammalian tissue and stem cell physiology. Development. 2011;138(16):3343–3356. doi: 10.1242/dev.058230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bellacosa A, Kumar CC, Di Cristofano A, Testa JR. Activation of AKT kinases in cancer: implications for therapeutic targeting. Adv Cancer Res. 2005;94:29–86. doi: 10.1016/S0065-230X(05)94002-5. [DOI] [PubMed] [Google Scholar]
- 49.Inoki K, Corradetti MN, Guan KL. Dysregulation of the TSC-mTOR pathway in human disease. Nat Genet. 2005;37(1):19–24. doi: 10.1038/ng1494. [DOI] [PubMed] [Google Scholar]
- 50.Podsypanina K, Ellenson LH, Nemes A, et al. Mutation of Pten/ Mmac1 in mice causes neoplasia in multiple organ systems. Proc Natl Acad Sci U S A. 1999;96(4):1563–1568. doi: 10.1073/pnas.96.4.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krymskaya VP, Shipley JM. Lymphangioleiomyomatosis: a complex tale of serum response factor-mediated tissue inhibitor of metalloproteinase- 3 regulation. Am J Respir Cell Mol Biol. 2003;28(5):546–550. doi: 10.1165/rcmb.F267. [DOI] [PubMed] [Google Scholar]
- 52.Li J, Yen C, Liaw D, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275(5308):1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 53.Makowski L, Hayes DN. Role of LKB1 in lung cancer development. Br J Cancer. 2008;99(5):683–688. doi: 10.1038/sj.bjc.6604515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hollander MC, Blumenthal GM, Dennis PA. PTEN loss in the continuum of common cancers, rare syndromes and mouse models. Nat Rev Cancer. 2011;11(4):289–301. doi: 10.1038/nrc3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eng CP, Sehgal SN, Vézina C. Activity of rapamycin (AY-22,989) against transplanted tumors. J Antibiot (Tokyo) 1984;37(10):1231–1237. doi: 10.7164/antibiotics.37.1231. [DOI] [PubMed] [Google Scholar]
- 56.Muthukkumar S, Ramesh TM, Bondada S. Rapamycin, a potent immunosuppressive drug, causes programmed cell death in B lymphoma cells. Transplantation. 1995;60(3):264–270. doi: 10.1097/00007890-199508000-00010. [DOI] [PubMed] [Google Scholar]
- 57.Withers DJ, Seufferlein T, Mann D, Garcia B, Jones N, Rozengurt E. Rapamycin dissociates p70(S6K) activation from DNA synthesis stimulated by bombesin and insulin in Swiss 3T3 cells. J Biol Chem. 1997;272(4):2509–2514. doi: 10.1074/jbc.272.4.2509. [DOI] [PubMed] [Google Scholar]
- 58.Oh CD, Kim SJ, Ju JW, et al. Immunosuppressant rapamycin inhibits protein kinase C alpha and p38 mitogen-activated protein kinase leading to the inhibition of chondrogenesis. Eur J Pharmacol. 2001;427(3):175–185. doi: 10.1016/s0014-2999(01)01241-9. [DOI] [PubMed] [Google Scholar]
- 59.Kim JE, Chen J. Regulation of peroxisome proliferator-activated receptor-gamma activity by mammalian target of rapamycin and amino acids in adipogenesis. Diabetes. 2004;53(11):2748–2756. doi: 10.2337/diabetes.53.11.2748. [DOI] [PubMed] [Google Scholar]
- 60.Brown RE, Boyle JL. Mesenchymal chondrosarcoma: molecular characterization by a proteomic approach, with morphogenic and therapeutic implications. Ann Clin Lab Sci. 2003;33(2):131–141. [PubMed] [Google Scholar]
- 61.Hosoi H, Dilling MB, Shikata T, et al. Rapamycin causes poorly reversible inhibition of mTOR and induces p53-independent apoptosis in human rhabdomyosarcoma cells. Cancer Res. 1999;59(4):886–894. [PubMed] [Google Scholar]
- 62.Mateo-Lozano S, Tirado OM, Notario V. Rapamycin induces the fusion-type independent downregulation of the EWS//FLI-1 proteins and inhibits Ewing’s sarcoma cell proliferation. Oncogene. 2003;22(58):9282–9287. doi: 10.1038/sj.onc.1207081. [DOI] [PubMed] [Google Scholar]
- 63.Lipskar AM, Glick RD, Huang J, et al. Cyclooxygenase 2 mediates the antiangiogenic effect of rapamycin in Ewing sarcoma. J Pediatr Surg. 2009;44(6):1139–1146. doi: 10.1016/j.jpedsurg.2009.02.037. discussion 1146–1147. [DOI] [PubMed] [Google Scholar]
- 64.Hu J, Rao UN, Jasani S, Khanna V, Yaw K, Surti U. Loss of DNA copy number of 10q is associated with aggressive behavior of leiomyosarcomas: a comparative genomic hybridization study. Cancer Genet Cytogenet. 2005;161(1):20–27. doi: 10.1016/j.cancergencyto.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 65.Hernando E, Charytonowicz E, Dudas ME, et al. The AKT-mTOR pathway plays a critical role in the development of leiomyosarcomas. Nat Med. 2007;13(6):748–753. doi: 10.1038/nm1560. [DOI] [PubMed] [Google Scholar]
- 66.Scotlandi K, Picci P. Targeting insulin-like growth factor 1 receptor in sarcomas. Curr Opin Oncol. 2008;20(4):419–427. doi: 10.1097/CCO.0b013e328302edab. [DOI] [PubMed] [Google Scholar]
- 67.Toretsky JA, Kalebic T, Blakesley V, LeRoith D, Helman LJ. The insulin-like growth factor-I receptor is required for EWS/FLI-1 transformation of fibroblasts. J Biol Chem. 1997;272(49):30822–30827. doi: 10.1074/jbc.272.49.30822. [DOI] [PubMed] [Google Scholar]
- 68.Riggi N, Cironi L, Provero P, et al. Development of Ewing’s sarcoma from primary bone marrow-derived mesenchymal progenitor cells. Cancer Res. 2005;65(24):11459–11468. doi: 10.1158/0008-5472.CAN-05-1696. [DOI] [PubMed] [Google Scholar]
- 69.Sun Y, Gao D, Liu Y, Huang J, Lessnick S, Tanaka S. IGF2 is critical for tumorigenesis by synovial sarcoma oncoprotein SYT-SSX1. Oncogene. 2006;25(7):1042–1052. doi: 10.1038/sj.onc.1209143. [DOI] [PubMed] [Google Scholar]
- 70.Chen J, Zheng XF, Brown EJ, Schreiber SL. Identification of an 11-kDa FKBP12-rapamycin-binding domain within the 289-kDa FKBP12- rapamycin-associated protein and characterization of a critical serine residue. Proc Nat Acad Sci U S A. 1995;92(11):4947–4951. doi: 10.1073/pnas.92.11.4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Podsypanina K, Lee RT, Politis C, et al. An inhibitor of mTOR reduces neoplasia and normalizes p70/S6 kinase activity in Pten+/− mice. Proc Natl Acad Sci U S A. 2001;98(18):10320–10325. doi: 10.1073/pnas.171060098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Porcher R, Kerob D, Dupin N, et al. Multicentric phase II clinical trial evaluating the role of everolimus (RAD001) in endemic or classic Kaposi’s sarcoma (C06-46) J Clin Oncol. 2011;29:1067. [Google Scholar]
- 73.Richter S, Pink D, Hohenberger P, et al. Multicenter, triple-arm, single-stage, phase II trial to determine the efficacy and safety of everolimus (RAD001) in patients with refractory bone or soft tissue sarcomas including GIST. J Clin Oncol. 2010;28(15s):10038. [Google Scholar]
- 74.Ridaforolimus. Drugs R D. 2010;10(3):165–178. doi: 10.2165/11586010-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hartford CM, Desai AA, Janisch L, et al. A phase I trial to determine the safety, tolerability, and maximum tolerated dose of deforolimus in patients with advanced malignancies. Clin Cancer Res. 2009;15(4):1428–1434. doi: 10.1158/1078-0432.CCR-08-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mita MM, Mita AC, Chu QS, et al. Phase I trial of the novel mammalian target of rapamycin inhibitor deforolimus (AP23573; MK-8669) administered intravenously daily for 5 days every 2 weeks to patients with advanced malignancies. J Clin Oncol. 2008;26(3):361–367. doi: 10.1200/JCO.2007.12.0345. [DOI] [PubMed] [Google Scholar]
- 77.Chawla SP, Staddon AP, Baker LH, et al. Phase II study of the mammalian target of rapamycin inhibitor ridaforolimus in patients with advanced bone and soft tissue sarcomas. J Clin Oncol. 2012;30(1):78–84. doi: 10.1200/JCO.2011.35.6329. [DOI] [PubMed] [Google Scholar]
- 78.Liu LZ, Zhou XD, Qian G, Shi X, Fang J, Jiang BH. AKT1 amplification regulates cisplatin resistance in human lung cancer cells through the mammalian target of rapamycin/p70S6K1 pathway. Cancer Res. 2007;67(13):6325–6332. doi: 10.1158/0008-5472.CAN-06-4261. [DOI] [PubMed] [Google Scholar]
- 79.Wendel HG, Lowe SW. Reversing drug resistance in vivo. Cell Cycle. 2004;3(7):847–849. [PubMed] [Google Scholar]
- 80.Quek R, Wang Q, Morgan JA, et al. Combination mTOR and IGF-1R inhibition: Phase I trial of everolimus and figitumumab in patients with advanced sarcomas and other solid tumors. Clin Cancer Res. 2011;17(4):871–879. doi: 10.1158/1078-0432.CCR-10-2621. [DOI] [PubMed] [Google Scholar]
- 81.Naing A, LoRusso P, Fu S, et al. Cixutumumab combined with temsirolimus in patients with refractory Ewing’s sarcoma. J Clin Oncol. 2011;29:10031. [Google Scholar]
- 82.Benjamin D, Colombi M, Moroni C, Hall MN. Rapamycin passes the torch: a new generation of mTOR inhibitors. Nat Rev Drug Discov. 2011;10(11):868–880. doi: 10.1038/nrd3531. [DOI] [PubMed] [Google Scholar]
- 83.Fletcher CDM, Unni KK, Mertens F, editors. Pathology and Genetics of Tumours of Soft Tissue and Bone. Lyon, France: IARC Press; 2002. World Health Classification of Tumours. [Google Scholar]