Abstract
We report on three children from two families with a new pattern recognition malformation syndrome consisting of severe congenital microcephaly (MIC), intractable epilepsy including infantile spasms, and generalized capillary malformations that was first reported recently in this journal [Carter et al. (2011); Am J Med Genet A 155: 301–306]. Two of our reported patients are an affected brother and sister, suggesting this is an autosomal recessive severe congenital MIC syndrome.
Keywords: microcephaly with simplified gyri, capillary malformations, infantile spasms, optic nerve hypoplasia
INTRODUCTION
Microcephaly (MIC) is traditionally defined as an occipitofrontal circumference (OFC) two standard deviations (SD) or more below the mean [Opitz and Holt, 1990], while we define severe MIC as OFC three SD or more below the mean [Dobyns, 2002]. It can result from both genetic and environmental etiologies, and can be congenital or postnatal. Most individuals with severe congenital MIC have a reduced number of gyri separated by shallow sulci, and thin or normal cortical thickness, a pattern we have designated “microcephaly with simplified gyral pattern” [Dobyns and Barkovich, 1999; Dobyns, 2002]. This relatively common malformation may occur as an isolated disorder designated “primary MIC,” with any of several additional brain malformations such as cortical malformations, disproportionate cerebellar hypoplasia, and periventricular nodular heterotopia [Barkovich et al., 1998; Dobyns and Barkovich, 1999; Sztriha et al., 2004; Basel-Vanagaite and Dobyns, 2010], or with a growing number of malformation syndromes.
Here we report three children from two families with a new pattern recognition malformation syndrome consisting of severe congenital MIC, intractable epilepsy including infantile spasms, and generalized capillary malformations that was first reported only recently in this journal [Carter et al., 2011]. Our observation of an affected brother and sister also supports autosomal recessive inheritance, which is unsurprising for severe congenital MIC.
CLINICAL REPORTS
All patients are enrolled under an IRB-approved research protocol at Seattle Children’s Research Institute. Patients 1 and 2 are siblings of African-American ancestry, with no known consanguinity. The clinical and neuroimaging features of the three patients are summarized in the Table I.
TABLE 1.
Comparison of the Clinical and Neuroimaging Features of the Five Reported Patients With MIC-CM Syndrome
| Patient | Patient 1 (LR07-155a1) |
Patient 2 (LR07-155a2) |
Patient 3 (LR10-168) |
Patient 1 (Carter et al., 2011) |
Patient 2 (Carter et al., 2011) |
|---|---|---|---|---|---|
| Clinical Data | |||||
| Sex | F | M | M | M | M |
| Short stature | − | − | − | + | + |
| Birth OFC | −6 | −4 | −2 | −2 | −4 |
| Last OFC (Age) | −8 (2.5y) | −6 (9m) | −6 (12m) | −4 (17m) | −3 (22d) |
| DYSM | + | + | + | + | + |
| Generalized CMs | + | + | + | + | + |
| Hypoplastic fingers/toes | + | + | − | + | + |
| Cerebellar angiomata | − | − | − | + | − |
| Cardiac abnormalities | − | + | − | + | + |
| Urinary tract malformations | − | − | − | − | + |
| Intractable neonatal epilepsy | + | + | + | + | + |
| ISS | + | − | + | ND | ND |
| Spastic quadriparesis | + | + | + | + | + |
| Myoclonus | + | − | + | + | − |
| Severe GDD | + | + | + | + | + |
| Small for gestational age | + | + | + | + | + |
| Brain Imaging | |||||
| MSG | + | + | + | + | + |
| Enlarged xax | + | + | + | + | + |
| Small HIP | + | + | + | ND | + |
CMs, capillary malformations; DYSM, dysmorphic features; GDD, global developmental delay; F, female; HIP, hippocampus; ISS, infantile spasms; MSG, microcephaly with simplified gyri; M, male; ND, no data; MSG, microcephaly with simplified gyri; OFC, occipito-frontal circumference; SD, standard deviations; xax, extra-axial space.
Patient 1 (DB#LR07-155a1)
This girl is the first born to her 17-year-old mother and 18-year-old father. Prenatal history is significant for lack of prenatal care. She was delivered via normal spontaneous vaginal delivery at 39 weeks of gestation with Apgar scores of 8 and 8 at 1 and 5 min, respectively. Birth weight was 2,578 g (3–10th centile for gestational age), birth length 43 cm (−4 SD below the mean for gestational age), and birth OFC 28 cm (−6 SD below the mean for gestational age). Besides obvious severe MIC, she was noted to have numerous generalized capillary malformations, dysmorphic facial features and an umbilical hernia.
Focal seizures started shortly after birth, and were treated with phenobarbital and topiramate. At 2–3 months, flexion movements of the extremities with EEG correlate consistent with infantile spasms appeared. She subsequently developed multiple seizure types including complex partial seizures in addition to occasional myoclonus. Seizures were refractory to multiple anti-epileptic medications and required hospitalization for several months.
Medical history was also complicated by gastroesophageal reflux, with poor oral feeding, failure to thrive, and multiple episodes of pneumonia necessitating further hospitalizations. The patient subsequently underwent G-tube placement and a Nissen fundoplication at 3 months of age, and remains fully G-tube fed thereafter. Ophthalmology exam revealed mild bilateral optic nerve hypoplasia. Renal ultrasound was normal. Hearing was thought to be normal with no formal hearing assessment on record. Her mood was described as stable without excessive or unexplained irritability or laughter, and no reported sleep problems.
On physical exam at 2.5 years, weight was 16 kg (90–97th centile) and OFC 37.8 cm (−8 SD). She had a very low sloping forehead and a wide nasal bridge. Inner canthal distance (ICD) was 3.4 cm (+3–4 SD above the mean), interpupillary distance was 6 cm (+4 SD), and outer canthal distance (OCD) was 8–8.2 cm (75–97th centile). Of note, the patient’s last recorded length was at 76.2 cm at 13 months of age (50–75th centile). Numerous generalized capillary malformations involving the trunk and extremities were clearly apparent, measuring 2–10 mm in size (Fig. 1A–C). Digits were tapered with hypoplastic distal phalanges with complete aplasia of the right first toe nail, and overlapping left 3rd and 4th toes. (Fig. 2A,B). Neurological exam revealed a severely handicapped child, with no gross or fine motor skills, no visual tracking or social interaction. Facial expression was minimal and limited to spontaneous eye movements and tongue protrusion, with frequent drooling. Muscle tone was markedly increased with brisk (+4) deep tendon reflexes in the upper and lower extremities, and clonus.
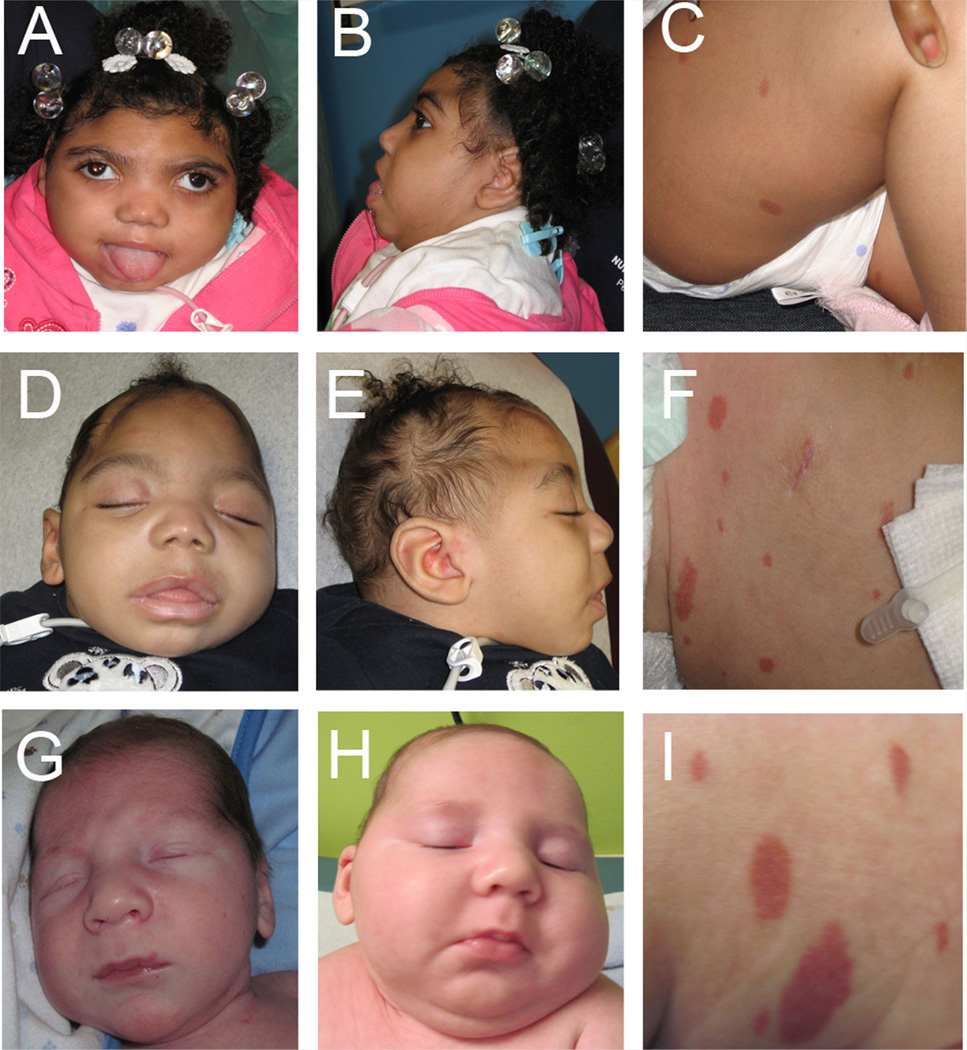
FIG. 1.
Images of Patient 1 (LR#07-155a1), (A–C) at 2.5 years of age, Patient 2 (LR#07-155a2) (D–F) at 9 months of age, and Patient 3 (LR10-168) at birth (G) and 2 months of age (H, I). Note severe microcephaly, bitemporal narrowing with a low-sloping forehead. Images (C, F, I) demonstrate generalized capillary malformations.
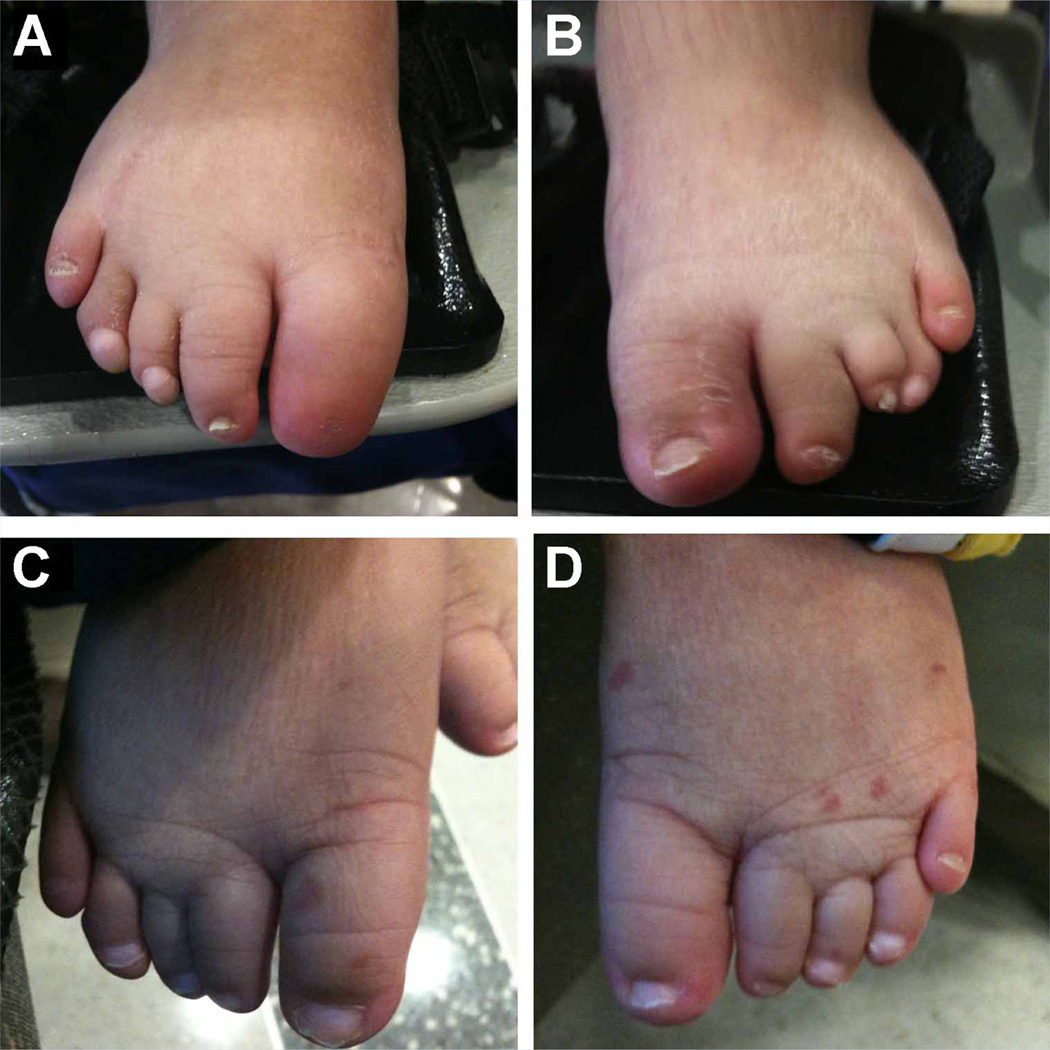
FIG. 2.
Images of the feet of Patient 1 (LR07-155a1), (A, B) at 3.5 years of age, and Patient 2 (LR07-155a2), (C, D) at 1 year 8 months. Note the short toes and hypoplastic toe nails, with complete nail aplasia of the right first toe and marked overlapping of the left 3rd–4th toes in Patient 1 (A, B).
Due to severe spasticity and poor control of oral secretions, which led to worsening upper airway obstruction and multiple aspiration events, a tracheostomy was placed at 3 years of age. Her current medications include valproic acid, phenobarbital, topiramate, and diazepam for seizures, and baclofen for spasticity.
Laboratory studies conducted with normal results include high-resolution chromosome analysis, urine organic acids, very long chain fatty acids, quantitative CSF amino acids analysis, congenital disorders of glycosylation screen and workup for an infectious etiology (including TORCH titers).
Patient 2 (DB#LR07-155a2)
This is the younger brother of Patient 1. Fetal ultrasound at 29 weeks gestation demonstrated small head size with measurements below the 5th centile for gestational age, closely apposed lateral ventricles and absent septum pellucidum. Prenatal labs were positive for Group B streptococcus (GBS) testing, for which the mother was adequately treated.
Delivery was a normal spontaneous vaginal one at 39 weeks of gestation with Apgar scores of 8 and 9 at 1 and 5 min, respectively. Birth weight was 2,575 g (3–10th centile for gestational age), birth length 44.5 cm (−2 SD below the mean for gestational age), and birth OFC 30.2 cm (−4 SD below the mean for gestational age). Similarly to his sister, he was noted to have severe MIC, multiple scattered capillary malformations, dysmorphic facial features, and an inguinal hernia.
EEG-confirmed seizures started shortly after birth. He was treated with fosphenytoin and phenobarbital, but developed intractable epilepsy with multiple seizure types occurring several times per week. His current seizure medications are phenobarbital, levetiracetam, and topiramate.
Ophthalmologic exam revealed bilateral optic nerve hypoplasia. Echocardiogram showed a patent foramen ovale, mild concentric right ventricular hypertrophy, possible right ventricular noncompaction, dilated median pulmonary artery, and a small pericardial effusion. Abdominal ultrasound was normal, and hearing assessment was normal. Given significant feeding difficulties and secondary respiratory complications, he underwent G-tube placement and Nissen fundoplication at 1 month of age. Despite this, he continued to have failure to thrive, and underwent multiple G-tube revisions, a repeat Nissen fundoplication and lysis of intestinal adhesions. Postnatal course was also complicated by congenital hip dysplasia.
On physical exam at 9 months of age, weight was 6.4 kg (−4 SD below the mean), and OFC was 34.7 cm (−8 SD below the mean). Dysmorphic features included a low sloping forehead, abnormally wide nasal bridge, slightly posteriorly rotated ears, a mildly short neck, and dysplastic palmar creases that extended to the 2nd–3rd finger web space. Inner canthal distance (ICD) was 3.3 cm (+4 SD), and outer canthal distance (OCD) 7.3 cm (50–75th centile). Multiple generalized capillary malformations were present, ranging in size from 2 to 10 mm in size (Fig.1D–F). The toes were short, with hypoplasia of the toe nails bilaterally (Fig. 2C, D). Neurologically, he was severely handicapped with spastic quadriparesis, brisk deep tendon reflexes (+4), and clonus. He was not visually tracking, had no social interaction or appreciable facial movements, and had significant drooling.
At 12 months of age, a tracheostomy was performed because of a chronic respiratory difficulties including obstructive apnea. His current medications include topiramate, levetiracetam, phenobarbital for seizures, lorazepam for irritability, and famotidine for gastroesophageal reflux. An Agilent oligonucleotide array was normal.
Patient 3 (DB# LR10-168)
This boy was born at 36 + 5 weeks of gestation to his 24-year-old mother. Pregnancy was complicated by oligohydramnios and intrauterine growth restriction. Delivery was induced with artificial rupture of membranes, and was complicated by failure to progress, fetal decelerations, and presumed chorioamnionitis that was adequately treated. Delivery was via caesarian, with Apgar scores of 9 and 9 at 1 and 5 min, respectively. Birth weight was 2.43 kg (3–10th centile for gestational age), birth length 47 cm (10–50th centile for gestational age), and OFC 29 cm (−2 SD below the mean for gestational age). He was noted to have congenital MIC with numerous capillary malformations.
Fifteen minutes after birth, the patient was noted to have EEG-confirmed seizure activity. He received multiple doses of lorazepam, phenobarbital, and fosphenytoin. Subsequent EEGs showed a markedly abnormal background including burst suppression type pattern, and multifocal epileptiform activity. No change in clinical behavior or EEG pattern was noted with administration of pyridoxine. Over the ensuing weeks, he began to experience clinical and intractable seizures, with intermittent myoclonus. Video EEG at 6 months of age demonstrated infantile spasms (Fig. 3). Seizures remain intractable despite multiple anti-epileptic medications that include topirmate, rufinamide, vigabatrin, clobazam, felbamate, levetiracetam, and clonazepam for clusters of seizures.
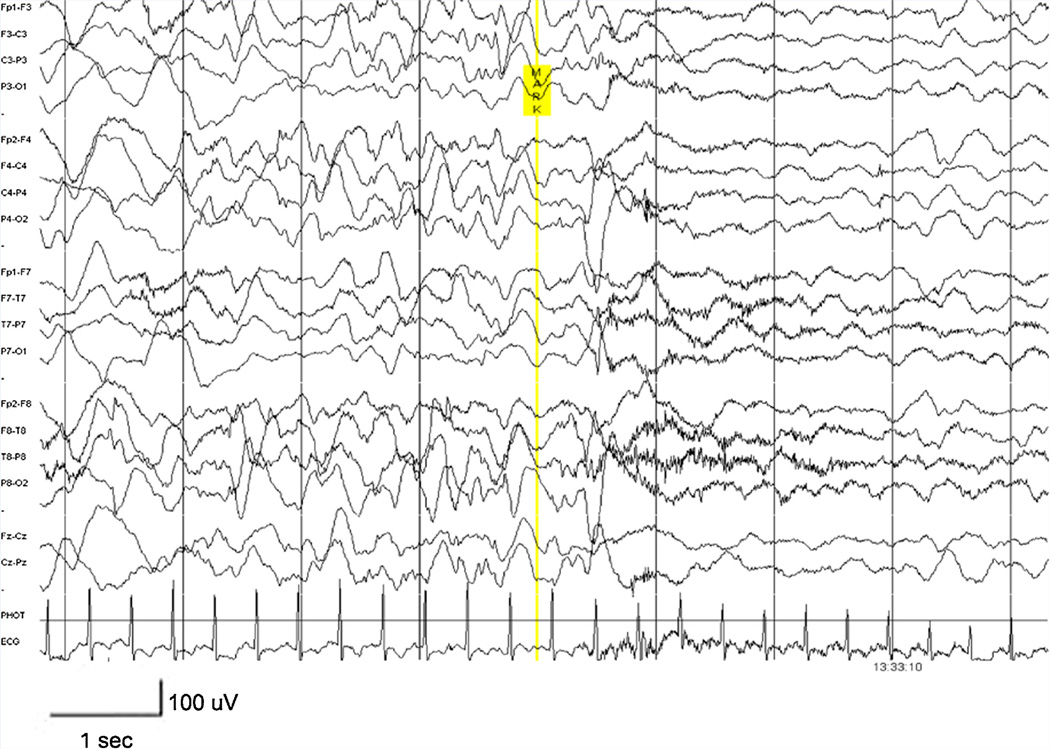
FIG. 3.
EEG of patient 3 (LR10-168) at 7 months of age, showing high voltage (up to 200 µV) and multifocal sharp waves consistent with hypsarrhythmia. A generalized electrodecrement was associated with clinical spasms (marked).
Ophthalmologic exam at 3 months demonstrated optic nerve pallor with increased cup-to-disc ratio consistent with optic atrophy, as well as mild hyperopic refractive error and intermittent exotropia. Visual evoked potential testing at 6 months of age also revealed visual cortical impairment. Neonatal echocardiogram, abdominal ultrasound, and swallow study were normal. A repeat swallow study at 8 months of age demonstrated aspiration, and he underwent gastrostomy tube placement at 9 months of age.
On physical exam at 12 months of age, weight was 10.8 kg (50–75th centile), length 71.8 cm (10–25%), and OFC 38.7 cm (−6 SD). The patient had a right pre-auricular skin tag, bitemporal narrowing, and mild micrognathia. Multiple capillary malformations scattered over the face, torso, and extremities, and ranging in size from 1–15 mm in size were appreciated (Fig.1G–I). Neurologic exam revealed spastic quadriparesis, with brisk deep tendon reflexes. Roving eye movements were seen but visual fixation and tracking were absent. He had purposeless spontaneous movements of his extremities, with constant fisting of the hands. The patient had global developmental delay, with inability to roll over or sit independently, and limited interaction with his environment.
Laboratory tests conducted with negative results include a high resolution chromosome analysis, chromosomal microarray (Affymetrix 6.0), RASA1 sequencing, serum and cerebrospinal fluid (CSF) lactate and pyruvate, serum amino acids, very long chain fatty acids, urine organic acids, urine sulfites, and normal quantitative CSF amino acid, and neurotransmitter levels.
RESULTS
Brain Imaging
Brain MRI studies were performed at 2 months, 6 days, and 20 days of life in Patients 1, 2, and 3, respectively (Fig. 4). All showed obvious MIC with a low-sloping forehead, diffusely reduced number of gyri with shallow sulci consistent with a severe simplified gyral pattern, and moderately enlarged extra-axial space. The hippocampus was small in all three patients, and the cerebellum was slightly small but proportionate to the overall brain size. Patient 3 had thin and immature white matter. The MRI findings in all three patients were strikingly similar and consistent with congenital MIC with simplified gyral pattern and enlarged extra-axial space, a known variant of MIC [Basel-Vanagaite and Dobyns, 2010].
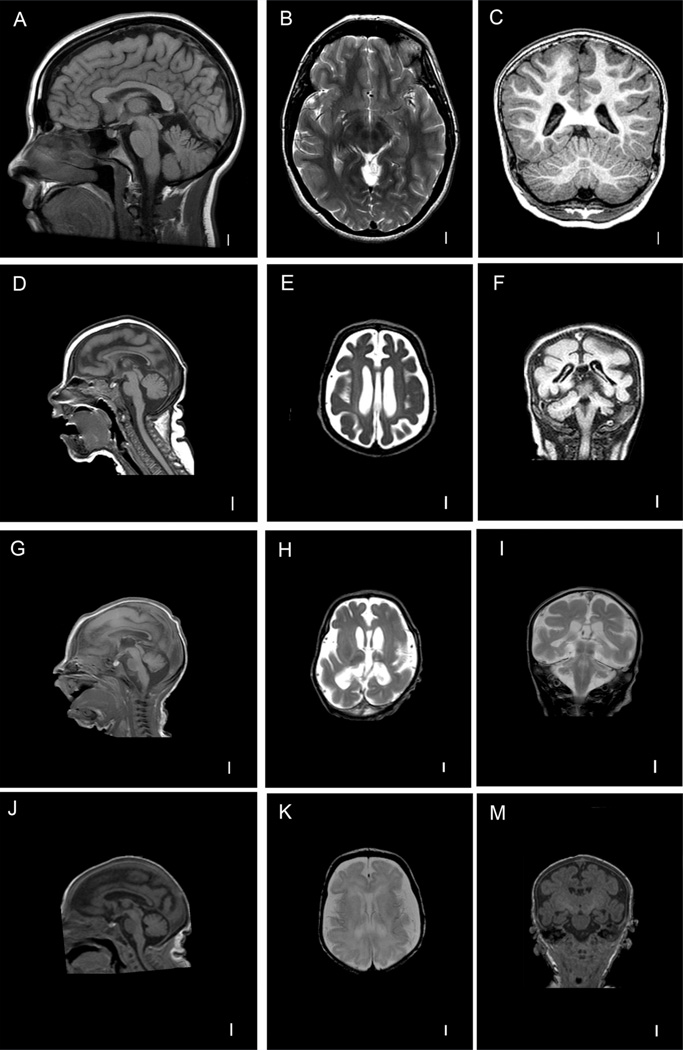
FIG. 4.
T1-weighted mid-sagittal and coronal and T2-weighted axial images of a normal patient (A–C) and Patient 1 (LR#07-155a1, D-F), T1-weighted mid-sagittal and T2-weighted axial and coronal images of Patient 2 (LR07-155a2, G–I), T1-weighted mid-sagittal and coronal and T2-weighted axial images of Patient 3 (LR10-168, J–M). Note obvious microcephaly, low-sloping forehead, with diffusely reduced number of gyri, shallow wide sulci consistent with microcephaly with simplified gyri, as well as moderately enlarged extra-axial space and thin cortex in all affected patients.
Skin Pathology
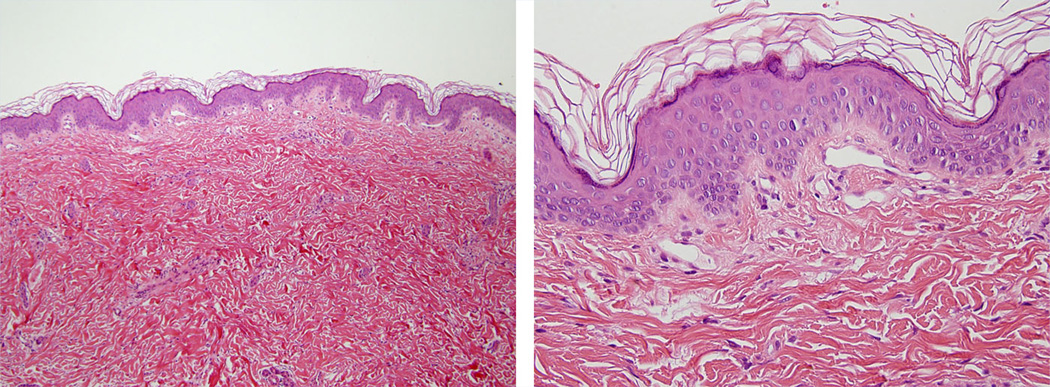
Skin biopsies were performed in Patients 2 and 3. Both showed dilated small-caliber blood vessels in the papillary dermis, consistent with capillary malformations (CMs) (Fig. 5).
FIG. 5.
Skin biopsy images of Patient 3 (LR10-168). Routine hematoxylin and eosin stained slides at 100× and 400× magnification show a punch biopsy of skin with an unremarkable epidermis and reticular dermis. The capillaries in the papillary dermis are increased in number and are dilated as compared to normal. These biopsy findings are diagnostic of capillary malformations.
DISCUSSION
We report three children with the recently reported microcephalycapillary malformation (MIC-CM) syndrome. The essential features of MIC-CM in all five children include (1) severe congenital MIC—although 2/5 had mild congenital and severe postnatal MIC. MIC was clearly progressive in 4/5 patients (2) profound mental retardation, (3) severe spastic quadriparesis, (4) neonatal-onset intractable epilepsy that included infantile spasms in 2/5 children, and (5) diffuse small capillary malformations of the skin. Several less consistent abnormalities may occur including (6) hypoplasia of the distal phalanges and of the fingers and toes as described in 4/5 (with 2–3 toe cutaneous syndactyly in 1/5), and (7) minor heart malformations in 3/5 including a small atrial septal defect in one, and a small muscular VSD in another [Carter et al., 2011].
The birth OFC was small in all children and usually very small, measured at −6 SD in 1/5, −4 SD in 2/5 children, and only −2 SD in 2/5. Postnatal head growth was also very slow, and our children had OFC measured at −6 to −8 SD, as early as 6 months of age. Brain imaging showed the low forehead and “simplified” gyral pattern with reduced numbers of gyri and shallow sulci. But additional abnormalities were more severe than seen in more common forms of primary MIC. For example, the “simplified” pattern was severe and diffuse rather than frontal predominant, and the extra-axial space was mildly widened suggesting a component of atrophy. In support of this, the two patients reported by Carter et al., had evidence of progressive and generalized cerebral atrophy brain MRI at 19 months of age (Patient 1), and autopsy at 17 months of age (Patient 2), with relative sparing of the cerebellum.
All reported patients had evidence of severe global developmental delay. The eldest of the siblings reported here was nonambulatory and non-verbal at 2.5 years of age. This phenotype contrasts with children with ASPM mutations or classic primary MIC who tend to have relatively mild neurologic and cognitive impairment with mild-moderate intellectual disability, most of whom are ambulatory with slightly delayed motor milestones, delayed yet existent speech, and normal tone [Basel-Vanagaite and Dobyns, 2010]. This syndrome is therefore clearly a distinct and more severe congenital MIC syndrome.
All reported children have intractable epilepsy of neonatal onset. While seizures with primary MIC have been reported in patients with ASPM mutations [Shen et al., 2005], the association is other-wise unusual. Furthermore, we are not aware of any reports of infantile spasms with any of the primary MIC syndromes. Both patients previously reported had intractable epilepsy, but the association of this congenital MIC syndrome with infantile spasms appears to be novel.
All five patients also had striking generalized capillary malformations scattered over the trunk, extremities, and head that ranged in size from 2 to 15 mm with no evidence of regression, and growth commensurate with the children’s overall body growth. Their nature was histologically confirmed in 3/5 patients. The diffuse pattern of capillary malformations seen in all five children resembles the pattern seen in the “capillary malformation-arteriovenous malformation” (CM-AVM) syndrome associated with mutations of RASA1. However, sequencing of the RASA1 gene was pursued and found negative in two patients. Of note, Patient 1 from Carter et al., additionally had two angiomas in the left cerebellar hemisphere, but the remaining four patients did not have evidence of other vascular malformations besides the generalized CMs.
Congenital MIC
Congenital MIC is caused by a vast array of genetic and environmental factors and has been reported in association with multiple congenital anomalies, but disorders characterized by severe congenital MIC (below −3 SD at birth) are mostly genetic with autosomal recessive inheritance [Ashwal et al., 2009; Woods et al., 2005]. Our observation of two affected sibs of different sexes further supports this observation.
Vascular Anomalies
Vascular anomalies are broadly divided into vascular tumors characterized by rapid growth followed by involution, such as hemangiomas of infancy, and non-progressive vascular malformations [Enjolras, 1997; Hand and Frieden, 2002; Cohen, 2006]. The latter group includes capillary malformations (some subtypes are also known as “port-wine stains”), venous, lymphatic, and arteriovenous malformations [Hand and Frieden, 2002]. Capillary malformations (CMs), specifically, are congenital lesions formed of dysplastic vascular channels with no potential for endothelial proliferation or spontaneous regression. They can be found in 0.3% of newborns and maybe sporadic or familial [Mulliken and Young, 1988; Enjolras and Mulliken, 1997]. On gross examination, they are flat macular, cutaneous lesions ranging from pink/red to dark purple, and are mostly localized on the face or limbs [Burns et al., 2009].
Disorders combining vascular anomalies and deregulated growth are numerous and often involve partial or generalized hypertrophy or overgrowth, local hypotrophy, or disproportionate growth [Oduber et al., 2011]. Some of these are associated with megalencephaly or hemimegalencephaly such as Klippel–Trenaunay syndrome (OMIM#149000) [Torregrosa et al., 2000], macrocephaly-capillary malformation syndrome (OMIM#602501) [Moore et al., 1997; Clayton-Smith et al., 1997], and PTEN-related disorders such as Bannayan–Riley–Ruvalcaba syndrome OMIM#153480), Cowden syndrome (MIM#158350), and Proteus syndrome (OMIM#176920) [Eng, 2003]. Clearly, this is a novel syndrome of generalized capillary malformations and severe congenital microcephaly with poor somatic growth. We hypothesize it may be caused by one or more genes involved in vasculogenesis and neuronal and growth regulation.
In summary, this is a novel autosomal recessive multiple congenital anomalies syndrome characterized by severe congenital MIC, diffuse capillary malformations, intractable epilepsy, including infantile spasms, and severe developmental handicap with spastic quadriparesis.
ACKNOWLEDGMENTS
We thank the families for allowing us to share their children’s medical information.
REFERENCES
- Ashwal S, Michelson D, Plawner L, Dobyns WB. Practic parameter: Evaluation of the child with microcephaly (an evidence-based review): Report of the quality standards subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2009;73:887–897. doi: 10.1212/WNL.0b013e3181b783f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkovich AJ, Ferriero DM, Barr RM, Gressens P, Dobyns WB, Truwit CL, Evrard P. Microlissencephaly: A heterogeneous malformation of cortical development. Neuropediatrics. 1998;29:113–119. doi: 10.1055/s-2007-973545. [DOI] [PubMed] [Google Scholar]
- Basel-Vanagaite L, Dobyns WB. Clinical and brain imaging heterogeneity of severe microcephaly. Pediatr Neurol. 2010;43:7–16. doi: 10.1016/j.pediatrneurol.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Burns AJ, Navarro JA, Cooner RD. Classification of vascular anomalies and the comprehensive treatment of hemangiomas. Plast Reconstr Surg. 2009;124:69e–81e. doi: 10.1097/PRS.0b013e3181aa1015. [DOI] [PubMed] [Google Scholar]
- Carter MT, Geraghty MT, De La Cruz L, Reichard RR, Boccuto L, Schwartz CE, Clericuzio CL. A new syndrome with multiple capillary malformations, intractable seizures, and brain and limb anomalies. Am J Med Genet A. 2011;155:301–306. doi: 10.1002/ajmg.a.33841. [DOI] [PubMed] [Google Scholar]
- Clayton-Smith J, Kerr B, Brunner H, Tranebjaerg L, Magee A, Hennekam RC, Mueller RF, Brueton L, Super M, Steen-Johnsen J, Donnai D. Macrocephaly with cutis marmorata, hemangioma and syndactyly-a distinctive overgrowth syndrome. Clin Dysmorphol. 1997;6:291–302. doi: 10.1097/00019605-199710000-00001. [DOI] [PubMed] [Google Scholar]
- Cohen MM., Jr Vascular update: Morphogenesis, tumors, malformations, and molecular dimensions. Am J Med Genet A. 2006;140:2013–2038. doi: 10.1002/ajmg.a.31333. [DOI] [PubMed] [Google Scholar]
- Dobyns WB. Primary microcephaly: New approaches for an old disorder. Am J Med Genet 1. 2002;112:318–326. doi: 10.1002/ajmg.10580. [DOI] [PubMed] [Google Scholar]
- Dobyns WB, Barkovich AJ. Microcephaly with simplified gyral pattern (oligogyric microcephaly) and microlissencephaly: Reply. Neuropediatrics. 1999;30:104–106. [Google Scholar]
- Eng C. PTEN: One gene, many syndromes. Hum Mutat. 2003;22:183–198. doi: 10.1002/humu.10257. [DOI] [PubMed] [Google Scholar]
- Enjolras O. Classification and management of the various superficial vascular anomalies: Hemangiomas and vascular malformations. J Dermatol. 1997;24:701–710. doi: 10.1111/j.1346-8138.1997.tb02522.x. [DOI] [PubMed] [Google Scholar]
- Enjolras O, Mulliken JB. Vascular tumors and vascular malformations (new issues) Adv Dermatol. 1997;13:375–423. [PubMed] [Google Scholar]
- Hand JL, Frieden IJ. Vascular birthmarks of infancy: Resolving nosologic confusion. Am J Med Genet. 2002;108:257–264. doi: 10.1002/ajmg.10161. [DOI] [PubMed] [Google Scholar]
- Moore CA, Toriello HV, Abuelo DN, Bull MJ, Curry CJ, Hall BD, Higgins JV, Stevens CA, Twersky S, Weksberg R, Dobyns WB. Macrocephaly-cutis marmorata telangiectatica congenital: A distinct disorder with developmental delay and connective tissue abnormalities. Am J Med Genet. 1997;70:67–73. [PubMed] [Google Scholar]
- Mulliken JB, Young AE. Vascular birthmarks: Hemangiomas and malformations. Philadelphia: W.B. Saunders; 1988. pp. 264–265. [Google Scholar]
- Oduber CE, van der Horst CM, Sillevis Smitt JH, Smeulders MJ, Mendiratta V, Harper JI, van Steensel MA, Hennekam RC. A proposal for classification of entities combining vascular malformations and deregulated growth. Eur J Med Genet. 2011;26:262–271. doi: 10.1016/j.ejmg.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Opitz JM, Holt MC. Microcephaly: General considerations and aids to nosology. J Craniofac Genet Dev Biol. 1990;10:175–204. [PubMed] [Google Scholar]
- Shen J, Eyaid W, Mochida GH, Al-Moayyad F, Bodell A, Woods CG, Walsh CA. ASPM mutations identified in patients with primary microcephaly and seizures. Am J Med Genet. 2005;42:725–729. doi: 10.1136/jmg.2004.027706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztriha L, Dawodu A, Gururaj A, Johansen JG. Microcephaly associated with abnormal gyral pattern. Neuropediatrics. 2004;35:346–352. doi: 10.1055/s-2004-830430. [DOI] [PubMed] [Google Scholar]
- Torregrosa A, Martí-Bonmatí L, Higueras V, Poyatos C, Sanchís A. Klippel-Trenaunay syndrome: Frequency of cerebral and cerebellar hemihypertrophy on MRI. Neuroradiology. 2000;42:420–423. doi: 10.1007/s002340000310. [DOI] [PubMed] [Google Scholar]
- Woods CG, Bond J, Enrad W. Autosomal recessive primary microcephaly (MCPH): A review of clinical, molecular, and evolutionary findings. Am J Hum Genet. 2005;76:717–728. doi: 10.1086/429930. [DOI] [PMC free article] [PubMed] [Google Scholar]