Abstract
Biodegradable nanopolymers are believed to offer great potential in cancer therapy. Here, we report the characterization of a novel, targeted, nanobiopolymeric conjugate based on biodegradable, nontoxic, and nonimmunogenic PMLA [poly(β-l-malic acid)]. The PMLA nanoplatform was synthesized for repetitive systemic treatments of HER2/neu-positive human breast tumors in a xenogeneic mouse model. Various moieties were covalently attached to PMLA, including a combination of morpholino antisense oligonucleotides (AON) directed against HER2/neu mRNA, to block new HER2/neu receptor synthesis; anti-HER2/neu antibody trastuzumab (Herceptin), to target breast cancer cells and inhibit receptor activity simultaneously; and transferrin receptor antibody, to target the tumor vasculature and mediate delivery of the nanobiopolymer through the host endothelial system. The results of the study showed that the lead drug tested significantly inhibited the growth of HER2/neu-positive breast cancer cells in vitro and in vivo by enhanced apoptosis and inhibition of HER2/neu receptor signaling with suppression of Akt phosphorylation. In vivo imaging analysis and confocal microscopy demonstrated selective accumulation of the nanodrug in tumor cells via an active delivery mechanism. Systemic treatment of human breast tumor-bearing nude mice resulted in more than 90% inhibition of tumor growth and tumor regression, as compared with partial (50%) tumor growth inhibition in mice treated with trastuzumab or AON, either free or attached to PMLA. Our findings offer a preclinical proof of concept for use of the PMLA nanoplatform for combination cancer therapy.
Introduction
Humanized anti-HER2/neu monoclonal antibody (mAb) trastuzumab (Herceptin, Genentech Inc. ) is used alone or combined with chemotherapy for treatment of patients with advanced breast cancer overexpressing HER2/neu (1–3). Despite significant antitumor effects of Herceptin, it also causes serious adverse effects on normal organs (4, 5). Moreover, many patients develop resistance to Herceptin within 1 year of treatment, which renders this treatment ineffective (6). Therefore, the new generation of drugs with specific tumor targeting and high accumulation in tumor cells with minimal side effects for nontumor tissues is urgently needed to improve HER2/neu-positive tumor therapy.
Antisense oligonucleotides (AON) that bind specifically to mRNA and block protein synthesis are well established as powerful and specific tools for gene/protein inhibition. Efficient delivery of AONs as well as siRNAs with systemic tumor treatment still presents significant problems (7, 8). However, recent preclinical studies of AON for cancer treatment showed promising results, and AONs' stability in plasma makes them feasible for systemic treatment (9–11). Morpholino AONs to dystrophin were also delivered to dystrophic muscle cells in vivo in Duchenne muscular dystrophy mouse model and patients (12, 13). Importantly, AON against HER2/neu appears to be more potent in inhibiting neoplastic cell proliferation in vitro than mAb inhibition of HER2/neu receptor (14). Combination treatment of HER2/neu-positive breast cancer cells in vitro with HER2/neu AON and conventional chemotherapeutic agents results in synergistic inhibition of tumor cell growth by activation of apoptosis (15, 16). We engineered novel nanobiopolymeric drugs on the basis of PMLA [poly(β-l-malic acid)] platform that were specifically designed for delivery into HER2/neu-positive tumors. PMLA is a natural polymer of the slime mold Physarum polycephalum (17, 18). PMLA is nontoxic, nonimmunogenic, and biodegradable in vitro and in vivo, stable in the bloodstream, and highly water soluble (18–20). We recently showed that systemic delivery of morpholino AONs against α4- and β1-chains of a tumor vasculature-specific protein, laminin-411 (formerly, laminin-8), to intracranial glioblastoma resulted in a marked inhibition of tumor angiogenesis and growth (21, 22). The lead drug tested here was designed to inhibit the synthesis of new HER2/neu receptors with AON and to block the activity of existing HER2/neu on the tumor cell membrane with Herceptin. To target tumor vasculature, a mAb to transferring receptor (TfR) was attached to the same nanoplatform. We investigated whether the new nanobiopolymer carrying both anti-HER2/neu antibody (Herceptin), anti-TfR antibody, and AON to HER2/neu would enhance the specificity and anti-tumor effect toward HER2/neu-positive breast cancer.
Materials and Methods
Reagents
Two versions of morpholino-30-NH2 AONs to HER2/neu
Version 1: 5′-AGGGAGCCGCAGCTTCATGTCTGTG-3′
Version 2: 5′-CATGGTGCTCACTGCGGCTCCGGC-3′
were custom made by Gene Tools.
Highly purified, endotoxin-free PMLA, weight-averaged molecular weight (Mw) = 100 kDa, polydispersity = 1. 1, was obtained from the culture broth of P. polycephalum. Rat antimouse TfR mAb R17217 (mTfR) was purchased from Southern Biotech. Cysteamine (2–mercaptoethyl-1-amine hydrochloride), N-hydroxysuccinimide, other reagents and solvents were of highest available purity and purchased from Sigma-Aldrich.
Synthesis of polymalic acid nanobiopolymers
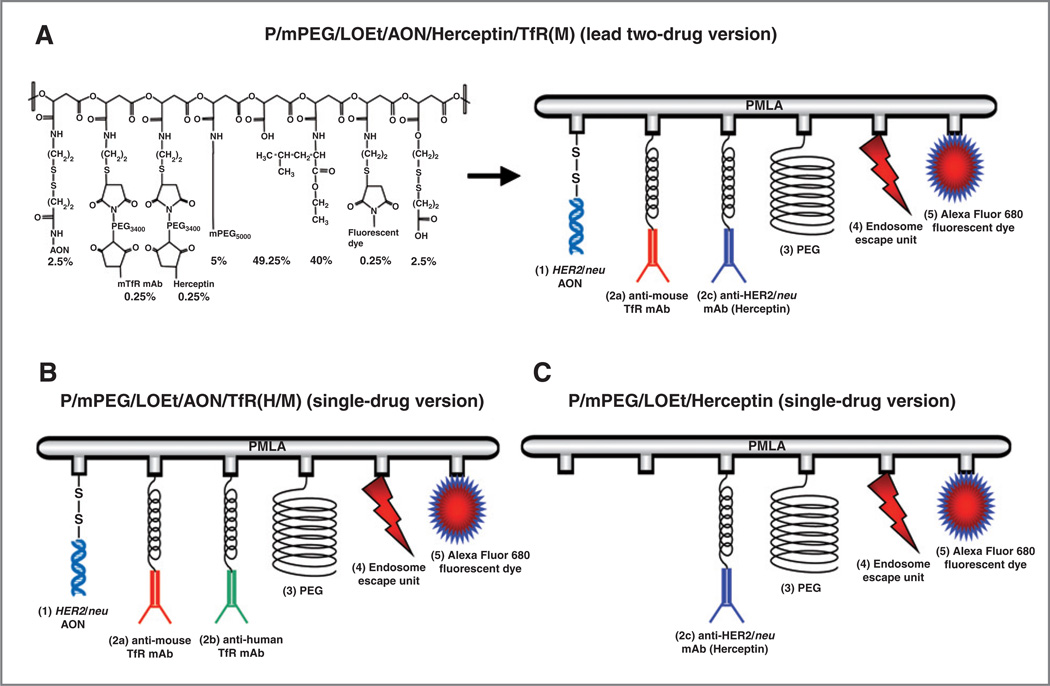
The nanobiopolymers contain 5 to 6 key components (Fig. 1): PMLA as the backbone; morpholino AON to inhibit HER2/neu protein synthesis; targeting anti-TfR mAb; antitumor Herceptin; 40% leucine ethyl ester (LOEt) as endosome escape unit to achieve cytoplasmic AON delivery, and 5% PEG5000 (polyethylene glycol, M = 5, 000) to increase stability in the bloodstream. Anti-TfR mAb on Herceptin-containing conjugate was only anti-mouse to target tumor vasculature. When the conjugate had only AON without Herceptin, an anti-human TfR mAb was also attached to it to ensure drug binding to human tumor cells and its internalization. The preconjugate containing 40% LOEt, 5% PEG5000, and 10% cysteamine (% referring to the total amount of pendant carboxyl groups in PMLA) was synthesized by the methods described previously (17). The antibodies conjugated with the preconjugate were qualitatively and quantitatively assayed by size exclusion high-performance liquid chromatography (HPLC). ELISA with purified TfR and HER2/neu was used to verify functional reactivity of attached antibodies as described (23).
Figure 1.
The nanobiopolymer schematic. The nanobiopolymeric conjugate was designed to inhibit HER2/neu expression by AON and to attenuate HER2/neu-mediated cell signaling by Herceptin. The modules are HER2/neu morpholino AON (1) conjugated to the PMLA scaffold by disulfide bonds (S-S) that are cleaved by cytoplasmic glutathione to release the free drugs; targeting and/or effector antibodies comprising anti-TfR either alone or combination of mAbs to mouse TfR (2a), human TfR (2b), and Herceptin (2c) for tumor endothelial and cancer cell targeting, receptor-mediated endocytosis, and antitumor effect, PEG for drug protection (3), stretches of conjugated LOEt for endosomal escape of the drug (4), and optional fluorescent reporter dye (Alexa Fluor 680) for imaging (5). The nanopolymer also contained free, unsubstituted, pendant carboxyl groups for enhancing solubility and nonfunctional disulfide originating from chemical masking of excess sulfhydryls.
Conjugates for imaging were fluorescently labeled with Alexa Fluor 680 C2-maleimide (Invitrogen) by forming thioether with sulfhydryl groups. Antibody conjugates were then allowed to react with HER2/neu AON (Fig. 1). The control conjugate contained Herceptin (Fig. 1) but was devoid of HER2/neu-specific AON.
The nanobiopolymer characterization
Chemical and physical characterization of polymeric nanobioconjugate was performed by various methods, including l-malate dehydrogenase assay, after nanobiopolymer hydrolysis at 100°C in the presence of 6 mol/L HCl, PEG colorimetric determination and protein quantification, size and ζ potential, HPLC, and ELISA. HPLC was performed on a Hitachi analytical Elite LaChrom HPLC-UV system (Hitachi) and size exclusion on a BioSep-SEC-S 3000 column (Phenomenex). The nanobiopolymer variants were characterized by their size (hydrodynamic diameter) on the basis of noninvasive back-scattering (NIBS), and ζ potential from electrophoretic mobility on the basis of the Helmholtz–Smoluchowski formula, using electrophoresis M3-PALS (19). Both measurements were performed in a Zetasizer Nano System ZS90 (Malvern Instruments). Data on molecular size and ζ potential represent mean ± SD obtained from 3 independent measurements.
Cell lines and culture conditions
Human breast cancer cell lines BT-474, SKBR-3, MDA-MB-231, MDA-MB-435, MDA-MB-468, and MCF-7 were obtained from American Type Culture Collection. BT-474, MDA-MB-231, MDA-MB-435, MDA-MB-468, and MCF-7 were cultured in DMEM with 10% FBS and antibiotics. SKBR-3 was cultured in McCoy's 5A medium with 10% FBS and antibiotics.
Nomenclature
The term "nanobiopolymer" denotes the drug delivery system with PMLA as nanoplatform and various functional groups covalently attached to it, specifically AON, rat anti-mouse and mouse anti-human targeting TfR mAbs (M and H, respectively), and LOEt as the endosomal escape unit. The newly synthesized nanobiopolymer versions (Fig. 1Table 1) to treat HER2/neu-positive breast cancer contained either a single drug (HER2/neu AON or Herceptin) or 2 drugs (HER2/neu AON þ Herceptin).
Table 1.
Nanobiopolymer versions, their sizes, and ζ potentials
| Nanobiopolymer variant | Version | Size, nm | ζ potential, mV |
|---|---|---|---|
| P/mPEG/LOEt/AON/Herceptin/TfR(M) | Lead version with AON, Herceptin, and anti-TfR(M) | 22.1 ± 2.3 | −5.2 ± 0.4 |
| P/mPEG/LOEt/AON/TfR(H/M) | With AON and anti-TfR(H/M) | 20.1 ± 2.4 | −5.7 ± 0.6 |
| P/mPEG/LOEt/Herceptin | With Herceptin alone | 15.1 ± 1.2 | −4.1 ± 0.4 |
| P/mPEG/LOEt/IgG | Control version for imaging study with IgG | N/A | N/A |
N/A, Not applicable.
Cell proliferation assay
HER2/neu-overexpressing breast cancer cells (BT-474 and SKBR-3) were seeded into 6-well plates at 3 × 105 cells per well. The next day, cells were treated with PBS (control), Endoporter (4 µmol/L; control), Herceptin (40 µg/mL), P/mPEG/LOEt/Herceptin (40 µg/mL), Endoporter (4 µmol/L) plus AON (4 µmol/L), P/mPEG/LOEt/AON/TfRH/M), and P/mPEG/LOEt/AON/Herceptin/TfRM) (at 4 µmol/L AON and 40 µg/mL each antibody for both conjugates). Seventy-two hours after treatment, the cells were stained with trypan blue. Cell viability was determined by calculating the mean of cell counts for each treatment group (in triplicate) and expressed as a percentage of the total number of cells treated to the number of cells treated with PBS.
Western blotting
BT-474 and SKBR-3 breast cancer cells were treated with controls (PBS or 4 µmol/L Endoporter); Herceptin (40 µg/mL), P/mPEG/LOEt/Herceptin (40 µg/mL Herceptin), Endoporter (4 µmol/L) plus AON (4 µmol/L), P/mPEG/LOEt/AON/TfRH/M), and P/mPEG/LOEt/AON/Herceptin/TfRM) (at 4 µmol/L AON and 40 µg/mL each antibody for both conjugates). Cell lysates were collected in 72 hours and analyzed by Western blotting as described previously (24). Lysates of excised breast tumors after various treatments were analyzed in the same way. The following anti-human primary antibodies were used: HER2/neu, Akt, phosphorylated Akt (p-Akt), glyceraldehyde 3-phosphate dehydrogenase (GAPDH, to normalize gel loading; all from Cell Signaling Technology), and PARP [poly(ADP ribose) polymerase; BD Biosciences].
Tumor xenografts in nude mice
Animal experiments were performed in accordance with the protocols approved by the Cedars-Sinai Medical Center Institutional Animal Care and Use Committee. Athymic mice (CrTac: NCr-Foxn1nu Homozygous; Taconic) were used. A 0. 72-mg, 90-day release 17β-estradiol pellet (Innovative Research of America) was inserted subcutaneously on the back of mice 7 days before cell injection. A total of 1 × 107 BT-474 cells suspended in 150 µL of Matrigel (BD Biosciences) were injected into the right flanks of 35 mice (5 mice per group), and treatment began when tumors reached an average size of greater than 120 mm3 (21 days after injection). Mice were divided into 5 treatment groups and given either sterile PBS (control), Herceptin (4. 5 µg/kg dose), P/mPEG/LOEt/Herceptin, P/mPEG/LOEt/AON/TfRH/M) or P/mPEG/LOEt/AON/Herceptin/TfRM) from the tail vein twice a week. All nanobiopolymers were given at the same concentrations of AON (2. 5 µg/kg) and of each antibody (4. 5 µg/kg) in 160 µL per injection. Herceptin dose was determined based on FDA approved clinical dose and was equivalent to Herceptin alone. Treatments were performed 6 times (for 3 weeks).
Tumor xenografts were measured with calipers twice a week, and tumor volumes were determined using the formula: (length × width2) × (π/6).
Eighteen days after the last treatment, the animals were anesthetized with 3% isoflurane–air mixture and killed by cervical dislocation. Tumor samples were stained with hematoxylin and eosin (H&E) for morphologic observation. The data were averaged from 2 independent experiments.
Confocal microscopy
Three different versions of Alexa Fluor 680–labeled nanobiopolymers (P/mPEG/LOEt/IgG, control); P/mPEG/LOEt/Herceptin, or P/mPEG/LOEt/AON/Herceptin/TfRM)were injected through the tail vein of mice at the same dose as for treatment. Twenty-four hours after drug administration, mice were euthanized; the tumors were harvested to detect the fluorescent signal, snap-frozen in liquid nitrogen, and embedded in OCT (optimum cutting temperature) compound for confocal microscopy (TCS SP5 X microscope; Leica Microsystems).
In vivo imaging study
BT-474 human breast cancer cells were implanted into the right thigh of mice as described in the Tumor Xenografts in Nude Mice section. When tumors grew up to 120 mm3, 160 µL of Alexa Fluor 680–labeled nanobiopolymers were injected intravenously at the same doses as for treatment. P/mPEG/LOEt/IgG (4. 5 mg/kg IgG dose) was used as a negative control. Drug distribution and localization was assessed in tumor-bearing mice using Xenogen IVIS 200 imager (Caliper Life Sciences), at different time points (before drug administration, 1, 3, 6, and 24 hours after the drug injection). Twenty-four hours after drug administration, mice were euthanized and the circulating drugs were eliminated by intra-arterial PBS perfusion. The tumor and major organs were harvested to detect the fluorescent signal.
Statistical analysis
Student's t test (for 2 groups) and ANOVA (for ≥3 groups) were used to calculate statistical significance of the experimental results. GraphPad Prism4 program (GraphPad Software) was utilized for all calculations. Data are presented as mean ± SEM. The significance level was set at P < 0.05.
Results
Synthesis of polymer conjugates
Of the two HER2/neu-specific AON sequences (see the Materials and Methods section), version 1 did not inhibit HER2/neu expression well in comparison with version 2 (data not shown); therefore, only version 2 was conjugated to the polymer platform. The absolute molecular weight of leading version of nanobiopolymer (Fig. 1) was 1, 300 kDa by light scattering and close to the calculated value on the basis of design. Hydrodynamic diameters (nanosizes) and ζ potentials of the nanobiopolymers in Figure 1 are summarized in Table 1. Parameters for ζ potentials in the range of −4. 1 to −5. 7 mV have been reported for other nanoparticles as compatible with cell membrane attachment and nanoparticle internalization (25, 26).
The lead nanobiopolymer carrying both Herceptin and HER2/neu AON [P/mPEG/LOEt/AON/Herceptin/TfR (M)] inhibits growth of breast cancer cells in vitro
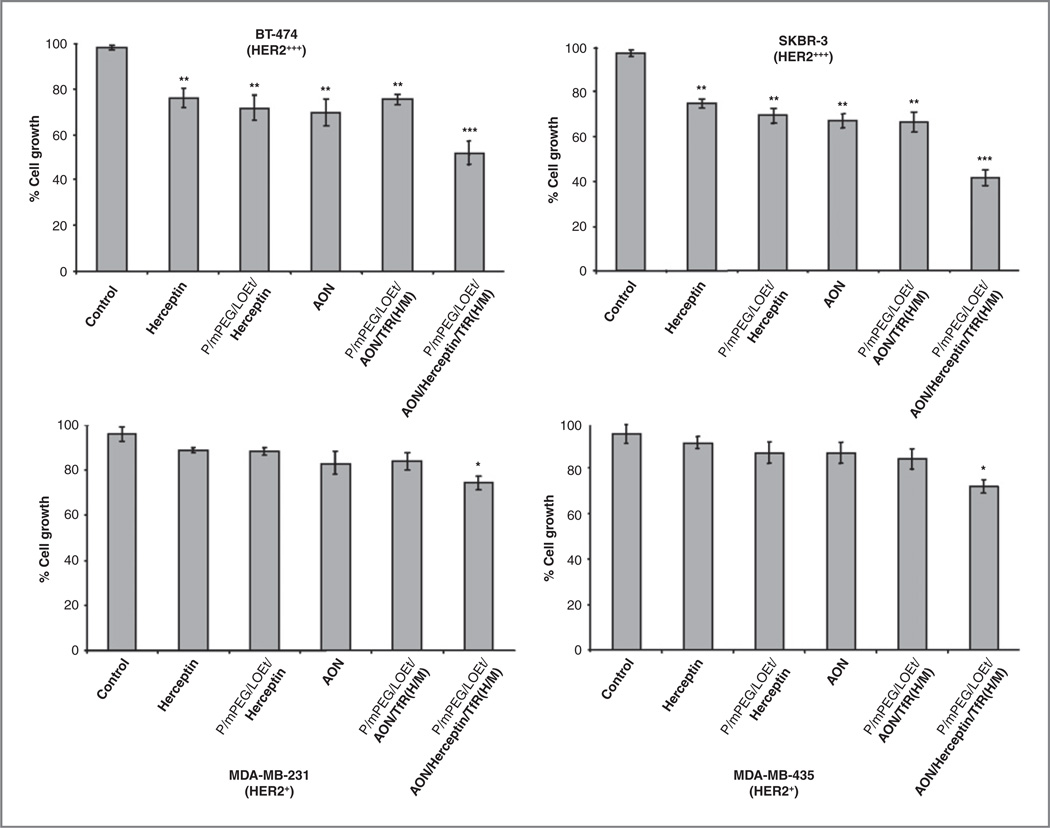
We have first examined breast cancer cell growth inhibition with HER2/neu AON and Herceptin. On the basis of optimization experiments, we tested AON at 4 µmol/L with 4 µmol/L Endoporter (in vitro AON delivery agent, Gene Tools), and Herceptin, at 40 µg/mL. Results in Figure 2 are shown for HER2/neu high-expressing cells BT-474 and SKBR-3, as well as for low-expressing cells, MDA-MB-231 and MDA-MB-435. At the concentrations used, both free AON and Herceptin showed some growth inhibition in HER2/neu high-expressing cells; although, low-expressing cell lines were significantly less sensitive to both treatments. Three versions of nanobiopolymeric conjugate (one 2-drug lead variant and two single-drug variants shown in Fig. 1) were then tested for tumor cell growth inhibitory effect. All nanobiopolymers, Herceptin, and free AON caused significant growth inhibition compared with PBS control in HER2/neu high-expressing cells (Fig. 2 top; P < 0. 01). The lead version produced the strongest inhibitory effect that was significantly higher than that of other drugs including Herceptin (P < 0. 005 vs. all groups). Importantly, in HER2/neu low-expressing cells, only the lead drug was able to induce modest but statistically significant growth inhibition compared with PBS (Fig. 2 bottom, P < 0. 02).
Figure 2.
In vitro cell viability assay. HER2/neu overexpressing breast cancer cells (BT-474 and SKBR-3; Fig. 3A) were treated with various drugs (top row). After 72 hours, cell viability was determined using trypan blue exclusion assay. Percentages of cell growth were calculated as the average of cell counts for each group and expressed relative to parallel samples treated with PBS (control) set to 100%. Growth of tumor cells treated with P/mPEG/LOEt/AON/Herceptin/TfRM) was significantly inhibited compared with other treatments in both cell lines. In cell lines expressing low amounts of HER2/neu (Fig. 3A), the only drug active in cell growth inhibition was the lead compound (bottom row). *, P < 0. 05; **, P < 0. 01; ***, P < 0. 003 compared with PBS. The lead drug also showed significant differences at P < 0. 005 when compared with all treatment groups in top row, and at P < 0. 02 when compared with Herceptin in bottom row.
The lead drug inhibits HER2/neu and p-Akt expression and induces apoptosis of HER2/neu-overexpressing breast cancer cells in vitro
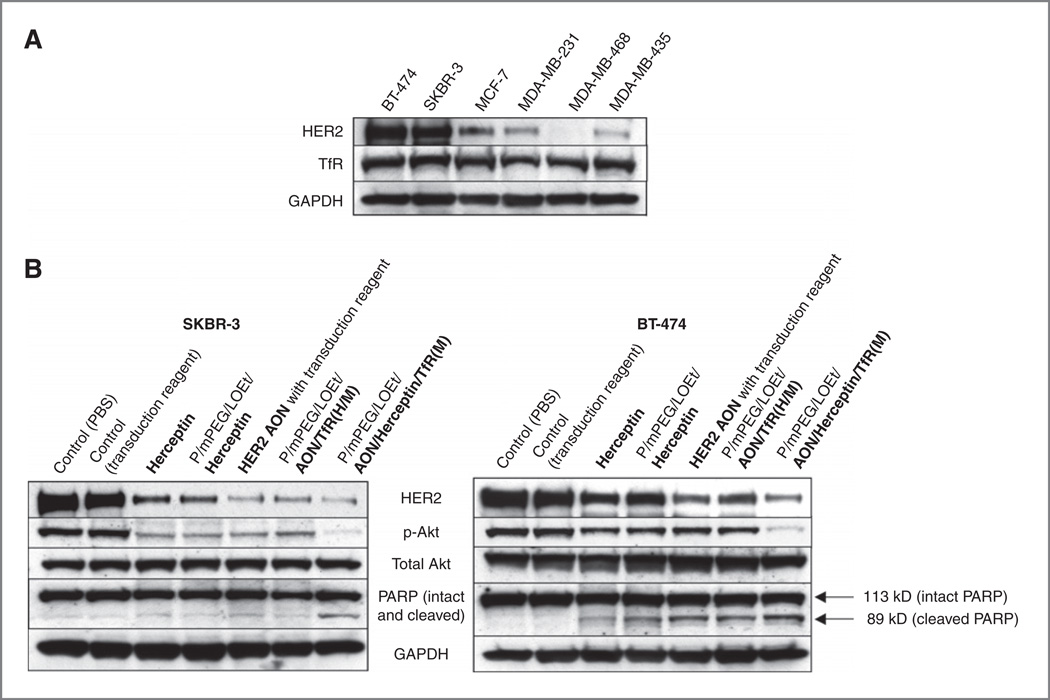
A phosphatidylinositol-3 kinase (PI3K) and its downstream target, the serine/threonine kinase Akt, play an important roll in HER2/neu-positive breast cancer cell growth and proliferation, as well as in antitumor effect of Herceptin (6, 27). HER2/neu signaling can activate the PI3K/Akt/mTOR cascade, and activated Akt stimulates increases in cell size, metabolism, and survival (28). To examine the mechanism responsible for the enhanced growth inhibitory effect of the lead nanobiopolymer, we assessed drug effects on the expression and phosphorylation of pertinent signaling markers, HER2/neu, Akt, and p-Akt. HER2/neu high-expressing cell lines BT-474 and SKBR-3 were used (Fig. 3A). To further determine whether the nanobiopolymer carrying both HER2/neu AON and Herceptin induces apoptosis, PARP cleavage was also examined by Western blot analysis.
Figure 3.
Changes of HER2/neu expression, Akt phosphorylation, and apoptosis upon various treatments of breast cancer cells in vitro. A, HER2/neu and TfR expression in cell lines. B, expression analysis of various markers. HER2/neu overexpressing breast cancer cells (a) were treated with various drugs. Western blot analysis showed decreased HER2/neu and phosphorylated Akt in Herceptin, P/mPEG/LOEt/Herceptin, AON or P/mPEG/LOEt/AON/TfR (H/M)-treated tumor cells but not in PBS or Endoporter-treated cells. P/mPEG/LOEt/AON/Herceptin/TfRM) further reduced both HER2/neu and p-Akt. Generation of cleaved PARP as a measure of apoptosis was best seen in P/mPEG/LOEt/AON/Herceptin/TfRM)-treated cells. GAPDH was used as an internal loading control. Breast cancer cell lines used in this study expressed high levels of TfR (A).
In both HER2/neu high-expressing cell lines, HER2/neu expression was inhibited to different degrees by Herceptin, AON, and two single-drug versions of the nanobiopolymer [P/mPEG/LOEt/Herceptin and P/mPEG/LOEt/AON/TfRH/M)] in comparison with controls. The strongest inhibition of HER2/neu expression was observed upon treatment with the lead nanobiopolymer that had 2 drugs (AON and Herceptin) attached to the same PMLA carrier molecule.
The expression of p-Akt, a key downstream mediator of HER2/neu signaling (6), was inhibited to a different extent in tumor cells treated with Herceptin, AON, or single-drug versions of nanobiopolymer as compared with control cells treated with PBS or AON transduction reagent Endoporter. Importantly, the p-Akt signal upon treatment of both breast cancer cell lines with the lead drug was markedly lower than after treatment with any other reagent (Fig. 3B). The amount of total Akt on Western blots was unchanged after all treatments.
Apoptosis assessed by PARP cleavage was induced to some extent by Herceptin, AON, and single-drug nanobiopolymers in HER2/neu high-expressing cells, especially in BT-474 cell line. However, the lead version, P/mPEG/LOEt/AON/Herceptin/TfRM), triggered apoptosis more significantly in both cell lines as evidenced by increased PARP cleavage as compared with the other drugs (Fig. 3B).
The lead drug specifically accumulates in HER2/neu-overexpressing breast tumors in vivo
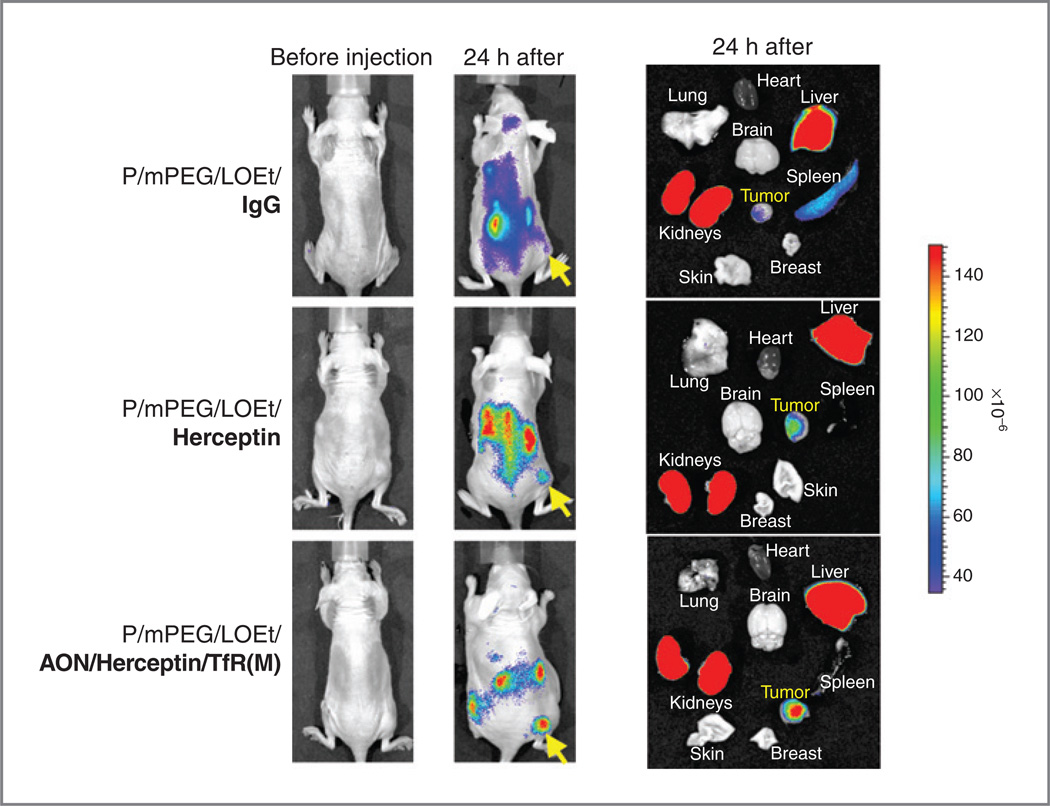
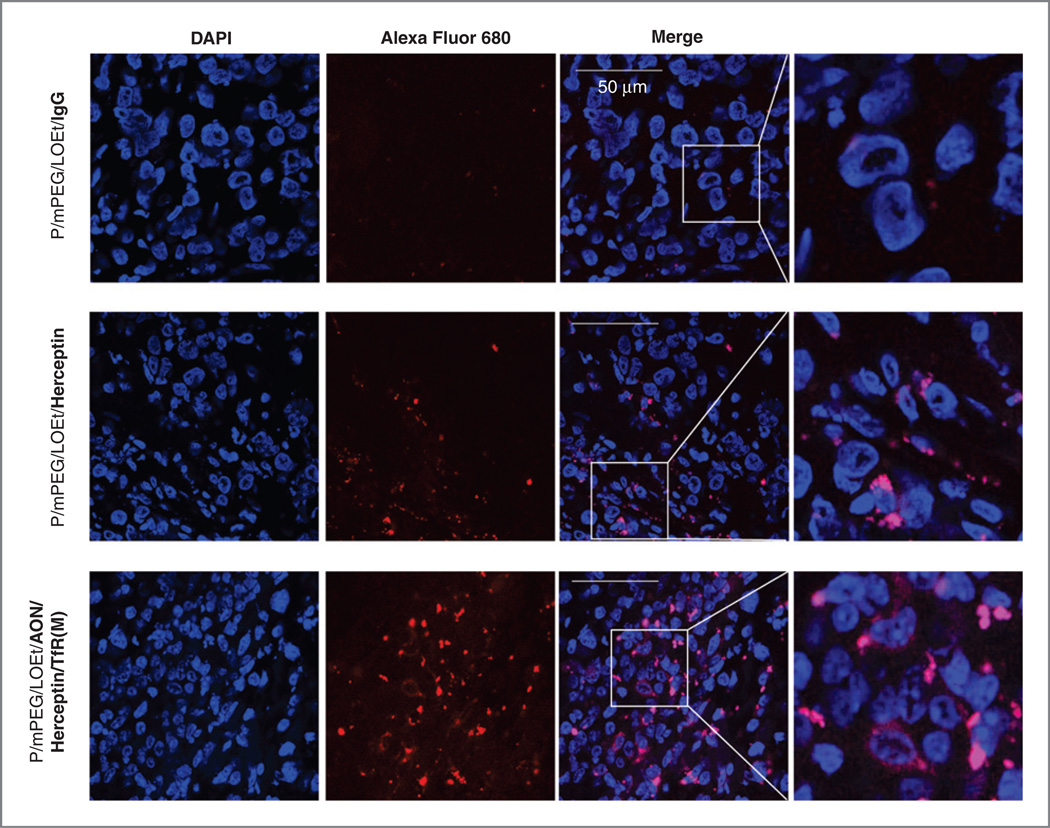
Imaging studies in vivo showed that anti-mouse TfR and anti-human HER2/neu combined on the same PMLA molecule provided tumor-specific drug delivery through host endothelial system into subcutaneous human breast tumors. Twenty-four hours after injection of drugs, they accumulated mostly in the tumor and draining organs, kidney and liver (Fig. 4). The nanobiopolymer with only Herceptin showed less tumor accumulation than the version with Herceptin, AON, and anti-TfR mAb (the lead drug), possibly because of the enhanced targeting of tumor vasculature with anti-TfR mAb compared with Herceptin. Control nanobiopolymer with IgG showed only little tumor accumulation (Fig. 4).
Figure 4.
Distribution of various drugs labeled with Alexa Fluor 680 in live mice with BT-474 breast tumors and in isolated organs. Twenty-four hours after intravenous injection (left), control conjugate with IgG instead of targeting antibodies (top row) had little BT-474 tumor accumulation, confirmed after major organs analysis (right). Most of this control polymer accumulated in drug clearing organs, liver and kidneys. Polymer with Herceptin alone had a moderate tumor accumulation (middle row). The highest accumulation in breast tumor was observed with the lead drug. Arrows mark tumor implantation site.
Confocal microscopy was performed on sections of brain tumors removed 24 hours after intravenous injection of Alexa Fluor 680–labeled drugs. A significantly stronger signal in tumor cells for P/mPEG/LOEt/Herceptin was observed than for the control conjugate P/mPEG/LOEt/IgG, and the highest tumor accumulation was observed with the lead drug compared with other nanobiopolymers (Fig. 5).
Figure 5.
Distribution of various drugs in BT-474 breast tumor cells. Animals treated intravenously with different drugs (Fig. 4) were sacrificed 24 hours after drug injection, their tumors were excised, and sections analyzed by confocal microscopy. Control conjugate P/mPEG/LOEt/IgG with attached Alexa Fluor 680 tracking dye (red) showed little if any tumor cell accumulation (top row). P/mPEG/LOEt/Herceptin displayed considerable accumulation in tumor cells, whereas the highest accumulation was observed for the lead drug P/mPEG/LOEt/AON/Herceptin/TfRM), in complete accordance with live animal imaging (Fig. 4). Nuclei were counterstained with DAPI (blue). Scale bar = 50 µm.
The lead drug significantly inhibits HER2/neu-positive breast tumor growth in vivo
We next investigated the therapeutic effect of lead drug upon intravenous treatment using subcutaneous mouse models of human breast tumor xenografts. Weselected BT-474 cell line for in vivo experiments because of its high HER2/neu expression and tumorigenicity. Treatment of BT-474 tumor–bearing mice with Herceptin, a single-drug nanobiopolymer and the lead drug, was performed in comparison with PBS. No decrease in body weight, morbidity or death was observed, indicating that all treatments were well tolerated (data not shown).
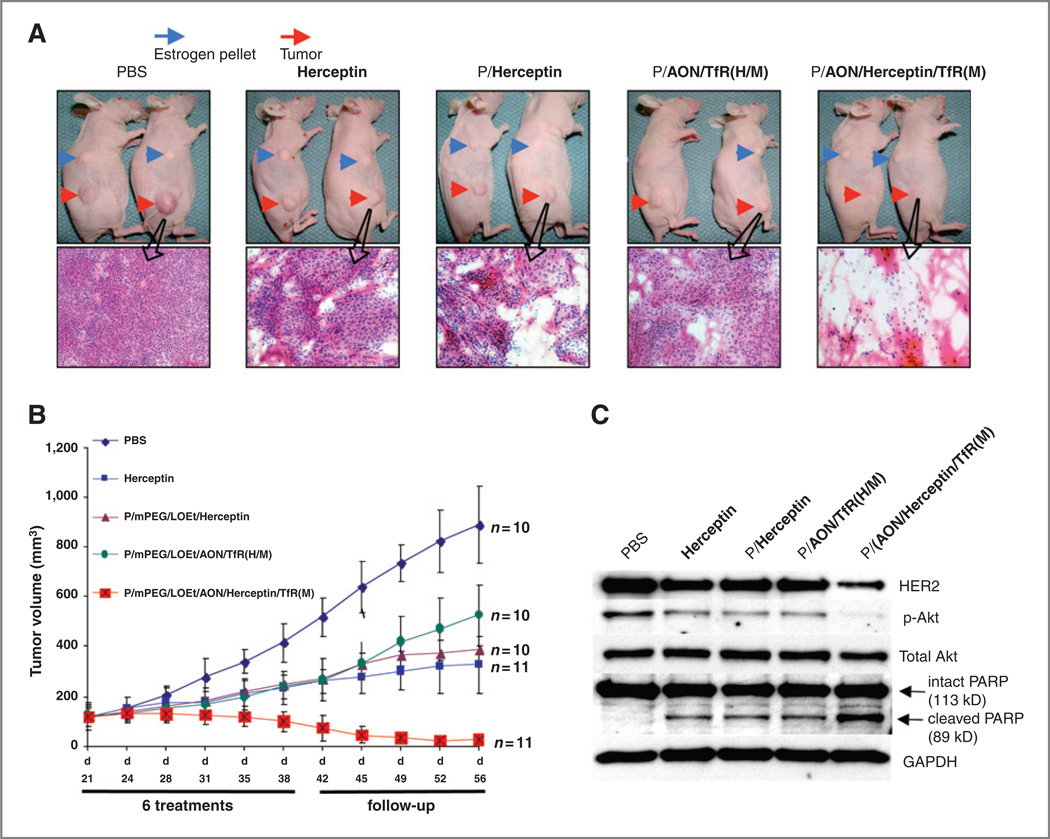
All the drugs inhibited tumor growth after 6 treatments (from days 21–38 posttumor implantation) and during follow-up to 56 days (Fig. 6B). Herceptinalone showeda similar tumor growth inhibition over time as PMLA-bound Herceptin. Both these drugs produced a somewhat stronger effect than HER2/neu AON bound to PMLA (Fig. 6B). This effect was significant for all 3 drugs (P< 0. 03 vs. PBS). When both Herceptin and HER2/neu AON were combined on one nanobiopolymer, it showed the highest degree of inhibition of tumor growth, with a clear synergistic effect compared with single-drug treatments (Fig. 6B; P < 0. 001 vs. PBS;P<0. 03 vs. all other treatment groups). The tumor regression after lead drug treatment ranged from 80% at the start of follow-up to 95% at the end of this period(day 56; Fig. 6B). Moreover, tumors in the group treated with lead drug started to regress within the first 2 weeks after the initial treatment, and all tumors in this group remained suppressed for an additional 20 days, at which time the experiment was terminated.
Figure 6.
Mouse tumor inhibition, pathology, signaling, and apoptosis marker expression. A, 2 representative animals for each group and histopathologic analysis of respective tumors (H&E staining) are shown after treatment with different drug variants. Variable amounts of dead tissue are present in all nanobiopolymer-treated groups. In accordance with tumor size reduction data, the lead drug P/mPEG/LOEt/AON/Herceptin/TfRM) caused pronounced disappearance of tumor cells with mostly necrotic areas present. B, tumor growth inhibition inmice. Animals treated with Herceptin, P/mPEG/LOEt/Herceptin, or P/mPEG/LOEt/AON/TfRH/M) showed significant inhibition compared with PBS control (P < 0. 03). P/mPEG/LOEt/AON/Herceptin/TfRM) treatment produced the highest inhibition of tumor growth resulting in 80% to 95% tumor regression during the follow-up period compared with other treatment groups (P < 0. 02 vs. Herceptin and other drugs; P < 0. 001 vs. PBS). Error bars denote SEM. C, Expression of select markers after treatment of HER2/neu-positive tumors in vivo. Western blot analysis revealed the decrease in HER2/neu and p-Akt (but not total Akt) expression in Herceptin-, P/mPEG/LOEt/Herceptin-, or P/mPEG/LOEt/AON/TfRH/M)-treated mice compared with PBS-treated ones. P/mPEG/LOEt/AON/Herceptin/TfRM) further inhibited HER2/neu expression, with near disappearance of p-Akt band. PARP cleavage as a measure of apoptosis was also the most pronounced in P/mPEG/LOEt/AON/Herceptin/TfRM)-treated mice compared with other groups. GAPDH was an internal control to normalize gel loading.
H&E staining revealed that the tumors treated with Herceptin, P/mPEG/LOEt/Herceptin, or P/mPEG/LOEt/AON/TfR (H/M) showed some areas of cell death compared with PBS (control)-treated tumor. However, treatment with the lead drug led to the appearance of massive morphologically necrotic areas with little unaffected tumor tissue remaining (Fig. 6A).
The mechanism of this antitumor effect was further investigated by Western blot analysis using lysates of subcutaneous BT-474 breast tumors after different treatments. Tumor HER2/neu expression was partially inhibited by Herceptin, AON, and single-drug versions of the nanobiopolymer [P/mPEG/LOEt/Herceptin and P/mPEG/LOEt/AON/TfRH/M)] in comparison with PBS controls (Fig. 6C). The lead drug produced the highest inhibition of HER2/neu tumor expression, in accordance with the in vitro Western blot analysis. The phosphorylated Akt was also reduced after drug treatments. Again, the lead drug caused the most pronounced decrease, with little p-Akt signal left (Fig. 6C). Total Akt remained unchanged upon treatments, as in the in vitro experiments.
Apoptosis assessed by PARP cleavage was induced to some extent by all drugs in HER2/neu high-expressing tumors compared with PBS treatment. However, the lead version, P/mPEG/LOEt/AON/Herceptin/TfRM), markedly increased PARP cleavage compared with all other treatments suggesting that this nanobiopolymer induced apoptosis more significantly than the other used drugs (Fig. 6C).
Discussion
Trastuzumab (Herceptin) is one of the most effective treatments of HER2/neu-overexpressing breast cancer. However, in 66% to 88% of cases, HER2/neu-overexpressing tumors demonstrate primary resistance to Herceptin (2, 29). This resistance may be due to epitope masking by overexpressed mucins, loss of receptor ability to influence prosurvival signaling through PI3K–Akt axis, or loss of protein phosphatase PTEN leading to the activation of PI3K-Akt signaling (6, 30–32). Combining Herceptin with other agents, such as paclitaxel and docetaxel, increased response rates, time to disease recurrence, and overall survival (33–35). Superior antitumor effects of these or new drug combinations can be reproduced by an emerging class of new drugs, the nanobiopolymeric conjugates. These compounds offer enhanced cancer cell specificity because of the presence of tumor targeting antibodies, bypass drug resistance by delivering polymer-bound drugs into cancer cell cytoplasm, and can carry multiple drugs on a single platform (36). Efficient delivery of nanobiopolymer-attached drugs to tumors is also increased because these complexes exploit passive targeting through enhanced permeability and retention (EPR) effect typical for tumors (37) and, in addition, active targeting using antibodies such as anti-TfR (37–39). Moreover, as Table 1 shows, the size (<30 nm) of these conjugates and their slightly negative ζ potential promote their interaction with the cell membrane and enhance intracellular internalization. This property is widely known for anionic nanoparticles (26). A general problem with anticancer drugs is lack of specific tumor targeting, resulting in rather random tissue accumulation and significant side effects for normal tissues (40, 41). To circumvent this drawback, tumor-targeting antibodies have been used as drug carriers or directly as therapeutics (e. g. , Herceptin). Dendrimer nanoconjugates with attached Herceptin displayed enhanced accumulation in breast cancer cells in animal models (41). Methotrexate-loaded dendrimers produced a cytotoxic effect in tumor cells in vitro resulting from Herceptin-mediated complex internalization (40). However, the efficacy of these nanodrugs was limited because of lack of efficient endosome release unit (40). The polymers we have previously designed specifically delivered drugs to cancer cells and inhibited tumor growth and angiogenesis in brain glioma–bearing animals (22, 42–44). Their efficiency was due to tumor targeting, use of AON drugs to more than one tumor marker at the same time, and the presence of endosome disruption moiety ensuring drug release inside the target cell (20).
In the present study, a new generation of this nanobiopolymeric conjugate specifically tailored for HER2/neu-expressing breast cancer treatment was designed and tested in vitro and in vivo. The drug was based on HER2/neu inhibition by simultaneously blocking the synthesis of HER2/neu with specific AON and internalizing the receptor by binding to Herceptin. The lead drug was thus meant to more efficiently inhibit HER2/neu expression and function.
Our in vitro data showed that, indeed, the lead drug P/mPEG/LOEt/AON/Herceptin/TfRM) suppressed proliferation of HER2/neu-positive breast cancer cell lines significantly more than Herceptin, P/mPEG/LOEt/Herceptin or P/mPEG/LOEt/AON/TfRH/M) (Fig. 2). Interestingly, the lead nanobiopolymer was effective on both HER2/neu high- and low-expressing breast cancer cell lines. With regard to HER2/neu low-expressing cells, our lead drug was also superior to previously used HER2/neu AON, which did not inhibit their growth in vitro (14). Moreover, this lead drug produced the highest inhibition of both HER2/neu expression and Akt phosphorylation, as well as enhanced tumor cell apoptosis, compared with other treatments. Herceptin mediates antiproliferative effects in HER2/neu-positive cells by facilitating either HER2/neu degradation or endocytic destruction of the HER2/neu receptor or downregulation of PI3K-Akt signaling (45) by inhibiting HER2/neu receptor dimerization, and also by inducing immune activation (46). In line with these mechanisms, our data suggest that the in vitro growth-inhibiting effect of the lead drug on tumor cells was enhanced by simultaneous AON-mediated inhibition of HER2/neu synthesis and by downregulation of surface HER2/neu through its binding to Herceptin. The stronger inhibition of Akt phosphorylation in this case could result from a significant attenuation of HER2/neu signaling.
The lead drug readily accumulated in breast tumors and dramatically inhibited human breast cancer growth in nude mice (Fig. 6). Importantly, the magnitude of antitumor effect of this lead drug suggests synergy between HER2/neu AON and Herceptin in vivo (Fig. 6). In comparison, the in vitro effect was more modest, showing only about 50% growth inhibition in HER2/neu high-expressing cells, in contrast to nearly complete in vivo inhibition. The synergistic antitumor action in vivo could result from a combination of several effects: enhanced reduction in HER2/neu-mediated tumor growth by AON together with Herceptin, preferential tumor accumulation mediated by combined EPR effect (37) and active targeting with antibodies (43), and maintenance of effective drug concentration due to multiple treatments. Compared with the previously used combination of HER2/neu AON with doxorubicin that was similarly effective against xenogeneic BT-474 tumors (14), our nanobiopolymeric conjugate would be free of side effects because of absence of toxic doxorubicin and of its efficient tumor targeting via Herceptin and anti-TfR.
Our experiments confirmed that a proper design of the lead nanobiopolymer was possible for efficient blocking of HER2/neu-positive breast tumor growth through dual inhibition of HER2/neu and Akt phosphorylation, and as a result, promoting enhanced tumor cell apoptosis. The nanobiopolymer's unique combination of features resulted in highly specific drug accumulation in the tumor tissue and inside tumor cells.
Our nanobiopolymer can accommodate virtually any antibody, drug, or AON, alone or in combination. By this virtue, the nanodrug can be tailored to target simultaneously different molecular tumor markers typical of particular tumor cells to become highly efficient against various tumors. This novel nanobiopolymer-based therapy against HER2/neu expressing cancer cells should make a significant clinical impact.
Acknowledgements
The authors thank Dr. J. D. Young, Director of the Department of Comparative Medicine, Cedars-Sinai Medical Center, for help and advice with animals, Dr. K. A. Wawrowsky for help with confocal microscopy, and A. Paradise and J. Massey from Leica Microsystems for technical support.
Grant Support
This work was supported by grants from NIH (R01 CA123495 to J. Y. Ljubimova, R01 CA CA136841 to J. Y. Ljubimova and M. L. Penichet, and R01 EY13431 to A. V. Ljubimov), Winnick Family Foundation and M01 RR00425 (to J. Y. Ljubimova).
Footnotes
Disclosure of Potential Conflicts of Interest
S. Inoue, E. Holler, K. L. Black, and J. Y. Ljubimova are inventors on a relevant patent application filed by Cedars-Sinai Medical Center in December 2010.
References
- 1.Baselga J. Targeting tyrosine kinases in cancer: the second wave. Science. 2006;312:1175–1178. doi: 10.1126/science.1125951. [DOI] [PubMed] [Google Scholar]
- 2.Baselga J, Tripathy D, Mendelsohn J, Baughman S, Benz CC, Dantis L, et al. Phase II study of weekly intravenous trastuzumab (Herceptin) in patients with HER2/neu-overexpressing metastatic breast cancer. Semin Oncol. 1999;26:78–83. [PubMed] [Google Scholar]
- 3.Slamon DJ, Press MF. Alterations in the TOP2A and HER2 genes: association with adjuvant anthracycline sensitivity in human breast cancers. J Natl Cancer Inst. 2009;101:615–618. doi: 10.1093/jnci/djp092. [DOI] [PubMed] [Google Scholar]
- 4.Keefe DL. Trastuzumab-associated cardiotoxicity. Cancer. 2002;95:1592–1600. doi: 10.1002/cncr.10854. [DOI] [PubMed] [Google Scholar]
- 5.Vahid B, Marik PE. Pulmonary complications of novel antineoplastic agents for solid tumors. Chest. 2008;133:528–538. doi: 10.1378/chest.07-0851. [DOI] [PubMed] [Google Scholar]
- 6.Tseng PH, Wang YC, Weng SC, Weng JR, Chen CS, Brueggemeier RW, et al. Overcoming trastuzumab resistance in HER2-overexpressing breast cancer cells by using a novel celecoxib-derived phosphoinositide-dependent kinase-1 inhibitor. Mol Pharmacol. 2006;70:1534–1541. doi: 10.1124/mol.106.023911. [DOI] [PubMed] [Google Scholar]
- 7.Patil SD, Rhodes DG, Burgess DJ. DNA-based therapeutics and DNA delivery systems: a comprehensive review. AAPS J. 2005;7:E61–E77. doi: 10.1208/aapsj070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thierry AR, Vives E, Richard JP, Prevot P, Martinand-Mari C, Robbins I, et al. Cellular uptake and intracellular fate of antisense oligonucleotides. Curr Opin Mol Ther. 2003;5:133–138. [PubMed] [Google Scholar]
- 9.Busch RK, Perlaky L, Valdez BC, Henning D, Busch H. Apoptosis in human tumor cells following treatment with p120 antisense oligodeoxynucleotide ISIS 3466. Cancer Lett. 1994;86:151–157. doi: 10.1016/0304-3835(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 10.Sekhon HS, London CA, Sekhon M, Iversen PL, Devi GR. c-MYC antisense phosphosphorodiamidate morpholino oligomer inhibits lung metastasis in a murine tumor model. Lung Cancer. 2008;60:347–354. doi: 10.1016/j.lungcan.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 11.Garbuzenko OB, Saad M, Pozharov VP, Reuhl KR, Mainelis G, Minko T. Inhibition of lung tumor growth by complex pulmonary delivery of drugs with oligonucleotides as suppressors of cellular resistance. Proc Natl Acad Sci U S A. 2010;107:10737–10742. doi: 10.1073/pnas.1004604107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu B, Lu P, Benrashid E, Malik S, Ashar J, Doran TJ, et al. Dose-dependent restoration of dystrophin expression in cardiac muscle of dystrophic mice by systemically delivered morpholino. Gene Ther. 2010;17:132–140. doi: 10.1038/gt.2009.120. [DOI] [PubMed] [Google Scholar]
- 13.Kinali M, Arechavala-Gomeza V, Feng L, Cirak S, Hunt D, Adkin C, et al. Local restoration of dystrophin expression with the morpholino oligomer AVI-4658 in Duchenne muscular dystrophy: a single-blind, placebo-controlled, dose-escalation, proof-of-concept study. Lancet Neurol. 2009;8:918–928. doi: 10.1016/S1474-4422(09)70211-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roh H, Pippin J, Drebin JA. Down-regulation of HER2/neu expression induces apoptosis in human cancer cells that overexpress HER2/neu. Cancer Res. 2000;60:560–565. [PubMed] [Google Scholar]
- 15.Rait AS, Pirollo KF, Rait V, Krygier JE, Xiang L, Chang EH. Inhibitory effects of the combination of HER-2 antisense oligonucleotide and chemotherapeutic agents used for the treatment of human breast cancer. Cancer Gene Ther. 2001;8:728–739. doi: 10.1038/sj.cgt.7700359. [DOI] [PubMed] [Google Scholar]
- 16.Lewis Phillips GD, Li G, Dugger DL, Crocker LM, Parsons KL, Mai E, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008;68:9280–9290. doi: 10.1158/0008-5472.CAN-08-1776. [DOI] [PubMed] [Google Scholar]
- 17.Lee BS, Fujita M, Khazenzon NM, Wawrowsky KA, Wachsmann-Hogiu S, Farkas DL, et al. Polycefin, a new prototype of a multifunctional nanoconjugate based on poly(β-L-malic acid) for drug delivery. Bioconjug Chem. 2006;17:317–326. doi: 10.1021/bc0502457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee BS, Vert M, Holler E. Water-soluble aliphatic polyesters: poly (malic acid)s. In: Doi Y, Steinbüchel A, editors. Biopolymers. Volume 3a: Polyesters. Weinheim, Germany: Wiley-VCH; 2002. pp. 75–103. [Google Scholar]
- 19.Gasslmaier B, Holler E. Specificity and direction of depolymerization of β-poly(L-malate) catalysed by polymalatase from Physarum polycephalum–fluorescence labeling at the carboxy-terminus of β-poly(Lmalate) Eur J Biochem. 1997;250:308–314. doi: 10.1111/j.1432-1033.1997.0308a.x. [DOI] [PubMed] [Google Scholar]
- 20.Gasslmaier B, Krell CM, Seebach D, Holler E. Synthetic substrates and inhibitors of β-poly(L-malate)-hydrolase (polymalatase) Eur J Biochem. 2000;267:5101–5105. doi: 10.1046/j.1432-1327.2000.01573.x. [DOI] [PubMed] [Google Scholar]
- 21.Ljubimova JY, Fujita M, Ljubimov AV, Torchilin VP, Black KL, Holler E. Poly(malic acid) nanoconjugates containing various antibodies and oligonucleotides for multitargeting drug delivery. Nanomedicine. 2008;3:247–265. doi: 10.2217/17435889.3.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding H, Inoue S, Ljubimov AV, Patil R, Portilla-Arias J, Hu J, et al. Inhibition of brain tumor growth by intravenous poly(β-L-malic acid) nanobioconjugate with pH-dependent drug release. Proc Natl Acad Sci U S A. 2010;107:18143–18148. doi: 10.1073/pnas.1003919107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujita M, Lee BS, Khazenzon NM, Penichet ML, Wawrowsky KA, Patil R, et al. Brain tumor tandem targeting using a combination of monoclonal antibodies attached to biopoly(β-L-malic acid) J Control Release. 2007;122:356–363. doi: 10.1016/j.jconrel.2007.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inoue S, Branch CD, Gallick GE, Chada S, Ramesh R. Inhibition of Src kinase activity by Ad-mda7 suppresses vascular endothelial growth factor expression in prostate carcinoma cells. Mol Ther. 2005;12:707–715. doi: 10.1016/j.ymthe.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 25.Lorenz MR, Holzapfel V, Musyanovych A, Nothelfer K, Walther P, Frank H, et al. Uptake of functionalized, fluorescent-labeled polymeric particles in different cell lines and stem cells. Biomaterials. 2006;27:2820–2828. doi: 10.1016/j.biomaterials.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 26.Wilhelm C, Billotey C, Roger J, Pons JN, Bacri JC, Gazeau F. Intracellular uptake of anionic superparamagnetic nanoparticles as a function of their surface coating. Biomaterials. 2003;24:1001–1011. doi: 10.1016/s0142-9612(02)00440-4. [DOI] [PubMed] [Google Scholar]
- 27.Yakes FM, Chinratanalab W, Ritter CA, King W, Seelig S, Arteaga CL. Herceptin-induced inhibition of phosphatidylinositol-3 kinase and Akt Is required for antibody-mediated effects on p27, cyclin D1, and antitumor action. Cancer Res. 2002;62:4132–4141. [PubMed] [Google Scholar]
- 28.Plas DR, Thompson CB. Akt-dependent transformation: there is more to growth than just surviving. Oncogene. 2005;24:7435–7442. doi: 10.1038/sj.onc.1209097. [DOI] [PubMed] [Google Scholar]
- 29.Nahta R, Takahashi T, Ueno NT, Hung MC, Esteva FJ. P27(kip1) down-regulation is associated with trastuzumab resistance in breast cancer cells. Cancer Res. 2004;64:3981–3986. doi: 10.1158/0008-5472.CAN-03-3900. [DOI] [PubMed] [Google Scholar]
- 30.Nagy P, Bene L, Balazs M, Hyun WC, Lockett SJ, Chiang NY, et al. EGF-induced redistribution of erbB2 on breast tumor cells: flow and image cytometric energy transfer measurements. Cytometry. 1998;32:120–131. doi: 10.1002/(sici)1097-0320(19980601)32:2<120::aid-cyto7>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 31.O’Brien NA, Browne BC, Chow L, Wang Y, Ginther C, Arboleda J, et al. Activated phosphoinositide 3-kinase/AKT signaling confers resistance to trastuzumab but not lapatinib. Mol Cancer Ther. 2010;9:1489–1502. doi: 10.1158/1535-7163.MCT-09-1171. [DOI] [PubMed] [Google Scholar]
- 32.Tanner M, Kapanen AI, Junttila T, Raheem O, Grenman S, Elo J, et al. Characterization of a novel cell line established from a patient with Herceptin-resistant breast cancer. Mol Cancer Ther. 2004;3:1585–1592. [PubMed] [Google Scholar]
- 33.Esteva FJ, Valero V, Booser D, Guerra LT, Murray JL, Pusztai L, et al. Phase II study of weekly docetaxel and trastuzumab for patients with HER-2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:1800–1808. doi: 10.1200/JCO.2002.07.058. [DOI] [PubMed] [Google Scholar]
- 34.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 35.Wardley AM, Pivot X, Morales-Vasquez F, Zetina LM, de Fatima Dias Gaui M, Reyes DO, et al. Randomized phase ii trial of first-line trastuzumab plus docetaxel and capecitabine compared with trastuzumab plus docetaxel in HER2-positive metastatic breast cancer. J Clin Oncol. 2009;49:976–983. doi: 10.1200/JCO.2008.21.6531. [DOI] [PubMed] [Google Scholar]
- 36.Wu K, Liu J, Johnson RN, Yang J, Kopecek J. Drug-free macromolecular therapeutics: induction of apoptosis by coiled-coil-mediated cross-linking of antigens on the cell surface. Angew Chem Int Ed Engl. 2010;49:1451–1455. doi: 10.1002/anie.200906232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maeda H, Bharate GY, Daruwalla J. Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect. Eur J Pharm Biopharm. 2009;71:409–419. doi: 10.1016/j.ejpb.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 38.Liu X, Wang Y, Nakamura K, Kubo A, Hnatowich DJ. Cell studies of a three-component antisenseMORF/tat/Herceptin nanoparticle designed for improved tumor delivery. Cancer Gene Ther. 2008;15:126–132. doi: 10.1038/sj.cgt.7701111. [DOI] [PubMed] [Google Scholar]
- 39.Peterson CM, Shiah JG, Sun Y, Kopeckova P, Minko T, Straight RC, et al. HPMA copolymer delivery of chemotherapy and photodynamic therapy in ovarian cancer. Adv Exp Med Biol. 2003;519:101–123. doi: 10.1007/0-306-47932-X_7. [DOI] [PubMed] [Google Scholar]
- 40.Shukla R, Thomas TP, Desai AM, Kotlyar A, Baker JR. HER2 specific delivery of methotrexate by dendrimer conjugated anti-HER2 mAb. Nanotechnology. 2008;19:1–7. doi: 10.1088/0957-4484/19/29/295102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shukla R, Thomas TP, Peters JL, Desai AM, Kukowska-Latallo J, Patri AK, et al. HER2 specific tumor targeting with dendrimer conjugated anti-HER2 mAb. Bioconjug Chem. 2006;17:1109–1115. doi: 10.1021/bc050348p. [DOI] [PubMed] [Google Scholar]
- 42.Fujita M, Khazenzon NM, Ljubimov AV, Lee BS, Virtanen I, Holler E, et al. Inhibition of laminin-8 in vivo using a novel poly(malic acid)-based carrier reduces glioma angiogenesis. Angiogenesis. 2006;9:183–191. doi: 10.1007/s10456-006-9046-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ljubimova JY, Fujita M, Khazenzon NM, Lee BS, Wachsmann-Hogiu S, Farkas DL, et al. Nanoconjugate based on polymalic acid for tumor targeting. Chem Biol Interact. 2008;171:195–203. doi: 10.1016/j.cbi.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patil R, Portilla-Arias J, Ding H, Inoue S, Konda B, Hu J, et al. Temozolomide delivery to tumor cells by a multifunctional nano vehicle based on poly(β-L-malic acid) Pharm Res. 2010;27:2317–2329. doi: 10.1007/s11095-010-0091-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clark AS, West K, Streicher S, Dennis PA. Constitutive and inducible Akt activity promotes resistance to chemotherapy, trastuzumab, or tamoxifen in breast cancer cells. Mol Cancer Ther. 2002;1:707–717. [PubMed] [Google Scholar]
- 46.Hudis CA. Trastuzumab – mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]