SUMMARY
The ability of natural-killer cells to regulate adaptive immunity is not well understood. Here we define an interaction between the class Ib major histocompatibility complex (MHC) molecule Qa-1–Qdm on activated T cells responsible for adaptive immunity and CD94–NKG2A inhibitory receptors expressed by natural-killer cells by using Qa-1-deficient and Qa-1 knockin mice containing a point mutation that selectively abolishes Qa-1–Qdm binding to CD94–NKG2A receptors. The Qa-1–NKG2A interaction protected activated CD4+ T cells from lysis by a subset of NKG2A+ NK cells and was essential for T cell expansion and development of immunologic memory. Antibody-dependent blockade of this Qa-1–NKG2A interaction resulted in potent NK-dependent elimination of activated autoreactive T cells and amelioration of experimental autoimmune encephalomyelitis. These findings extend the functional reach of the NK system to include regulation of adaptive T cell responses and suggest a new clinical strategy for elimination of antigen-activated T cells in the context of autoimmune disease and transplantation.
INTRODUCTION
There is increasing evidence that maintenance of immunological self-tolerance is an active process that requires the participation of cells belonging to both the adaptive and innate immune systems. Although considerable progress has been made in understanding the regulatory role of subsets of T lymphocytes in this process (Paust and Cantor, 2005), the contribution of innate immune mechanisms to self-tolerance is less well defined.
The ability of natural-killer (NK) lymphocytes to recognize and efficiently lyse activated T cells in vitro may allow these cells to participate in this process, e.g., through inhibition of clonal expansion of T cells activated by foreign or self-antigens in vivo (Rabinovich et al., 2003; Xu et al., 2005). NK cells may also downregulate adaptive immune responses through elimination of antigen-presenting dendritic cells (DCs) or through production of inhibitory cytokines (Piccioli et al., 2002). One approach to defining the regulatory role of NK cells comes from the finding that NK cell activation is tightly constrained by an interaction between Qa-1–Qdm (Qa-1 determinant modifier, AMAPRTLLL) ligands on target cells and inhibitory CD94–NKG2A receptors on activated NK cells, here termed the Qa-1–NKG2A interaction (Vance et al., 1998).
The Qa-1–Qdm ligand is a heterodimer composed of the major histocompatibility complex (MHC) class Ib molecule Qa-1 (H2-T23; the murine homolog of human leukocyte antigen-E [HLA-E]), β2m, and peptides derived in TAP-dependent fashion from the MHC class I leader sequences (Qdm) (Aldrich et al., 1992; Lo et al., 1999, 2000; Sullivan et al., 2002). The interaction between Qa-1–Qdm and CD94–NKG2A generally inhibits NK (or CD8+ CTL) activity (Moser et al., 2002). Although Qa-1 is expressed in many cell types at the RNA level (Transy et al., 1987), expression of the Qa-1–Qdm surface protein is restricted to activated T and B lymphocytes and dendritic cells (Soloski et al., 1995; Sullivan et al., 2002), allowing this ligand to selectively mark the central triad of immunological cells.
We tested the hypothesis that an inhibitory interaction between the class Ib MHC molecule Qa-1–Qdm on activated T cells and the CD94–NKG2A receptor on NK cells (1) protects activated T cells from NK lysis and (2) is essential for clonal expansion and development of immunologic memory by self-reactive T cells. We found that Qa-1-deficient CD4+ T cells failed to undergo antigen-induced or homeostatic expansion secondary to increased susceptibility to lysis by a defined subset of NK cells. Further, lentiviral-mediated expression of Qa-1–Qdm on autoreactive CD4+ T cells rescued expansion of self-reactive T cell clones whereas antibody-dependent interruption of the Qa-1–NKG2A interaction dampened development of experimental autoimmune encephalomyelitis (EAE).
RESULTS
Defective Expansion of Qa-1-Deficient CD4+ T Cells by NK Lysis
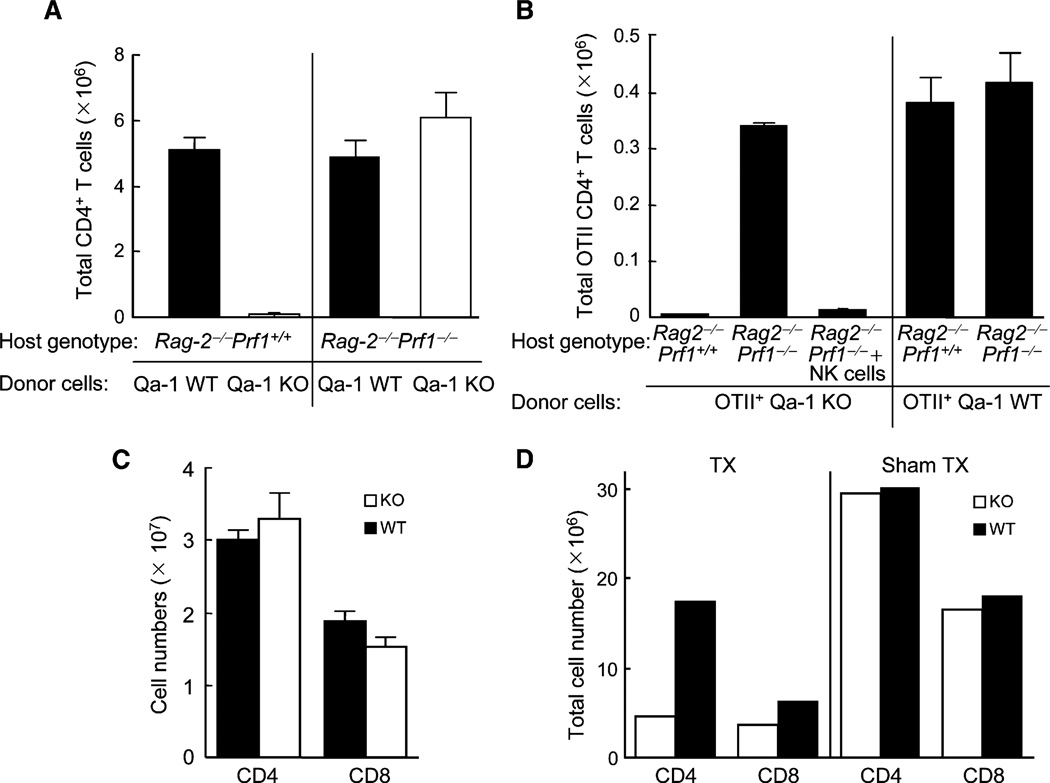
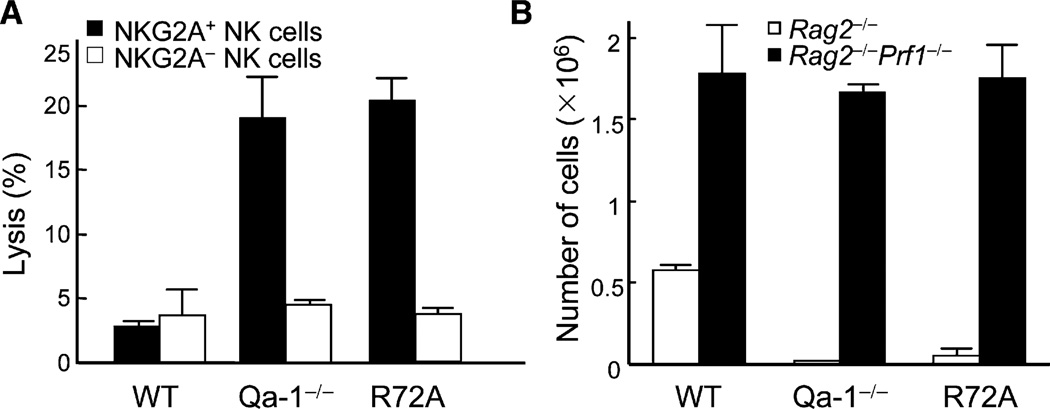
We initially defined the contribution of Qa-1 to protect expanding CD4+ T cells from NK lysis in Rag2-deficent hosts. Qa-1-deficient CD4+ T cells failed to undergo homeostatic expansion over a 2 week period in Rag2−/− hosts, in contrast to vigorous expansion by Qa-1 wild-type (WT) CD4 cells. Defective expansion of Qa-1-deficient CD4 cells in Rag2−/− (NK cell-sufficient) hosts was restored in Rag2−/−Prf1−/− (NK cell-deficient) hosts (Figure 1A). Expansion of Qa-1-deficient CD4+ T cells expressing the OTII T cell receptor (TCR), specific for chicken ovalbumin (OVA) 323–339 peptide bound to MHC class IIb, was also severely impaired in Rag2−/− hosts and restored in Rag2−/−Prf1−/− hosts. This model measures antigen-dependent expansion of CD4 cells because significant numbers of OTII CD4+ T cells were not recovered at day 7 and 14 unless OVA peptide was provided at day 0. Perforin-dependent reduction of Qa-1-deficient CD4+ T cells in Rag2−/− hosts depended on NK cells, because Rag2−/−Prf1−/− hosts reconstituted with purified NK cells prevented antigen-induced expansion of Qa-1-deficient OTII+ CD4 cells (Figure 1B). Thus, expression of Qa-1 protected activated CD4 cells from NK lysis and was essential for both homeostatic and antigen-induced CD4-cell expansion.
Figure 1. Proliferation of Qa-1 WT and Qa-1-Deficient CD4+ T Cells.
(A) CD4+ T cells isolated from either C57BL/6 (B6) Qa-1WTor B6 Qa-1-deficient (N11) mice were transferred into syngeneic Rag2−/− or Rag2−/−Prf1−/− hosts. 14 days later, CD4+ T cells from spleen and LN were enumerated. Data are from one representative experiment of three in which each group consisted of 3–4 mice. Graphs show mean ± SD (n = 3).
(B) Purified Qa-1 WT or Qa-1-deficient OTII CD4+ T cells were transferred (106/mouse) into Rag2−/− or Rag2−/−Prf1−/− hosts along with immunization of 50 µg OVA peptide emulsified in CFA. Proliferation of OTII cells was monitored by enumeration of CD4+Vβ5+ cells in LN and spleen 14 days later. NK cells (0.5 × 106) purified from splenocytes of Rag2−/− mice were cotransferred with Qa-1-deficient OTII cells into Rag2−/− Prf1−/− hosts, as indicated. Data are from one representative experiment of three in which each group consisted of 3–4 mice. Graphs show mean ± SD (n = 3).
(C) Peripheral T cell numbers in B6 Qa-1 WT and Qa-1-deficient mice: Total numbers of CD4+ and CD8+ T cells from pooled spleen and lymph node of 5- to 6-week-old Qa-1-deficient (KO) mice or its wild-type (WT) littermates (4–6 mice/group). Graphs show mean ± SD (n = 4).
(D) Qa-1-deficient or WT mice were thymectomized (TX) or sham thymectomized at around 10 weeks age. The numbers of CD4+ and CD8+ cells in peripheral lymphoid tissues 6 weeks after either thymectomy or sham thymectomy of Qa-1 WT or Qa-1-deficient mice at 10 weeks of age (3 mice/group) were measured. Data shown represent the average of 3 pooled mice.
Decreased Lifespan of Qa-1-Deficient CD4+ T Cells
An important prediction of the hypothesis that Qa-1 expression on CD4 cells protects them from NK-dependent destruction in vivo is that Qa-1-deficient mice should harbor reduced numbers of CD4 cells. However, examination of adult Qa-1-deficient mice did not reveal this phenotype (Figure 1C). Possibly, continuous resupply of T cells from the thymus compensated for NK-dependent elimination of Qa-1-deficient T cells in peripheral tissues. If so, removal of the thymus from adult Qa-1-deficient mice might uncover this phenotype. This was indeed the case. Within 6 weeks after thymic ablation, Qa-1-deficient mice displayed a substantial (>85%) reduction in CD4+ T cells compared with Qa-1 WT mice (Figure 1D). Thus, expression of Qa-1 was essential to ensure an unimpaired lifespan of CD4 cells in peripheral tissues.
The Contribution of Qa-1 Expression to Memory Development by CD4 Cells
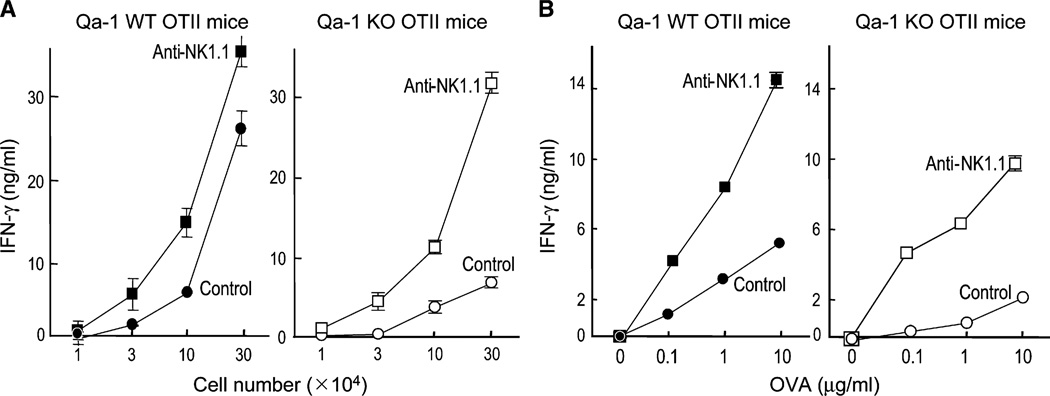
Impaired antigen-induced expansion of Qa-1-deficient OTII CD4+ T cells in Rag2−/− but not Rag2−/−Prf1−/− mice suggested that Qa-1 expression protects activatedCD4+ T cells from perforin-dependent killing. We studied memory development in mice that express the OTII TCR. Virtually all peripheral T cells in OTII C57BL/6 (B6) mice are CD4+ T cells, and these mice lack CD8+ T cells. After in vitro OVA peptide restimulation of purified CD4+ T cells from OVA-immune OTII donor mice, Qa-1-deficient CD4+ T cells displayed a >90% reduction in IFN-γ responses. This reflected increased susceptibility of Qa-1-deficient CD4+ T cells to NK lysis, because antibody-dependent depletion of NK cells during in vivo priming allowed Qa-1-deficient CD4+ T cells to develop unimpaired peptide-recall responses (Figures 2A and 2B).Analysis of the cytokine response of Qa-1-deficient or Qa-1 WT OTII CD4+ T cells 7–10 days after injection into Rag2−/−Prf1−/− hosts along with OTII peptide showed that the recall IFN-γ response of the two groups of CD4+ T cells was indistinguishable (Figure S1 in the Supplemental Data available online). Thus, Qa-1 expression was essential for the development of CD4+ T cell memory responses after antigen stimulation in vivo.
Figure 2. Cytokine Recall Response upon OTII Peptide Immunization.
Qa-1 WT or Qa-1-deficient mice bearing the OTII TCR transgene were immunized with 25 µg OVA peptide emulsified in CFA. 14 days later, OTII CD4+ T cells were purified from draining LN. To deplete NK cells, one group of mice was injected i.v. with NK1.1 antibody (200 µg/ mouse) at day −1 and day 6 after peptide immunization. Control mice were injected with PBS (3–4 mice/group).
(A) Increasing numbers of purified CD4+ T cells were stimulated in vitro with OVA peptide (10 µg/ml) and irradiated splenocytes (4 × 105). Supernatants were collected 48 hr later and tested for IFN-γ by ELISA. Data shown represent mean ± SD (n = 3).
(B) Purified OTII CD4+ T cells (1 × 105) were stimulated with the indicated concentrations of OVA peptide before collection of culture supernatant 48 hr later and testing for IFN-γ by ELISA. Data shown represent mean ± SD (n = 3).
Defective Responses of Qa-1-Deficient CD4+ T Cells to Antigen in BM Chimeras
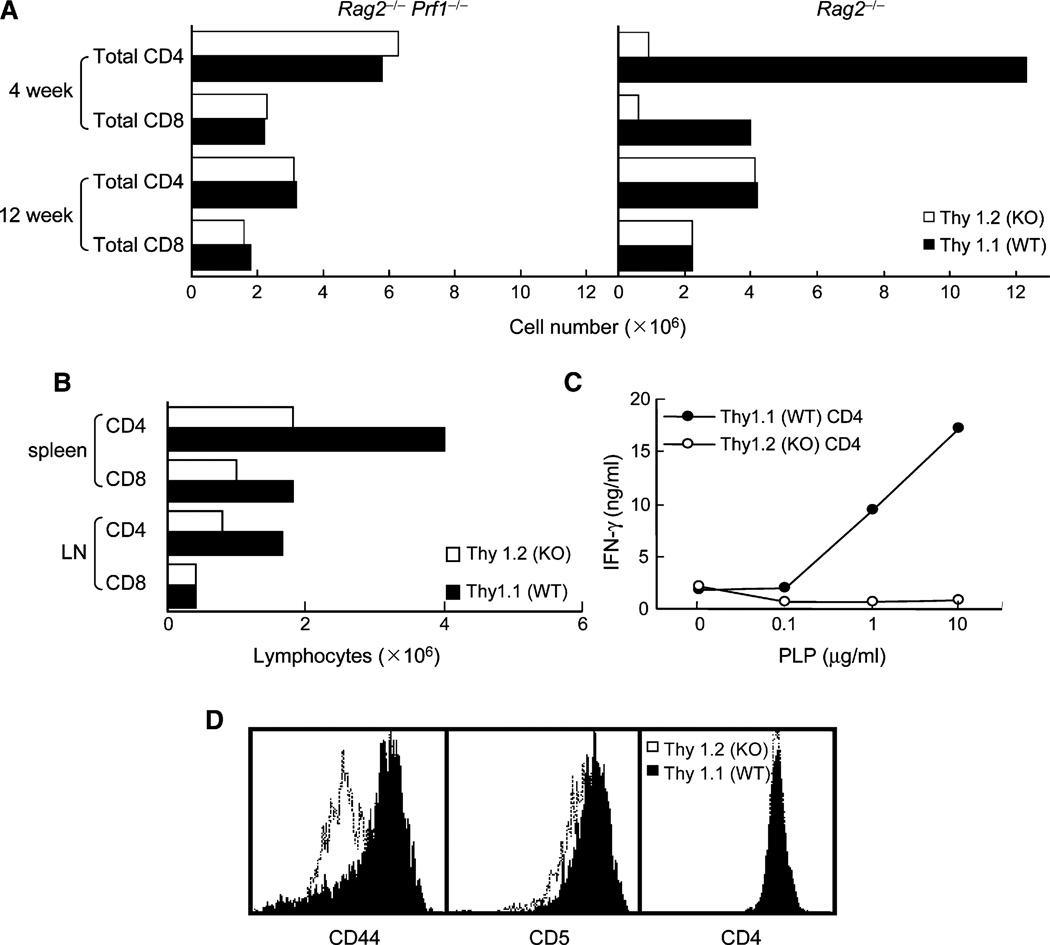
Loss of Qa-1 expression by hematopoietic cells in Qa-1-deficient mice might enhance NK reactivity, as noted for MHC class Ia antigens, or otherwise alter NK-cell development. To address this issue, we analyzed mixed bone-marrow (BM) chimeras containing both Qa-1+ and Qa-1− hematopoietic cells. Lethally irradiated Rag2−/− mice were reconstituted with Qa-1 WT (Thy1.1) plus Qa-1-deficient (Thy1.2) BM and analyzed 4 or 12 weeks later. The numbers of Qa-1-deficient CD4+ (and CD8+) lymphocytes were reduced at 4–6 weeks in Rag2−/− but not Rag2−/−Prf1−/− hosts. However, by 12 weeks of BM reconstitution, the numbers of Qa-1-deficient CD4+ T cells were similar to that of Qa-1 WT CD4+ T cells (Figure 3A), similar to studies of intact Qa-1-deficient mice that indicated continuous T cell replenishment by the thymus (see Figures 1C and 1D). We then compared the impact of antigen stimulation on the Qa-1-deficient and Qa-1 WT cohorts of CD4+ T cells in these BM chimeras. Although both Qa-1 WT and Qa-1-deficient CD4 cells were well represented in these chimeric mice (Figure 3B), after immunization with PLP peptide, only Qa-1 WT CD4+ T cells responded to peptide restimulation as judged by IFN-γ production (Figure 3C). Further analysis of CD4+ T cells in these BM chimeras showed that the Qa-1+ fraction of CD4+ T cells expressed substantially higher amounts of CD44 and CD5 (Figure 3D). Thus, expression of Qa-1 was essential to allow antigen-stimulated CD4+ T cells to develop memory activity as judged by surface markers and cytokine recall responses.
Figure 3. Development and Function of Qa-1 WT and Qa-1-Deficient T Cells in Mixed Bone-Marrow Chimeras.
(A) Hematopoietic stem cell (HSC)-enriched bone-marrow cells (106/mouse) from Qa-1 WT (Thy1.1) and Qa-1-deficient (KO; Thy1.2) mice was cotransferred into Rag2−/− or Rag2−/−Prf1−/− hosts and sacrificed at 4 or 12 weeks after transfer (4 mice/group). The total number of peripheral CD4+ and CD8+ T cells from spleen and LN were enumerated. Data shown represent the average of 3 pooled mice.
(B) BM chimeric hosts were immunized subcutaneously with PLP peptide 12 weeks after adoptive transfer of BM cells. 14 days after immunization, total CD4+ and CD8+ T cell numbers in draining LN were enumerated. Data shown represent the average of 3 pooled mice.
(C) Pooled (n = 3) Qa-1 WT (Thy1.1) and Qa-1-deficient (Thy1.2) CD4+ T cells were purified from draining LN and stimulated with PLP peptide together with irradiated splenocytes. IFN-γ secretion was measured after 48 hr.
(D) The indicated subset of CD4+ T cells from the draining LN were analyzed for CD44 and CD5 expression by FACS. Gated on CD4 cells: the solid line represents Thy1.1 (WT); the dotted line represents Thy1.2 (KO).
Defective EAE Induction by Qa-1-Deficient CD4+ T Cells Reflects Susceptibility to NK Lysis
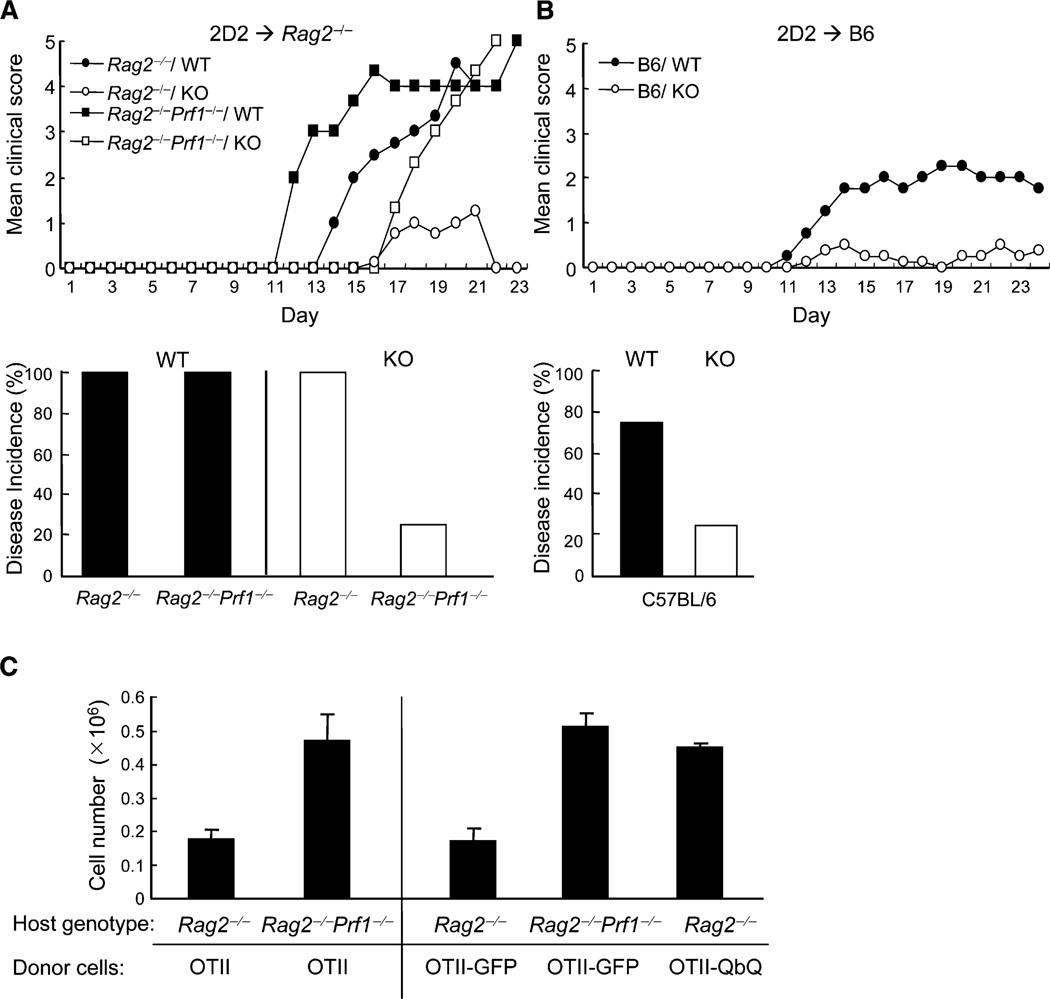
To investigate the potential impact of Qa-1–NKG2A interactions on expansion of autoreactive CD4+ T cells and consequent autoimmune disease, we analyzed the response of CD4+ T cells that express a transgenic TCR (2D2) specific for myelin oligodendrocyte glycoprotein (MOG) autoantigen and induce EAE in adoptive hosts (Bettelli et al., 2003). Transfer of 2D2 CD4+ T cells (106) into Rag2−/− hosts stimulated with 10 µg of MOG peptide and pertussis toxin resulted in fulminating EAE that was lethal within 3 weeks. In contrast, Rag2−/− mice reconstituted with Qa-1-deficient 2D2 CD4+ T cells developed little or no disease after challenge with MOG–Complete Freund’s Adjuvant (CFA) and pertussis toxin (Figure 4A). Defective responses of Qa-1-deficient autoreactive CD4 cells reflected increased susceptibility to perforin-dependent lysis, because Rag2−/−Prf1−/− hosts reconstituted with 2D2+ Qa-1− or Qa-1+ CD4 cells developed robust EAE that led to death in approximately 3 weeks after disease induction. Finally, Qa-1-dependent protection of autoreactive T cells from NK lysis was not limited to lymphopenic Rag2−/− hosts. Intravenous transfer of 2D2 Qa-1 WT CD4+ T cells but not 2D2 Qa-1-deficient CD4+ T cells induced EAE in C57BL/6 recipients (Figure 4B). Thus, Qa-1 expression by pathogenic autoreactive CD4+ T cells was essential for protection of these cells against perforin-dependent lysis and allowed them to induce EAE in both C57BL/6 WT and C57BL/6 Rag2−/− hosts.
Figure 4. Reduced Ability of Qa-1-Deficient 2D2 CD4+ T Cells to Transfer EAE into Rag2−/− Mice.
(A) 2D2 CD4+ T cells (1 × 106) from either Qa-1 WT or Qa-1-deficient mice were transferred into syngeneic Rag2−/− or Rag2−/−Prf1−/− mice (5 mice/group) immunized with MOG 35–55 and CFA and pertussis toxin. Development of EAE was scored as described in Experimental Procedures. The bar graph represents the disease incidence of the different groups.
(B) 2D2 CD4+ T cells (1 × 106) from either Qa-1 WT or Qa-1-deficient mice were transferred into C57BL/6 hosts (5 mice/group) followed by immunization with (150 µg) MOG 35–55 peptide with pertussis toxin on the same day and day 2 to induce EAE. The development of EAE is shown, as in (A), and disease incidence of the different groups is shown in the bar graph.
(C) Antigen-driven proliferation of OTII cells in Rag2−/− and Rag2−/−Prf1−/− hosts. OTII CD4+ T cells (1 × 106) were transferred into Rag2−/− or Rag2−/− Prf1−/− mice (4 mice/group) before immunization with OVA peptide (75 µg peptide–CFA) immediately after transfer. Mice were taken down 72 hr later, and total OTII cells in the draining LN and spleen were enumerated. On the right panel, OTII CD4+ T cells infected with lentivirus expressing either GFP control or Qdm-β2m-Qa-1 fusion protein were also transferred into Rag2−/− or Rag2−/−Prf1−/− hosts (4 mice/group) before immunization with OVA peptide (75 µg peptide–CFA). 72 hr later, expansion of OTII cells was analyzed as described above. Data shown represent mean ± SD of the recovery OTII CD4+ T cells from draining lymph nodes.
Lentiviral-Mediated Expression of Qa-1–Qdm Confers Protection to CD4+ T Cells
The findings that (1) responses of Qa-1-deficient CD4+ T cells to foreign and self-antigens were defective in Rag2−/−Prf1−/− hosts and (2) depletion of NK cells restored peptide-recall responses of Qa-1-deficient mice and enhanced the CD4 response in Qa-1 WT OTII mice suggest that Qa-1–Qdm expression by CD4+ T cells may protect them from NK lysis. To further evaluate this hypothesis, we analyzed antigen-dependent responses of Qa-1 WT OTII CD4+ T cells in Rag2−/− or Rag2−/− Prf1−/− hosts. Expansion of OTII cells in Rag2−/− was delayed compared to Rag2−/−Prf1−/− hosts, and the numbers of OTII cells in draining LN of Rag2−/− mice were reduced by more than 50% on day 3 after stimulation compared with Rag2−/−Prf1−/− hosts (Figure 4C). More importantly, expression of a Qdm–β2m–Qa-1 fusion protein by OTII cells restored proliferation of OTII cells in Rag2−/− hosts to amounts similar to that observed in Rag2−/−Prf1−/− hosts (Figure 4C, right), directly supporting the hypothesis that antigen-induced proliferation of T cells requires Qa-1–Qdm-dependent protection from NK lysis.
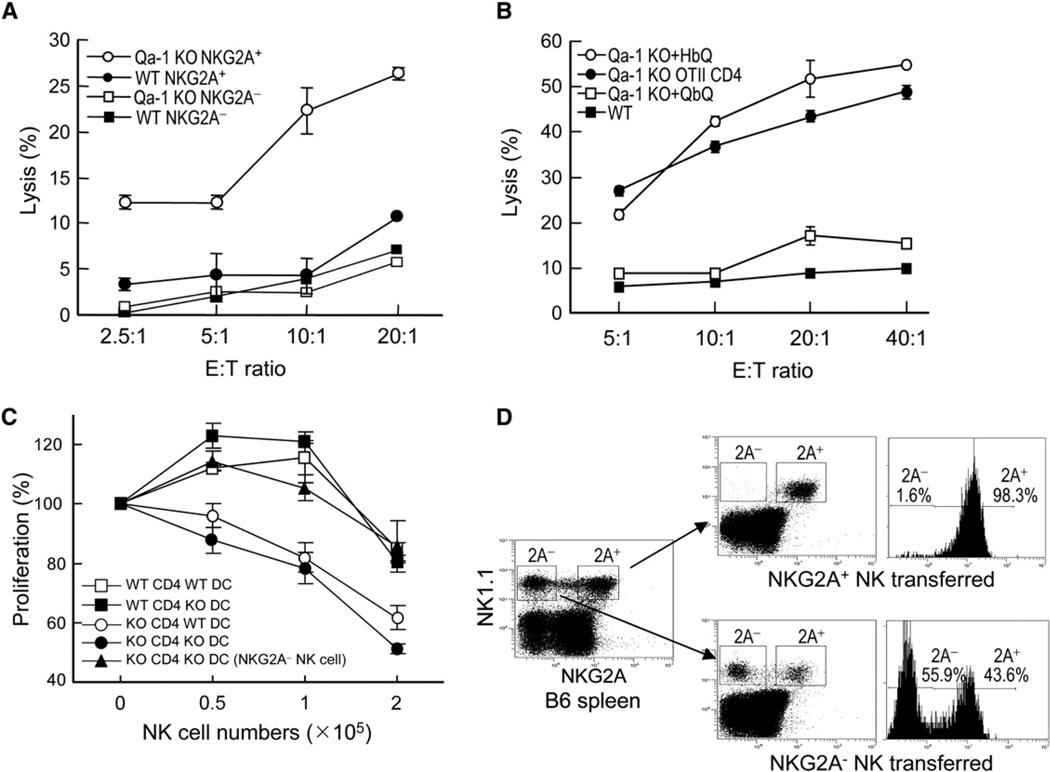
Qa-1-Deficient Activated CD4+ T Cells Are Regulated by the NKG2A+ Subset of NK Cells
About half of NK cells from C57BL/6 mice expressed NKG2A (NKG2A+ NK cells) and half did not (NKG2A− NK cells); both NKG2A phenotypes were stable after 1 week of in vitro culture with IL-2 (Figure S2A). We then tested these cells for lysis of Qa-1-deficient and WT CD4 cells (see Experimental Procedures). The NKG2A+ but not NKG2A− NK-cell subset lysed Qa-1-deficient cells (Figure 5A). This finding indicated that lytic activity is invested in an NK subset that expresses NKG2A+ and therefore can bind to Qa-1–Qdm. The implications of this finding require further investigation. Although NKG2A− NK1.1+ cells did not lyse Qa-1-deficient CD4+ T cells, they efficiently lysed H-2Kb-deficient CD4+ T cells in a Qdm peptide-independent fashion (Figure S1). The NKG2A+ subsets also lysed H-2Db-deficient cells because of the lack of Qdm peptide. Lysis was inhibited by addition of Qdm peptide (which reconstituted the Qa-1–Qdm complex on H2-Db-deficient cells). The addition of Qdm peptide also partially inhibited lysis of H-2KbDb double-deficient cells by NKG2A+ NK cells but not by NKG2A− NK cells (Figures S2B and S2C). In addition, the lytic activity of NKG2A+ and NKG2A− NK cells against MHC-deficient target L cells was similar to that exerted against H-2KbDb-deficient target cells: both NK subsets lysed L cells equally well, and lysis by NKG2A+ cells was partially inhibited by expression of Qa-1–Qdm (Figure S2D).
Figure 5. In Vitro Lysis of Qa-1-Deficient CD4+ T Cells by IL-2-Activated NKG2A+ NK Cells.
(A) NKG2A+ and NKG2A− NK cells isolated from B6 mice and activated individually by IL-2 were used at the indicated E:T ratios as effector killer cells to kill ConA-activated CD4+ T cells from Qa-1 wild-type and Qa-1-deficient mice in a standard killing assay. This experiment is representative of a total of four experiments. Data shown represent mean ± SD (n = 3).
(B) Protection of Qa-1-deficient CD4 cells from NK lysis by lentiviral-mediated surface expression of a covalent Qdm-β2m-Qa-1 complex. Qa-1-deficient (Qa-1 KO) OTII cells were infected with lentivirus expressing either a Qdm-β2m-Qa-1 fusion protein (QbQ) or a HSP60 peptide-β2m-Qa-1 fusion protein (HbQ) and used as target cell in the killing assay by NKG2A+ NK cells. Qa-1 WT and Qa-1-deficient OTII cells were used as control. Percentage of killing is shown at the indicated E:T ratios.
(C) CD4 cells were purified from Qa-1 WT and Qa-1-deficient OTII TCR transgenic mice, and splenic DCs were purified from Qa-1 WT and Qa-1-deficient mice and activated by anti-CD40. NKG2A+ and NKG2A− NK cells were purified from B6 mice and stimulated with 1000 U/ml IL-2 for 5 days. OTII CD4+ T cells (5 × 104) were stimulated with 1 µg/ml OVA peptide and 2.5 × 104 activated DC. The indicated numbers of IL-2-activated NK cells were added to cultures 48 hr before proliferation of CD4+ T cells was measured. Data shown represent mean ± SD (n = 3).
(D) Adoptive transfer and homeostatic expansion of NK cells. NKG2A− and NKG2A+ NK cells were purified from the spleens of B6 mice by FACS sorting and injected i.v. (5 × 105) into Rag2−/− γc-deficient mice. 10 days later, spleens of host mice were analyzed for NK cells by staining with antibodies against NK1.1 and NKG2A. This result represents three different experiments with 3–4 mice/group.
Increased susceptibility of Qa-1-deficient CD4+ T cells to NK-cell lysis reflected defective expression of surface Qa-1–Qdm (rather than altered development of CD4+ T cells in Qa-1-deficient mice), because lentiviral-dependent re-expression of Qa-1 heavy chain covalently attached to a Qdm peptide efficiently restored resistance of Qa-1-deficient cells to NK lysis (Figure 5B). In contrast, lentiviral introduction of Qa-1 heavy chain covalently linked to a different peptide (from HSP60) did not protect these cells (Figure 5B). These findings indicated that protection of CD4+ T cells was mediated by the Qa-1–Qdm ligand rather than Qa-1 complexed to other peptides. The hypothesis that the NKG2A+ fraction of NK cells is selectively equipped to monitor cellular Qa-1–Qdm expression was supported by an examination of the regulatory activity of NK-cell subsets. Titration ofNKG2A+ but not NKG2A− NK cells into cultures containing Qa-1-deficient or Qa-1 WT OTII CD4 cells revealed a dose-dependent inhibition of the former response by NKG2A+ but not NKG2A− NK cells (Figure 5C).
The surface phenotype and function of both NK subsets were stable after 5 days in culture in the presence of IL-2 in vitro (Figure S1). Because NK cells, like other lymphocytes, undergo homeostatic expansion in lymphopenic hosts, we transferred NKG2A+ or NKG2A− NK cells into Rag2−/− common γ chain (γc)-deficient hosts to evaluate the stability of the NKG2A+ subset in vivo. 2 weeks after adoptive transfer of purified NKG2A+ NK1.1 cells into Rag2−/− γc-deficient hosts, these cells maintained expression of NKG2A on their surface, indicating that the NKG2A+ phenotype marks a stable subset of NK cells in vivo (Figure 5D). Because NK cells developing from fetal progenitors acquire expression of NKG2A and Ly49 in a random manner, differential expression of Ly49 gene products on NKG2A+ and NKG2A− NK cells might account for their ability to lyse “activated self” targets. NKG2A+ and NKG2A− NK cells may also express different amounts of activating receptors such as NKG2D (Rabinovich et al., 2003). Examination of Ly49C+I expression by NKG2A+ and NKG2A− NK cells indicated that roughly 2/3 of each NK subset is Ly49I+C. Whether the inhibitory Ly49C+I receptor might regulate lysis of MHC class I+–Qa-1-deficient target cells by NKG2A+ NK cells requires further study.
Selective Disruption of the Interaction between Qa-1 and CD94-NKG2A by a Point Mutation
We have shown that Qa-1-deficient CD4+ T cells were susceptible to lysis by NKG2A+ NK cells and failed to develop immunological memory in vivo. However, genetic deletion of Qa-1 and insertion of a Neor gene into the MHC class I locus might affect expression of linked MHC genes or alter surface protein display in a way that increases cellular susceptibility to lysis. A direct molecular approach to this question came from the definition of a Qa-1 amino acid mutation that eliminated binding activity to the CD94–NKG2A receptor but spared other Qa-1-dependent biological activities.
The solved structure of the human HLA-E molecule and mutagenic analysis of its interaction with CD94–NKG2 molecules (Wada et al., 2004) allowed us to identify potential residues that may be essential for the Qa-1 interaction with CD94–NKG2A but do not interfere with its interaction with the T cell receptor. These residues included R65, D69, Q72, R75, R79, H155, D162, and E166, which are conserved in the mouse Qa-1 molecule. Five Qa-1 mutations (D69A, R72A, R75A D162A, and E166A) were tested for their impact on the functional interaction of Qa-1 with CD94–NKG2A. The R72 residue at the top of the α1 domain of Qa-1 was the most critical for functional interactions with the CD94–NKG2A receptor: Qa-1 R72A mutants failed to protect L cells from lysis by NKG2A+ NK cells but were fully able to target Qa-1-specific alloreactive CD8 T cell lysis (Figures S3A and S3C). Mutations of Qa-1 at R75, D162, and E166 did not impair Qa-1–NKG2A interactions, and these Qa-1 mutants fully protected L cells from NKG2A+ NK-cell lysis (Figures S3A and S3B). Finally, although the D69A mutation impaired protection from NK lysis, this mutation also interfered with detection of the molecule by Qa-1 antibody and was therefore not chosen for further study (Figures S3A and S3B).
Qa-1 R72A Mutant Knockin Mice Display the Qa-1-Deficient Phenotype
We generated Qa-1 R72A mutant knockin mice by using the cre-loxP system (Figures S4A and S4B). Although the R72A mutant was expressed on activated T cells at amounts similar to the WT protein in WT mice (Figure S4C), Qa-1 R72A mutant T cells were no longer protected from NK lysis and were as vulnerable as Qa-1-deficient T cells (Figure 6A). Next, CD4+ T cells from Qa-1 R72A mutant mice were transferred into Rag2−/− and Rag2−/−Prf1−/− hosts and examined for homeostatic expansion. CD4+ T cells expressing the Qa-1 R72A point mutation expressed the phenotype of Qa-1-deficient cells and failed to expand in Rag2−/− hosts but expanded as well as Qa-1 WT cells in Rag2−/−Prf1−/− hosts (Figure 6B). These results reinforced findings with Qa-1-deficient mice that Qa-1-dependent protection of CD4+ T cell expansion and adaptive memory responses depends entirely on its interaction with CD94–NKG2A. The generation and validation of Qa-1 R72A mutant mice also provided a unique mouse model to examine the functional outcome of defective Qa-1–NKG2A interactions on immune responses without affecting other antigen-presenting properties of the Qa-1 molecule.
Figure 6. NK Killing Susceptibility of Qa-1-Deficient Cells Can Be Mimicked by a Point Mutation at R72 that Disrupts Its Binding to NKG2A.
(A) NK cell susceptibility of R72A mutant CD4 cells. CD4+ T cells from Qa-1 WT, Qa-1-deficient, and Qa-1-R72A mutant mice were activated by ConA for 48 hr, labeled with 51Cr, and used as targets for IL-2-activated NKG2A+ and NKG2A− NK cells in a standard 4 hr killing assay. Percentage of killing at E:T ratio of 20:1 is shown. Data shown represent mean ± SD (n = 3).
(B) Homeostatic expansion of R72A mutant CD4 cells. CD4+ T cells (1 × 106) were isolated from Qa-1 WT, Qa-1-deficient, and Qa-1-R72A mutant mice and i.v. transferred into Rag2−/− and Rag2−/−Prf1−/− host (3 mice/group). 14 days later, host mice were killed and total CD4+ T cells were enumerated from spleen and LN. Data shown represent mean ± SD (n = 3).
Antibody-Dependent Blockade of Qa-1–NKG2A Interaction Inhibits Development of EAE
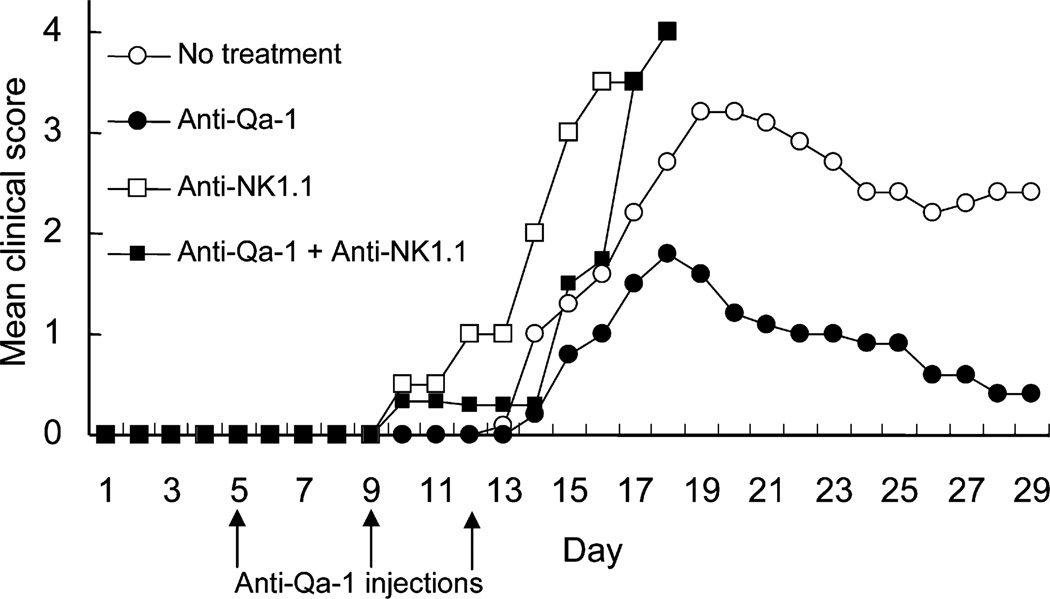
To determine whether blockade of this interaction with Qa-1 antibody inhibits the development of EAE, we administered Qa-1 antibody to mice during the period preceding clinical signs of disease and preceding the development of Qa-1-restricted CD8+ regulatory T cells (Jiang et al., 1992; Hu et al., 2004). This protocol markedly reduced the intensity of EAE. The therapeutic effects of Qa-1-antibody injection depended on the action of NK cells, because hosts depleted of NK cells with NK1.1 antibody did not display a therapeutic response to treatment with Qa-1 antibody (Figure 7). These observations indicate that the autoimmune CD4+ T response responsible for EAE can be almost fully ameliorated by antibody-dependent blockade of the Qa-1–NKG2A interaction and associated NK-dependent elimination of pathogenic T cells. Although murine Qa-1 is structurally similar to human HLA-E, potential differences in surface expression (Lee et al., 1998) and increased numbers of MHC peptides stabilized by HLA-E compared with murine Qa-1 needs to be taken into consideration when applying these murine findings to studies of the interaction of HLA-E and human NK cells.
Figure 7. Antibody Blockade of Qa-1–NK Cell Interaction Inhibits the Development of EAE.
EAE were induced in C57BL/6 mice as described in Experimental Procedures via MOG peptide. Qa-1 antibody was injected i.v. (200 µg/injection) at days 5, 9, and 12 after peptide immunization. In a separate group, anti-NK1.1 was administered (150 µg, i.v.) at days 3, 6, and 10 to deplete NK cells with or without injection of Qa-1 antibody on days 5, 9, and 12, as above. Control groups were injected with either PBS or mouse IgG1 isotype control antibody (5 mice/group). The development of disease was monitored as described in Experimental Procedures. Data shown represent two independent experiments.
DISCUSSION
Activation of T cells is thought to be regulated by cells belonging to both the adaptive and innate immune systems. Although the role of CD25+ Foxp3+ CD4+ regulatory T cells has been the object of intense study, the regulatory activity of cells belonging to the innate immune system has been given less experimental attention. Although analyses of murine autoimmune disease models have indicated that depletion of NK cells can augment EAE (Zhang et al., 1997; Xu et al., 2005), whereas activation of NK cells can inhibit disease (Wang et al., 2006), the mechanism responsible for these effects is not well understood. NK cells are equipped to recognize and lyse activated T cells (Rabinovich et al., 2003; Gasser et al., 2005), and interactions between NK cells and antigen-presenting cells may inhibit the activation of T helper cells (Gerosa et al., 2002; Martin-Fontecha et al., 2004). NK cells also produce inhibitory cytokines that can modulate immunity (Mehrotra et al., 1998).
To define the molecular basis of Qa-1-dependent protection, we generated Qa-1 knockin mice that express an amino acid exchange mutation (R72A) that eliminates Qa-1 binding to CD94–NKG2A but spares binding to the TCR and CD8 coreceptor. Activated Qa-1 R72A CD4+ T cells were highly susceptible to lysis by NKG2A+ NK cells and failed to expand in vivo, which provides molecular confirmation of the role of the Qa-1–NKG2A interaction in protection of CD4+ T cells from NK lysis. Dissection of the NK cell population revealed that the NKG2A+ but not NKG2A− subset of NK cells mediate lysis of Qa-1-deficient CD4 cells. An important therapeutic corollary of these findings is that blockade of the Qa-1–NKG2A interaction that constrains NK lysis of activated T cells after immunization should result in potent NK-dependent elimination of activated autoreactive T cells and amelioration of autoimmune disease in EAE. This proved to be the case. These findings extend the functional reach of the NK system to include regulation of adaptive T cell responses and suggest that antibody-mediated blockade of Qa-1–NKG2A interactions represents a new clinical approach for elimination of antigen-activated T cells in the context of autoimmune disease or transplantation.
Qa-1 associated with its dominant peptide Qdm is the major ligand of the CD94–NKG2A receptor. Expression of Qa-1–Qdm on the surface of activated T cells may provide a sensitive monitor of altered or diminished MHC expression to NK cells after viral infection or transformation. This system is analogous to the classical interaction of Ly49 receptor isoforms with endogenous class I molecules that lead to a negative signal for NK cytotoxicity (Karlhofer et al., 1992; Litwin et al., 1994) and which may regulate NK interactions with developing thymocytes (Schott et al., 2003). Although decreased MHC class Ia expression after viral infection or cell transformation can increase susceptibility to NK lysis, this process may not be as efficient as alterations in Qa-1–Qdm expression, which has an increased off rate and shorter membrane half-life (Kraft et al., 2000). As a result, reductions in newly synthesized MHC molecules are likely to be sensed relatively early by NKG2A+ NK cells that monitor the MHC signal peptide (Qdm) complexed to Qa-1. We have previously documented a role for Qa-1-peptide ligands as a target for regulatory CD8+ T cells (Hu et al., 2004). Here we used cellular and genetic experimental approaches to study the interaction between Qa-1–Qdm and CD94–NKG2A through selective disruption of contact residues responsible for Qa-1 binding to CD94–NKG2A but not to CD8. A second Qa-1 mutant knockin mouse defective in mediating Qa-1 CD8 binding but not CD94–NKG2A has been generated to provide a simplified in vivo system that will allow further dissection of the role of the Qa-1–NKG2A and Qa-1–TCR interactions in immune regulation.
Because the level of activated NKG2A+ NK cells after infection or deliberate immunization determined the potency of NK-dependent inhibitory activity, certain infectious stimuli associated with strong NK-cell activation may be particularly susceptible to NK-dependent regulation (Wang et al., 1998). Alterations in the surface Qa-1–Qdm expression on individual clones of CD4 cells after immunization will also determine their ability to expand in the face of activated NK cells. Naive T cells, which do not express significant surface levels of Qa-1–Qdm, are not lysed by NKG2A+ NK cells, presumably because they fail to express stimulatory ligand(s) for NK-cell lysis (Raulet and Vance, 2006). CD4+ T cells become transiently susceptible to NK-dependent elimination shortly after activation (Rabinovich et al., 2003), perhaps through reductions in the ratio of Qa-1–Qdm to Qa-1 bound to other peptides that fail to interact with CD94–NKG2A (Lu et al., 2006). This window of susceptibility is apparent in vivo from enhanced CD4 responses to foreign or self-antigens after NK-cell depletion.
In addition to the protective effects of Qa-1–Qdm on the development of immunological memory, we have uncovered a division of labor between NKG2A+ and NKG2A− NK cells. The ability to monitor T cells for expression of an intact Qa-1–Qdm complex but diminished MHC class I is invested in both subsets of NK cells. The NKG2A+, but not NKG2A−, NK subset is equipped to detect missing Qa-1–Qdm-labeled complex on activated T cells and lyse these target cells. Although NKG2A− NK cells are not constrained by CD94–NKG2A-dependent inhibitory signals, these cells are unable to lyse Qa-1-deficient target cells. This does not reflect differential expression of Ly49 inhibitory and/or activation receptors on their surface (data not shown). Although Ly49-dependent regulation of NK-cell activity depends on in vivo education against the relevant H-2 molecules, this may not to be the case for the Qa-1 ligand under the experimental conditions used here. NKG2A+ NK cells from Qa-1-deficient mice displayed unimpaired lytic activity against autologous activated CD4 cells, consistent with the substantially reduced levels of T cells in Qa-1-deficient mice after elimination of new thymic immigrants by thymectomy. What about the contribution of the NKG2A protein to lysis? It may be relevant that human NK cells clones from hepatitis C virus-infected human liver are divisible into functionally distinct subsets according toNKG2A expression. NKG2A+ but not NKG2A− NK clones lyse vaccinia-infected autologous BLCL cells, while both NKG2A+ and NKG2A− clones lyse a MHC class I-negative parent BLCL cell line (Brooks et al., 2006). Although NK cells from DBA/2J mice are naturally CD94 deficient, a preliminary analysis of NK cells from DBA/2Ncr (CD94:NKG2A+) and DBA/2J (CD94:NKG2A−) failed to show a difference in NK lytic activity against autologous activated CD4+ T cells in the presence of Qa-1 antibody (not shown). Possibly, NKG2A− NK cells either tolerate Qa-1-deficient cells or are not educated to recognize and lyse Qa-1-deficient target cells (Raulet, 2006).
Monoclonal antibody therapy to selectively deplete or suppress activation of pathogenic T cells has had limited success because of poor efficacy and potentially severe side effects (Bach, 2006). These approaches have included antibodies against CD3, which can induce activation-induced apoptosis of antigen-activated T cells, and anti-CD28 or anti-CD40L, which can deplete activated CD4+ T cells. Because these antibodies often retain stimulatory or costimulatory properties, a frequent outcome of their use is wholesale and sometimes massive release of cytokines, leading to a “cytokine storm” (Suntharalingam et al., 2006). Other nonselective antibodies that have been tested in the clinic, including anti-CD52-dependent depletion of T, B, NK, and monocytes, can lead to generalized immunosuppression and associated opportunistic infections (Chatenoud, 2004), whereas anti-CD25-dependent depletion of activated T cells is now known to affect regulatory T cells as well.
Our findings suggest a new clinical approach for selective inhibition of activated pathogenic T cells that exploits the NK-dependent physiological regulatory mechanism delineated in this report. The use of antibody-dependent blockade of the Qa-1–NKG2A interaction to allow NK-cell-mediated elimination of activated CD4+ T cells has several features that distinguish it from current therapies. Current antibody-based approaches depend on direct activation or depletion of pathogenic lymphocytes, whereas interruption of the inhibitory Qa-1–NKG2A interaction to unleash NK cell-dependent elimination of activated potentially-pathogenic autoreactive cells (1) does not deliver costimulatory signals and thus avoids the risk of cytokine storm and (2) will not affect naive T cell populations and thus should preserve immunocompetence. Because propagation and exacerbation of disease is likely to depend on continuous recruitment and activation of new T cells and their subsequent differentiation into pathogenic memory and effector cells, Qa-1–NKG2A blockade after established disease may reduce disease severity by inhibition of the continued development of effector cells from newly arising autoreactive precursors.
The therapeutic effects of Qa-1–NKG2A blockade may depend in part on the levels of NK-cell activation at different stages of autoimmune inflammatory disease. Indeed, the ability of IFN-α and IFN-β to ameliorate MS in humans (Willenborg et al., 1996; Jacobs et al., 2000; Steinman and Zamvil, 2006) and of IFN-γ to inhibit EAE in mice (Durelli et al., 1995) may reflect the ability of these cytokines to transiently activate NK-dependent regulatory responses (Edwards et al., 1985). However, because IFN treatment also upregulates Qa-1 expression on T cells (Ota et al., 2005), the short duration and usually modest nature of these therapeutic effects may reflect a Qa-1-dependent decrease in NK activation and associated immunoregulatory activity. Combined treatment with recombinant IFN along with antibody-mediated interruption of Qa-1–NKG2A interactions may allow more efficient and durable therapeutic responses in MS patients. Therapeutic disruption of Qa-1–NKG2A interaction in the context of autoimmune disease might also inhibit recently activated T cells specific for foreign antigens. Therefore, vaccination against pathogens should be avoided during anti-Qa-1-mediated therapy, and vaccine-induced memory responses against pathogens should be closely monitored by skin testing. These and other therapeutic approaches to autoimmune disease depend on additional molecular insight into the regulatory interactions that govern NK cell-dependent regulation of adaptive immune responses described in this report.
EXPERIMENTAL PROCEDURES
Mice
C57BL/6, Rag2−/−, Rag2−/−perforin (Prf1)−/−, Rag2−/− common γ chain (γc)-deficient mice, H-2KbDb-deficient, H-2Kb-deficient, and H-2Db-deficient mice were purchased from Taconic Laboratories. Qa-1-deficient mice were backcrossed onto C57BL/6 for at least 11 generations and have been described previously (Hu et al., 2004). OTII TCR-transgenic mice provided by H. Ploegh (Harvard Medical School, Boston, MA) were crossed with B6.Qa-1b-deficient mice. C57BL/6 2D2 TCR transgenic mice express a TCR recognizing the CNS auto-antigen MOG (Bettelli et al., 2003) and were crossed with B6.Qa-1b-deficient mice. Mice were housed in a specific pathogen-free, viral antibody-free animal facility at the Dana-Farber Cancer Institute. All experiments were done in compliance with federal laws and institutional guidelines and have been approved by the Dana Farber Cancer Institute Animal Care and Use Committee.
Antibodies
Purified mAbs against CD3ε (145-2C11), CD8 (53-6.7), CD4 (GK1.5), B220 (RA3-6B2), MAC-1 (M1/70), GR-1 (RB6-8C5), NK1.1 (PK136), Ly49I/C, and FITC-conjugated anti-NKG2A/C/E were purchased from Pharmingen. Purified and biotinylated-anti-NKG2AB6 were purchased from eBiosicience. Anti-biotin and anti-DX5 beads were purchased from Miltenyi Biotec (Auburn, CA).
Antigens
The peptide MOG 35–55 (MEVGWYRSPFSRVVHLYRNGK), OVA peptide (amino acids 323–339; ISQAVHAAHAEINEAGR), and PLP peptide (amino acids 172–183; PVYIYFNTwtTC) were synthesized by New England Peptide (Gardner, MA).
CD4 and CD8 Cell Purification
CD4 and CD8 T cells were purified by negative selection after incubation for 30 min with rat anti-mouse CD8 (anti-CD8) or anti-CD4, in addition to anti-B220, anti-Mac-1, anti-Gr-1, and anti-NK1.1 (BD Pharmingen). After washing, cells were incubated for 30 min with magnetic beads coated with sheep anti-rat IgG (Dynal) before isolation of CD4 and CD8 T cells by magnetic separation.
Adoptive CD4 T Cell Transfer and Induction and Assessment of EAE
2D2 CD4 T cells purified from either Qa-1 WT or Qa-1-deficient mice as described above were transferred i.v. (106) into Rag2−/− or Rag2−/− Prf1−/− hosts that were immunized s.c. with 10 µg MOG 35–55 emulsified in CFA (supplemented with 4 mg/ml of Mycobacterium tuberculosis) and injected i.p. on days 0 and 2 with 200 ng pertussis toxin to induce EAE. Clinical assessment of EAE was performed daily and scoring was as follows: 0, no disease; 1, decreased tail tone; 2, hind limb weakness or partial paralysis; 3, complete hind limb paralysis; 4, front and hind limb paralysis; 5, moribund state.
Bone-Marrow Transfer
BM cells were harvested from femur and tibia under sterile conditions from Qa-1 WT (Thy1.1) or Qa-1-deficient (Thy1.2) mice, and erythrocytes were lysed and enriched for hematopoietic stem cells by depletion of lineage-positive cells with anti-CD4, anti-CD8, anti-CD3, anti-Gr1, anti-Mac1, anti-CD19, and anti-DX5 followed by sheep anti-rat IgG-conjugated immunomagnetic beads (Dynal). Cells (1 × 106) were then injected into irradiated (400 rads) Rag2−/− or Rag2−/− Prf1−/− mice.
Immunization and In Vitro Recall Response
OTII TCR transgenic Qa-1 WT or Qa-1-deficient mice were immunized s.c. with 50 µg of OVA peptide (amino acids 323–339; ISQAVHAAHAEINEAGR) in CFA. For NK-cell depletion, mice were injected with 200 µg purified NK1.1 antibody at day −1 and day 6. 12 days after peptide immunization, CD4 cells from draining lymph node were incubated with different concentrations of OVA peptide and irradiated splenocytes from B6 mice in RPMI 1640 medium supplemented with 10% fetal calf serum and 50 µM β-mercaptoethanol. Culture supernatants were collected after 48 hr of culture, and cytokine concentrations in supernatants were determined by enzyme-linked immunosorbent assay (ELISA) kit (BD Pharmingen).
NK-Cell Purification
Splenocytes were incubated with CD4, CD8, and B220 antibodies and mixed with magnetic beads and separated by magnetic cell sorting. For purification of NKG2A+ NK cells, the CD4−, CD8−, and B220− splenocytes were stained with biotinylated NKG2AB6 mAb (eBioscience), followed by incubation with magnetic microbeads coated with biotin antibody (Mytech) before magnetic cell sorting with MACS. DX5 mAb was then used to further purify the NKG2A− NK cell population from the flow through cells. Purified NK cells were cultured in RPMI-1640 supplemented with 10% FCS in the presence of 1000 U/ml human recombinant IL-2 (BD Pharmingen) for 5 days.
NK Cytotoxic Assay
Target cells were labeled with 50 µCi of Na2(51Cr)O4 for 1 hr at 37°C, washed 3 times with PBS before mixing (1 × 104/well) with effector cells in U-bottomed 96-well plates at different E–T ratios (10:1, 20:1, 40:1) in triplicate. After 4 hr of incubation, cell-free supernatants were collected and radioactivity measured by Micro-β counter (Wallac). Percentage of lysis is calculated by (sample release – spontaneous release)/(maximum release – spontaneous release).
Lentiviral Expression of Qa-1
Full-length ORF of Qa-1 was amplified by RT-PCR from ConA-activated C57BL/6 spleen RNA, digested with BamHI-XhoI, and cloned into pLenti6/V5 (Invitrogen) by using BamHI and XhoI restriction sites. The construct was confirmed by sequencing. Enhanced GFP alone was cloned into pLenti6/V5 as a negative control. For mutation screening, a point mutation was introduced by site-directed mutagenesis according to manufacturer’s instructions (Stratagene). For expression of peptide sewed in Qa-1 molecules, Qdm–β2m–Qa-1 and HSP60–β2m–Qa-1 fusion proteins were made by overlapping PCR (see Supplemental Data for protocol). Lentiviral stocks were generated by cotransfection of 293 T cells with the packaging plasmids pLP1, pLP2, and pLP/VSVG (Invitrogen) via Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol. Viral supernatants were collected 72 hr after transfection and the viral titer for all transfections was approximately 107 plaque-forming units/ml. Naive Qa-1-deficient CD4 T cells were infected with lentivirus at a moi of 5–10 for 3–6 hr at 37°C with 5% CO2 and either washed and used directly after a 3 hr infection or washed and rested for an additional 15 hr at 37°C with 5% CO2.
Anti-Qa-1 Treatment and Induction of EAE
C57BL/6 mice were immunized s.c. with 150 µg MOG 35–55 emulsified in CFA (supplemented with 4 mg/ml of Mycobacterium tuberculosis) and injected i.p. on day 0 and day 2 with 200 ng pertussis toxin to induce EAE. To block the Qa-1–NKG2A interaction, Qa-1 antibody was administered three times at day 5, 9, and 12 after immunization (200 µg, i.v.). In a separate group, anti-NK1.1 was administered (150 µg, i.v.) at day 3, 7, and 10 to deplete NK cells before administration of Qa-1 antibody on days 5, 9, and 12 to determine whether altered disease development was NK-cell dependent or not. A control group was given either PBS or mouse IgG1 isotype control (5 mice per group), and clinical assessment of EAE was performed daily and scored as described above.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the NIH (AI 37562) and National Multiple Sclerosis Society to H.C.; L.L. is a Claudia Adams Barr Investigator and K.I. was supported by a fellowship from Taiho Pharmaceuticals. We thank V.K. Kuchroo for provision of 2D2 TCR transgenic mice, D.H. Raulet for helpful discussion, D. Laznik for technical assistance, and A. Angel for manuscript and graphics preparation.
Footnotes
Supplemental Data
Four figures are available at http://www.immunity.com/cgi/content/full/26/5/593/DC1/.
REFERENCES
- Aldrich CJ, Waltrip R, Hermel E, Attaya M, Lindahl KF, Monaco JJ, Forman J. T cell recognition of QA-1b antigens on cells lacking a functional Tap-2 transporter. J. Immunol. 1992;149:3773–3777. [PubMed] [Google Scholar]
- Bach JF. Induction of immunological tolerance using monoclonal antibodies: applications to organ transplantation and autoimmune disease. C.R. Biol. 2006;329:260–262. doi: 10.1016/j.crvi.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Pagany M, Weiner HL, Linington C, Sobel RA, Kuchroo VK. Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J. Exp. Med. 2003;197:1073–1081. doi: 10.1084/jem.20021603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks CR, Elliott T, Parham P, Khakoo SI. The inhibitory receptor NKG2A determines lysis of vaccinia virus-infected autologous targets by NK cells. J. Immunol. 2006;176:1141–1147. doi: 10.4049/jimmunol.176.2.1141. [DOI] [PubMed] [Google Scholar]
- Chatenoud L. Anti-CD3 antibodies: towards clinical antigen-specific immunomodulation. Curr. Opin. Pharmacol. 2004;4:403–407. doi: 10.1016/j.coph.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Durelli L, Bongioanni MR, Cavallo R, Ferrero B, Ferri R, Verdun E, Bradac GB, Riva A, Geuna M, Bergamini L. Interferon alpha treatment of relapsing-remitting multiple sclerosis: long-term study of the correlations between clinical and magnetic resonance imaging results and effects on the immune function. Mult. Scler. 1995;1(Suppl 1):S32–S37. [PubMed] [Google Scholar]
- Edwards BS, Merritt JA, Fuhlbrigge RC, Borden EC. Low doses of interferon alpha result in more effective clinical natural killer cell activation. J. Clin. Invest. 1985;75:1908–1913. doi: 10.1172/JCI111905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436:1186–1190. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G. Reciprocal activating interaction between natural killer cells and dendritic cells. J. Exp. Med. 2002;195:327–333. doi: 10.1084/jem.20010938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Ikizawa K, Lu L, Sanchirico ME, Shinohara ML, Cantor H. Analysis of regulatory CD8 T cells in Qa-1-deficient mice. Nat. Immunol. 2004;5:516–523. doi: 10.1038/ni1063. [DOI] [PubMed] [Google Scholar]
- Jacobs LD, Beck RW, Simon JH, Kinkel RP, Brownscheidle CM, Murray TJ, Simonian NA, Slasor PJ, Sandrock AW. Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. CHAMPS Study Group. N. Engl. J. Med. 2000;343:898–904. doi: 10.1056/NEJM200009283431301. [DOI] [PubMed] [Google Scholar]
- Jiang H, Zhang S-L, Pernis B. Role of CD8+ T cells in murine experimental allergic encephalomyelitis. Science. 1992;256:1213–1215. doi: 10.1126/science.256.5060.1213. [DOI] [PubMed] [Google Scholar]
- Karlhofer FM, Ribaudo RK, Yokoyama WM. MHC class I alloantigen specificity of Ly-49+ IL-2-activated natural killer cells. Nature. 1992;358:66–70. doi: 10.1038/358066a0. [DOI] [PubMed] [Google Scholar]
- Kraft JR, Vance RE, Pohl J, Martin AM, Raulet DH, Jensen PE. Analysis of Qa-1b peptide binding specificity and the capacity of CD94/NKG2A to discriminate between Qa-1-peptide complexes. J. Exp. Med. 2000;192:613–623. doi: 10.1084/jem.192.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N, Llano M, Carretero M, Ishitani A, Navarro F, Lopez-Botet M, Geraghty DE. HLA-E is a major ligand for the natural killer inhibitory receptor CD94/NKG2A. Proc. Natl. Acad. Sci. USA. 1998;95:5199–5204. doi: 10.1073/pnas.95.9.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwin V, Gumperz J, Parham P, Phillips JH, Lanier LL. NKB1: a natural killer cell receptor involved in the recognition of polymorphic HLA-B molecules. J. Exp. Med. 1994;180:537–543. doi: 10.1084/jem.180.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo WF, Ong H, Metcalf ES, Soloski MJ. T cell responses to gram-negative intracellular bacterial pathogens: a role for CD8+T cells in immunity to Salmonella infection and the involvement of MHC class Ib molecules. J. Immunol. 1999;162:5398–5406. [PubMed] [Google Scholar]
- Lo WF, Woods AS, DeCloux A, Cotter RJ, Metcalf ES, Soloski MJ. Molecular mimicry mediated by MHC class Ib molecules after infection with gram-negative pathogens. Nat. Med. 2000;6:215–218. doi: 10.1038/72329. [DOI] [PubMed] [Google Scholar]
- Lu L, Werneck MB, Cantor H. The immunoregulatory effects of Qa-1. Immunol. Rev. 2006;212:51–59. doi: 10.1111/j.0105-2896.2006.00418.x. [DOI] [PubMed] [Google Scholar]
- Martin-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, Sallusto F. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat. Immunol. 2004;5:1260–1265. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- Mehrotra PT, Donnelly RP, Wong S, Kanegane H, Geremew A, Mostowski HS, Furuke K, Siegel JP, Bloom ET. Production of IL-10 by human natural killer cells stimulated with IL-2 and/or IL-12. J. Immunol. 1998;160:2637–2644. [PubMed] [Google Scholar]
- Moser JM, Gibbs J, Jensen PE, Lukacher AE. CD94-NKG2A receptors regulate antiviral CD8(+) T cell responses. Nat. Immunol. 2002;3:189–195. doi: 10.1038/ni757. [DOI] [PubMed] [Google Scholar]
- Ota T, Takeda K, Akiba H, Hayakawa Y, Ogasawara K, Ikarashi Y, Miyake S, Wakasugi H, Yamamura T, Kronenberg M, et al. IFN-gamma-mediated negative feedback regulation of NKT-cell function by CD94/NKG2. Blood. 2005;106:184–192. doi: 10.1182/blood-2004-11-4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paust S, Cantor H. Regulatory T cells and autoimmune disease. Immunol. Rev. 2005;204:195–207. doi: 10.1111/j.0105-2896.2005.00247.x. [DOI] [PubMed] [Google Scholar]
- Piccioli D, Sbrana S, Melandri E, Valiante NM. Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J. Exp. Med. 2002;195:335–341. doi: 10.1084/jem.20010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovich BA, Li J, Shannon J, Hurren R, Chalupny J, Cosman D, Miller RG. Activated, but not resting, T cells can be recognized and killed by syngeneic NK cells. J. Immunol. 2003;170:3572–3576. doi: 10.4049/jimmunol.170.7.3572. [DOI] [PubMed] [Google Scholar]
- Raulet DH. Missing self recognition and self tolerance of natural killer (NK) cells. Semin. Immunol. 2006;18:145–150. doi: 10.1016/j.smim.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Raulet DH, Vance RE. Self-tolerance of natural killer cells. Nat. Rev. Immunol. 2006;6:520–531. doi: 10.1038/nri1863. [DOI] [PubMed] [Google Scholar]
- Schott E, Bonasio R, Ploegh HL. Elimination in vivo of developing T cells by natural killer cells. J. Exp. Med. 2003;198:1213–1224. doi: 10.1084/jem.20030918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloski MJ, DeCloux A, Aldrich CJ, Forman J. Structural and functional characteristics of the class IB molecule, Qa-1. Immunol. Rev. 1995;147:67–89. doi: 10.1111/j.1600-065x.1995.tb00088.x. [DOI] [PubMed] [Google Scholar]
- Steinman L, Zamvil SS. How to successfully apply animal studies in experimental allergic encephalomyelitis to research on multiple sclerosis. Ann. Neurol. 2006;60:12–21. doi: 10.1002/ana.20913. [DOI] [PubMed] [Google Scholar]
- Sullivan BA, Kraj P, Weber DA, Ignatowicz L, Jensen PE. Positive selection of a Qa-1-restricted T cell receptor with specificity for insulin. Immunity. 2002;17:95–105. doi: 10.1016/s1074-7613(02)00343-6. [DOI] [PubMed] [Google Scholar]
- Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, Panoskaltsis N. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N. Engl. J. Med. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- Transy C, Nash SR, David-Watine B, Cochet M, Hunt SW, Hood LE, Kourilsky P. A low polymorphic mouse H-2 class I gene from the Tla complex is expressed in a broad variety of cell types. J. Exp. Med. 1987;166:341–361. doi: 10.1084/jem.166.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance RE, Kraft JR, Altman JD, Jensen PE, Raulet DH. Mouse CD94/NKG2A is a natural killer cell receptor for the nonclassical MHC class I molecule Qa-1b. J. Exp. Med. 1998;188:1841–1847. doi: 10.1084/jem.188.10.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada H, Matsumoto N, Maenaka K, Suzuki K, Yamamoto K. The inhibitory NK cell receptor CD94/NKG2A and the activating receptor CD94/NKG2C bind the top of HLA-E through mostly shared but partly distinct sets of HLA-E residues. Eur. J. Immunol. 2004;34:81–90. doi: 10.1002/eji.200324432. [DOI] [PubMed] [Google Scholar]
- Wang M, Ellison CA, Gartner JG, HayGlass KT. Natural killer cell depletion fails to influence initial CD4 T cell commitment in vivo in exogenous antigen-stimulated cytokine and antibody responses. J. Immunol. 1998;160:1098–1105. [PubMed] [Google Scholar]
- Wang J, Sun R, Wei H, Dong Z, Gao B, Tian Z. Poly I:C prevents T cell-mediated hepatitis via an NK-dependent mechanism. J. Hepatol. 2006;44:446–454. doi: 10.1016/j.jhep.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Willenborg DO, Fordham S, Bernard CCA, Cowden W, Ramshaw IA. IFN-gamma plays a critical downregulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. J. Immunol. 1996;157:3223–3227. [PubMed] [Google Scholar]
- Xu W, Fazekas G, Hara H, Tabira T. Mechanism of natural killer (NK) cell regulatory role in experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2005;163:24–30. doi: 10.1016/j.jneuroim.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Zhang B, Yamamura T, Kondo T, Fujiwara M, Tabira T. Regulation of experimental autoimmune encephalomyelitis by natural killer (NK) cells. J. Exp. Med. 1997;186:1677–1687. doi: 10.1084/jem.186.10.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.