Abstract
The purpose of this pilot study was to assess the association between 25-hydroxyvitamin D (25[OH]D) concentrations, vitamin D intake and sunlight exposure in patients with HF compared to healthy volunteers. Fourteen healthy volunteers 50 and older were recruited to compare with 14 patients with HF. Healthy volunteers were compared to HF patients by serum 25(OH)D concentrations, dietary vitamin D intake, weekly sunlight exposure, and other covariates. Independent samples t-tests and linear regression models were used to compare differences between HV and patients with HF. The mean serum 25(OH)D concentration was not significantly different between groups (Healthy volunteers 25.7±11.1 ng/ml, HF 20.4±10.2 ng/ml, p=.2) and no group effect was found in any multivariable models. BMI regardless of group was found to be inversely associated with serum 25(OH)D concentrations (p=.025). There was no difference in the dietary intake of vitamin D or calcium between groups. The healthy volunteers had significantly greater amount of sunlight exposure but this did not result in higher 25(OH)D when compared to those with HF. Our findings suggest that BMI has an important relationship with 25(OH)D concentrations regardless of a person being healthy or having HF.
Introduction
Vitamin D, a prosteroid hormone, is essential to bone and muscle health, and growing observational evidence indicates that vitamin D deficiency may be associated with increased risk of cardiovascular diseases such as heart failure (HF).1 Vitamin D may be of particular importance to patients with HF since these patients develop a myopathy,2 and are prone to osteoporosis and fractures. 3, 4, 5
Vitamin D insufficiency is rising across all ages, genders, races, and ethnicities in the United States.6 Based on data from the third report of the National Health and Nutrition Examination Survey and NHANES 2001–2004, the prevalence of Americans with serum concentrations who met minimum requirements for general health decreased from 45% to 23% during 1988–1994 to 2001–2004.6 Mean 25(OH)D concentrations decreased with older age.7
Patients with HF have been noted to have low 25(OH)D concentrations,8 and this has been associated with poor aerobic capacity and frailty.4 Specifically, African American patients with HF have a higher prevalence of hypovitaminosis D (25(OH)D concentrations <30 ng/ml).7 Vitamin D3 supplementation to HF patients has been found to be potentially cardioprotective by improving the imbalance between proinflammatory and anti-inflammatory cytokines involved in HF pathogenesis.9 Vitamin D has been shown to downregulate the renin angiotensin system in animal models.10 There have been few trials on vitamin D supplementation in patients with HF. A recent trial of vitamin D vs placebo for patients with HF found no improvement in the 6 minute walk or health status in those given vitamin D compared with placebo.11
It remains unclear if HF pre-disposes people to vitamin D deficiency. Vitamin D deficiency has well known health effects such as osteoporosis and muscle weakness. Patients with HF may be confined to indoors due to increased shortness of breath with activity and intolerance to extremes of temperature. Their lack of activity tolerance may put them at risk for year-round sunlight deprivation. Lack of sunlight itself may also be harmful by contributing to vitamin D deficiency, particularly for depression.12
The purpose of this pilot study was to assess the association between 25(OH)D concentrations, dietary vitamin D intake and sunlight exposure in patients with HF compared to healthy volunteers. We hypothesized that patients with HF would be more susceptible to vitamin D deficiency based on having a chronic illness which effects aerobic capacity and activity level and that age, race, and sex would be of lesser importance.
Methods
Study Population
The study was reviewed and approved by University Hospitals Case Medical Center (UHCMC) Institutional Review Board. All participants provided written informed consent. Fourteen HF patients (50 years and older) were compared to a group of 14 healthy volunteers. Data for HF patients was available from the baseline evaluation of those who participated in a randomized controlled trial of vitamin D therapy. HF patients were excluded from the randomized controlled trial for the following: (1) primary hyperparathyroidism or hypercalcemia, (2) nephrolithiasis, (3) current treatment for or a diagnosis of osteoporosis by bone mineral density, (4) hemo or peritoneal dialysis and/or creatinine of > 2.5, (5) 25(OH)D concentration ≥ 37.5 ng/ml (6) current use of daily vitamin D > 400 IU, corticosteroids, parathyroid hormone, androgen or estrogen, (7) current illicit drug user or consumption of ≥ 3 alcoholic drinks a day, (8) metastatic/advanced cancer , and/or (9) myocardial infarction in the preceding 6 months. Also, individuals were excluded if they were taking medications that are known to lower vitamin D concentrations or bioavailability of oral vitamin D administration including: ketoconazole, colestipol, cholestyramine, mineral oil, phenobarbitol, and/or phenytoin. Healthy volunteers were recruited from within and around (UHCMC) including businesses within 5 miles of the medical campus. HV were excluded if they had any chronic illness including: (1) cardiovascular disease or risk factors, such as hypertension, HF, history of stoke or myocardial infarction, (2) chronic kidney, lung, or liver disease, (3) diabetes, (4) cancer, (5) systemic rheumatological or connective tissue disease, (6) history of bariatric surgery, and/or (7) any other illnesses for which HF patients were excluded.
Study Visits
During study visits, participants completed the Nutrition Quest Block calcium/vitamin D screener, which was developed based on NHANES 1999 to 2001 dietary recall data and calculates the intake frequency of vitamin D and calcium rich foods (including fortification) and supplements.6 We assessed weekly sunlight exposure by asking the following questions: (1) On average, how many hours do you spend in the sun on a weekly basis? and (2)Have you traveled to a sunny destination lately (in the last 4 months)? Blood was collected and stored at 2 to 8°C. 25(OH)D was measured by chemilluminescence immunoassay (ARUP Salt Lake City, Utah) with an intra-assay CV of 3% and 6% and a between assay variability of 6% to 11%.
Statistical Methods
Descriptive statistics were used to describe the study participants using means (± standard deviations) for continuous variables and proportions for categorical variables. Student t-tests were used to compare demographic variables between the two study groups (HF vs. healthy volunteers). Simple and multivariable linear regression modeling was used to determine the factors associated with 25(OH)D concentration. In assessing group effects (HF vs. healthy volunteers) on 25(OH)D concentration, interactions with the other covariates of interest were considered.
Results
Fourteen patients with HF were enrolled in the study. Eighty-two healthy individuals volunteered for screening (66 women and 16 men). Fifty-one healthy volunteers qualified and were 14 matched by age (± 5 years), race, and sex with a HF patient (Table I). Healthy volunteers were disqualified from the study for the following reasons: 16 for hypertension, 6 for osteoporosis, 3 for cancer, 3 for previous bariatric surgeries, 1 for valve disease, 1 for Crohns disease, and 1 for hepatitis C. Both HF and healthy volunteers groups had 1:1 ratio of men to women as well as Caucasian to African Americans. Mean age of the healthy volunteers was 58.5±7.1 years compared with 61.0 ±7.7 years for the patients with HF. The mean body mass index (BMI) was 28.2±7.9 kg/m2 for healthy volunteers and 35.2±8.4 kg/m2 for patients with HF.
Table I.
Demographics of Heart Failure Patients (HF) and Healthy Volunteers (HV)
| HF n=14 |
HV n=14 |
p-value | |
|---|---|---|---|
| Age (yrs) | 61 ± 7.7 | 58.5 ± 7.1 | .373 |
| Women, % | 50 | 50 | - |
| Race (Caucasian %) | 50 | 50 | - |
| BMI, kg/m2 | 35.2 ±8.4 | 28.2±7.9 | 0.032 |
| Cause of HF: Ischemic % | 50 | N/A | – |
| NYHA Class, mean, % | II: 64 III: 36 |
N/A | – |
| EF, mean, % | 32.5 | N/A | – |
| Years with HF | 7.9 | N/A | – |
| Hyperlipidemia, No. (%) Hypertension, No. (%) Previous/current tobacco use, No. (%) Coronary Artery Disease, No. (%) Pulmonary Disease, No. (%) Diabetes, No. (%) Depression, No. (%) Thromboembolic disease, No. (%) |
(14) 100 (12) 86 (10) 71 (7) 50 (5) 36 (4) 29 (3) 21 (2) 14 |
N/A | – |
| Serum 25-hydroxyvitamin D, ng/ml | 20.4 ± 10.2 | 25.7 ± 11.1 | .194 |
| Daily Dietary Vitamin D IU | 89.1 ± 72.0 | 110.8 ± 146.4 | .624 |
| Daily Dietary Calcium mg | 486.9 ± 469.1 | 550.8 ± 453.8 | .717 |
| Visit to a sunny destination, No. (%)a | 6 (43) | 0 | .007 |
| Study visit month Dec. – Oct. | 8 (57%) | 14 (100%) | 0.006 |
Abbreviations: BMI, body mass index; EF, ejection fraction; N / A, not applicable; NYHA, New York Heart Association.
One missing value in the HF group.
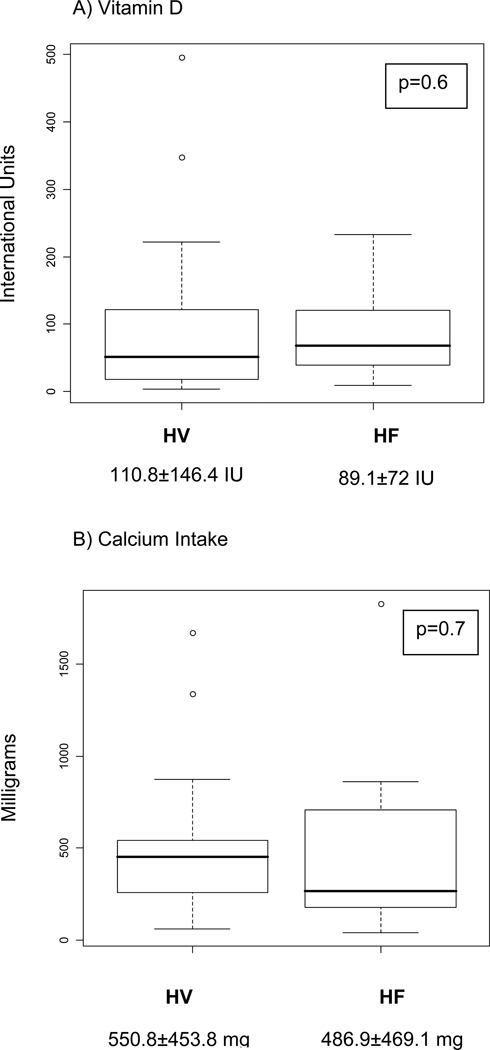
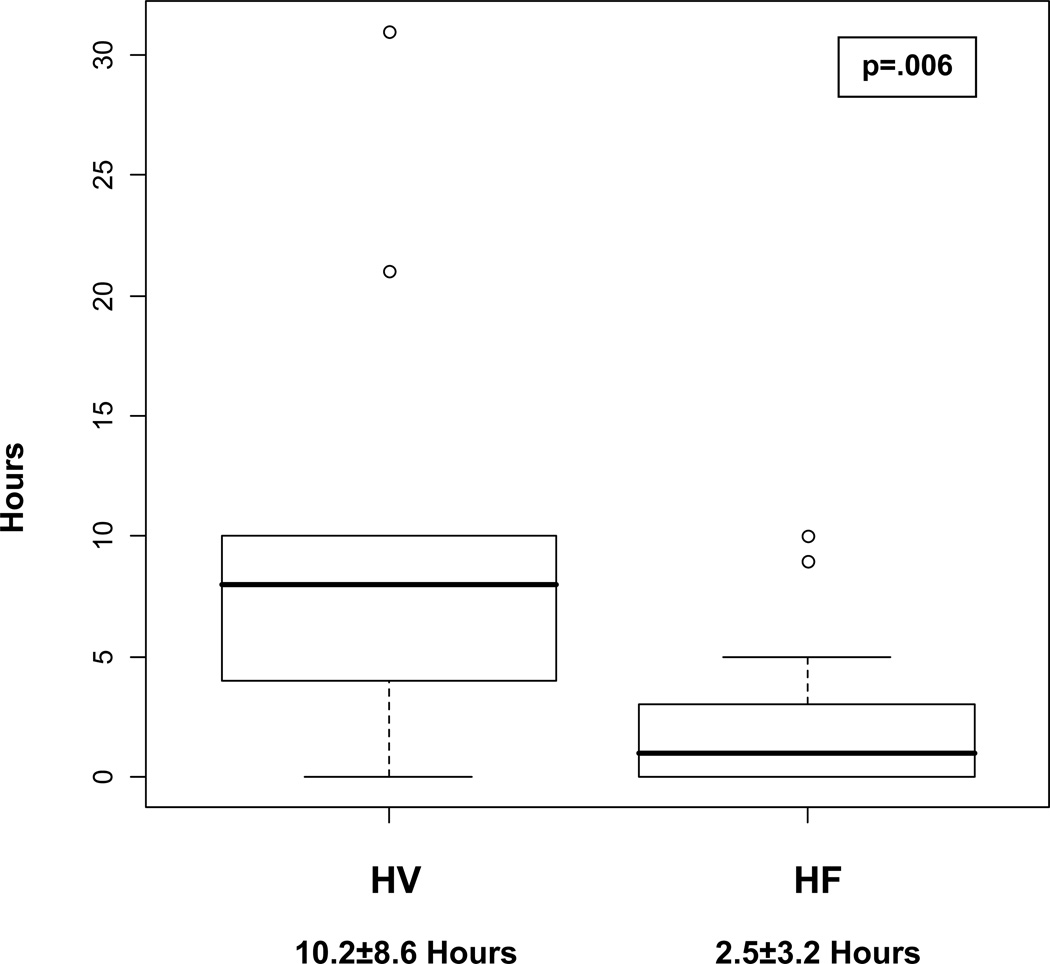
Serum 25(OH)D concentrations were not found to be significantly different on average between the HF and healthy volunteers groups (HV 25.7±11.1 ng/ml, HF 20.4±10.2 ng/ml, p = 2). Daily dietary vitamin D intake and daily calcium intake did not significantly differ between healthy volunteers and those with HF (Figure 1). On average, healthy volunteers spent significantly more time in the sun on a weekly basis than those with HF (Figure 2).
Figure 1.
Daily Dietary Vitamin D (A) and Calcium Intake (B)
Figure 2.
Average Weekly Sunlight Exposure of healthy volunteers (HV) vs heart failure patients (HF).
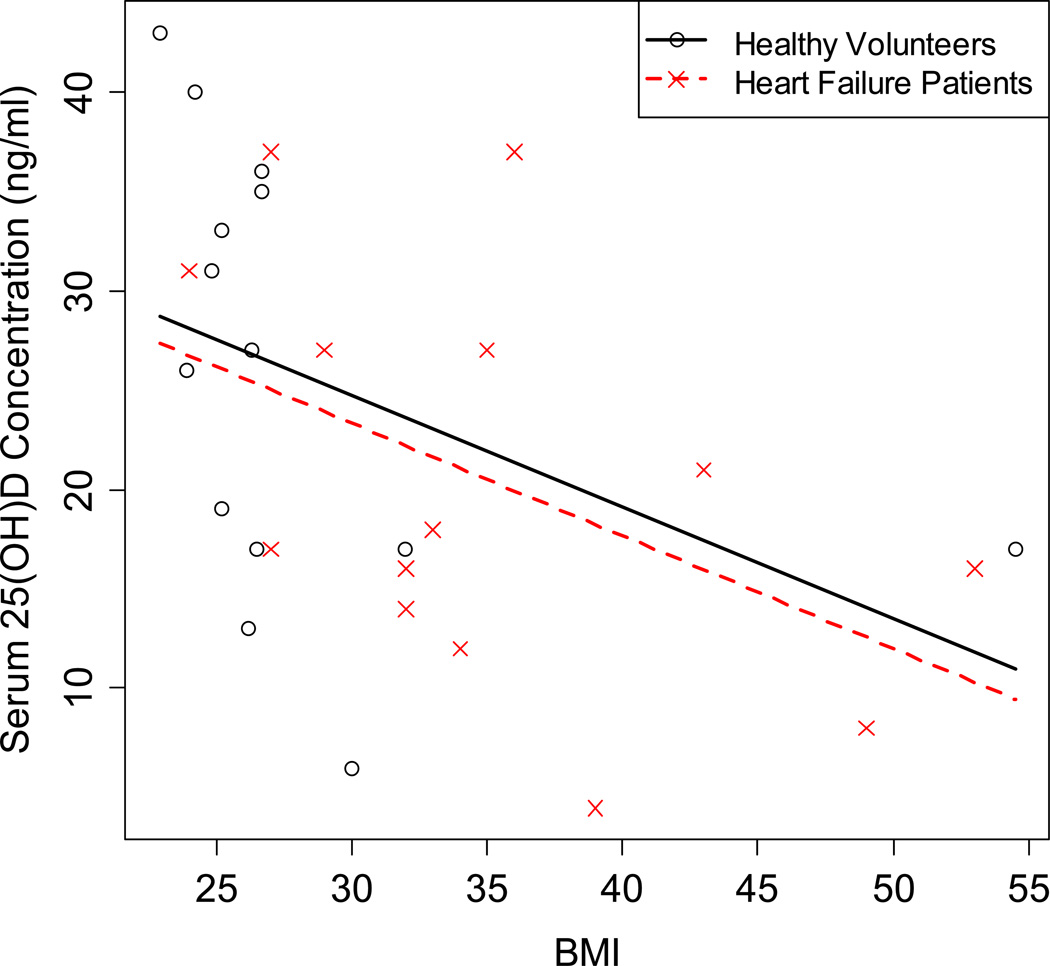
In multivariable modeling (including, sex, race, age, BMI, month of blood draw, sun exposure, dietary vitamin D intake, with serum 25(OH)D concentration as the dependant variable, BMI was the only significant predictor of serum 25(OH)D concentrations (Table II). Modeling indicated for every 5 unit increase in BMI, 25(OH)D decreased by 2.8 ng/ml on average. There was no effect due to healthy volunteers versus HF group (Figure 3).
Table II.
Multivariable Modeling indicating predictors of serum 25(OH)D concentration
| Covariates | Effect of covariate on Serum 25(OH)D |
p-value |
|---|---|---|
| Categorical (effect between groups) | ||
| Group, HF | −5.4 | .194 |
| Women | −5.8 | .154 |
| Caucasian | 5.8 | .154 |
| Sunny Destinationa | 7.2 | .226 |
| Visit in month 5–10b | 4.4 | .453 |
| Continuous (effect per unit increase of covariate) | ||
| BMI | −0.56 | .025 |
| Age | 0.02 | .541 |
| Sun Exposure | −0.07 | .834 |
| Vitamin D from Diet | 0.003 | .881 |
One missing value of sunny destination in the heart failure (HF) group.
Highest possible UVB exposure occurs from May to October.
Each model is a multiple regression model with serum 25-hydroxyvitamin D as the dependent variable, HF group as the covariate of interest, and one additional covariate corresponding to the row of the table (exception is row 1 with only HF group as a covariate).
Figure 3.
Body mass index (BMI) serum 25(OH)D concentrations in healthy volunteers and heart failure patients.
Serum 25(OH)D concentrations are plotted against BMI and grouped by HV versus HF. Reference lines are for a full interaction model. The regression lines are parallel, indicating no interaction between BMI and HF. The two lines are not significantly different from each other, indicating no HF group effect. The slope with BMI is significant.
Discussion
In this pilot study, healthy volunteers were exposed to more weekly sunlight, but this presumed advantage did not translate into higher 25(OH)D concentrations. Healthy volunteers and patients with HF were equally insufficient in vitamin D. We hypothesized that HF which limits functional capacity and therefore outdoor activities, would be the strongest predictor of 25(OH)D concentrations. However, in this small sample, BMI is the strongest predictor of 25(OH)D serum concentrations in multivariable modeling. Similarly, Lagunova and colleagues13 concluded that vitamin D deficiency was most prevalent in individuals with higher BMI. Adipose acts as a reservoir for vitamin D, a fat-soluble vitamin. Obese individuals with more subcutaneous fat produce similar amounts of vitamin D3 in the skin but sequester more of the cutaneously synthesized vitamin D in fat, decreasing its bioavailability by >50%.14 Orally supplemented vitamin D is more bioavailable than cutaneously produced vitamin D in obese patients, although it is also subject to storage in fat once in the bloodstream.14 Accordingly, larger amounts or longer duration of oral vitamin D supplementation may be necessary to replete these obese patients, which challenges the recommendation that the extent of vitamin D repletion should be governed by baseline vitamin D levels alone.15 Ultimately, fat loss could be an important adjunct to vitamin D deficiency in obese patients.
Race was not significantly associated with the serum 25(OH)D concentrations of our cohort, although population studies have shown that African Americans exhibit lower levels of 25(OH)D compared to Caucasians.16 Increased skin pigmentation plays a role in this phenomenon, in that the melanocytes in darker skin block UV-B light more effectively. Vitamin D intake has been reported as below the requirement in all age groups of the African American population.16 Interestingly, African Americans have a lower risk of fragility and osteoporotic fractures despite low 25(OH)D.17 Our cohort’s poor UV-B exposure from living in Cleveland, Ohio (latitude of 41°) may be responsible for the lack of difference in 25(OH)D concentrations between races.
Patients with HF had significantly lower sunlight exposure, which may reflect lower activity levels. The low amount of sunlight exposure in HF patients is a concern, regardless of its impact on vitamin D concentrations. Associations have been found between the lack of sunlight and depression.12 HF patients have higher rates of depression18 and poorer quality of life than patients with other illnesses such as chronic hepatitis C and lung disease.19, 20 Bright light therapy has been suggested as a nonpharmacological option for depression in older institutionalized patients with a year round lack of sunlight.21 Sunlight exposure or light therapy may be a therapeutic option to consider for depressed older adults with HF.
Clinical Implications
Observational evidence has indicated that vitamin D deficiency may contribute to cardiovascular risk and disease. Presently, observational evidence has demonstrated an association between low 25(OH)D concentrations and HF. Studies are ongoing to investigate the effects of vitamin D supplementation on functional or pathophysiological outcomes for patients with HF. From this study, even with its small sample, it is evident that obesity plays an important role in low 25(OH)D serum concentrations. Although this was not hypothesized at the outset of the study, it is an important finding. Individuals who are obese may require significantly higher oral doses of vitamin D. Sunlight exposure may be important to consider as an adjunct to oral vitamin D supplements in these patients.
Study Limitations
The sample size in this study is small. Although, there was no statistical significance in serum 25(OH)D concentrations between the healthy volunteers and HF, there was a trend for a lower level in those with HF. Evaluation in a larger sample may reveal a difference in 25(OH)D concentrations between healthy individuals and those with HF as well as the contribution of sunlight exposure between the two groups. HF patients and healthy volunteers were not matched on the time of the year of evaluation, although participants were asked to estimate the amount of time exposed to sunlight on average. HF patients were screened by 25(OH)D concentrations as part of the randomized controlled trial. However, only 9 out of 301 (3%) of screened HF patients had 25(OH)D concentrations ≥37.5 ng/ml. Due to many variables such as geographic location, season, skin type, and time of day of sun exposure, attempting to convert one’s UVB light exposure to the equivalent amount of oral vitamin D supplementation is nearly impossible.22 Therefore, associating higher sunlight exposure with higher 25(OH)D levels may not be a valid assumption because of the numerous variables that affect the production of vitamin D by the skin. The production of vitamin D by the skin occurs faster during the summer and for individuals with lighter skin types.22 The sunlight exposure of our study participants varied across season, time of day of exposure, and skin type, which, may in part, explain the lack of correlation between sunlight exposure and 25(OH)D concentration.
Acknowledgements
Christina DiCarlo was supported by a National Institutes of Health (NIH) T35 Training Grant HL082544 of the National Heart, Lung, and Blood Institute. Dr. Boxer and this work are supported by the KL2RR024990 and in part by the American Heart Association Scientist Development Grant 0635055N. The project described was supported by the Clinical and Translational Science Collaborative and utilized the REDCap database. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of National Center for Research Resources (NCRR) or NIH. Grant Number UL1 RR024989 from the NCRR, a component of the National Institutes of Health (NIH).
Contributor Information
Christina DiCarlo, Case Western Reserve University School of Medicine.
Brian Schmotzer, Case Western Reserve University School of Medicine.
Marianne Vest, Department of Medicine, University Hospitals-Case Medical Center.
Rebecca Boxer, Department of Medicine, University Hospitals-Case Medical Center.
References
- 1.Judd SE, Tangpricha V. Vitamin D deficiency and risk for cardiovascular disease. Am J Med Sci. 2009 Jul;338(1):40–44. doi: 10.1097/MAJ.0b013e3181aaee91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anker SD, Chua TP, Ponikowski P, et al. Hormonal changes and catabolic/anabolic imbalance in chronic heart failure and their importance for cardiac cachexia. Circulation. 1997 Jul 15;96(2):526–534. doi: 10.1161/01.cir.96.2.526. [DOI] [PubMed] [Google Scholar]
- 3.Abou-Raya S, Abou-Raya A. Osteoporosis and congestive heart failure (CHF) in the elderly patient: double disease burden. Arch Gerontol Geriatr. 2009 Sep-Oct;49(2):250–254. doi: 10.1016/j.archger.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Boxer RS, Dauser DA, Walsh SJ, Hager WD, Kenny AM. The association between vitamin D and inflammation with the 6-minute walk and frailty in patients with heart failure. J Am Geriatr Soc. 2008 Mar;56(3):454–461. doi: 10.1111/j.1532-5415.2007.01601.x. [DOI] [PubMed] [Google Scholar]
- 5.Lyons K, Majumdar SR, Ezekowitz JA. The Unrecognized Burden of Osteoporosis-Related Vertebral Fractures in Patients with Heart Failure. Circ Heart Fail. May 10; doi: 10.1161/CIRCHEARTFAILURE.111.961185. [DOI] [PubMed] [Google Scholar]
- 6.Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch Intern Med. 2009 Mar 23;169(6):626–632. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim DH, Sabour S, Sagar UN, Adams S, Whellan DJ. Prevalence of hypovitaminosis D in cardiovascular diseases (from the National Health and Nutrition Examination Survey 2001 to 2004) Am J Cardiol. 2008 Dec 1;102(11):1540–1544. doi: 10.1016/j.amjcard.2008.06.067. [DOI] [PubMed] [Google Scholar]
- 8.Pilz S, Marz W, Wellnitz B, et al. Association of vitamin D deficiency with heart failure and sudden cardiac death in a large cross-sectional study of patients referred for coronary angiography. J Clin Endocrinol Metab. 2008 Oct;93(10):3927–3935. doi: 10.1210/jc.2008-0784. [DOI] [PubMed] [Google Scholar]
- 9.Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2006 Apr;83(4):754–759. doi: 10.1093/ajcn/83.4.754. [DOI] [PubMed] [Google Scholar]
- 10.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002 Jul;110(2):229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Witham MD, Crighton LJ, Gillespie ND, Struthers AD, McMurdo ME. The effects of vitamin D supplementation on physical function and quality of life in older patients with heart failure: a randomized controlled trial. Circ Heart Fail. Mar;3(2):195–201. doi: 10.1161/CIRCHEARTFAILURE.109.907899. [DOI] [PubMed] [Google Scholar]
- 12.Lambert GW, Reid C, Kaye DM, Jennings GL, Esler MD. Effect of sunlight and season on serotonin turnover in the brain. Lancet. 2002 Dec 7;360(9348):1840–1842. doi: 10.1016/s0140-6736(02)11737-5. [DOI] [PubMed] [Google Scholar]
- 13.Lagunova Z, Porojnicu AC, Lindberg F, Hexeberg S, Moan J. The dependency of vitamin D status on body mass index, gender, age and season. Anticancer Res. 2009 Sep;29(9):3713–3720. [PubMed] [Google Scholar]
- 14.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000 Sep;72(3):690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 15.Lee P, Greenfield JR, Seibel MJ, Eisman JA, Center JR. Adequacy of vitamin D replacement in severe deficiency is dependent on body mass index. Am J Med. 2009 Nov;122(11):1056–1060. doi: 10.1016/j.amjmed.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 16.Harris SS. Vitamin D and African Americans. J Nutr. 2006 Apr;136(4):1126–1129. doi: 10.1093/jn/136.4.1126. [DOI] [PubMed] [Google Scholar]
- 17.Aloia JF. African Americans, 25-hydroxyvitamin D, and osteoporosis: a paradox. Am J Clin Nutr. 2008 Aug;88(2):545S–550S. doi: 10.1093/ajcn/88.2.545S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Connor CM, Joynt KE. Depression: are we ignoring an important comorbidity in heart failure? J Am Coll Cardiol. 2004 May 5;43(9):1550–1552. doi: 10.1016/j.jacc.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Juenger J, Schellberg D, Kraemer S, et al. Health related quality of life in patients with congestive heart failure: comparison with other chronic diseases and relation to functional variables. Heart. 2002 Mar;87(3):235–241. doi: 10.1136/heart.87.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hobbs FD, Kenkre JE, Roalfe AK, Davis RC, Hare R, Davies MK. Impact of heart failure and left ventricular systolic dysfunction on quality of life: a cross-sectional study comparing common chronic cardiac and medical disorders and a representative adult population. Eur Heart J. 2002 Dec;23(23):1867–1876. doi: 10.1053/euhj.2002.3255. [DOI] [PubMed] [Google Scholar]
- 21.Sumaya IC, Rienzi BM, Deegan JF, 2nd, Moss DE. Bright light treatment decreases depression in institutionalized older adults: a placebo-controlled crossover study. J Gerontol A Biol Sci Med Sci. 2001 Jun;56(6):M356–M360. doi: 10.1093/gerona/56.6.m356. [DOI] [PubMed] [Google Scholar]
- 22.Terushkin V, Bender A, Psaty EL, Engelsen O, Wang SQ, Halpern AC. Estimated equivalency of vitamin D production from natural sun exposure versus oral vitamin D supplementation across seasons at two US latitudes. J Am Acad Dermatol. Jun;62(6):929. doi: 10.1016/j.jaad.2009.07.028. e921–929. [DOI] [PubMed] [Google Scholar]