Abstract
We present a fabrication process to create bifunctional microparticles displaying two distinct proteins that are spatially segregated onto the surface hemispheres. Silica and polystyrene microparticles with 2.0 μm, 4.1 μm, and 4.7 μm diameters are processed with metal deposition to form two chemically distinct and segregated hemispheres. The surface of each hemisphere is then separately derivatized with biological proteins using different chemical conjugation strategies. These bifunctional Janus particles possess biologically relevant, native conformation proteins attached to a biologically-unreactive and safe substrate. They also display high densities of each type of proteins which may enable a range of capabilities that monofunctional particles cannot, such as improved targeting of drugs and bioimaging agents.
Keywords: Janus particle, segregated protein, bifunctional
Introduction
Bifunctional particles with biologically or chemically distinct hemispheres, also known as Janus particles,1–5 have a wide range of applications3–7 in the fields of biotechnology, nanotechnology, electronics, and renewable energy.4,5,8 Biologically bifunctional Janus particles can potentially be utilized as advanced pharmaceutical agents which can enhance targeting of tissues and cells through ligands on one hemisphere while simultaneously stimulating biological pathways with ligands from the second hemisphere.
Biologically bifunctional Janus particles are nonetheless a challenge to fabricate as the synthesis technique requires consideration of protein functionality and stability.9 Monofunctional and spatially uniform microparticles are routinely produced through the surface modification of microparticles.4,10,11 These methods result in microparticles coated with a nominally uniform distribution of proteins that create a single targeting or stimulating capability.10–12
Chemical Janus particles or single-sided biological Janus particles have been successfully fabricated using several techniques, including microfluidics1,5,7,13 and selective surface modifications.2,4–6,14 The latter modifications can be created through microfabrication techniques such as evaporation15 and sputtering8,16 in which a hemisphere is temporarily or permanently masked while the exposed surface is modified.1,3–6,14
Currently, there are no described methods to produce biological Janus particles with two types of separated proteins. In this study, we have developed several procedures to modify uniform microparticles with a metal coating on one hemisphere and to then conjugate different types of spatially segregated proteins onto the microparticles using different chemistries for the two hemispheres. The microparticles were modified by depositing a gold film on one side such that each particle displays two conjugation moieties on each hemisphere: a gold surface and the original surface consisting of either biotinylated polystyrene, carboxylated polystyrene, or silica. While all of the methods were successful at producing biologically bifunctional Janus particles, we have found that the highest yield and highest reproducibility is obtained by gold-coating silica microparticles, followed by gold functionalization, silanization of silica, and then protein attachments to the chemically active surfaces. Bifunctional biological activities are obtained through the robust attachment of native-conformations of protein onto the spatially segregated surfaces. The possible uses of these particles are a result of the biological functions of proteins, in addition to the physical and chemical properties of the particles. Two recognition proteins could be used to bring different biological effectors in close proximity to facilitate their activation or breakdown by cellular or enzymatic activity. A recognition protein plus an activation molecule could simultaneously bind a cell and stimulate the immune system or facilitate the breakdown of toxic products.17,18 Alternatively, a protein drug plus a targeting and internalization motif could target treatment to a specific subset of cells and reduce nonspecific effects of drugs with severe side effects.11 Bifunctional Janus particles can be used to create an entirely novel class of smart particle capable of high avidity targeting to and stimulation of multiple cell types. With these new particles, we can potentially improve the range, specificity, and capabilities of therapeutic interventions.
Experimental Section
Materials
1-Ethyl-3(dimethylaminopropyl)carbodiimide (EDAC), PolyLink coupling buffer solution, and silica and carboxyl polystyrene particles with 4.7 μm and 4.1 μm diameters respectively were purchased from Bangs Laboratories Inc. (Fishers, IN). Biotin-conjugated polystyrene microparticles with 2 μm diameters were purchased from Polysciences (Warrington, PA). (3-Aminopropyl)triethoxysilane (APTES), rabbit IgG, fluorescein isothiocyanate (FITC) conjugated bovine serum albumin (BSA), and unlabeled BSA were purchased from Sigma-Aldrich (St. Louis, MO). Alexa Fluor 488 conjugated goat anti-rabbit IgG secondary antibody, Alexa Fluor 647 conjugated with streptavidin (SA), tetramethylrhodamine-6-isothiocyanate (TRITC), phosphate-buffered saline (PBS), and natural human fibronectin (FN) were purchased from Invitrogen (Carlsbad, CA). 8-Amino-1-octanethiol was purchased from Dojindo Molecular Technologies Inc. (Rockville, MD). Thiol-poly(ethylene glycol)-biotin, a heterobifunctional PEG derivative, was purchased from Nanocs (New York, NY). Ethanol, acetone, and other chemicals were purchased and used as received from VWR (Radnor, Pennsylvania).
Four different proteins were used as models to demonstrate particle bifunctionalization: BSA represents an important passivation protein, FN and immunoglobulin G antibody are important adhesive proteins, and SA is a useful cross-linking protein which binds with a high affinity to biotinylated molecules. The BSA was received fluorescently unlabeled as well as pre-labeled with FITC. The SA was also received pre-labeled with Alexa Fluor 647. FN was labeled with TRITC according to the manufacturer’s instructions. Goat anti-rabbit IgG conjugated with Alexa Fluor 488 was used to later label the non-fluorescent rabbit IgG.
Methods
Chemical modification of microparticles through gold deposition
A monolayer of microparticles was deposited onto glass slides cut into slivers by spotting 1–2 μL droplets of microparticles suspended in deionized water and drying the solution at room temperature. Prior to deposition, the microparticles were washed repeatedly with deionized water. The concentration of the microparticles in the droplets was chosen to reduce the formation of overstacking clusters19. Concentrations of 0.05 mg/mL, 0.7 mg/mL, and 20 mg/mL were used for the biotin-conjugated polystyrene, carboxyl polystyrene, and silica microparticles respectively. After the monolayer preparation, the microparticles were coated with a layer of gold using a metal evaporation process (CHA E-Beam Evaporator).15 The gold was deposited to a thickness of 150 nm with a rate of 2 Å/s following a 15 nm titanium adhesion layer. After the gold deposition, the glass slivers were placed into microcentrifuge tubes filled with solvent for the next processing step and were gently sonicated (Haier Ultrasonic Cleaner) to remove the gold-coated particles from the glass substrate. Atomic force microscopy (AFM) imaging (Asylum Research MFP-3D) was used to characterize the density of the microparticle monolayer by examining the remnant gold layer after the microparticles were removed. The density on the glass sliver with the 4.7 μm diameter silica microparticles was determined from AFM analysis to be 50,000 particles per mm2, with a slightly lower density of particles in the central region of the monolayer. The gold-coated silica particles were further characterized with scanning electron microscopy (SEM, Hitachi S-3700 VP-SEM) and bright field microscopy to verify the hemispherical gold coverage.
Protein conjugations to the particle surface
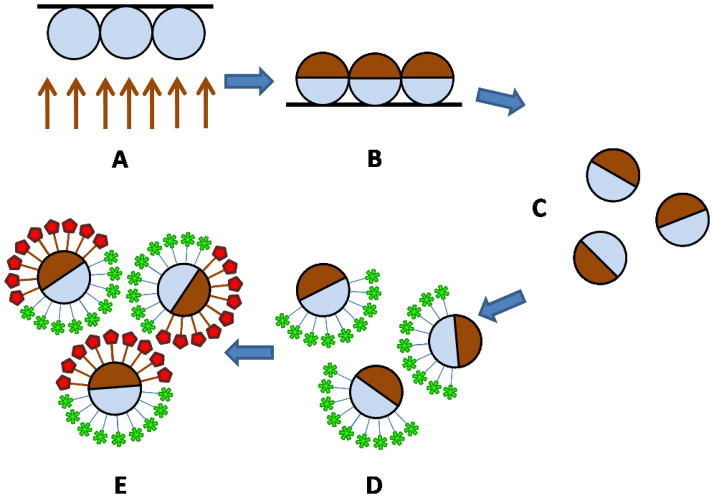
The gold hemisphere spatially defined a region for selective conjugation of one type of protein onto the surface of the microparticles. The gold hemispherical shell is selectively modified via an amino-alkanethiol reagent2,10,11,20 or thiol-PEG-biotin.21 The other hemisphere was modified by selectively activating the original particle surface via several alternate procedures described below. Figure 1 shows a general schematic of the process flow for biological bifunctional particle fabrication. (FIGURE 1)
Figure 1.
General schematic of the bifunctional Janus particle fabrication process, including (A) particles before gold deposition on a glass substrate, (B) particles after gold deposition, (C) particle removal from glass substrate, (D) original particle surface functionalization, and (E) gold functionalization.
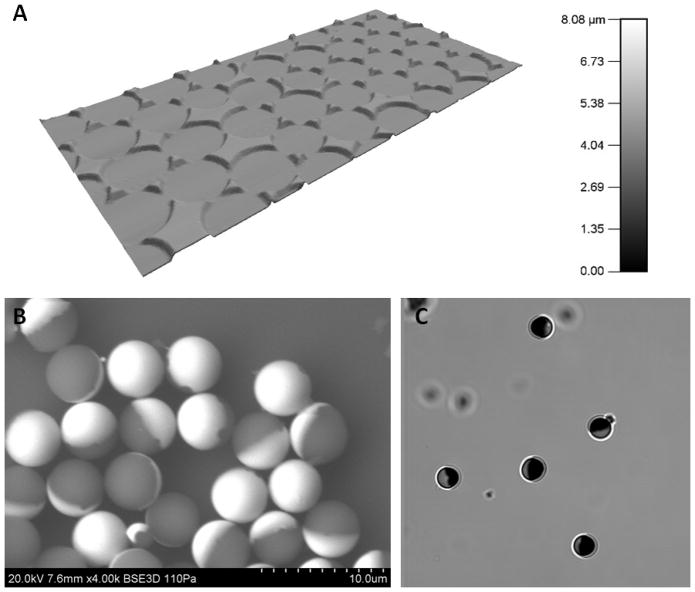
Before protein functionalization, polystyrene and silica particles were processed with a metal deposition technique to obtain a thin layer of gold on one hemisphere as previously described. The characterization of the gold deposition process on silica microparticles is shown in Figure 2 through imaging by atomic force microscopy, scanning electron microscopy, and bright field optical microscopy. (FIGURE 2)
Figure 2.
(A) AFM surface topography of the remnant gold on the glass substrate after particle removal, (B) SEM of 4.7 μm gold-coated silica particles after sonication removal from the glass substrate, and (C) bright field microscopy of the particles.
Gold-coated silica microparticles were incubated for 4 hours with a 1 mM solution of the thiol-PEG-biotin reagent in PBS to chemically activate the gold hemispherical shell. The microparticles were cleansed with ethanol and air-dried to prepare for the silanization of the silica hemisphere. The microparticles were suspended in a 2% APTES solution and rinsed with water followed by oven drying to complete the silanization process. The activated microparticles were then incubated with BSA for one hour, cross-linking the BSA to the silica surface. This process was also repeated using the rabbit IgG (rIgG) antibody attached to the silica surface followed by one hour incubation with fluorescently conjugated anti-rabbit IgG antibody. After functionalization of the silica hemisphere, the particles were incubated with SA for one hour to functionalize the gold hemisphere. Gold-coated polystyrene particles were also functionalized with thiol-PEG-biotin and EDAC activation followed by BSA cross-linking onto the polystyrene surface and SA cross-linking onto the gold surface. Table 1 compiles the various particle compositions and the respective modification and proteins. (TABLE 1)
Table 1.
Various particle compositions with the respective cross-linking chemistry and protein modification.
| Particle Surfaces | Cross-linking Chemistry | Proteins on Surface |
|---|---|---|
| Biotin-conjugated Polystyrene/Gold | None/Amino-alkanethiol | SA/BSA |
| Silica/Gold | APTES/Amino-alkanethiol | BSA/Fn |
| APTES/Thiol-PEG-Biotin | BSA/SA | |
| APTES/Thiol-PEG-Biotin | rIgG/SA | |
| Polystyrene/Gold | EDAC/Amino-alkanethiol | BSA/Fn |
| EDAC/Thiol-PEG-Biotin | BSA/SA |
Carboxylated polystyrene microparticles were functionalized with BSA via EDAC activation while silica particles were functionalized with BSA via APTES silanization. In both methods, the particles were then resuspended in a 1 mM amino-alkanethiol solution for 2 hours and functionalized with FN onto the gold surface. Biotin-conjugated polystyrene microparticles were also functionalized after a 1 mM amino-alkanethiol solution functionalization of the gold hemisphere followed by BSA cross-linking and SA attachment to the gold and biotinylated hemispheres respectively. Silica particles were functionalized with APTES and BSA cross-linking, followed by 1mM amino-alkanethiol solution functionalization of the gold hemisphere and FN cross-linking. Monofunctional silica and polystyrene particles, which did not undergo the gold deposition step, were also functionalized with BSA. Mixtures of functionalization where equal ratios of labeled BSA and unlabeled BSA were conjugated to microparticles through either EDAC cross-linking in the case of polystyrene microparticles or through APTES in the case of silica microparticles.
Microscopy and flow cytometry
Confocal laser microscopy (Zeiss LSM 510 VIS Confocal Microscope) was used to visually demonstrate the spatial segregation of the two proteins onto hemispherical surfaces of individual particles. We used the provided software (Zen Lite 2011) to determine the mean fluorescence intensity (MFI), defined as the integrated intensity divided by the cross sectional area, both for particles with spatially segregated proteins and for particles possessing a mixed distribution of proteins on the surface. Flow cytometry was performed using the Accuri C6 and FlowJo software to verify the intensity distribution of the fluorophore-conjugated proteins for a population of 100,000 microparticles. The intensity was determined for each particle in the FL-1 and FL-4 channels, corresponding to the FITC/Alexa Fluor 488 and Alexa Fluor 647 respectively and debris were excluded from analysis using a forward scatter-triggered gating. Four experimental groups of gold-coated particles were analyzed: particles that were processed as previously described with thiol-PEG-biotin and APTES; particles that were not further processed and were entirely non-fluorescent (Supplementary Figure 1); particles that were processed with either the FITC or Alexa Fluor 488 fluorescence on a single hemisphere for BSA or rIgG, respectively, and particles that were processed with the Alexa Fluor 647 conjugated SA cross-linked to the gold hemisphere.
The protein surface density of the bifunctional Janus particles was measured with confocal microscopy and compared to the particles which did not possess spatially segregated proteins. Mean fluorescence intensity (MFI) of confocal images, defined as the intensity per unit functionalized area, was measured using Zen Lite 2011 as a surrogate for protein density measurements on the surface of the gold-coated particles with a single protein type and the microparticles functionalized with a mixture of labeled and unlabeled BSA. A two-sample T-test (Minitab) demonstrates significance between the fully-coated microparticles opsonized with labeled BSA and the fully-coated microparticles opsonized with an equal mixture of labeled and unlabeled BSA.
Results
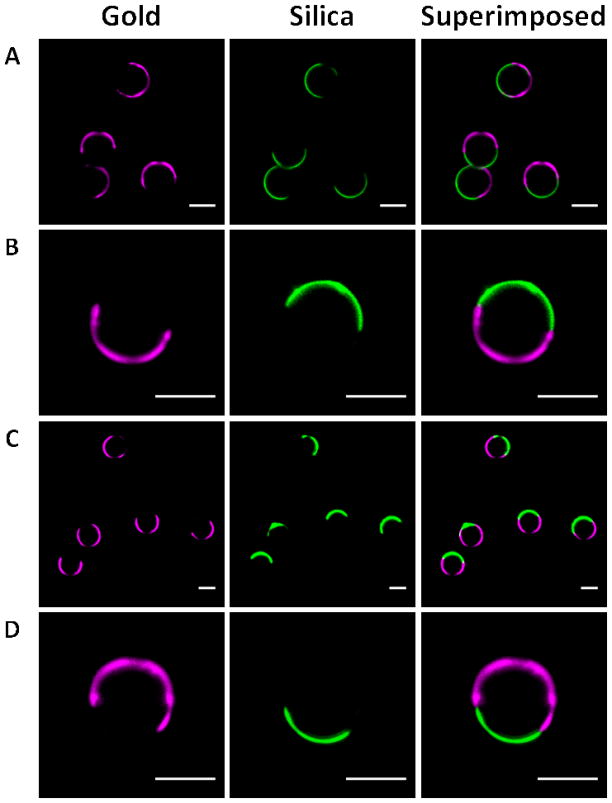
A variety of Janus particles were created with distinct protein types spatially segregated onto two hemispheres. Two types of silica particles processed with BSA/SA and rIgG/SA are imaged with confocal laser scanning microscopy and bright field microscopy as shown in Figure 3. (FIGURE 3) The observed fluorescent regions corresponding to the microparticles’ hemispheres indicate the bifunctionalized surfaces. A video which shows the confocal image of a bifunctional Janus microparticle in rotating view is provided in the supplementary materials.
Figure 3.
Confocal images of 4 μm silica particles processed to create biological Janus particles with APTES and thiol-PEG-biotin. (A) Zoomed-out and (B) magnified view of particles with FITC conjugated BSA and Alexa Fluor 647 conjugated SA on gold and silica surfaces respectively. (C) Zoomed-out and (D) magnified view of particles with Alexa Fluor 488 conjugated goat anti-rabbit IgG and Alexa Fluor 647 conjugated SA on gold and silica surfaces respectively. The scale bar in each image corresponds to 4 μm.
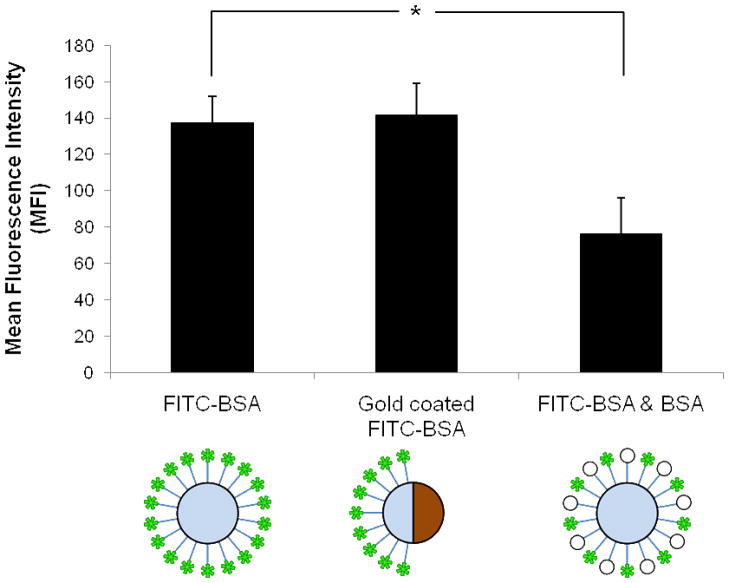
We analyzed the fluorescent confocal images to calculate the MFI to compare the density of fluorescently labeled proteins in beads fully coated with FITC-BSA, beads that were half coated with FITC-BSA and beads that were coated with a mixture of labeled and unlabeled BSA. The results in Figure 4 show that particles with spatially segregated proteins do not possess a significantly different MFI compared with particles fully coated with FITC-BSA. (FIGURE 4) However, the MFI measurements from both particles were both significantly higher than particles coated with an equal mixture of fluorescent and non-fluorescent BSA (p=4×10−10, N = 15). The significant difference in fluorescence was expected due to the lower densities of fluorescent protein. Spatial segregation of protein functionalization therefore is important to maintain a high density of ligands in the creation of bifunctional particles. This should improve the biological activity of the bifunctional Janus particles due to multivalent interactions that will increase interaction efficiency or may be required for some biological effects.
Figure 4.
Bar plot of the MFI for different procedures of particles functionalization of particles. Measurements are shown for single protein coating, single protein Janus particles, and mixed protein coating, as depicted by the schematics below each bar plot. The mixed protein coating consist of a mixture of FITC conjugated BSA and unlabeled BSA. The error bars represent the standard error.
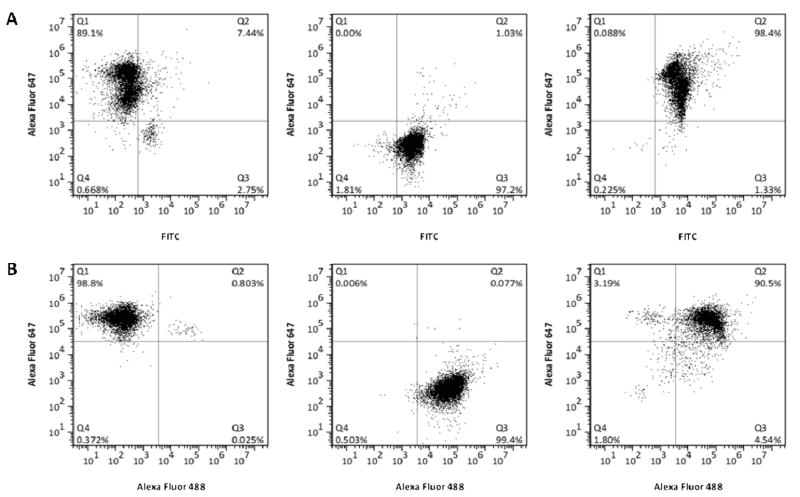
The yield of bifunctional particle production varied with each processing method. Two yields are defined. The first is the process yield that measures the percentage of particles with two types of spatially segregated proteins compared to the total number of particles present after the processing has been completed, as determined from flow cytometer analysis. The process yield is 90.5% for the silica particles processed with the thiol-PEG-biotin reagent and the rIgG/SA and 98.5% for BSA/SA, as shown in Figure 5. (FIGURE 5) Confocal microscopy verified that the vast majority of these microparticles had spatially segregated proteins. The different processing yields are summarized in Table 2. (TABLE 2)
Figure 5.
Fluorescence analysis of bifunctional Janus particles using flow cytometery for silica particles processed with thiol-PEG-biotin and APTES. The particles were conjugated with (A) BSA/SA and (B) rIgG/SA.
Table 2.
Summarized results with different procedures of biological Janus fabrication process. We conjugated BSA/SA and rIgG/SA to the Silica/Gold microparticles via APTES/Thiol-PEG-biotin cross-linking chemistry, which provided a range in processing yield.
| Particle Surface | Cross-linking Chemistry | Process Yield |
|---|---|---|
| Carboxylated Polystyrene/Gold | EDAC/Amino-alkanethiol | < 5% |
| Biotin-conjugated Polystyrene/Gold | None/Amino-alkanethiol | 11% |
| Silica/Gold | APTES/Amino-alkanethiol | < 5% |
| Silica/Gold | APTES/Thiol-PEG-biotin | 90.5–98% |
The total yield is defined as the number of bifunctional particles created compared to the initial number of particles that were used prior to processing. This number is by definition lower than the process yield since some particles are lost in the gold deposition and washing steps. The total yield is approximately 30% for the silica particles and was below 10% for polystyrene particles.
Discussion
As seen from the processing yield results, we have found that the thiol-PEG-biotin/APTES functionalization process with the silica particles provides the highest yield of bifunctional Janus particles. A high process yield is important to making Janus particles with increased homogeneity and reduced manufacturing costs. We hypothesize that the higher yield is in part due to the PEG derivative inhibiting non-specific adsorption to the gold surface while biotinylation exhibits a high selectivity for SA. The net result is that the microparticles have two selective surfaces to which proteins stably bind.
Another advantage of this functionalization strategy is that both the silica hemispherical surface and the gold coating are chemically active prior to protein immobilization. Protein instability in harsh solvents or conditions is therefore avoided during all subsequent protein conjugation steps which should help maintain the native conformation of proteins as they are conjugated to the particle surfaces. This assumption is supported by the binding of the secondary antibody to rIgG. Also, this procedure helps to increase the total yield due to fewer particle processing steps.
We achieved a higher total yield of spatially segregated proteins using the silica particles compared with the carboxylated polystyrene particles. The lower total yield for polystyrene particles may be a result of increased nonspecific binding caused by surface charges as well as the hydrophobic nature of polystyrene. We also observed increased aggregation of carboxylated polystyrene particles during the EDAC conjugation, especially for particles with low carboxyl densities on the surface, resulting in a total yield is less than 10% for the polystyrene-based particles. The silica particles on the other hand were more hydrophilic, which helped to reduce adsorption onto the container’s sides during incubation. The biotin-conjugated polystyrene particles on the other hand have a relatively higher process yield of 11% compared to the carboxylated polystyrene particles, due to the high affinity binding to SA and lower non-specific adsorption.
These studies were conducted as a “proof of principle” for the creation of biologically bifunctional Janus particles. By attaching fluorophore-conjugated proteins onto two chemically-distinct hemispheres of a microparticle, we showed that two different proteins can be deposited in their native-conformation on a microparticle, creating bifunctional capabilities. Creating bifunctional microparticles with a high density of proteins can be important in the development of more advanced therapeutic particles by incorporating multiple targeting and stimulation capabilities in one particle or adding a therapeutic capability imparted by one or more protein types to each hemisphere. Spatially segregated bifunctionality, in comparison to mixed distributions on a surface, leads to a higher density of ligands that will increase adhesive capabilities through multivalent binding22 or improve signaling capabilities through cross-linking of receptors or other sterically-sensitive processes.23 Bifunctionality can therefore greatly improve the effectiveness of microparticle technologies for applications where multifunctionality and high avidity are necessary.
Conclusion
We have created the first bifunctional Janus particles with biologically relevant, native conformation proteins attached to a biologically-unreactive and safe substrate.10,20 These particles have many potential biomedical applications, including multifunctional drug targeting, bioimaging, and adhesive crosslinking of cells. Suspension method, functionalization times, and the protein immobilization methods can be optimized to achieve higher yields in future studies.
Supplementary Material
Fluorescence analysis of gold-coated silica particles with no proteins. The particles show low fluorescence in both channels.
Rotating silica microparticle processed with thiol-PEG-biotin/APTES followed by BSA/SA cross-linking.
Acknowledgments
The authors thank the Bill and Melinda Gates Foundation and the National Institutes of Health (grant number 1R21EB013413-01) for support of this research. The authors also thank Candace Derenge and the Summer Undergraduate Research Fellowship (SURF) from the National Science Foundation for support in proof of concept studies.
Footnotes
SUPPORTING INFORMATION AVAILABLE
The following data is available as supporting data, including flow cytometry fluorescence analysis of particles with no protein and a video of rotating silica microparticle processed with thiol-PEG-biotin/APTES followed by BSA/SA cross-linking. This information is available free of charge via the Internet at http://pubs.acs.org/.
References
- 1.Hong L, Jiang S, Granick S. Langmuir3: the ACS journal of surfaces and colloids. 2006;22:9495–9. doi: 10.1021/la062716z. [DOI] [PubMed] [Google Scholar]
- 2.Perro A, Meunier F, Schmitt V, Ravaine S. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2009;332:57–62. [Google Scholar]
- 3.Perro A, Reculusa S, Ravaine S, Bourgeat-Lami E, Duguet E. Journal of Materials Chemistry. 2005;15:3745. [Google Scholar]
- 4.Wang B, Dong B, Li B, Zhao B, Li CY. Polymer. 2010;51:4814–4822. [Google Scholar]
- 5.Lee KJ, Yoon J, Lahann J. Current Opinion in Colloid & Interface Science. 2010 [Google Scholar]
- 6.Chen RT, Muir BW, Such GK, Postma A, McLean KM, Caruso F. Chemical communications (Cambridge, England) 2010;46:5121–3. doi: 10.1039/c0cc00474j. [DOI] [PubMed] [Google Scholar]
- 7.Nie Z, Li W, Seo M, Xu S, Kumacheva E. Journal of the American Chemical Society. 2006;128:9408–12. doi: 10.1021/ja060882n. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki D, Kawaguchi H. Colloid and Polymer Science. 2006;284:1471–1476. [Google Scholar]
- 9.Liu R, Qin P, Wang L, Zhao X, Liu Y, Hao X. Journal of biochemical and molecular toxicology. 2010;24:66–71. doi: 10.1002/jbt.20314. [DOI] [PubMed] [Google Scholar]
- 10.Duncan B, Kim C, Rotello VM. Journal of controlled release3: official journal of the Controlled Release Society. 2010 doi: 10.1016/j.jconrel.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghosh P, Han G, De M, Kim CK, Rotello VM. Advanced drug delivery reviews. 2008;60:1307–15. doi: 10.1016/j.addr.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 12.Hong R, Han G, Fernández JM, Kim B-jin, Forbes NS, Rotello VM. Journal of the American Chemical Society. 2006;128:1078–9. doi: 10.1021/ja056726i. [DOI] [PubMed] [Google Scholar]
- 13.Roh KH, Yoshida M, Lahann J. Materialwissenschaft und Werkstofftechnik. 2007;38:1008–1011. [Google Scholar]
- 14.Adams DJ, Adams S, Melrose J, Weaver AC. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2008;317:360–365. [Google Scholar]
- 15.Bao Z, Chen L, Weldon M, Chandross E, Cherniavskaya O, Dai Y, Tok JBH. Chemistry of Materials. 2002;14:24–26. [Google Scholar]
- 16.Paunov VN, Cayre OJ. Advanced Materials. 2004;16:788–791. [Google Scholar]
- 17.Zhang Y, Hoppe AD, Swanson JA. PNAS. 2010 [Google Scholar]
- 18.Cheng Y, Liu Y, Huang J, Li K, Zhang W, Xian Y, Jin L. Talanta. 2009;77:1332–6. doi: 10.1016/j.talanta.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 19.Deckman HW. Applied Physics Letters. 1982;41:377. [Google Scholar]
- 20.Bhumkar DR, Joshi HM, Sastry M, Pokharkar VB. Pharmaceutical research. 2007;24:1415–26. doi: 10.1007/s11095-007-9257-9. [DOI] [PubMed] [Google Scholar]
- 21.Heo DN, Yang DH, Moon HJ, Lee JB, Bae MS, Lee SC, Lee WJ, Sun IC, Kwon IK. Biomaterials. 2012;33:856–66. doi: 10.1016/j.biomaterials.2011.09.064. [DOI] [PubMed] [Google Scholar]
- 22.Sulchek Ta, Friddle RW, Langry K, Lau EY, Albrecht H, Ratto TV, DeNardo SJ, Colvin ME, Noy A. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:16638–43. doi: 10.1073/pnas.0505208102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stone JD, Cochran JR, Stern LJ. Biophysical Journal. 2001;81:2547–2557. doi: 10.1016/S0006-3495(01)75899-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fluorescence analysis of gold-coated silica particles with no proteins. The particles show low fluorescence in both channels.
Rotating silica microparticle processed with thiol-PEG-biotin/APTES followed by BSA/SA cross-linking.