Abstract
Background
In the normal heart, phosphodiesterase type 5 (PDE5) hydrolyzes cGMP coupled to nitric oxide (specifically from NOS3) but not natriuretic peptide (NP) stimulated guanylyl cyclase. PDE5 is upregulated in hypertrophied and failing hearts and is thought to contribute to their pathophysiology. Since NO signaling declines whereas NP-derived cGMP rises in such diseases, we hypothesized that PDE5 substrate selectivity is retargeted to blunt NP-derived signaling.
Methods and Results
Mice with cardiac myocyte inducible PDE5 overexpression (P5+) were crossed to those lacking NOS3 (N3−), and each model, the double cross, and controls were subjected to transaortic constriction (TAC). P5+ mice developed worse dysfunction and hypertrophy, and enhanced NP stimulation, whereas N3− mice were protected. However, P5+/N3− mice behaved similarly to P5+ despite the lack of NOS3-coupled cGMP generation, with PKG activity suppressed in both models. PDE5 inhibition did not alter ANP-stimulated cGMP in the resting heart, but augmented it in the TAC heart. This functional retargeting was associated with PDE5 translocation from sarcomeres to a dispersed distribution. P5+ hearts exhibited higher oxidative stress whereas P5+/N3− hearts had low levels (likely due to the absence of NOS3 uncoupling). This highlights the importance of myocyte PKG activity as a protection to pathological remodeling.
Conclusions
These data provide the first evidence for functional retargeting of PDE5 from one compartment to another, revealing a role for NP-derived cGMP hydrolysis by this esterase in diseased heart myocardium. Retargeting likely impacts the pathophysiologic consequence and also therapeutic impact of PDE5 modulation in heart disease.
Keywords: cyclic nucleotides, hypertrophy, oxidative stress, phosphodiesterases receptors
Introduction
Phosphodiesterase type 5 (PDE5) is one of an eleven-member superfamily of enzymes responsible for the hydrolysis of cyclic nucleotides to their 5’monophosphate form. It was the first to be identified as relatively selective for cyclic guanosine monophosphate (cGMP), and is expressed in vascular smooth muscle particularly in the corpus cavernosum and pulmonary circulation. PDE5 inhibition stimulates protein kinase G-dependent vasodilation, forming the basis for its clinical use to treat erectile dysfunction and pulmonary hypertension1. Recent studies have revealed PDE5 is also expressed in cardiac muscle2–5, and growing evidence supports its contribution to ventricular disease in experimental models6, 7 and in humans8. While expression is normally quite low, it rises considerably in experimental4 and human cardiac hypertrophy and failure5, 9, 10, a change that has been linked to oxidative stress4. Higher levels of PDE5 expression can participate in cardiac pathophysiology, worsening remodeling and dysfunction after myocardial infarction5 or following sustained pressure-overload7. Such findings have led to clinical trials testing the utility of PDE5 inhibition in dilated cardiomyopathy (DCM) 8, 11, heart failure with a preserved ejection fraction (HFpEF)12, and Duchenne and Becker muscular dystrophy13. Initial results support the efficacy of sildenafil in human DCM for improving breathlessness and exercise capacity14, 15, and cardiac remodeling and function8, without altering systemic arterial pressures (i.e. afterload).
In the ventricular myocyte, cGMP is generated by two different pathways: by stimulating nitric oxide synthase (NOS) to generate NO and activate soluble guanylate cyclase (sGC)16, or by natriuretic peptide activation of particulate GC attached to the cognate receptor17. These are not redundant pathways, but display different constellations of distal targets and regulatory PDEs. In particular, myocyte PDE5 normally targets NOS3-sGC derived cGMP, while other PDEs (e.g. PDE1, PDE2) may target pGC related pools18, 19. PDE5 inhibition in the normal heart (including human) and myocytes suppresses acute β-adrenergic-stimulated contractility; however, this is not observed when NOS3 is genetically deleted or NOS inhibited pharmacologically2, 20. Moreover, PDE5 inhibition enhances PKG activity upon NO but not ANP stimulation in the normal heart18, has no impact on membrane localized cGMP induced by ANP in rat myocytes19, and mimics NO- but not ANP modulated compartmentation of PKA isoform activity21.
In many forms of heart disease, NO-derived cGMP declines due in part to NOS uncoupling22 and sGC oxidation23 whereas NP-derived cGMP is markedly enhanced. If increased PDE5 activity with HF remained solely targeted to NO-sGC derived pools, its impact would be likely diminished given less available substrate. However, if the esterase could be retargeted to NP-cGMP pools, its pathophysiological role and the therapeutic impact from its inhibition would be enhanced. The current study tested this hypothesis by studying mice with a tetracycline controllable myocyte-specific PDE5 overexpression (P5+)7 and crossing them into a background lacking NOS3 (N3−). We previously reported that P5+ mice exhibit worsened hypertrophy, fibrosis, and LV dysfunction when subjected to trans-aortic constriction (TAC), and this reverses once myocyte PDE5 expression is reduced (adding doxycycline to the drinking water to suppress transgene expression)7. N3− mice, by contrast, develop concentric adaptive hypertrophy with severe TAC, coupled to a lack of oxidative stress that is otherwise generated by NOS uncoupling22, a process whereby NOS produces superoxide rather than NO24. If PDE5 remained targeted only to NOS3-sGC derived cGMP, one would predict a model lacking NOS3 but with myocyte PDE5 enhanced would behave like the N3− itself. However, if alternative targeting to NP-pGC derived cGMP occurrs, the pathophysiology should be worse. Our results support the latter, providing the first evidence for functional PDE retargeting to a completely different cyclic nucleotide pool with in vivo consequences. We further reveal that suppressing myocyte PKG activity exacerbates pressure-overload remodeling at organ and molecular levels, even despite low levels of myocardial oxidative stress.
Methods
Mouse Models and Pressure-Overload Stress
Bi-genic P5+ mice have been described previously7; they overexpress PDE5 in myocytes (αMHC promoter) but only in the absence of doxycycline (tet-off system). We used the line with 7× PDE5 protein overexpression. P5+ mice were further crossed into a N3− background (Jackson Labs, C57BL/6) to generate tri-genic mice. Four groups were studied (mean age 2–3 months when heart function and molecular signaling are similar to controls): controls (littermates expressing NOS3 and lacking PDE5 transgene), P5+, N3−, and the cross (P5+/N3−). Mice were subjected to 7–8 days of trans-aortic constriction (TAC), a duration previously shown to induce marked chamber dilation/dysfunction and early mortality in P5+ mice7.
Cardiac Function
Intact cardiac function was assessed in conscious animals by echocardiography to derive indexes of LV dimension, fractional shortening, and wall thickness as described7.
Confocal Immunohistochemistry
The myocyte distribution of PDE5 followed previously reported protocols and equipment, using both a primary antibody to PDE5 (Cell Signaling) and to Flag (tagged with transgene, Sigma Aldrich)7. Myocyte size was detected by wheat germ agglutinin (WGA) staining7, using a semi-automated method to assess mean cross sectional area.
Molecular Analysis
Myocardial expression of fetal genes (nppa, nppb), genes related to nuclear factor of activated T-cell (NFAT) signaling (regulator of calcineurin, Rcan-1, transient receptor potential canonical channel 6, Trpc6), and fibrosis/remodeling genes (transforming growth factor beta-1 Tgfb1, connective tissue growth factor, ctgf) were determined by real-time quantitative PCR (primers in Supplemental Methods). Cyclic GMP was assessed by EIA and PKG activity by fluorescent polarization assay as described7. PDE5 protein expression in subcellular and cytosolic compartments was determined in myocardial tissue subjected to gradient centrifugation.
Oxidative Stress
Myocardial superoxide formation was determined by HPLC assay of 2-hydroxyethidium formation from frozen tissue extracts as described25. Tissue level of reduced and oxidized glutathione (GSH/GSSG) was measured using a Bioxytech GSH/GSSG-412 kit (Oxis International Inc.). For measurement of GSSG, the thiol-scavenging reagent, 1-methyl-2-vinylpyridinium trifluoromethanesulfonate (M2VP), was added to the homogenization buffer to minimize oxidation of GSH to GSSG. Gene expression of NADPH oxidases, NOX2 and NOX4 was determined by qPCR.
Statistics
Results are presented as mean ± SEM. For analysis between the four experimental groups, we used a 1-way ANOVA with a Tukey-Kramer multiple comparisons test. In experiments where the data distribution failed normality tests, a Kruskal-Wallis non-parametric test and Dunn’s multiple comparison test was performed. All p-values reported for within-group differences are adjusted for multiple comparisons. We also used 2-way ANOVA to test for TAC-group or drug-drug interactions, often employing a subsequent within-group 1-way ANOVA and multiple comparisons test as appropriate. The specific tests and significant differences between groups as well as relevant sample sizes are provided in the figure legends. Analysis was performed using Systat® Version 10.2.
Results
Cardiac function and remodeling are worse after TAC in P5+ mice with or without NOS3
Baseline cardiac morphology, function, and LV and lung mass were similar in all four groups (Supplemental Fig. S1). Resting PKG activity was lower in the two groups carrying the P5+ transgene in myocytes versus controls or N3− (Supplemental Fig. S2). Normal basal PKG activation in hearts lacking NOS3 (N3−) is consistent with prior studies showing enhanced NP-signaling in this model26.
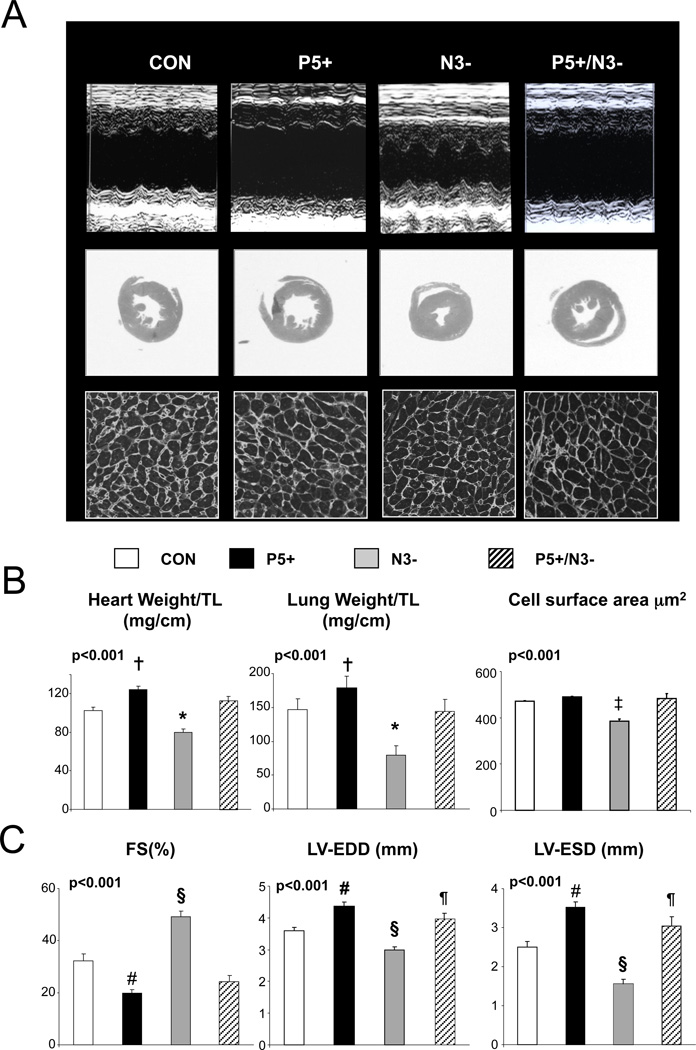
In contrast to resting function, the cardiac response to TAC differed markedly among the four groups. Figure 1A displays representative echocardiograms, cross sectional histology, and WGA stained myocardium from hearts after TAC. LV dilation and function were worse and myocyte hypertrophy increased in P5+. N3− hearts were protected, displaying no dilation but rather concentric hypertrophy, with less net increase in LV mass and myocyte enlargement. However, in P5+/N3− mice, the protection afforded by an absence of NOS3 was lost, and LVs again developed marked dilation, dysfunction, and myocyte and organ hypertrophy. Summary data are provided in Figure 1B.
Figure 1.
Influence of myocyte PDE5 upregulation with and without NOS3 expression on hearts subjected to trans-aortic constriction (TAC). A) Top panels show example M-mode echocardiograms, Middle panels show histologic cross-sections (H&E stain) at the mid-chamber level, and lower panels show myocyte cross sectional area (WGA stain, average of several hundred cells from multiple views in each heart, n=3–5 hearts per group). B) Summary results for heart and lung weight normalized to tibia length (TL), myocyte cross sectional area, fractional shortening (%FS), and left ventricular end-diastolic and end-systolic short axis dimension (LV-EDD, LVESV) for the four models after TAC (n=25, 15, 27, 15 for each model respectively, left to right). P-values shown at the upper left are results for either a 1-way ANOVA or non-parametric Kruskal-Wallis test. Symbols above the bars denote results of multiple comparisons test: * p<0.05 vs P5+ and P5+/N3−; † p<0.05 vs control (Dunn’s test); ‡ p<0.01 vs all other groups; § p<0.001 vs all other groups; # p<0.05 vs CON; and ¶ p<0.05 versus P5+; all Tukey-Kramer test.
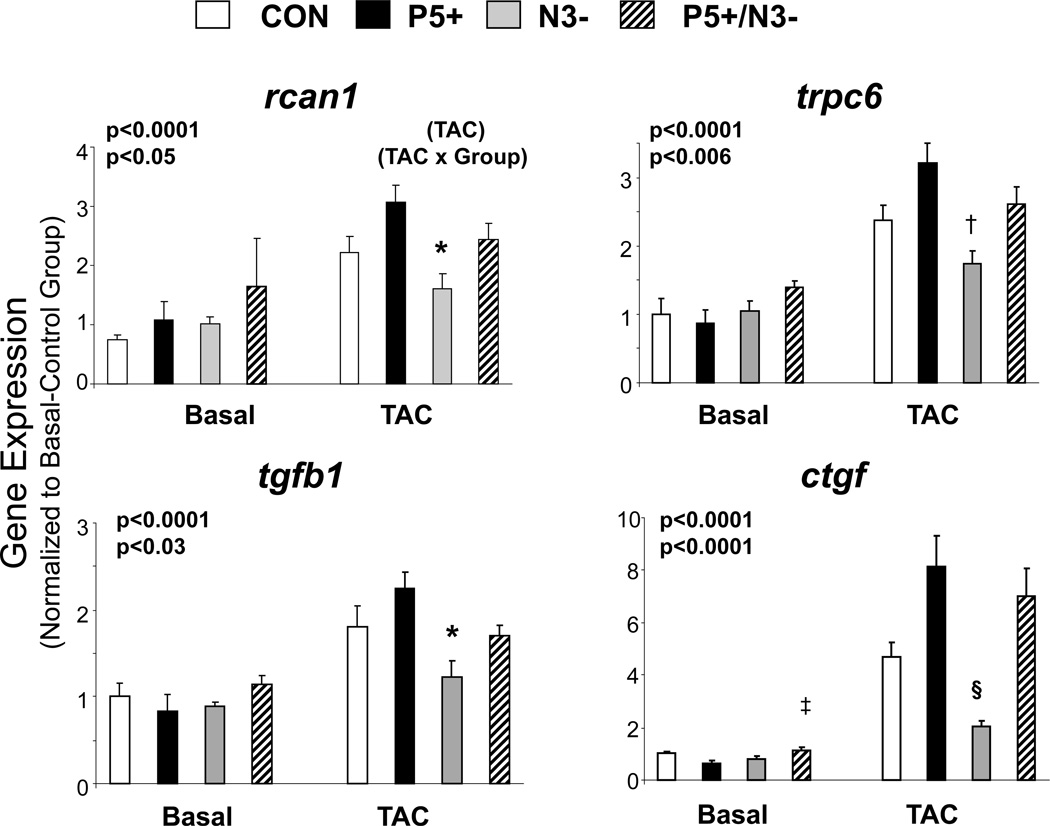
The differences in chamber remodeling were accompanied by corresponding disparities in molecular signaling that is targeted by PKG activation7. Gene expression markers of calcineurin upregulation (Rcan1, Trpc6), and growth/fibrosis signaling (tgfb1, ctgf), were significantly enhanced after TAC particularly in models with PDE5 overepression (Figure 2). Importantly, this occurred whether NOS3 was expressed in the heart or not.
Figure 2.
Impact of myocyte PDE5 upregulation with or without NOS3 expression on myocardial genes coupled to hypertrophy/fibrosis signaling in hearts at rest and after TAC. Rcan-1 – regulator of calcineurin, trpc6 – transient receptor potential canonical channel 6, tgfb1 – transforming growth factor beta, ctgf – connective tissue growth factor. P values are for a combined 2-way ANOVA, with presence or absence of TAC and the experimental group being the two factors. Results for the influence of TAC (upper p-value) and interaction between TAC and group (lower value) are provided. Individual symbols are results of post-hoc Tukey-Kramer MCT. * p<0.01 vs P5+, † p<0.002 vs P5+, p<0.05 vs P5+/N3−, ‡, =0.02 vs P5+; §p<0.001 vs P5+ and P5/N3. N=4–8 samples for each group and time point.
PDE5 is functionally retargeted to NP-derived cGMP in TAC-stressed hearts
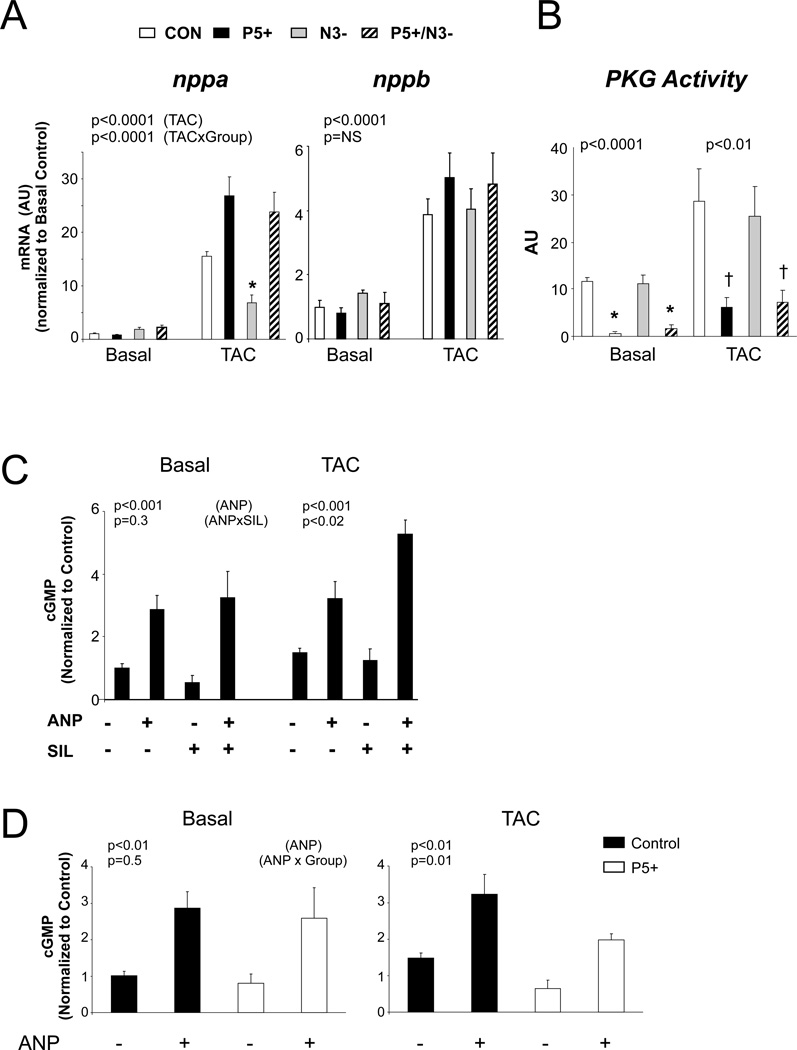
The finding that PDE5 upregulation worsened organ and molecular responses to TAC despite a lack of NOS3 suggested an alternative pool of cGMP was being targeted, such as from NP signaling. Myocardial ANP and BNP gene expression rose in all models, with ANP increasing even more in P5+ and P5+/N3− (Fig 3A). Corresponding PKG activity is shown in Fig. 3B. While TAC increased activity overall (p<0.05 by 2-way ANOVA), there remained striking differences between groups, with activation remaining reduced in both models with with enhanced PDE5 activity. This suggested that NP-generated cGMP was being hydrolyzed. To test this more directly, sham and TAC mice were administered ANP (10 µg), sildenafil (12.5 µg), or both 19 (Figure 3C). In sham controls, myocardial cGMP rose similarly with ANP alone or when combined with sildenafil. However after TAC, cGMP rose disproportionately more with the combination than with ANP itself (p<0.02 for SIL/ANP interaction).
Figure 3.
PDE5 is retargeted to NP-derived pools in hearts subjected to TAC. A) mRNA expression for ANP (nppa) and BNP (nppb) genes at basal state and after TAC in four models. Both were markedly upregulated by TAC (p<0.001) particularly in models with upregulated PDE5. N3− alone showed a blunted ANP response. N=3–8/group. P-values are results of a 2-way ANOVA, top value showing the effect from TAC effect, and the lower value the interaction of TAC × experimental group. As in the prior figures, symbols denote results of multiple comparisons test. * p<0.05 versus P5+ and P5/N3. B) PKG activity increases with TAC, but is suppressed in hearts with enhanced PDE5 levels with or without NOS3 expression. N=4–5/group. P-values for 1-way ANOVA within each group (CON, TAC). * p=0.01 versus CON and N3−, † p<0.05 versus CON and N3− by Kruskal Wallis test. C) cGMP in myocardium from hearts exposed to ANP, sildenafil (SIL), or both. N=3–9/group. P-values from 2-way ANOVA: top value for effect from ANP, lower: ANP × SIL interaction. In sham controls, ANP increased cGMP similarly with or without SIL, whereas after TAC, the ANP+SIL response was greater than with ANP alone. D) cGMP in myocardium from CON or P5+ hearts before and after exposure to ANP, at basal condition and after TAC. P- values 2-way ANOVA: top ANP effect, bottom, ANP × Genotype interaction. N=3–9/group.
A potential concern with any transgenic model is that the overexpressed protein may exhibit promiscuous targeting. To test this, we administered ANP to both WT and P5+ hearts at baseline and after TAC (Figure 3D). ANP augmented cGMP similarly in both models at baseline, indicating that PDE5 overexpression alone did not result in off-target cGMP hydrolysis. After TAC, however, the ANP-stimulated rise in cGMP was blunted more in P5+ hearts (p=0.01 for interaction of ANP and genetic model), supporting retargeting.
We also examined gene expression levels for the other NOS isoforms (Supplemental Fig. S3). Expression of both NOS1 (neuronal) and NOS2 (inducible) isoforms was similar at baseline in all four models. After TAC, NOS2 rose slightly more in mice expressing NOS3 versus those that did not (p<0.05, 3-way ANOVA). NOS1 by contrast remained similar among the groups, though expression was somewhat lower overall after TAC (p<0.002, 3-way ANOVA). Thus, while some changes were observed, they did not correlate with cardiac remodeling, cGMP or PKG activity.
Functional retargeting of PDE5 is accompanied by a loss of normal myocyte localization
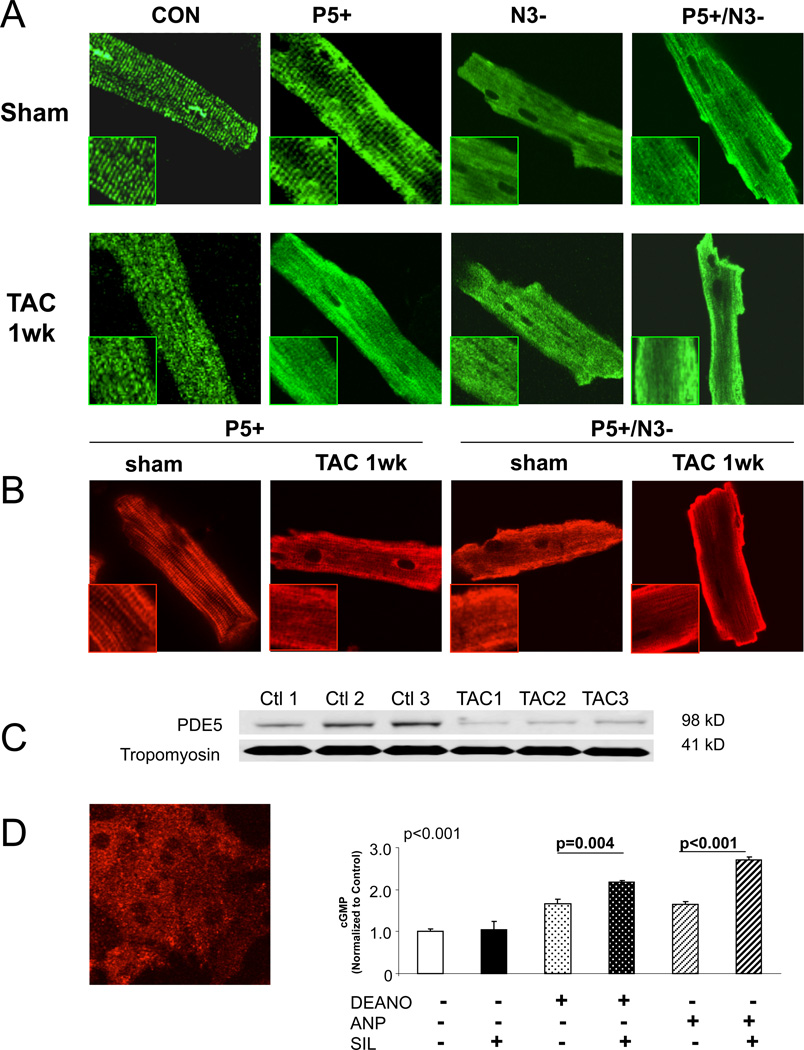
Cardiomyocyte PDE5 is normally distributed throughout the cell with enhanced striated pattern reflecting sarcomere localization2, 20. This becomes diffuse if NOS3 activity is inhibited2, 20, behavior also observed in canine dilated cardiomyopathy27. We speculated that a similar change might accompany hypertrophy/dilation coupled with functional enzyme targeting. Figure 4A shows confocal images of myocytes from each model at baseline and after TAC. A striated pattern was present in CON and P5+ cells, but this became diffuse throughout the cell after TAC. Both N3− and P5+/N3− hearts displayed a diffuse pattern at baseline and after TAC. However, PKG activation was preserved in N3− after TAC, despite a diffuse PDE5 distribution, likely due to persistent low levels of PDE5 activation in these hearts (Supplemental Fig. S4). In contrast, P5+/N3+ hearts had enhanced PDE5 activity due to the tet-off transgene. The TAC stress-specific change in localization of the PDE5 transgene was confirmed by staining with anti-Flag antibody (Fig. 4B), and immuneblot of PDE5 in myofibrillar sub-fractions, with tropomyosin used as a loading control (Figure 4C).
Figure 4.
Altered myocyte PDE5a localization in myocytes from pressure-overload hearts. A) confocal images of myocytes from each experimental group at baseline and after TAC, staining for PDE5a. Control and P5+ cells show striated localization at rest, but diffuse distribution after TAC. Models lacking NOS3 show a diffuse pattern of staining before and after TAC. B) confocal images from P5+ and P5/N3 cells before and after TAC, using Flg Antibody to detect transgene only. This shows similar change in localization after TAC. C) Gel electrophoresis from myofibrillar sub-fraction probed with PDE5, and tropomyosin as a loading control. With TAC, there is a decline in PDE5 expression in this subfraction. D) PDE5 is diffuse in neonatal myocytes (left), and this is coupled to an enhancement of both an ANP or NO-stimulated cGMP response (right). P values for unpaired t-test between groups.
When neonatal myocytes are stained for PDE5, they show a diffuse staining pattern (Figure 4D) analogous to that observed in adult myocytes after TAC, suggesting this latter change may be part of fetal recapitulation. Interestingly, diffuse staining in neonatal cells was also coupled to dual targeting of PDE5 to NO and ANP-stimulated cGMP pools (Fig. 4D).
PDE5 is itself potently activated by PKG phosphorylation at S92, and in platelets this modification has been coupled to compartmentation28. We therefore speculated that loss of this signal from NOS3-PKG activation might lead to unmooring of the enzyme from its sarcomeric location. PDE5 mutants with either inactivated (S92A) or pseudophosphor-ylated (S92D) modifications fused with DsRed tag were incorporated into adenoviral vectors and injected into the heart in vivo20. Myocytes isolated 48 hours later were examined by con-focal imaging. Neither mutant altered the normal striated pattern of PDE5 distribution (Supplemental Fig S5).
Hypertrophy/failure in P5+/N3− hearts occurs despite minimal ROS activation
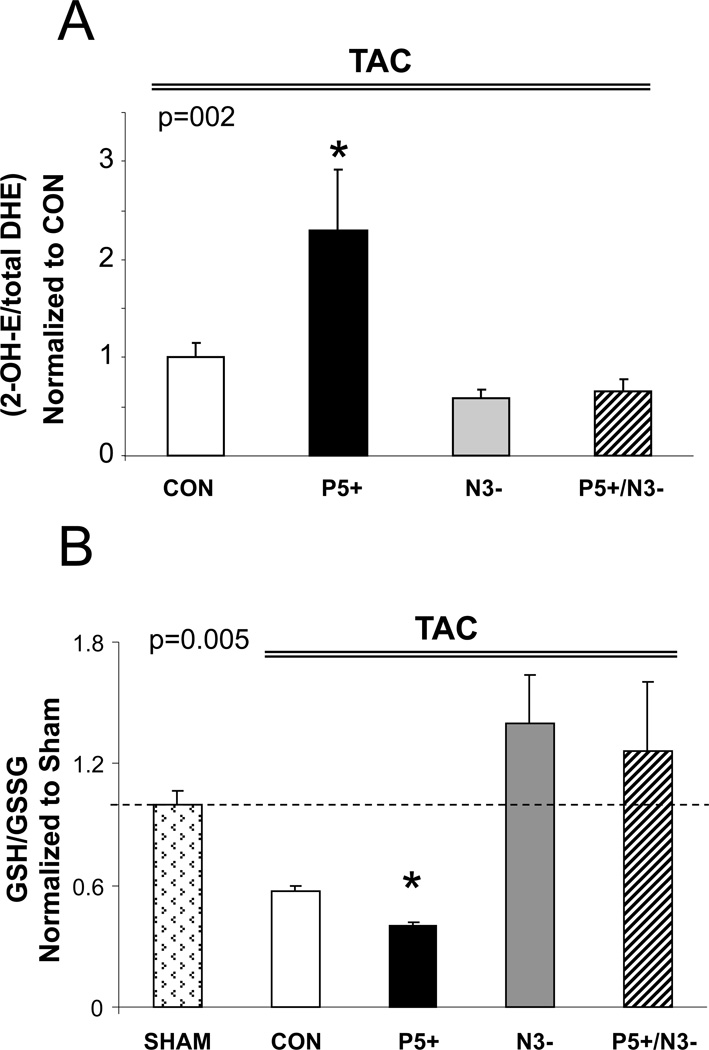
In N3− hearts, protection against maladaptive (dilated) hypertrophy is coupled to the absence of NOS3-derived reactive oxygen species that otherwise occurs via NOS uncoupling22. PDE5 up-regulation has itself been linked to ROS4, and we hypothesized that consequent declines in cGMP/PKG signaling would further amplify ROS, perhaps contributing to the dysfunction/dilation observed in P5+ as well as P5+/N3− hearts. The first prediction proved correct, but the second surprisingly not. Myocardial superoxide determined by HPLC assay for 2-OH DHE showed a >2-fold increase in P5+ over controls. Levels were below control in N3− myocardium, yet were similarly low in P5+/N3− (Figure 5A). These results were supported by the ratio of reduced/oxidized glutathione (GSH/GSSG) that reflects overall redox. This ratio declined in Con and P5+, but was similar to control in models lacking NOS3 (Figure 5B). Despite differences in ROS, the expression of NADPH oxidases (NOX2 and NOX4) rose in all models after TAC, with NOX2 increasing equally and NOX4 rising more in both models with greater PDE5 expression and worse remodeling (Supplemental Fig. S6).
Figure 5.
Enhanced PDE5 expression increases myocardial oxidative stress, but this is diminished in hearts lacking NOS3. A) Myocardial superoxide was detected by 2-OH-ET, and increased in P5+ tissue compared to control. Both N3− and P5/N3 myocardium showed a similar and much lower level. * p<0.02 vs all other groups. (n=8–10/group) B) Ratio of GSH/GSSG in the four models. The ratio was reduced most in P5+ (* p<0.05 versus Sham) consistent with increased oxidative stress, but was at control levels in the two models lacking NOS3(n=3–4/group). P values in graph are for 1-way ANOVA between groups.
Discussion
The major new finding of this study is that cardiac stress remodeling associated with pressure-overload re-targets PDE5 hydrolytic activity to the cGMP pool generated via natriuretic peptide signaling rather than solely from NOS3-NO-sGC dependent cGMP as found in the normal heart. This supported by NP-PDE5A interaction data in hearts subjected to TAC, and by the finding that genetic deletion of NOS3 did not eliminate the adverse impact of PDE5 upregulation. Retargeting was accompanied by a redistribution of the protein from a sarcomeric pattern to one that is diffuse, similar to that observed in neonatal myocytes. As a result, potentially protective effects from NP-signaling are blunted by PDE5 upregulation, worsening maladaptive remodeling, but also increasing the therapeutic impact from PDE5 inhibition. Lastly, we show that myocyte PKG is a key suppressor of maladaptive cardiac hypertrophy and dilation, even in settings with reduced oxidative stress.
Compartmentation of cyclic nucleotide signaling
In order for cyclic nucleotides to perform their broad array of intracellular signaling roles, concentrations and protein targeting must be constrained within microdomains. This is accomplished by compartmentalizing the cyclase with its corresponding kinase and hydrolyzing phosphodiesterase. Such micodomains have been best studied for cAMP regulation in which all three components are often further coordinated by A-kinase anchoring proteins (AKAPs) which serve as complex scaffolds29. PDEs also play a primary role in this compartmentation30 by limiting cyclic nucleotide diffusion beyond a micro-domain and/or preventing distal targeting. Among these, PDE4 appears particularly suited for regional regulation due to a variable N-terminal region that acts as a zip code, directing expression to specific locations31. Other PDEs are targeted by different and less well clarified mechanisms. Examples are the local confinement to the plasma membrane of cAMP coupled to β2-AR stimulation by PDE3 and PDE432, post-activation of the β2-AR by PDE4D5 which shifts it from Gs to Gi-coupled signaling33, and modulation of the ryanodine receptor by PDE4D334.
Compartmentation of cGMP signaling is equally important, although the molecular mechanisms remain less well understood in part due to challenges in creating sensitive fluorescent probes to detect cGMP or PKG activity in microdomains within adult myocytes. cGMP signaling can been detected at the plasma membrane by means of cGMP-sensitive olfactory ion channels, and this has yielded evidence of tight compartmentation. Membrane localized cGMP rises with ANP but not NO stimulation, and the ANP response is enhanced by inhibition of PDE2 but not PDE519. This has functional consequences as PDE5 inhibition but not ANP blunts β -AR stimulated contraction in both myocytes and intact hearts, despite much greater increases in measurable myocardial cGMP from ANP stimulation18. Cyclic GMP compartmentation has been reported in studies conducted in neonatal myocytes as well, for example in the work by Stangherlin et al21 who found differential regulation by cGMP on cAMP-mediated PKA activation depending upon the guanylate cyclase stimulated, the PKA-isoform activated, and the specific PDE regulating the signaling. However, the exact details differ somewhat from adult cells, perhaps reflecting greater maturation of compartments as sarcomere structure develops. For example, they found both ANP and NO-stimulated cGMP regulated cAMP-PKA signaling21, with the former coupled to PDE2. However, in adult myocytes, ANP has minimal effects on β -adrenergic modulation with or without PDE2 inhibition35.
PDE5 retargeting as a mechanism for altered signaling
The normal myocyte localizes PDE5 in the cytosol as well as the sarcomere. In the latter location, its inhibition blunts β-AR stimulated contractility via β3-AR coupled, PKG dependent troponin I phosphorylation35. This is consistent with observed declines in cell shortening without concomitant changes in the calcium transient, i.e. calcium desensitization. As noted, NP ± PDE2 inhibition does not mimic this effect, supporting the notion that myofilament PDE5 serves as a local containment mechanism for contractile regulation. There are only few data describing PDE5 compartmentation in other cell types, an example being an ER localized pool in platelets that regulates thrombin-mediated calcium28. To our knowledge, the cardiac myoycte is the only cell type in which redistribution of the enzyme has been reported, first in a canine model of heart failure27, and subsequently in mice lacking NOS3 or with chronic NOS inhibition2, 20. In the latter case, altered localization was reversible by either chronically restoring NOS activity or by activating sGC even with persistent NOS inhibition2, 20. Importantly, only when PDE5 was localized to the sarcomere did its inhibition blunt β -AR stimulation.
The present study reveals a second and arguably more important consequence of PDE5 dyslocalization; functional retargeting from one cGMP pool to another. This is particularly relevant to heart disease where NP-stimulated cGMP often rises markedly whereas NOS-derived cGMP declines. Staining patterns did not reveal enhancement at the plasma membrane, and whether direct protein-interaction of PDE5 with the NPRA (or NPRB) receptor occurs remains unknown. However, the functional results showed that a diffuse cytosolic diffusion was sufficient to impact non sGC-derived cGMP pools. Further work is ongoing to assess different PDE5 sub-proteomes in normal and stressed hearts.
The decline in PKG activity under basal conditions in both the P5+ and P5+/N3- myocardium (Fig. 3B) might suggest that functional retargeting results from PDE5 upregulation itself, and not following pressure-overload stress. However, loss of NOS3 itself results in dispersion of PDE5 from the z-disk (Fig. 4A), supporting a key role of sGC-derived cGMP to normal PDE5 regulation. Without concomitant PDE5 upregulation, this does not impact basal PKG activity; however when both are combined, this activity declines substantially as observed. In addition, the P5+ model itself did not display basal PDE5 hydrolytic interaction with ANP-stimulated cGMP pools (Fig 3D). This was only observed after TAC, further supporting the importance of stress-remodeling.
PDE5 retargeting could explain the efficacy of its inhibition even when this is used to treat late-stage hypertrophy/dilation disorders36, where sGC and NOS activity are often blunted. Importantly, NP-derived cGMP signaling in the heart has also been shown to be anti-hypertrophic37, and to inhibit RGS2, TRPC6, and other signaling pathways coupled to pathological remodeling38. More promiscuous targeting by upregulated and dyslocalized PDE5 would suppress this modulation and enhance the impact of PDE5 inhibition. Enhanced NP-targeting is also consistent with studies performed in kidney that found reduced NPR-A coupled cGMP generation despite increased gene expression39 and unaltered or slightly reduced protein expression40. Augmented PDE5 activity has been linked to renal and lung NP desensitization41, 42 and our findings now support this in the diseased heart as well. Impaired PKG activity linked to upregulated and retargeted PDE5 could further impair ANP signaling by suppressing the PKG-dependent phosphorylation/activation of the NPRA itself43.
Retargeting of cyclic nucleotide signaling is not unique to the cGMP/PKG pathway, as studies have also reported changes in cAMP signaling under disease or pharmacogical conditions. For example, Nikolaev et al44 found β2-ARs that normally lie within deep transverse-tubular membranes in the cardiac myocyte shifted to surface membrane crest territories where β1-AR are usually found, in the diseased heart. This was coupled with loss of membrane containment of the cAMP signal, perhaps due to a change in the PDE microenvironment. PDE4A4 has been observed to move into accretion foci upon binding of the inhibitor rolipram to its catalytic site45, although to our knowledge, retargeting of PDE4 as a component of heart disease has not been reported.
Movement and functional retargeting of PDE5 can be added to other members of the PKG signalosome that also move with cardiac stress. For example, Gq-agonist stimulation in vascular smooth muscle46 and cardiac myocytes47 results in rapid migration of PKG1α from the cytosol to plasma membrane, coupled to activation and co-migration of regulator of G-protein signaling2. This blunts the Gq-stimulus, and appears coupled to early therapeutic efficacy of sildenafil to counter pressure-overload47. More recently, soluble GC was observed to translocate from caveolin-3 enriched membrane domains into non-lipid raft domains23. This had functional significance, as sGC in the latter was more oxidized and thus far less responsive to NO than that within Cav-3 domains, and pressure-overload induced a shift out of the latter, contributing to reduced NO responsiveness.
Study Limitations
The proteins involved with PDE5 migration remain unknown. One of the difficulties of studying this is that adult myocytes are required – as the compartmentation is lacking in the neonatal cells. Using the PDE5tg-model to enhance pull down assays, we attempted a broad proteomic screen to define the interactome, and proteomic analysis with 2-D gel electrophoresis and mass spectroscopy revealed several heat shock proteins (HSC70 and HSP27) that were confirmed by IP. However, given the multitude of protein interactions, their role in localization is difficult to define. This analysis remains a work in progress.
While our models excluded NOS3 stimulation of sGC as a source of cGMP, it did not eliminate NOS1 or NOS2. However, neither were augmented in models lacking NOS3 before or after TAC, and their expression did not correlate with cGMP or PKG activity. Current data suggests myocyte NOS1 largely signals via cGMP-independent pathways48, and though its decline with TAC could exacerbate disease and oxidative stress49, differences among groups did not reach significance. As shown in prior work, NOS2 does not seem a major modulator of TAC-induced disease50. Lastly, we recognize that N3− mice reflect an embryonic knockout that may induce adaptions to alter signaling. However, prior studies regarding PDE5 regulation of the β-AR cascade have revealed behavior very similar between N3− and WT controls administered L-NAME.
Conclusion
In conclusion, we have shown that upregulation of PDE5 in conjunction with pressure-overload stress and accompanying hypertrophy/dilation results in a change in the protein’s myocyte sub-cellular distribution, retargeting from cGMP generated by NO-dependent sGC to that from NP-derived rGC. This likely plays a key role in the pathophysiological consequences of increased PDE5 expression observed in experimental and human heart disease, and also contributes to the ameliorative effects of its inhibition in heart diseases where NOS and sGC activity are impaired.
Supplementary Material
Clinical Summary.
Phosphodiesterase type 5 (PDE5) hydrolyzes cyclic GMP and its inhibition is widely used to treat erectile dysfunction and pulmonary hypertension. Recent studies have expanded its role to the cardiomyocyte, as PDE5 inhibitors also suppress remodeling and improve function in heart diseases such as hypertrophy, infarction, and dilated heart failure. These preclinical data have spawned clinical trials in heart failure. In the normal myocyte, PDE5 degrades cGMP generated by nitric-oxide stimulated soluble guanylate cyclase but has virtually no impact on natriuretic peptide (NP) derived cGMP. Yet in heart failure, cGMP from NO-signaling declines whereas NP-generated cGMP rises. This poses a paradox for the inhibitor studies, since if normal PDE5 cGMP-targeting persisted in heart failure, its impact and efficacy of inhibitors should both decline. Here we reveal an explanation, showing in the hypertrophied/dilated heart, PDE5 is retargeted to degrade NP-cGMP. Even in mice in which NOS3 is genetically deleted to remove the normal source of PDE5-targeted cGMP, upregulated myocyte PDE5 still worsened hypertrophy and LV dilation/dysfunction to pressure-overload coupled to increased NP-cGMP hydrolysis. This was associated with physical displacement of PDE5 from its normal sarcomere localization to a diffuse cytosolic distribution. Enhanced myocyte PDE5 expression also stimulated reactive oxygen species (ROS). However, this was not required for its pathological effects, as ROS was greatly reduced in mice with co-deletion of NOS3 despite similar adverse remodeling to pressure-overload. PDE5 retargeting likely contributes to cardiac NP-desensitization, and suggests a combined benefit from PDE5 inhibition and NP stimulation in cardiac disease.
Acknowledgments
Funding Sources: This work was supported by National Institute of Health Grants HL-089297, HL-093432, T32-HL-07227, HHS268201000032C-3-0-1 (The Johns Hopkins Proteomic Innovation Center in Heart Failure) American Heart Association Fellowships (MZ, DL), British Heart Foundation grants RG/08/011/25922 and RE/08/003, and a Leducq Foundation Transatlantic Network of Excellence
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: None
References
- 1.Francis SH, Busch JL, Corbin JD, Sibley D. Cgmp-dependent protein kinases and cgmp phosphodiesterases in nitric oxide and cgmp action. Pharmacol Rev. 2010;62:525–563. doi: 10.1124/pr.110.002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takimoto E, Champion HC, Belardi D, Moslehi J, Mongillo M, Mergia E, Montrose DC, Isoda T, Aufiero K, Zaccolo M, Dostmann WR, Smith CJ, Kass DA. Cgmp catabolism by phosphodiesterase 5a regulates cardiac adrenergic stimulation by nos3-dependent mechanism. Circ Res. 2005;96:100–109. doi: 10.1161/01.RES.0000152262.22968.72. [DOI] [PubMed] [Google Scholar]
- 3.Das A, Xi L, Kukreja RC. Phosphodiesterase-5 inhibitor sildenafil preconditions adult cardiac myocytes against necrosis and apoptosis. Essential role of nitric oxide signaling. J Biol Chem. 2005;280:12944–12955. doi: 10.1074/jbc.M404706200. [DOI] [PubMed] [Google Scholar]
- 4.Lu Z, Xu X, Hu X, Lee S, Traverse JH, Zhu G, Fassett J, Tao Y, Zhang P, Dos RC, Pritzker M, Hall JL, Garry DJ, Chen Y. Oxidative stress regulates left ventricular pde5 expression in the failing heart. Circulation. 2010;121:1474–1483. doi: 10.1161/CIRCULATIONAHA.109.906818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pokreisz P, Vandenwijngaert S, Bito V, Van den BA, Lenaerts I, Busch C, Marsboom G, Gheysens O, Vermeersch P, Biesmans L, Liu X, Gillijns H, Pellens M, Van Lommel A, Buys E, Schoonjans L, Vanhaecke J, Verbeken E, Sipido K, Herijgers P, Bloch KD, Janssens SP. Ventricular phosphodiesterase-5 expression is increased in patients with advanced heart failure and contributes to adverse ventricular remodeling after myocardial infarction in mice. Circulation. 2009;119:408–416. doi: 10.1161/CIRCULATIONAHA.108.822072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takimoto E, Champion HC, Li M, Belardi D, Ren S, Rodriguez ER, Bedja D, Gabrielson KL, Wang Y, Kass DA. Chronic inhibition of cyclic gmp phosphodiesterase 5a prevents and reverses cardiac hypertrophy. Nat Med. 2005;11:214–222. doi: 10.1038/nm1175. [DOI] [PubMed] [Google Scholar]
- 7.Zhang M, Takimoto E, Hsu S, Lee DI, Nagayama T, Danner T, Koitabashi N, Barth AS, Bedja D, Gabrielson KL, Wang Y, Kass DA. Myocardial remodeling is controlled by myocyte-targeted gene regulation of phosphodiesterase type 5. J Am Coll Cardiol. 2010;56:2021–2030. doi: 10.1016/j.jacc.2010.08.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guazzi M, Vicenzi M, Arena R, Guazzi MD. Pde5 inhibition with sildenafil improves left ventricular diastolic function, cardiac geometry, and clinical status in patients with stable systolic heart failure: Results of a 1-year, prospective, randomized, placebo-controlled study. Circ Heart Fail. 2011;4:8–17. doi: 10.1161/CIRCHEARTFAILURE.110.944694. [DOI] [PubMed] [Google Scholar]
- 9.Nagendran J, Archer SL, Soliman D, Gurtu V, Moudgil R, Haromy A, St Aubin C, Webster L, Rebeyka IM, Ross DB, Light PE, Dyck JR, Michelakis ED. Phosphodiesterase type 5 is highly expressed in the hypertrophied human right ventricle, and acute inhibition of phosphodiesterase type 5 improves contractility. Circulation. 2007;116:238–248. doi: 10.1161/CIRCULATIONAHA.106.655266. [DOI] [PubMed] [Google Scholar]
- 10.Shan X, Quaile MP, Monk JK, French B, Cappola TP, Margulies KB. Differential expression of pde5 in failing and non-failing human myocardium. Circ Heart Fail. 2012;5:79–86. doi: 10.1161/CIRCHEARTFAILURE.111.961706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guazzi M, Samaja M, Arena R, Vicenzi M, Guazzi MD. Long-term use of sildenafil in the therapeutic management of heart failure. J Am Coll Cardiol. 2007;50:2136–2144. doi: 10.1016/j.jacc.2007.07.078. [DOI] [PubMed] [Google Scholar]
- 12.Evaluating the effectiveness of sildenafil at improving health outcomes and exercise ability in people with diastolic heart failure (the relax study) clinicaltrials.Gov: Nct00763867
- 13.Revatio for heart disease in duchenne muscular dystrophy and becker muscular dystrophy (reverse-dbmd) clinicaltrials.Gov: Nct01168908
- 14.Guazzi M, Tumminello G, Di Marco F, Fiorentini C, Guazzi MD. The effects of phosphodiesterase-5 inhibition with sildenafil on pulmonary hemodynamics and diffusion capacity, exercise ventilatory efficiency, and oxygen uptake kinetics in chronic heart failure. J Am Coll Cardiol. 2004;44:2339–2348. doi: 10.1016/j.jacc.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 15.Lewis GD, Lachmann J, Camuso J, Lepore JJ, Shin J, Martinovic ME, Systrom DM, Bloch KD, Semigran MJ. Sildenafil improves exercise hemodynamics and oxygen uptake in patients with systolic heart failure. Circulation. 2007;115:59–66. doi: 10.1161/CIRCULATIONAHA.106.626226. [DOI] [PubMed] [Google Scholar]
- 16.Derbyshire ER, Marletta MA. Structure and regulation of soluble guanylate cyclase. Annu Rev Biochem. 2012;81:533–559. doi: 10.1146/annurev-biochem-050410-100030. [DOI] [PubMed] [Google Scholar]
- 17.Potter LR. Guanylyl cyclase structure, function and regulation. Cell Signal. 2011;23:1921–1926. doi: 10.1016/j.cellsig.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takimoto E, Belardi D, Tocchetti CG, Vahebi S, Cormaci G, Ketner EA, Moens AL, Champion HC, Kass DA. Compartmentalization of cardiac beta-adrenergic inotropy modulation by phosphodiesterase type 5. Circulation. 2007;115:2159–2167. doi: 10.1161/CIRCULATIONAHA.106.643536. [DOI] [PubMed] [Google Scholar]
- 19.Castro LR, Verde I, Cooper DM, Fischmeister R. Cyclic guanosine monophosphate compartmentation in rat cardiac myocytes. Circulation. 2006;113:2221–2228. doi: 10.1161/CIRCULATIONAHA.105.599241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagayama T, Zhang M, Hsu S, Takimoto E, Kass DA. Sustained soluble guanylate cyclase stimulation offsets nitric-oxide synthase inhibition to restore acute cardiac modulation by sildenafil. J Pharmacol Exp Ther. 2008;326:380–387. doi: 10.1124/jpet.108.137422. [DOI] [PubMed] [Google Scholar]
- 21.Stangherlin A, Gesellchen F, Zoccarato A, Terrin A, Fields LA, Berrera M, Surdo NC, Craig MA, Smith G, Hamilton G, Zaccolo M. Cgmp signals modulate camp levels in a compartment-specific manner to regulate catecholamine-dependent signaling in cardiac myocytes. Circ Res. 2011;108:929–939. doi: 10.1161/CIRCRESAHA.110.230698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takimoto E, Champion HC, Li M, Ren S, Rodriguez ER, Tavazzi B, Lazzarino G, Paolocci N, Gabrielson KL, Wang Y, Kass DA. Oxidant stress from nitric oxide synthase-3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J Clin Invest. 2005;115:1221–1231. doi: 10.1172/JCI21968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsai EJ, Liu Y, Koitabashi N, Bedja D, Danner T, Jasmin JF, Lisanti MP, Friebe A, Takimoto E, Kass DA. Pressure-overload-induced subcellular relocalization/oxidation of soluble guanylyl cyclase in the heart modulates enzyme stimulation. Circ Res. 2012;110:295–303. doi: 10.1161/CIRCRESAHA.111.259242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: From marvel to menace. Circulation. 2006;113:1708–1714. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- 25.Laurindo FR, Fernandes DC, Santos CX. Assessment of superoxide production and nadph oxidase activity by hplc analysis of dihydroethidium oxidation products. Methods Enzymol. 2008;441:237–260. doi: 10.1016/S0076-6879(08)01213-5. [DOI] [PubMed] [Google Scholar]
- 26.Gyurko R, Kuhlencordt P, Fishman MC, Huang PL. Modulation of mouse cardiac function in vivo by enos and anp. Am J Physiol Heart Circ .Physiol. 2000;278:H971–H981. doi: 10.1152/ajpheart.2000.278.3.H971. [DOI] [PubMed] [Google Scholar]
- 27.Senzaki H, Smith CJ, Juang GJ, Isoda T, Mayer SP, Ohler A, Paolocci N, Tomaselli GF, Hare JM, Kass DA. Cardiac phosphodiesterase 5 (cgmp-specific) modulates beta-adrenergic signaling in vivo and is down-regulated in heart failure. FASEB J. 2001;15:1718–1726. doi: 10.1096/fj.00-0538com. [DOI] [PubMed] [Google Scholar]
- 28.Wilson LS, Elbatarny HS, Crawley SW, Bennett BM, Maurice DH. Compartmentation and compartment-specific regulation of pde5 by protein kinase g allows selective cgmp-mediated regulation of platelet functions. Proc Natl Acad Sci U S A. 2008;105:13650–13655. doi: 10.1073/pnas.0804738105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McConnachie G, Langeberg LK, Scott JD. Akap signaling complexes: Getting to the heart of the matter. Trends Mol Med. 2006;12:317–323. doi: 10.1016/j.molmed.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 30.Mika D, Leroy J, Vandecasteele G, Fischmeister R. Pdes create local domains of camp signaling. J Mol Cell Cardiol. 2012;52:323–329. doi: 10.1016/j.yjmcc.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 31.Houslay MD. Underpinning compartmentalised camp signalling through targeted camp breakdown. Trends Biochem Sci. 2010;35:91–100. doi: 10.1016/j.tibs.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 32.Nikolaev VO, Bunemann M, Schmitteckert E, Lohse MJ, Engelhardt S. Cyclic amp imaging in adult cardiac myocytes reveals far-reaching beta1-adrenergic but locally confined beta2-adrenergic receptor-mediated signaling. Circ Res. 2006;99:1084–1091. doi: 10.1161/01.RES.0000250046.69918.d5. [DOI] [PubMed] [Google Scholar]
- 33.Baillie GS, Sood A, McPhee I, Gall I, Perry SJ, Lefkowitz RJ, Houslay MD. Beta-arrestin-mediated pde4 camp phosphodiesterase recruitment regulates beta-adrenoceptor switching from gs to gi. Proc Natl Acad Sci U S A. 2003;100:940–945. doi: 10.1073/pnas.262787199. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Lehnart SE, Wehrens XH, Reiken S, Warrier S, Belevych AE, Harvey RD, Richter W, Jin SL, Conti M, Marks AR. Phosphodiesterase 4d deficiency in the ryanodine-receptor complex promotes heart failure and arrhythmias. Cell. 2005;123:25–35. doi: 10.1016/j.cell.2005.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee DI, Vahebi S, Tocchetti CG, Barouch LA, Solaro RJ, Takimoto E, Kass DA. Pde5a suppression of acute beta-adrenergic activation requires modulation of myocyte beta-3 signaling coupled to pkg-mediated troponin i phosphorylation. Basic Res Cardiol. 2010;105:337–347. doi: 10.1007/s00395-010-0084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagayama T, Hsu S, Zhang M, Koitabashi N, Bedja D, Gabrielson K, Takimoto E, Kass DA. Sildenafil stops progressive chamber, cellular, and molecular remodeling and improves calcium handling and function in hearts with pre-existing advanced hypertrophy due to pressure-overload. J Am Coll Cardiol. 2009;53:207–215. doi: 10.1016/j.jacc.2008.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holtwick R, van Eickels M, Skryabin BV, Baba HA, Bubikat A, Begrow F, Schneider MD, Garbers DL, Kuhn M. Pressure-independent cardiac hypertrophy in mice with cardiomyocyte-restricted inactivation of the atrial natriuretic peptide receptor guanylyl cyclase-a. J Clin .Invest. 2003;111:1399–1407. doi: 10.1172/JCI17061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kinoshita H, Kuwahara K, Nishida M, Jian Z, Rong X, Kiyonaka S, Kuwabara Y, Kurose H, Inoue R, Mori Y, Li Y, Nakagawa Y, Usami S, Fujiwara M, Yamada Y, Minami T, Ueshima K, Nakao K. Inhibition of trpc6 channel activity contributes to the antihypertrophic effects of natriuretic peptides-guanylyl cyclase-a signaling in the heart. Circ Res. 2010;106:1849–1860. doi: 10.1161/CIRCRESAHA.109.208314. [DOI] [PubMed] [Google Scholar]
- 39.Brown LA, Nunez DJ, Wilkins MR. Differential regulation of natriuretic peptide receptor messenger rnas during the development of cardiac hypertrophy in the rat. J Clin Invest. 1993;92:2702–2712. doi: 10.1172/JCI116887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bryan PM, Xu X, Dickey DM, Chen Y, Potter LR. Renal hyporesponsiveness to atrial natriuretic peptide in congestive heart failure results from reduced atrial natriuretic peptide receptor concentrations. Am J Physiol Renal Physiol. 2007;292:F1636–F1644. doi: 10.1152/ajprenal.00418.2006. [DOI] [PubMed] [Google Scholar]
- 41.Margulies KB, Burnett JC., Jr Inhibition of cyclic gmp phosphodiesterases augments renal responses to atrial natriuretic factor in congestive heart failure. J Card Fail. 1994;1:71–80. doi: 10.1016/1071-9164(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 42.Forfia PR, Lee M, Tunin RS, Mahmud M, Champion HC, Kass DA. Acute phosphodiesterase 5 inhibition mimics hemodynamic effects of b-type natriuretic peptide and potentiates b-type natriuretic peptide effects in failing but not normal canine heart. J Am Coll Cardiol. 2007;49:1079–1088. doi: 10.1016/j.jacc.2006.08.066. [DOI] [PubMed] [Google Scholar]
- 43.Castro LR, Schittl J, Fischmeister R. Feedback control through cgmp-dependent protein kinase contributes to differential regulation and compartmentation of cgmp in rat cardiac myocytes. Circ Res. 2010;107:1232–1240. doi: 10.1161/CIRCRESAHA.110.226712. [DOI] [PubMed] [Google Scholar]
- 44.Nikolaev VO, Moshkov A, Lyon AR, Miragoli M, Novak P, Paur H, Lohse MJ, Korchev YE, Harding SE, Gorelik J. Beta2-adrenergic receptor redistribution in heart failure changes camp compartmentation. Science. 2010;327:1653–1657. doi: 10.1126/science.1185988. [DOI] [PubMed] [Google Scholar]
- 45.Terry R, Cheung YF, Praestegaard M, Baillie GS, Huston E, Gall I, Adams DR, Houslay MD. Occupancy of the catalytic site of the pde4a4 cyclic amp phosphodiesterase by rolipram triggers the dynamic redistribution of this specific isoform in living cells through a cyclic amp independent process. Cellular Signal. 2003;15:955–971. doi: 10.1016/s0898-6568(03)00092-5. [DOI] [PubMed] [Google Scholar]
- 46.Tang KM, Wang GR, Lu P, Karas RH, Aronovitz M, Heximer SP, Kaltenbronn KM, Blumer KJ, Siderovski DP, Zhu Y, Mendelsohn ME. Regulator of g-protein signaling-2 mediates vascular smooth muscle relaxation and blood pressure. Nat .Med. 2003;9:1506–1512. doi: 10.1038/nm958. [DOI] [PubMed] [Google Scholar]
- 47.Takimoto E, Koitabashi N, Hsu S, Ketner EA, Zhang M, Nagayama T, Bedja D, Gabrielson KL, Blanton R, Siderovski DP, Mendelsohn ME, Kass DA. Regulator of g protein signaling 2 mediates cardiac compensation to pressure overload and antihypertrophic effects of pde5 inhibition in mice. J Clin Invest. 2009;119:408–420. doi: 10.1172/JCI35620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang YH, Casadei B. Sub-cellular targeting of constitutive nos in health and disease. J Mol Cell Cardiol. 2012;52:341–350. doi: 10.1016/j.yjmcc.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 49.Otani H. The role of nitric oxide in myocardial repair and remodeling. Antioxid Redox Signal. 2009;11:1913–1928. doi: 10.1089/ars.2009.2453. [DOI] [PubMed] [Google Scholar]
- 50.Hataishi R, Rodrigues AC, Morgan JG, Ichinose F, Derumeaux G, Bloch KD, Picard MH, Scherrer-Crosbie M. Nitric oxide synthase 2 and pressure-overload-induced left ventricular remodelling in mice. Exp Physiol. 2006;91:633–639. doi: 10.1113/expphysiol.2005.033068. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.