Abstract
To date, few mutations are described to underlie highly-elevated HDLc levels in families. Here we sequenced the coding regions and adjacent sequence of the LIPG, CETP, and GALNT2 genes in 171 unrelated Dutch Caucasian probands with HDLc≥90th percentile and analyzed segregation of mutations with lipid phenotypes in family members. In these probands, mutations were most frequent in LIPG (12.9%) followed by GALNT2 (2.3%) and CETP (0.6%). A total of 6 of 10 mutations in these three genes were novel (60.0%), and mutations segregated with elevated HDLc in families. Interestingly, the LIPG mutations N396S and R476W, which usually result in elevated HDLc, were unexpectedly found in 6 probands with low HDLc (i.e., ≤10th percentile). However, 5 of these probands also carried mutations in ABCA1, LCAT, or LPL. Finally, no CETP and GALNT2 mutations were found in 136 unrelated probands with low HDLc. Taken together, we show that rare coding and splicing mutations in LIPG, CETP, and GALNT2 are enriched in persons with hyperalphalipoproteinemia and segregate with elevated HDLc in families. Moreover, LIPG mutations do not overcome low HDLc in individuals with ABCA1 and possibly LCAT and LPL mutations, indicating that LIPG affects HDLc levels downstream of these proteins.
Introduction
Coronary artery disease (CAD) is the leading cause of mortality in the industrialized world [1], demonstrating an urgent and unmet need to develop new therapies that reduce risk for CAD [2]. Epidemiological studies show that increased plasma HDLc confers significant protection against CAD [3], [4], in part by elevating reverse cholesterol transport and its anti-inflammatory properties [5], [6]. In contrast to common SNP alleles that associate with very small lipid changes in large populations [7], gene mutations that are extremely rare in the general population and result in major elevations in HDLc levels are more likely to mimic the effects of drugs such as CETP inhibitors that have major inhibitory effects on protein function [8], [9].
The successful identification of these rare mutations is often precluded by their low frequency in populations, which generally limits the power to test for associations [10]. Instead, they are often validated by assessing their segregation in families with apparent Mendelian forms of extreme HDLc traits [11]–[17]. However, while this approach has been very successful in identifying mutations in families with extremely low HDLc, few studies to date report mutations that lead to highly-elevated HDLc in families [10], [18].
HDLc turnover is significantly controlled by the gene products of LIPG and CETP [19]. LIPG encodes endothelial lipase (EL), a major regulator of HDL phospholipid catabolism. Loss-of-function of EL significantly increases HDLc levels in mice and humans, potentially through reduced catabolism of large, lipid-rich HDL particles [17], [20]–[22], while loss-of-function mutations in families do not affect other lipid measures [17], [23]. CETP encodes cholesteryl ester transfer protein, which exchanges triglycerides from VLDL and LDL particles for cholesterol esters from HDL and selectively enhances liver HDL cholesterol ester uptake [24]. In families, loss-of-function CETP mutations that inhibit cholesterol ester transfer result in larger HDL particles, reduced catabolism, and increased serum HDLc, in addition to reduced LDLc [11], [16], [25], [26].
Another strong candidate for HDLc regulation in humans is GALNT2, which regulates O-linked oligosaccharide biosynthesis [27]. Common GALNT2 SNPs associate with very small (e.g., ∼1–2%) changes in HDLc and triglycerides in large genome-wide association studies [7]. Moreover, Galnt2 over-expression in mouse liver reduces HDLc, while shRNA-based Galnt2 knockdown increases HDLc [7]. More recently, a rare familial GALNT2 mutation was observed to underlie accelerated postprandial triglyceride clearance and increased unsialylated apolipoprotein C-III (APOC3) levels, which in turn associated with elevated lipoprotein lipase (LPL) activity [28]. Mutations in both APOC3 and LPL also lead to large changes in HDLc [13], [29], [30], further supporting that mutations in GALNT2 may also affect HDLc in humans.
Here we performed a family-based study to assess the segregation of rare mutations with elevated HDLc levels. We first identified mutations by sequencing the coding regions and adjacent UTR and intronic sequences of LIPG, CETP, and GALNT2 in 171 unrelated individuals with extremely high HDLc (defined here as HDLc≥90th percentile adjusted for age and gender) [31], and compared this to sequence data from 136 persons with extremely low HDLc (HDLc≤10th percentile). We then assessed the family members of probands with rare mutations to determine the associations of these mutations with lipid phenotypes.
Materials and Methods
Patients
We identified 171 unrelated probands of Dutch Caucasian ancestry with HDLc≥90th percentile and 136 unrelated Dutch Caucasian probands with HDLc≤10th percentile (based on age- and sex-specific Lipid Research Clinic data and as described previously) [31], [32] with no other abnormal lipid measures. We also studied 199 family members of 29 total probands with mutations. The study protocol was approved by the Ethics Committees of the Academic Medical Center, Amsterdam. All subjects provided written informed consent. Lipoprotein measurements were performed on fresh plasma as described [33]. Cholesterol and triglyceride levels were determined in total plasma and plasma at density d<1.006 g/mL obtained after preparative ultracentrifugation, before and after precipitation with dextran manganese.
DNA sequencing and data analysis
The LIPG, CETP, and GALNT2 coding regions and adjacent intron and UTR sequence as defined in human genome hg18 were sequenced from genomic DNA in all probands using either standard fluorescent dye terminator chemistry (Seqwright, Houston TX) or next generation paired-end read sequencing (Illumina, San Diego CA). For standard sequencing, DNA primers were designed to flank LIPG, CETP, and GALNT2 exons and adjacent intron and UTR sequence and sequenced bidirectionally. Primer sequences are listed in Table S7. Sequence changes were identified using Sequencher v4.7 (Ann Arbor, MI) and confirmed in dbSNP build 130 and 1000Genomes November 2010 data release or by sample resequencing. For next generation sequencing, sequence changes were identified by alignment of sequence data to the human genome (NCBI Build 36.1) using CASAVA v.1.7 software (Illumina, San Diego CA). Sequence changes predicted to be damaging to protein function as defined below were then confirmed by standard sequencing as described above. In all cases, data analysis was performed by individuals who were blinded to the phenotypes of sequenced individuals. Reference DNA/Protein sequences used for data analysis include NM_006033.2/NP_006024 for LIPG, NM_000078.2/NP_000069 for CETP, NM_004481.3/NP_004472 for GALNT2, NM_005502.2/NP_005493 for ABCA1, NM_000039.1/NP_000030 for APOA1, NM_000229.1/NP_000220 for LCAT, and NM_000237.2/NP_000228 for LPL.
Mutations were defined as any sequence change that is predicted to be damaging to protein function as determined by Polyphen-2 (http://genetics.bwh.harvard.edu/pph2/) and/or Spliceview (http://zeus2.itb.cnr.it/~webgene/wwwspliceview_ex.html) in silico algorithms [34], [35]. Vertebrate sequence conservation of mutations was determined using the conservation tracks in the UCSC Genome Bioinformatics Human Genome Browser Gateway (http://genome.ucsc.edu/).
Segregation analysis of mutations in families
Family members of probands with mutations were genotyped using primers and standard sequencing techniques described above. Lipid parameters of mutation carriers were compared to first-degree relative controls, defined as all parents, siblings, and children of persons with a mutation that do not carry a known mutation themselves.
Statistical analyses
In total probands, single mutation burden effects for each gene were assessed by Fisher's exact test. Where appropriate, combined mutation burden effects for each gene were assessed by C-alpha test [36] and cohort allelic sum test (CAST) [37]. Combined mutation burden effects for all genes were also assessed by combined multivariate and collapsing (CMC) test [38]. In contrast to the collapsing and CMC methods, the C-alpha test allows for the joint presence of risk and protective variants. The CMC method is used for multiple predefined groups and expands on the collapsing method for a single group of markers. Groups were defined as genes for the present purpose. Fisher exact test was performed when only a single variant-test is considered. For all tests, a p value of 0.05 or less was considered significant.
In total families, significance of mutations with lipid profiles in carrier individuals vs. first-degree relative controls was determined by stratified conditional logistic regression. The matched sets (strata) were defined by the family number. For CETP and GALNT2, an exact stratified conditional logistic regression was performed due to the small sample size. Results of each lipid profile were adjusted for age and sex. A p value of 0.05 or less was considered significant.
Results
LIPG, CETP, and GALNT2 mutations in probands with high HDLc
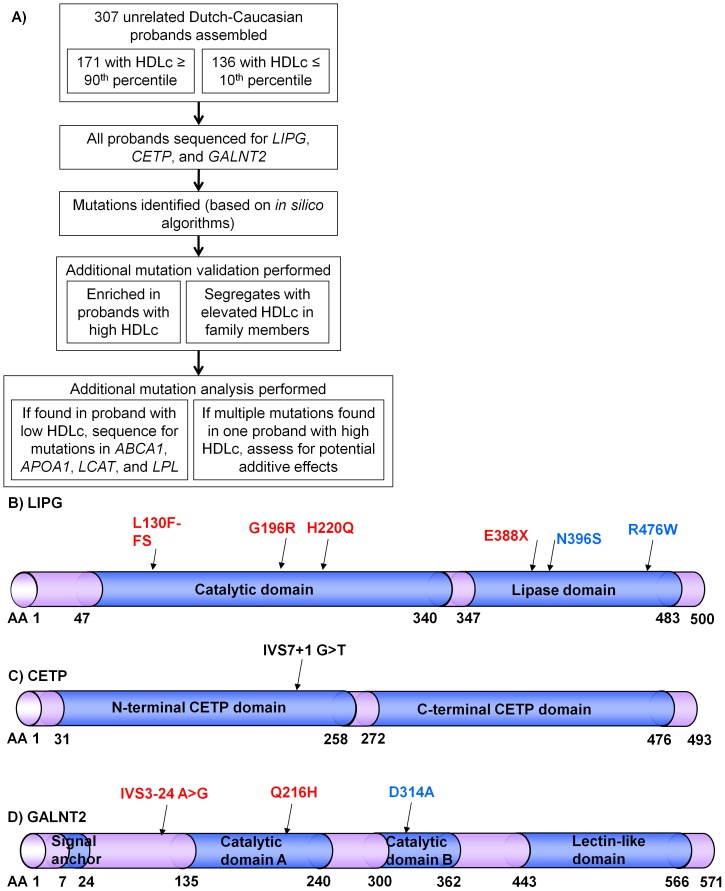
An overview of the study design is shown in Figure 1A. We assembled 171 unrelated Dutch probands with HDL≥90th percentile (Table 1) and sequenced the coding regions and adjacent UTR and intronic sequence of LIPG, CETP, and GALNT2. Mutations were defined as any sequence change that is predicted to be damaging to protein function as determined by Polyphen-2 and/or Spliceview in silico algorithms [34], [35]. In all individuals, mutations were most frequently identified in LIPG (22 of 171, 12.9%) but were rarely found in CETP (1 of 171, 0.6%) or GALNT2 (4 of 171, 2.3%) (Table 2).
Figure 1. Mutations identified in probands with high or low HDLc.
A) Overview of the study design. B–D) Predicted mutation effects on B) LIPG, C) CETP, and D) GALNT2 proteins. Red sequence changes are novel, blue are previously described [17], [23], [28], and black is previously described in a subset of this cohort [16].
Table 1. Demographics of unrelated probands with extreme HDLc.
| Measure | HDLc percentile | |
| ≥90th | ≤10th | |
| Total assessed | 171 | 136 |
| Age (y)a | 53.6 (14.1) | 51.8 (14.2) |
| Male individualsb | 85 (49.7%) | 88 (64.7%) |
| Total cholesterol (mmol/L)a | 5.94 (1.06) | 4.48 (1.36) |
| Triglycerides (mmol/L)a | 0.88 (0.44) | 1.68 (1.01) |
| HDLc (mmol/L)c | 2.23 (2.16–2.31) | 0.71 (0.68–0.71) |
| LDLc (mmol/L)a | 3.30 (0.92) | 3.01 (1.23) |
| BMI (kg/m2)a | 24.0 (3.1) | 27.0 (4.6) |
, Average (SD);
, N (%);
, Average (95% confidence interval).
Table 2. Mutation frequencies and burden effects in probands.
| Gene | Mutations | N (%) | p value | ||||
| ≥90th | ≤10th | C-alpha | CAST | CMC | Fisher's | ||
| N sequenced | 171 | 136 | |||||
| LIPG | Total | 22 (19.9%) | 3 (2.2%) | 2.7*10−3 | 5.5*10−4 | ||
| N396S | 11 (6.4%) | 2 (1.5%) | 0.04 | ||||
| R476W | 6 (3.5%) | 1 (0.7%) | 0.14 | ||||
| CETP | Total | 1 (0.6%) | 0 (0.0%) | 0.55 | |||
| GALNT2 | Total | 4 (2.3%) | 0 (0.0%) | 0.35 | 0.13 | ||
| All genes | Total | 25 (14.6%)a | 3 (2.2%) | 2.6*10−3 | 9.9*10−5 | 3.0*10−3 | |
| Novel | 8 (4.7%) | 0 (0.0%) | 0.30 | 9.9*10−3 | 0.04 | ||
, Two probands have rare LIPG+GALNT2 variants.
If mutations in LIPG, CETP, and GALNT2 underlie elevated HDLc, then they should be less frequent or absent in control probands with the opposite phenotype (i.e., extremely low HDLc). We therefore sequenced the same coding regions and adjacent sequence of these genes in 136 unrelated probands with HDLc≤10th percentile (Table 1). No CETP or GALNT2 mutations were found in probands with low HDLc (Table 2). In contrast, three probands with low HDLc were unexpectedly observed to carry the previously-described HDLc-raising LIPG mutations N396S or R476W [17], [23], raising the possibility of additional genetic factors that may cause low HDLc in the presence of these mutations (see below). Total LIPG mutations were significantly enriched in probands with elevated HDLc (e.g., p = 2.7*10−3 by C-alpha test; Table 2). In contrast, the frequencies of these very rare CETP and GALNT2 mutations did not reach statistical significance in our small and likely underpowered cohort of total probands, indicating the need to test the segregation of these mutations with elevated HDLc in family members.
The predicted functional consequences of all mutations are shown in Figure 1B–D and summarized in Table 3. Of 10 total mutations identified in three genes, 4 of 6 LIPG mutations and 2 of 3 GALNT2 mutations are not reported in previous literature, dbSNP, or 1000Genomes (Table 3). Notably, the novel LIPG mutation E388X was identified in two Dutch probands who have no additional sequence variation across all LIPG exons and adjacent sequence, implying a shared ancestral haplotype of at least 24.5 kb. Moreover, the GALNT2 Q216H mutation was also identified in two Dutch probands, and segregation analysis of common SNPs in close proximity to GALNT2 Q216H in family members indicated a shared ancestral haplotype of at least 7.2 kb (data not shown). No novel mutations were detected in 136 individuals with HDLc≤10th percentile (e.g., p = 9.9*10−3 by CAST and p = 0.04 by CMC test; Table 2).
Table 3. LIPG, CETP, and GALNT2 mutations.
| Gene | Mutation | Novel? | # of occurrences | Vertebrate conservation | Predicted effect | ||
| Position (hg18) | Effect | HDLc≥90th %ile | HDLc≤10th %ile | ||||
| LIPG | Chr18:45347918 C>del | L130F-FS | Yes | 1 | 0 | Completely conserved | Truncates protein |
| Chr18:45355751 G>A | G196R | Yes | 1 | 0 | Completely conserved | Probably damaging (Polyphen) | |
| Chr18:45355825 C>G | H220Q | Yes | 1 | 0 | Completely conserved | Probably damaging (Polyphen) | |
| Chr18:45362846 G>T | E388X | Yes | 2 | 0 | Completely conserved | Truncates protein | |
| Chr18:45363953 A>G | N396S | No [23] | 11 | 2 | Completely conserved | Possibly damaging (Polyphen) | |
| Chr18:45367163 C>T | R476W | No [17] | 6 | 1 | Lys, Gln tolerated | Possibly damaging (Polyphen) | |
| CETP | Chr16:55562795 G>T | IVS7+1 G>T | No [16] | 1 | 0 | Completely conserved | Abolishes splice donor site (Spliceview) |
| GALNT2 | Chr1:228438359 A>G | IVS3-24 A>G | Yes | 1 | 0 | Completely conserved | Introduces ectopic splice acceptor site (Spliceview) |
| Chr1:228445715 A>T | Q216H | Yes | 2 | 0 | Completely conserved | Possibly damaging (Polyphen) | |
| Chr1:228452861 A>C | D314A | No [28] | 1 | 0 | Completely conserved | Possibly damaging (Polyphen) | |
If rare, deleterious mutations in these genes are indeed identified to be enriched in probands with elevated HDLc, then non-deleterious DNA changes should occur with similar frequencies in both probands with extremely high or low HDLc. We therefore assessed the minor allele frequencies for all additional SNPs identified in GALNT2, in addition to all additional coding SNPs in LIPG and CETP, in a representative subset of 55 probands with HDLc≥90th and 55 probands with HDLc≤10th percentiles for which standard fluorescent dye terminator-based sequencing data were available (Tables S1 and S2). Of 15 additional SNPs found in GALNT2, none were predicted to be nonsynonymous or affect splicing with the exception of a known V554M polymorphism that is predicted to be functionally benign. Furthermore, no minor alleles for any GALNT2 SNP were observed to be significantly enriched in either proband population (Table S1). In LIPG, the synonymous SNP S21S and the previously-described nonfunctional T111I polymorphism [17] were identified, neither of which were significantly associated with high or low HDLc (Table S2). In CETP, two benign coding SNPs (F287F and I422V) were also identified with no significant minor allele associations with elevated HDLc (Table S2). Finally, for LIPG and CETP, no additional noncoding SNPs were identified with predicted deleterious effects on splicing (data not shown). Taken together, these observations further indicate that only LIPG, CETP, and GALNT2 mutations with predicted deleterious effects, but not non-deleterious sequence changes, are distinctly enriched in individuals with elevated HDLc.
Taken together, 25 of 171 (14.6%) total probands with HDLc≥90th percentile have mutations in ≥1 gene (Table 2), with 6 of 10 total mutations being novel (60.0%; Table 3). When total mutations across all sequenced exons and adjacent intronic sequence are considered, we found 1 LIPG mutation per 673 base pairs (bp) of sequence (6 mutations found in 4,036 total sequenced bp), 1 CETP mutation per 5,292 bp (1 mutation in 5,292 total sequenced bp), and 1 GALNT2 mutation per 1,488 bp (3 mutations in 4,465 total sequenced bp).
Mutation segregation with elevated HDLc in families
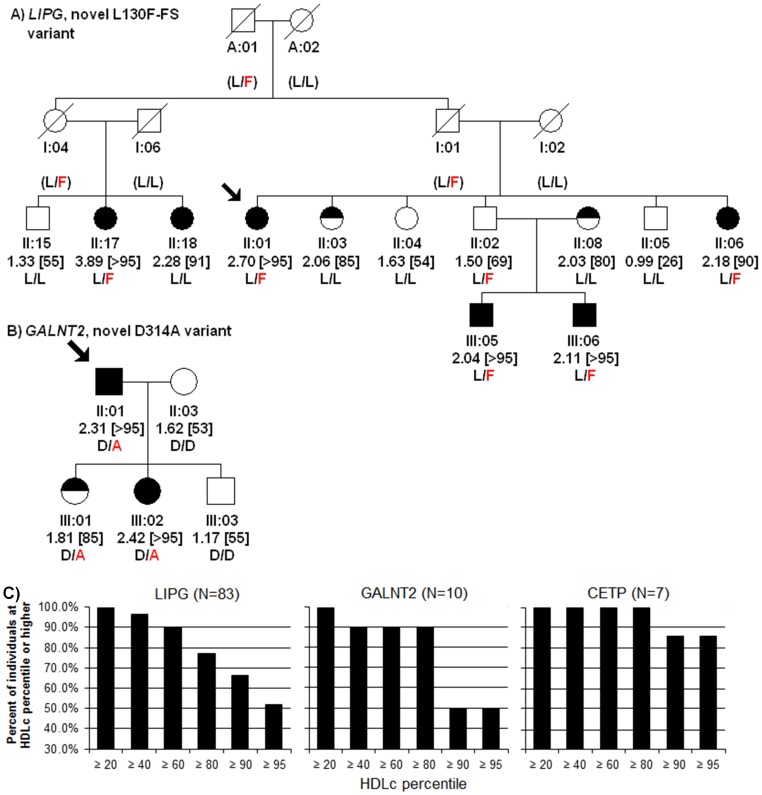
For 22 total probands with HDLc≥90th percentile and single mutations in LIPG, CETP, or GALNT2, we genotyped all available family members and assessed lipid phenotypes (Figure 2A–B; Figure S1; Table 4). For LIPG and CETP, significant segregation of mutations was observed with elevated HDLc, further supporting that these mutations are likely to be deleterious and confer large changes on HDLc. For example, assessment of 83 total LIPG mutation carriers in families revealed an average 0.33 mmol/L elevated HDLc compared to 80 first-degree relative controls that do not carry any known mutations (+19.3%, p = 7.47*10−6). Moreover, 7 CETP mutation carriers had an average 0.96 mmol/L increased HDLc vs. 9 first-degree relative controls (+62.3%, p = 0.027). For GALNT2, an average but borderline significant 0.52 mmol/L HDLc increase was observed in 10 total GALNT2 mutation carriers vs. 11 first-degree controls (+34.2%, p = 0.093), suggesting that either GALNT2 mutations have weaker or more variable effects than LIPG and CETP mutations on HDLc elevation and/or these families have insufficient power for detecting significant GALNT2 mutation associations with increased HDLc. In contrast, no significant changes in triglycerides, LDLc, or BMI were observed for mutations of any gene.
Figure 2. Representative segregation of mutations in families.
A, B) Segregation of (A) LIPG L130F-FS and (B) GALNT2 D314A with elevated HDLc. For each individual, the individual ID, HDLc (in mmol/L) plus [HDLc percentile], and genotype are shown. Squares, Males; Circles, Females; Arrow, proband. Filled shape, HDLc≥90th percentile; half-filled, HDLc between 80–89th percentiles; empty shape, HDLc<80th percentile. Slash = deceased. C) Percent of individuals in families with mutations at given HDLc percentiles or higher.
Table 4. Phenotypes of family members with single LIPG, CETP, and GALNT2 mutations.
| Gene | Measure | 1st-degree controls | Mutation carriers | % change | p value |
| LIPG | Total assessed | 80 | 83 | ||
| Age (y)a | 44.5 (19.0) | 43.9 (20.7) | 0.371 | ||
| Male individualsb | 32 (40.0%) | 46 (55.4%) | 0.074 | ||
| Total cholesterol (mmol/L)a | 5.40 (1.32) | 5.83 (1.41) | 7.9% | 0.224 | |
| Triglycerides (mmol/L)a | 1.05 (0.72) | 1.02 (0.64) | −2.9% | 0.338 | |
| HDLc (mmol/L)a | 1.71 (0.48) | 2.04 (0.64) | 19.3% | 7.47*10−6 | |
| LDLc (mmol/L)a | 3.21 (1.22) | 3.32 (1.21) | 3.4% | 0.368 | |
| BMI (kg/m2)a | 23.6 (4.5) | 22.7 (3.2) | −3.4% | 0.191 | |
| CETP | Total assessed | 9 | 7 | ||
| Age (y)a | 42.8 (15.4) | 34.3 (19.4) | 0.349 | ||
| Male individualsb | 4 (44.4%) | 2 (28.6%) | 0.632 | ||
| Total cholesterol (mmol/L)a | 6.05 (0.72) | 5.61 (0.70) | −7.3% | 0.378 | |
| Triglycerides (mmol/L)a | 2.11 (1.35) | 1.11 (0.29) | −47.4% | 0.135 | |
| HDLc (mmol/L)a | 1.54 (0.31) | 2.50 (0.45) | 62.3% | 0.027 | |
| LDLc (mmol/L)a | 3.53 (0.65) | 2.60 (0.33) | −26.5% | 0.135 | |
| BMI (kg/m2)a | 24.5 (3.0) | 21.6 (2.6) | −12.0% | 0.250 | |
| GALNT2 | Total assessed | 11 | 10 | ||
| Age (y)a | 31.5 (18.3) | 43.2 (15.1) | 0.170 | ||
| Male individualsb | 8 (72.7%) | 4 (40.0%) | 0.220 | ||
| Total cholesterol (mmol/L)a | 5.25 (0.95) | 6.15 (1.20) | 17.1% | 0.500 | |
| Triglycerides (mmol/L)a | 1.38 (1.30) | 1.21 (0.84) | −12.3% | 0.879 | |
| HDLc (mmol/L)a | 1.52 (0.29) | 2.04 (0.64) | 34.2% | 0.093 | |
| LDLc (mmol/L)a | 3.09 (0.75) | 3.55 (1.02) | 14.9% | 0.937 | |
| BMI (kg/m2)a | 21.4 (3.1) | 23.6 (2.1) | 10.3% | 0.400 |
, Average (SD) shown;
, N (%) shown.
We next estimated the relative expressivity of these mutations to elevate HDLc (Figure 2C). In total, only 55 of 83 individuals with only LIPG mutations manifested HDLc≥90th percentile (66.3%), while 5 of 10 (50.0%) GALNT2 mutation carriers exhibited HDLc≥90th percentiles. In contrast, 6 of 7 CETP mutation carriers had HDLc≥90th percentile (85.7%), suggesting that these CETP mutations may confer stronger average effects on HDLc elevation than LIPG mutations, which in turn may confer stronger effects than GALNT2 mutations.
Low HDLc in individuals with mutations in LIPG plus ABCA1, LCAT, or LPL
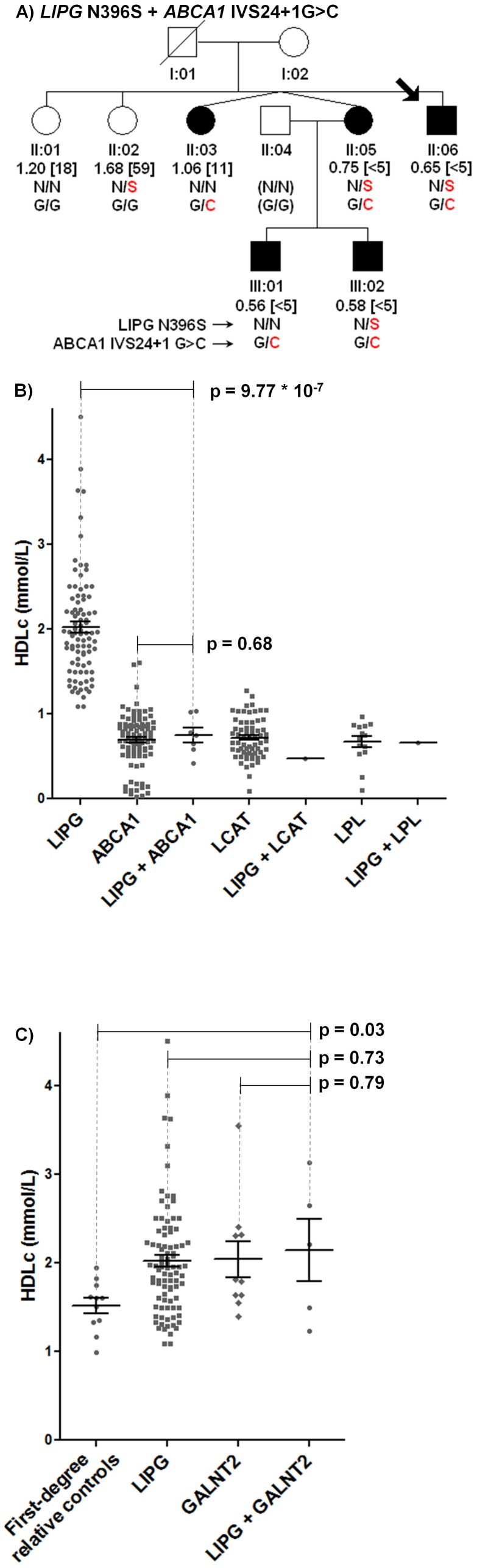
As described above, although LIPG mutations were significantly more prevalent in probands with high HDLc (e.g., p = 2.7*10−3 by C-alpha test, Table 2), 3 probands with low HDLc were unexpectedly observed to carry the previously-described HDLc-raising LIPG mutations N396S or R476W [17], [23]. Genotyping of an independent cohort of 34 unrelated, Dutch-Caucasian probands with HDLc≤10th percentile (data not shown) identified 3 more carriers of N396S or R476W, for a total of 6 individuals with these LIPG mutations and low HDLc (4 with N396S and 2 with R476W).
As the LIPG mutations N396S and R476W generally associate with elevated HDLc both here and elsewhere [17], [23], we asked whether additional genetic factors may cause low HDLc in the presence of these LIPG mutations. We therefore sequenced these 6 probands for ABCA1, APOA1, LCAT, and LPL, which are all well-established to harbor mutations that cause low HDLc [13], [14], [39], [40]. Strikingly, 5 of 6 probands with LIPG mutations and low HDLc also carried mutations in ABCA1 (3 of 6), LCAT (1 of 6), and LPL (1 of 6, Table 5). All mutations found in these genes are predicted to be damaging by Polyphen-2 or Spliceview [34], [35] with the exception of the LPL mutation N296S, which is previously reported to underlie low HDLc [13].
Table 5. Additional mutations in individuals with LIPG mutations and low HDLc.
| Gene | Mutation | Novel? | Vertebrate conservation | Predicted effect | |
| Position (hg18) | Effect | ||||
| ABCA1 | Chr9:106631215 T>A | M640L | No [47] | Completely conserved | Possibly damaging (Polyphen-2) |
| Chr9:106619433 C>G | IVS24+1G>C | No [15] | Completely conserved | Abolishes splice donor site (Spliceview) | |
| Chr9:106618441 G>A | S1181F | No [48] | Completely conserved | Probably damaging (Polyphen-2) | |
| LCAT | Chr16:66534158 G>A | T147I | No [12] | Completely conserved | Possibly damaging (Polyphen-2) |
| LPL | Chr8:19857809 A>G | N318S | No [13] | Ser tolerated | Reduced HDLc (Reymer et al., 1995) |
In addition, family members with the LIPG N396S mutation consistently manifested low HDLc in the presence of a known ABCA1 mutation (Figure 3A; Figure S2) [15]. In total, 7 individuals with LIPG+ABCA1 mutations from 3 families had an average 1.29 mmol/L lower HDLc vs. 83 unrelated individuals with only LIPG mutations (0.75 vs. 2.04 mmol/L, −63.2%, p = 9.77*10−7), but virtually no difference in HDLc vs. 93 independent, unrelated Dutch Caucasian individuals with ABCA1 mutations (0.75 vs. 0.70 mmol/L, +7.1%, p = 0.68; Figure 3B; Table S3). Moreover, one individual with both LIPG+LCAT mutations had 1.57 mmol/L lower HDLc vs. individuals with only LIPG mutations (0.47 mmol/L; −76.9%; Table S4). Similarly, one individual with both LIPG+LPL mutations had 1.38 mmol/L lower HDLc vs. individuals with only LIPG mutations (0.66 mmol/L; −67.6%; Table S5). Thus low HDLc in 5 of 6 probands with established HDLc-raising mutations in LIPG can be attributed to mutations in ABCA1, LCAT, and LPL.
Figure 3. Characterization of probands and families with multiple mutations.
A) Representative segregation of LIPG N396S and ABCA1 IVS24+1G>C mutations. Filled shape, HDLc<15th percentile; empty shape, HDLc≥15th percentile. B) HDLc levels of individuals with LIPG+/−ABCA1, LCAT, or LPL mutations. Individuals with LIPG mutations alone are described in Table 4. Individuals with single ABCA1, LCAT, and LPL mutations are from an independent Dutch-Caucasian population (Tables S1, S2, and S3 and data not shown). C) HDLc levels of individuals with mutations in LIPG, GALNT2, or LIPG+GALNT2. Individuals with single LIPG or GALNT2 mutations are described in Table 4.
No further HDLc elevation in individuals with LIPG+GALNT2 mutations
We also identified 2 probands and 3 family members with both GALNT2 Q216H and either LIPG N396S or R476W. These individuals had an average 0.62 mmol/L increased HDLc vs. 11 first-degree relative controls with no mutations (+40.8%, p = 0.032; Figure 3C; Table S6). However, no significant differences in HDLc or other lipid measures were observed in individuals with both LIPG+GALNT2 mutations when compared to individuals with mutations in either LIPG alone (0.11 mmol/L increased HDLc vs. 89 individuals with LIPG mutations, +5.9%, p = 0.67) or GALNT2 alone (0.10 mmol/L increased HDLc vs. 10 individuals with GALNT2 mutations, +4.9%, p = 0.79), raising the possibility that mutations in GALNT2 and LIPG may be redundant with respect to HDLc elevation.
Discussion
To date, few mutations have been described to lead to large changes in HDLc elevation in families [10], [18]. Here we performed a family-based study by initially sequencing the coding regions and adjacent sequence of LIPG, CETP, and GALNT2 in a series of probands with extreme HDLc levels and searching for rare mutations with expected large effects on HDLc elevation. Mutations were then further validated by genotyping available family members of each proband and by assessing segregation with extreme HDLc phenotypes. In 171 probands with HDLc≥90th percentile, mutations were most frequently identified in LIPG (22 of 171, 12.9%), followed by GALNT2 (4 of 171, 2.3%) and CETP (1 of 171, 0.6%), with significantly increased total and LIPG mutations in probands with HDLc≥90th percentile when compared to 136 probands with HDLc≤10th percentile. We also found no CETP or GALNT2 mutations in probands with HDLc≤10th percentile. Interestingly, and in contrast to these results, a recent sequencing study of 64 Thai individuals with hyperalphalipoprotenemia [41] identified 6 CETP mutations, 2 mutations in hepatic lipase, and no LIPG mutations, suggesting that enrichment of HDLc-elevating mutations in distinct genes may be dependent on geographic origin, a possibility which warrants further investigation in additional cohorts of different ancestries.
When assessed in family members, LIPG, CETP, and GALNT2 mutations described here segregated respectively with average HDLc elevations of 0.33, 0.66, and 0.52 mmol/L in carriers compared to first-degree relative controls, with no other significant changes in lipids. Segregation of LIPG and CETP mutations with elevated HDLc in families was significant, while GALNT2 mutations were borderline significant. Notably, mutations in LIPG (E388X) and GALNT2 (Q216H) were each found in 2 of 171 Dutch probands with HDLc≥90th percentile (1.2%). Evidence of founder effects for these mutations was obtained from shared ancestral haplotypes in probands and family members, suggesting that these two mutations may each potentially be present in ∼1% of HDLc≥90th percentile in the Dutch population. Finally, we show that the large majority (∼85%) of probands with HDLc≥90th percentile in this cohort have no known causes of elevated HDLc, thereby emphasizing the opportunity to discover novel genetic factors of hyperalphalipoproteinemia.
Recently, the GALNT2 D314A loss-of-function mutation was observed in humans to underlie non-sialyation of APOC3, which in turn led to increased LPL activity [28]. In that study, only 2 of 243 subjects with HDLc≥95th percentile had GALNT2 mutations, emphasizing that GALNT2 mutations are exceedingly rare. Similarly, we identify GALNT2 mutations in only 4 of 171 probands with HDLc≥90th percentile while identifying only 2 additional novel mutations. Although GALNT2 mutations in families associated with a large (average 0.52 mmol/L) elevation in HDLc in this study, this trend was borderline statistically significant, suggesting that either GALNT2 mutations have weaker or variable effects on HDLc elevation than LIPG or CETP mutations and/or these families have insufficient power for detecting a significant association. Regardless, our observations are consistent with mis-expression studies in mice, where Galnt2 mRNA levels are inversely proportional to HDLc levels [7]. Moreover, loss-of-function mutations in both APOC3 and LPL result in large increases and reductions in HDLc, respectively [13], [29], [30], which further support a role for GALNT2 loss-of-function mutations in HDLc elevation. We also observed that individuals with both GALNT2 and LIPG mutations had HDLc levels similar to individuals with mutations in either GALNT2 or LIPG alone, raising the possibility that mutations in GALNT2 and LIPG may be redundant with respect to HDLc elevation. Notably, we also did not observe a significant reduction in triglycerides in family members with GALNT2 mutations in this study. However, triglyceride levels in this study were measured in the fasting state, whereas triglyceride effects in GALNT2 mutation carriers becomes more apparent following oral fat challenge [28]. Additional phenotypic analysis of these and additional individuals with rare GALNT2 mutations is warranted to elucidate the exact mechanisms by which these mutations may lead to elevated HDLc in humans.
We unexpectedly observed the HDLc-elevating LIPG N396S and R476W mutations in 6 probands with HDLc≤10th percentile. However, 5 of these probands also carried mutations in ABCA1, LCAT, or LPL [13], [14], [39]. Moreover, family members with LIPG plus ABCA1 mutations consistently manifested phenotypes that resemble those of individuals with mutations in ABCA1 alone. These observations indicate that LIPG N396S and R476W do not overcome the loss-of-function effects of ABCA1, LCAT, and LPL mutations in these cohorts. They also suggest that patients with mutations in these genes are less likely to respond to HDLc-raising therapeutic strategies that target antagonism of LIPG.
How might ABCA1, LCAT, and LPL mutations overcome the HDLc-elevating effects of LIPG mutations? ABCA1 encodes ATP-binding cassette transporter A1, which is a cell membrane transporter that regulates cholesterol efflux from tissues [19]. LCAT, in turn, converts free cholesterol to cholesterol esters as part of mature, spherical HDL particle formation [39]. Finally, LPL encodes a triglyceride hydrolase that regulates delivery of apolipoproteins and phospholipids to HDL [42], [43]. ABCA1-mediated cholesterol efflux leads to lipidation and formation of pre-beta HDL particles, which mature into large spherical HDL particles via LCAT and LPL-mediated mechanisms. In contrast, EL is involved in downstream HDL phospholipid catabolism and thus acts on particles that are already modified by ABCA1, LCAT, and LPL. The inability of some LIPG mutations to overcome the effects of ABCA1, LCAT, and LPL mutations is therefore consistent with the sequential roles of the respective gene products in HDL metabolism. These studies exquisitely indicate how investigations of rare families provide novel insights with profound implications for understanding gene-gene interactions and elucidating the sequential roles of respective gene products in specific metabolic pathways.
When family members are assessed, we also observed that 85.7% of CETP mutation carriers assessed in this study have HDLc≥90th percentile, compared to 66.3% of individuals with LIPG mutations and only 50.0% with GALNT2 mutations. Conversely, only one individual with CETP mutations had HDLc between 80–89th percentiles, compared to 10.8% of individuals with LIPG mutations and 40.0% with GALNT2 mutations. These observations may imply differential effects on function for mutations in each of these genes. Computational assessment predicts damaging effects of all of these DNA changes on function but clearly functional studies are warranted to clarify this. Alternatively, these findings are also compatible with the concept that defects in cholesterol ester unloading from HDL may confer larger effects on HDLc elevation.
A total of 6 of 10 mutations identified in LIPG, CETP, and GALNT2 were novel. These mutations are likely to reduce protein activity and lead to elevated HDLc in humans based on the following. First, novel mutations largely occurred in highly conserved amino acid residues and/or were predicted to cause premature protein truncation or damage protein function. Second, they were detected in 8 of 171 probands with HDLc≥90th percentile (4.7%) but none of 136 probands with HDLc≤10th percentile (e.g., p = 9.9*10−3 by CAST; Table 2). Third, they segregated with elevated HDLc in family members of probands identified with mutations. These observations indicate that the majority of novel mutations described here are likely deleterious to protein function, although further validation awaits in vitro functional analysis.
Where data are available, the lipid phenotypes of individuals with mutations in this study bear a striking similarity to drug effects observed from emerging clinical trials. For example, individuals with heterozygous CETP mutations and expected 50% activity in this study had 62.3% increased HDLc and 26.4% reduced LDLc compared to first-degree relative controls (Table 4). These studies would predict that further inhibition should enhance these effects. Indeed, patients treated with anacetrapib, a CETP inhibitor that reduces CETP activity by ∼90% [44], had 138.1% increased HDLc and 39.8% reduced LDLc when compared to individuals treated with placebo [9], or roughly 1.5–2.5 times the effects observed with heterozygous, loss-of-function CETP mutations. Our results therefore suggest that detailed analysis of patients with rare, loss-of-function mutations may be a useful predictor of expected lipid changes with drugs conferring significant loss of function in this gene.
The following limitations of our study should be considered. First, the relatively small size of our initial proband cohort with a focus exclusively on Dutch-Caucasian individuals indicates that mutation frequencies and effects on HDLc elevation should be investigated in independent groups, and ideally in very large populations of Dutch or similar ancestries. This is particularly necessary to establish population-based mutation frequencies for CETP and GALNT2 mutations, as our cohort is underpowered to detect statistically significant enrichment of these rare events with elevated HDLc in unrelated probands. Second, our analyses do not correct for the effects of multiple testing. For example, if Bonferroni correction for the multiple comparisons of HDLc elevation in mutation carriers vs. first-degree relative controls across all genes is considered, a significant p value threshold should be 0.05/3 = 0.017, thereby making our observation of CETP mutation segregation with elevated HDLc borderline significant. However, while the Bonferroni correction is a robust but conservative method that can underestimate statistical significance, these observations further emphasize the need for additional assessment of these and other mutations in independent cohorts and families. Third, our sequence analysis is limited to the LIPG, CETP, and GALNT2 coding regions and adjacent sequence. Notably, a recent study identified rare gain-of-function promoter mutations in LIPG in individuals with low HDLc [22]. It is therefore of interest to determine whether rare gain-of-function mutations in the gene promoters may also underlie low HDLc in this cohort. Finally, while our human genetics-based approach successfully identifies exceedingly rare individuals with LIPG mutations plus mutations in ABCA1, LCAT, and LPL, in addition to LIPG plus GALNT2 mutations, these observations should be assessed in additional individuals, which may now be more readily enabled by the advent of next-generation sequencing technologies [45]. For example, exon capture technologies combined with next-generation sequencing can be used to simultaneously assess multiple genes that underlie HDLc levels in numerous probands [46], thereby increasing the probability of identifying individuals with more than one mutation. Alternatively, in probands with LIPG mutations and low HDLc, exome or genome sequencing may identify new mutations in addition to those already found in ABCA1, LCAT, and LPL.
In conclusion, we show that LIPG, CETP, and GALNT2 mutations are preferentially found in individuals with HDLc≥90th percentile and segregate with elevated HDLc in families. We also show that some LIPG mutations do not overcome the deleterious effects of ABCA1, LCAT, and LPL mutations. Moreover, we indicate that GALNT2 mutations do not further elevate HDLc in individuals with LIPG mutations, indicating that mutations in these genes may be redundant to HDLc elevation. These studies provide a foundation by which to assess the effects of LIPG, CETP, and GALNT2 mutations on CAD risk and the potential of these genes to serve as effective targets for drug development. Finally, we show that additional causes of elevated HDLc in this cohort are yet to be discovered.
Supporting Information
Segregation of LIPG , CETP , and GALNT2 mutations with elevated HDLc in pedigrees. For each individual, the individual ID, Age (in years), total cholesterol (mmol/L), triglycerides (mmol/L), HDLc (in mmol/L) plus [HDLc percentile], BMI, and genotype(s) for listed mutations are shown. Squares, Males; Circles, Females; Arrow, proband. Filled shape, HDLc≥90th percentile; half-filled, HDLc between 80–89th percentiles; empty shape, HDLc<80th percentile. Three probands for which no pedigree data are available are also shown.
(TIF)
Segregation of LIPG , mutations in pedigrees with ABCA1 , LCAT , and LPL mutations. Data are presented as described in Figure S1, except filled shape, HDLc≤10th percentile; half-filled, HDLc between 10–20th percentiles; empty shape, HDLc>20th percentile.
(TIF)
Additional GALNT2 SNPs found in 55 probands with HDLc≥90th vs. 55 probands with HDLc<10th percentiles. P values are calculated by Fisher's exact test.
(DOC)
Additional LIPG and CETP SNPs found in 55 probands with HDLc≥90th vs. 55 probands with HDLc≤10th percentiles. P values are calculated by Fisher's exact test.
(DOC)
Phenotypes of individuals with LIPG+ABCA1 mutations in families.
(DOC)
Phenotypes of individuals with LIPG+LCAT mutations in families.
(DOC)
Phenotypes of individuals with LIPG+LPL mutations in families.
(DOC)
Association of LIPG+GALNT2 mutations with HDLc in families.
(DOC)
PCR primer sequences.
(DOC)
Funding Statement
The funders, Xenon Pharmaceuticals Inc., did have a role in study design, data collection and analysis, decision to publish, and preparation of the manuscript, since some of the authors are employees of this company.
References
- 1.Ali M, Beusenberg M, Bloessner M, Boschi Pinto C, Briand S, et al. (2009) World health statistics 2009. World Health Organization. Available: http://www.who.int/whosis/whostat/EN_WHS09_Full.pdf.
- 2. Plump AS, Lum PY (2009) Genomics and cardiovascular drug development. J Am Coll Cardiol 53: 1089–1100. [DOI] [PubMed] [Google Scholar]
- 3. Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR (1977) High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med 62: 707–714. [DOI] [PubMed] [Google Scholar]
- 4. Muntner P. Lee F, Astor BC (2010) Association of high-density lipoprotein cholesterol with coronary heart disease risk across categories of low-density lipoprotein cholesterol: The Atherosclerosis Risk in Communities Study. Am J Med Sci 341: 173–180. [DOI] [PubMed] [Google Scholar]
- 5. von Eckardstein A, Hersberger M, Rohrer L (2005) Current understanding of the metabolism and biological actions of HDL. Curr Op Clin Nutr Metab Care 8: 147–152. [DOI] [PubMed] [Google Scholar]
- 6. Barter PJ, Puranik R, Rye KA (2007) New insights into the role of HDL as an anti-inflammatory agent in the prevention of cardiovascular disease. Curr Cardiol Rep 9: 493–498. [DOI] [PubMed] [Google Scholar]
- 7. Teslovich TM, Musunuru K, Smith AV, Edmondson AC, Stylianou IM, et al. (2010) Biological, clinical and population relevance of 95 loci for blood lipids. Nature 466: 707–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, et al. (2007) Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 357: 2109–2122. [DOI] [PubMed] [Google Scholar]
- 9. Cannon CP, Shah S, Dansky HM, Davidson M, Brinton EA, et al. (2010) Safety of Anacetrapib in patients with or at high risk for coronary artery disease. N Engl J Med 363: 2406–2415. [DOI] [PubMed] [Google Scholar]
- 10. Bauer RC, Stylianou IO, Rader DJ (2011) Functional validation of new pathways in lipoprotein metabolism identified by human genetics. Curr Opin Lipidol 22: 123–128. [DOI] [PubMed] [Google Scholar]
- 11. Inazu A, Brown ML, Hesler CB, Agellon LB, Koizumi J, et al. (1990) Increased high-density lipoprotein levels caused by a common cholesteryl-ester transfer protein gene mutation. N Engl J Med 323: 1234–1238. [DOI] [PubMed] [Google Scholar]
- 12. Funke H, von Eckardstein A, Pritchard PH, Albers JJ, Kastelein JJ, et al. (1991) A molecular defect causing fish eye disease: an amino acid exchange in lecithin-cholesterol acyltransferase (LCAT) leads to the selective loss of alpha-LCAT activity. Proc Natl Acad Sci 88: 4855–4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reymer PW, Gagné E, Groenemeyer BE, Zhang H, Forsyth I, et al. (1995) A lipoprotein lipase mutation (Asn291Ser) is associated with reduced HDL cholesterol levels in premature atherosclerosis. Nat Genet 10: 28–34. [DOI] [PubMed] [Google Scholar]
- 14. Brooks-Wilson A, Marcil M, Clee SM, Zhang LH, Roomp K, et al. (1999) Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat Genet 22: 336–345. [DOI] [PubMed] [Google Scholar]
- 15. Clee SM, Kastelein JJP, van Dam M, Marcil M, Roomp K, et al. (2000) Age and residual cholesterol efflux affect HDL cholesterol levels and coronary artery disease in ABCA1 heterozygotes. J Clin Invest 106: 1263–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van der Steeg WA, Hovingh GK, Klerkx AH, Hutten BA, Nootenboom IC, et al. (2007) Cholesteryl ester transfer protein and hyperalphalipoproteinemia in Caucasians. J Lipid Res 48: 674–682. [DOI] [PubMed] [Google Scholar]
- 17. Edmondson AC, Brown RJ, Kathiresan S, Cupples LA, Demissie S, et al. (2009) Loss-of-function variants in endothelial lipase are a cause of elevated HDL cholesterol in humans. J Clin Invest 119: 1042–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Klos KLE, Kullo IJ (2007) Genetic determinants of HDL: Monogenic disorders and contributions to variation. Curr Opin Cardiol 23: 344–351. [DOI] [PubMed] [Google Scholar]
- 19. Weissglas-Volkov D, Pajukanta P (2010) Genetic causes of high and low serum HDL-cholesterol. J Lipid Res 51: 2032–2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ishida T, Choi S, Kundu RK, Hirata K, Rubin EM, et al. (2003) Endothelial lipase is a major determinant of HDL level. J Clin Invest 111: 347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jin W, Millar JS, Broedl U, Glick JM, Rader DJ (2003) Inhibition of endothelial lipase causes increased HDL cholesterol levels in vivo. J Clin Invest 111: 357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Khetarpal SA, Edmondson AC, Raghavan A, Heeli H, Jin W, et al. (2011) Mining the LIPG allelic spectrum reveals the contribution of rare and common regulatory variants to HDL cholesterol. PLoS Genet 7: e1002393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. deLemos AS, Wolfe ML, Long CJ, Sivapackianathan R, Rader DJ (2002) Identification of genetic variants in endothelial lipase in persons with elevated high-density lipoprotein cholesterol. Circulation 106: 1321–1326. [DOI] [PubMed] [Google Scholar]
- 24. Gauthier A, Lau P, Zha X, Milne R, McPherson R (2005) Cholesteryl ester transfer protein directly mediates selective uptake of high density lipoprotein cholesteryl esters by the liver. Arterioscler Thromb Vasc Biol 25: 2177–2184. [DOI] [PubMed] [Google Scholar]
- 25. Koizumi J, Mabuchi H, Yoshimura A, Michishita I, Takeda M, et al. (1985) Deficiency of serum cholesteryl-ester transfer activity in patients with familial hyperalphalipoproteinaemia. Atherosclerosis 58: 175–186. [DOI] [PubMed] [Google Scholar]
- 26. Brown ML, Inazu A, Hesler CB, Agellon LB, Mann C, et al. (1989) Molecular basis of lipid transfer protein deficiency in a family with increased high-density lipoproteins. Nature 342: 448–451. [DOI] [PubMed] [Google Scholar]
- 27. Wandall HH, Hassan H, Mirgorodskaya E, Kristensen AK, Roepstorff P, et al. (1997) Substrate specificities of the three members of the human UDP-N-acetyl-alpha-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase family, GalNAc-T1, -T2, and –T3. J Biol Chem 272: 23503–23514. [DOI] [PubMed] [Google Scholar]
- 28. Holleboom AG, Karlsson H, Lin RS, Beres TM, Sierts JA, et al. (2011) Heterozygosity for a loss-of-function mutation in GALNT2 improves plasma triglyceride clearance in man. Cell Metab 14: 811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. von Eckardstein A, Holz H, Sandkamp M, Weng W, Funke H, et al. (1991) Apolipoprotein C-III(Lys58→Glu): Identification of an Apolipoprotein C-III variant in a family with hyperalphalipoproteinemia. J Clin Invest 87: 1724–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pollin TI, Damcott CM, Shen H, Ott SH, Shelton J, et al. (2008) A null mutation in human APOC3 confers a favorable plasma lipid profile and apparent cardioprotection. Science 322: 1702–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Heiss G, Johnson NJ, Reiland S, Davis CE, Tyroler HA (1980) The epidemiology of plasma high-density lipoprotein cholesterol levels. The Lipid Research Clinics Program Prevalence Study. Circulation 62: IV116–136. [PubMed] [Google Scholar]
- 32. Marcil M, Brooks-Wilson A, Clee SM, Roomp K, Zhang LH, et al. (1999) Mutations in the ABC1 gene in familial HDL deficiency with defective cholesterol efflux. Lancet 354: 1341–1346. [DOI] [PubMed] [Google Scholar]
- 33. Rogler G, Trumbach B, Klima B, Lackner KJ, Schmitz G (1995) HDL-mediated efflux of intracellular cholesterol is impaired in fibroblasts from Tangier disease patients. Arterioscler Thromb Vasc Biol 15: 683–690. [DOI] [PubMed] [Google Scholar]
- 34. Rogozin IB, Milanesi L (1997) Analysis of donor splice signals in different organisms. J Mol Evol 45: 50–59. [DOI] [PubMed] [Google Scholar]
- 35. Ramensky V, Bork P, Sunyaev S (2002) Humans non-synonymous SNPs: server and survey. Nucleic Acids Res 30: 3894–3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Neale BM, Rivas MA, Voight BF, Altshuler D, Devlin B, et al. (2011) Testing for an unusual distribution of rare variants. PLoS Genet 7: e1001322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Morgenthaler S, Thilly WG (2007) A strategy to discovery genes that carry multi-allelic or mono-allelic risk for common diseases: A cohort allelic sums test (CAST). Mutation Res 615: 28–56. [DOI] [PubMed] [Google Scholar]
- 38. Li B, Leal SM (2008) Methods for detecting associations with rare variants for common diseases: Application to analysis of sequence data. Am J Hum Genet 83: 311–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kuivenhoven JA, Pritchard H, Hill J, Frohlich J, Assmann G, et al. (1997) The molecular pathology of lecithin:cholesterol acyltransferase (LCAT) deficiency syndromes. J Lipid Res 38: 191–205. [PubMed] [Google Scholar]
- 40. Hovingh GK, Brownlie A, Bisoendial RJ, Dube MP, Levels JH, et al. (2004) A novel apoA-I mutation (L178P) leads to endothelial dysfunction, increased arterial wall thickness, and premature coronary artery disease. J Am Coll Cardiol 44: 1429–1435. [DOI] [PubMed] [Google Scholar]
- 41. Khovidhunkit W, Chartyingcharoen P, Siriwong S, Limumpornpetch P, Plengpanich W (2012) Resequencing CETP, LIPC, and LIPG genes in Thai subjects with hyperalphalipoproteinemia. Am J Cardiol doi:10.1016/j.amjcard.2012.02.052. [DOI] [PubMed] [Google Scholar]
- 42. Strauss JG, Frank S, Kratky D, Hammerle G, Hrzenjak A, et al. (2001) Adenovirus-mediated rescue of lipoprotein lipase-deficient mice. Lipolysis of triglyceride-rich lipoproteins is essential for high density lipoprotein maturation in mice. J Biol Chem 276: 36083–36090. [DOI] [PubMed] [Google Scholar]
- 43. Merkel M, Eckel RH, Goldberg IJ (2002) Lipoprotein lipase: genetics, lipid uptake, and regulation. J Lipid Res 43: 1997–2006. [DOI] [PubMed] [Google Scholar]
- 44. Krishna R, Garg A, Panebianco D, Cote J, Bergman AJ, et al. (2009) Single-dose pharmacokinetics and pharmacodynamics of anacetrapib, a potent cholesterol ester transfer protein (CETP) inhibitor, in healthy subjects. Br J Clin Pharmacol 68: 535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ku CS, Naidoo N, Pawitan Y (2011) Revisiting Mendelian disorders through exome sequencing. Hum Genet 129: 351–370. [DOI] [PubMed] [Google Scholar]
- 46. Gnirke A, Melnikov A, Maguire J, Rogov P, LeProust EM, et al. (2009) Solution hybrid selection with ultra-long oligonucleotides for massively parallel targeted sequencing. Nat Biotechnol 27: 182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tietjen I, Hovingh GK, Singaraja R, Radomski C, McEwen J, et al. (2012) Increased risk of coronary artery disease in Caucasians with extremely low HDL cholesterol due to mutations in ABCA1, APOA1, and LCAT. Biochim Biophys Acta 1821: 416–424. [DOI] [PubMed] [Google Scholar]
- 48. Cohen JC, Kiss RS, Pertsemlidis A, Marcel YL, McPherson R, et al. (2004) Multiple rare alleles contribute to low plasma levels of HDL cholesterol. Science 305: 869–872. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Segregation of LIPG , CETP , and GALNT2 mutations with elevated HDLc in pedigrees. For each individual, the individual ID, Age (in years), total cholesterol (mmol/L), triglycerides (mmol/L), HDLc (in mmol/L) plus [HDLc percentile], BMI, and genotype(s) for listed mutations are shown. Squares, Males; Circles, Females; Arrow, proband. Filled shape, HDLc≥90th percentile; half-filled, HDLc between 80–89th percentiles; empty shape, HDLc<80th percentile. Three probands for which no pedigree data are available are also shown.
(TIF)
Segregation of LIPG , mutations in pedigrees with ABCA1 , LCAT , and LPL mutations. Data are presented as described in Figure S1, except filled shape, HDLc≤10th percentile; half-filled, HDLc between 10–20th percentiles; empty shape, HDLc>20th percentile.
(TIF)
Additional GALNT2 SNPs found in 55 probands with HDLc≥90th vs. 55 probands with HDLc<10th percentiles. P values are calculated by Fisher's exact test.
(DOC)
Additional LIPG and CETP SNPs found in 55 probands with HDLc≥90th vs. 55 probands with HDLc≤10th percentiles. P values are calculated by Fisher's exact test.
(DOC)
Phenotypes of individuals with LIPG+ABCA1 mutations in families.
(DOC)
Phenotypes of individuals with LIPG+LCAT mutations in families.
(DOC)
Phenotypes of individuals with LIPG+LPL mutations in families.
(DOC)
Association of LIPG+GALNT2 mutations with HDLc in families.
(DOC)
PCR primer sequences.
(DOC)