Abstract
Angiotensin I-converting enzyme (ACE; kininase II) levels in humans are genetically determined. ACE levels have been linked to risk of myocardial infarction, but the association has been inconsistent, and the causality underlying it remains undocumented. We tested the hypothesis that genetic variation in ACE levels influences myocardial tolerance to ischemia. We studied ischemia-reperfusion injury in mice bearing 1 (ACE1c), 2 (ACE2c, wild type), or 3 (ACE3c) functional copies of the ACE gene and displaying an ACE level range similar to humans. Infarct size in ACE1c was 29% lower than in ACE2c (P<0.05). Pretreatment with a kinin B2 receptor antagonist suppressed this reduction. In ACE3c, infarct size was the same as in ACE2c. But ischemic preconditioning, which reduced infarct size in ACE2c (−63%, P<0.001) and ACE1c (−52%, P<0.05), was not efficient in ACE3c (−2%, NS, P<0.01 vs. ACE2c). In ACE3c, ischemic preconditioning did not decrease myocardial inflammation or cardiomyocyte apoptosis. Pretreatment with a renin inhibitor had no cardioprotective effect in ACE2c, but in ACE3c partially restored (38%) the cardioprotection of ischemic preconditioning. Thus, a modest genetic increase in ACE impairs myocardial tolerance to ischemia. ACE level plays a critical role in cardiac ischemia, through both kinin and angiotensin mediated mechanisms.—Messadi, E., Vincent, M.-P., Griol-Charhbili, V., Mandet, C., Colucci, J., Krege, J. H., Bruneval, P., Bouby, N., Smithies, O., Alhenc-Gelas, F., Richer, C. Genetically determined angiotensin converting enzyme level and myocardial tolerance to ischemia.
Keywords: renin inhibition, kinins, genetically modified mice
Angiotensin converting enzyme (ACE; kininase II) plays an important role in cardiovascular homeostasis by catalyzing the conversion of inactive angiotensin I into active angiotensin II and the inactivation of bradykinin (1). ACE is an ectoenzyme of vascular endothelial cells, also released into the circulation on cleavage of its membrane anchor. ACE levels vary largely among subjects. These levels are under strong genetic influence. Studies in nuclear families have documented genetic transmission of plasma ACE levels (2). Polymorphism of the ACE gene, especially an insertion/deletion (I/D) polymorphism located in intron 16, has been associated with plasma levels of ACE (3). ACE concentrations in tissues are also genetically determined, and associated with the ACE gene I/D polymorphism, including in the heart (4, 5).
Variation in plasma and tissue ACE concentration was generally considered to have no or little consequence for cardiovascular homeostasis, contrary to variation in renin secretion, because of high availability of endothelial ACE for angiotensin I conversion. However, this concept has been challenged following observation of heterogeneity in vascular ACE content among peripheral organs. In the kidney and the heart, endothelial ACE content is low, and moderate variation in ACE levels might influence the rate of angiotensin II formation (6, 7). In addition, ACE is the main enzyme inactivating kinins in the circulation. Theoretical and experimental evidence suggests that the ACE level influences kinin concentration (8).
In pathological situations, angiotensin II formation and/or kinin depletion may play a deleterious role, resulting in heart, artery, or kidney damage during hemodynamic, ischemic, or metabolic injury (6, 9). The effect of genetic variation in ACE levels on prognosis of cardiac and renal diseases has been studied in populations and cohorts of patients. Thus, genetic ACE levels are causally linked to severity of renal involvement in type 1 diabetes. Association between ACE levels and diabetic nephropathy has been replicated in major clinical studies, and causality between genetically high ACE levels and diabetic renal damage has been documented in genetically modified mice (10, 11). The ACE gene polymorphism and ACE levels have also been associated with risk of myocardial infarction (12, 13). Individuals harboring the deletion allele of this gene and having high ACE levels have been found to have an increased prevalence of myocardial infarction (11–15). However, association of the ACE gene with myocardial infarction has not been consistent across all studies (16, 17). In addition, the mechanism underlying the association remains unclear and causality has not been addressed.
As an attempt to clarify the effect of genetic variability in ACE levels in ischemic heart disease, we used genetically engineered mice carrying either an inactivation or a duplication of the ACE gene that we submitted to myocardial ischemia reperfusion (IR) injury and ischemic preconditioning (IPC). These mice display an ACE level range similar to humans (10, 18). IR injury has relevance to the human disease, and several studies have documented the role of at least one ACE substrate, kinins, in myocardial tolerance to ischemia (19–22). We especially studied the effect of a modest genetic increase in ACE levels above physiological value on outcome of experimental cardiac ischemia, and assessed the mechanism underlying this effect.
MATERIALS AND METHODS
Generation and characterization of mice with 1–3 copies of the ACE gene
Genetically engineered mice carrying either an inactivation or a duplication of the ACE gene on chromosome 11 were used for the study (18). Adult male mice carrying either 1 (ACE1c), 2 (ACE2c, identical to wild type), or 3 (ACE3c) functional ACE gene copies were studied. All the experimental procedures were performed in accordance with the European regulations for the care and use of laboratory animals (L 358-86/609/EEC) and were approved by the Université Paris Animal Care and Use Committee.
Blood pressure response to angiotensin I and angiotensin II
Blood pressure changes triggered by angiotensin II (300 ng/kg) or increasing doses of angiotensin I (0.3–30 μg/kg) (1 μl/g body weight bolus injected at 5-min intervals via the jugular vein) were measured in pentobarbital-anesthetized ACE1c (n=5–9), ACE2c (n=7–15), or ACE3c (n=7–10). At sacrifice, the animals were used for quantification of plasma ACE activity and ACE mRNA in the lungs and hearts.
Quantification of plasma ACE activity and ACE mRNA abundance in the lung and heart
Plasma ACE activity was measured spectrophotometrically with hippuryl-histidine-leucine as substrate (23). Relative changes in gene expression of ACE in heart and lung were quantified using real-time PCR (24).
Total RNA was isolated from the heart and lung (Trizol; Invitrogen/Life Technologies, Cergy Pontoise, France). cDNA was synthesized from 2 μg total RNA using 1 μg random hexamers and 40 U Superscript II MMLV-reverse transcriptase (Invitrogen/Life Technologies) in the presence of 200 U RNaseOUT in a 20 μl final volume. Real-time PCR was performed on the ABI PRISM 7000 Sequence Detection System by using SYBR green PCR Master Mix and assay-on-demand gene expression probes (Applied Biosystems; Applera France, Courtaboeuf, France). Reverse transcribed RNA (15 ng) was submitted to PCR in a 20-μl final volume. Each sample was tested in triplicate. DNA contamination was excluded by performing PCR amplification without the RT step for each RNA sample, and blank without sample but with all reagents. Data were normalized to GAPDH mRNA (24). Changes in the target gene relative to the mean expression in the wild-type control group were calculated by the 2−ΔΔCt comparative method for each sample (25).
In vivo mouse model of myocardial IR injury and IPC: effect of ACE gene titration
Surgical preparation
Mice were anesthetized with sodium pentobarbital (60 mg/kg, i.p.). The animals were intubated and ventilated with 100% oxygen (200 μl/breath at a rate of 170 breaths/min), using a Harvard rodent ventilator (Model 845, Harvard Apparatus, Les Ulis, France). Drugs were administered via a catheter inserted into the jugular vein. Body temperature was monitored with a rectal probe connected to a digital thermometer, and maintained at 37°C using a heating pad. The electrocardiogram (ECG) was recorded throughout the experiments on a Gould TA240 recorder (ECG biotech; Gould Instruments, Cleveland, OH, USA). A left thoracotomy was performed to expose the heart, and the pericardium was removed. The left anterior descending coronary artery was occluded with an 8.0 prolene suture, 2 mm from the tip of the left atrium for 30 min. Successful coronary occlusion was verified by the development of a pale color in the distal myocardium and by ST segment elevation and QRS widening on the ECG. After 30 min of sustained ischemia, coronary blood flow was restored by loosening the suture. Successful reperfusion was confirmed by visualization of hyperaemic response and restoration of normal ECG. The lungs were then reinflated by increasing positive end expiratory pressure, and the chest was closed. Reperfusion was maintained for a 3-h period (19). IPC was produced by a sequence of 3 successive cycles, each consisting of 3 min of coronary occlusion followed by 5 min of reperfusion, according to Yang et al. (26). Immediately after IPC, all the animals were subjected to IR injury (30 min of coronary occlusion followed by reperfusion). ACE1c, ACE2c (wild type), or ACE3c mice were subjected to the same myocardial IR, alone (ACE1c, n=8; ACE2c, n=12; ACE3C, n=8) or after IPC (ACE1c, n=8; ACE2c, n=14; ACE3C n=9).
Measurement of infarct size (IS)
After reperfusion, the chest was reopened, the coronary artery was reoccluded, and 0.5 ml of a 5% Evans blue solution was injected as a bolus into the jugular vein in order to delineate the area at risk (AR), which remained unstained by the Evans blue. The heart was excised, and the left ventricle (LV) was isolated, weighed, and sliced into 4 transverse pieces from base to apex, the first cutter blade being positioned at the site of the coronary occlusion. The slices were weighed, and color digital images of both sides of each slice were obtained with a Power Shot S50 zoom digital camera (Canon, Tokyo, Japan) connected to a microscope (Leica MZ 75; Leica Microsystems, Rueil-Malmaison, France), using the Adobe Photoshop software (Adobe Systems, San Jose, CA, USA). The slices were then incubated at 37°C with buffered 1% 2,3,5-triphenyltetrazolium chloride (TTC) solution for 20 min. Viable myocardium, which contained dehydrogenases, reacted with TTC and was stained brick red, whereas any necrotic tissue remained unstained due to the lack of active enzymes. The tissue sections were then fixed in a buffered 10% formalin solution for 24 h before being photographed again to delineate the IS (19). The cross-sectional area, the lumen area, the AR (unstained by Evans blue), and IS (unstained by TTC) of the LV were outlined on each color image and quantified by a masked observer using the Scion Image software (Scion Image for Windows; http://www.scioncorp.com). The absolute weights of AR and IS were then calculated for each slice. The sum of the absolute weight values of AR and IS of the 3 ischemic slices of each heart was calculated and expressed as a percentage of the total weight of the slice. The ratio of IS to AR was calculated from these absolute weight evaluations and expressed as a percentage of AR.
Plasma troponin determination
As an additional readout for myocardial infarct severity, we measured cardiac TroponinI (cTnI) levels in the plasma of mice with or without IPC after a 24-h reperfusion period so that IR lesions were full blown at this time (n=6–13/group). Blood samples were obtained from carotid artery at the end of reperfusion. Plasma cTnI levels were determined with a mouse cardiac quantitative cTnI assay (Life Diagnostics, West Chester, PA, USA) (27). Then, the hearts were excised and fixed for AR and IS determination, and assessment of myocardial apoptosis and inflammation.
Histological analysis of the heart: assessment of myocardial apoptosis and inflammation
The hearts were divided into several slices, apex and upper-medium slices, for paraffin embedding. Paraffin sections were stained with hematoxylin-eosin (H&E) for histological determination of necrosis. Myocardial apoptosis was assessed by the terminal deoxynucleotidyltransferase (TdT)-mediated dUTP nick-end labeling (TUNEL) technique (ApoTag kit; Chemicon-Millipore, Molsheim, France) as described previously (28).The percentage of positive apoptotic nuclei in cardiomyocytes was counted along the whole border zone surrounding the myocardial infarction with a light microscope at ×40 in the 2 sections from the heart of each mouse. The mean percentage of apoptotic cells per group was calculated. For inflammation, leukocyte infiltration was detected by immunohistochemistry using an anti-mouse CD45 antibody (BD Biosciences-Pharmingen, San Diego, CA, USA) reacting against most of leukocytes, polymorphonuclears being prominent at 24 h reperfusion time. The CD45 labeling was evaluated semiquantitatively in the infarcted area of the LV using a score from 0 (no leukocyte infiltration) to 5 (highest leukocyte infiltration in density and extension) with a light microscope at ×10 in the 2 sections from the heart of each mouse. The mean inflammation score per group was calculated.
Role of kinins in the cardioprotective effect of low ACE level: effect of B2 receptor blockade by icatibant
To investigate the role of kinins through the B2 receptor activation in the cardioprotective effect observed in ACE1c mice after IR, additional ACE1c (n=12) and ACE2c (n=17) mice were pretreated with the B2 receptor antagonist icatibant (HOE140; Aventis Pharma Deutschland GmbH, Frankfurt, Germany; 500 μg/kg i.v.) (ACE1c, n=4; ACE2c, n=5) or with saline (ACE1c, n=8; ACE2c, n=12) 5 min before the onset of ischemia.
Role of angiotensin II in myocardial IR injury and in IPC: effect of renin inhibition by aliskiren
To investigate the role of angiotensin II in the loss of cardioprotection observed in ACE3c mice, additional experimental groups of ACE2c and ACE3c mice were subjected to either IR alone (ACE2c, n=19; ACE3c n=14), or IR after IPC (ACE2c, n=18; ACE3c, n=12). The mice were pretreated with aliskiren (Novartis Pharma AG, Basel, Switzerland), a direct renin inhibitor (1 mg/kg, i.v. bolus 5 min before starting ischemia followed by an infusion 10 μg/kg/min) (ACE2c, n=23; ACE3c, n=13). Other mice received saline (ACE2c, n=14; ACE3c, n=13).
Preliminary experiments were designed to determine the proper dose of aliskiren. In a first set of experiments, the blood pressure-lowering effect of increasing doses of aliskiren from 0.5 to 10 mg/kg was assessed in pentobarbital anesthetized wild-type mice (n=8/group). Blood pressure was recorded in these mice up to 45 min after aliskiren or saline administration, and the maximal blood pressure variations were determined. Blood samples were collected into heparinized tubes, and plasma renin activity (PRA) was determined by radioimmunoassay of angiotensin I generated after incubating the plasma for 1 h at pH 7.4 (29). In a second set of experiments, systemic and coronary and renal vascular effects of aliskiren administered at the previously determined dose (1 mg/kg, i.v. bolus 5 min before starting ischemia followed by an infusion 10 μg/kg/min), were quantified in anesthetized wild-type mice (n=6–7/group) using the fluorescent microsphere technique (30).
Statistical analysis
Results are expressed as means ± se. Comparisons between genotypes and between treatments in each genotype were performed by 2-way ANOVA followed by post hoc analysis using the JMP software system (JMP, SAS Institute Inc., Cary, NC, USA). The effects of icatibant or aliskiren were assessed by unpaired Student's t test. Angiotensin I-pressor response [variation in mean blood pressure (MBP) expressed as absolute value] curves in genotype groups were compared by ANOVA for repeated measurements with the Greenhouse-Geisser adjustment (31). AUCs for pressor responses vs. log dose angiotensin I were compared by ANOVA. Values of P < 0.05 were considered to be statistically significant.
RESULTS
Characterization of mice with 1–3 copies of the ACE gene
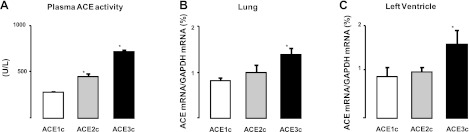
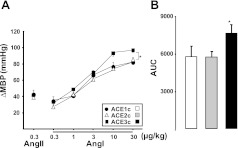
Plasma ACE activity was directly related to the ACE gene copy number (genotypic effect P<0.001, Fig. 1A) and ACE mRNA in the lung and in the LV were also dependent on ACE gene copy number (Fig. 1B, C; genotypic effect P<0.05). MBP and heart rate (HR) were not significantly affected by ACE gene copy number. ACE1c: MBP = 59.0 ± 7.8 mm Hg, HR = 365 ± 9 bpm; ACE2c: MBP = 59.2 ± 2.9 mm Hg, HR = 374 ± 14 bpm; and ACE3c: MBP = 65.0 ± 3.3 mm Hg, HR = 381 ± 13 bpm. The increases in blood pressure triggered by increasing doses of angiotensin I were significantly enhanced in ACE3c mice (P<0.05) and unaffected in ACE1c mice compared to ACE2c mice. Maximal pressor responses to angiotensin II did not significantly differ among the three genotype groups (Fig. 2).
Figure 1.
Plasma ACE activity (A, effect of genotype P<0.001) and ACE mRNA in lung (B, effect of genotype P<0.05) and LV (C, effect of genotype P<0.05) in ACE1c (n=9), ACE2c (n=10), or ACE3c (n=10) mice. Results are normalized to GAPDH mRNA level. Data are means ± se. *P < 0.05 vs. ACE2c.
Figure 2.
ACE gene titration and pressor responses to angiotensins. A) Maximal pressor responses (ΔMBP, absolute variation of MBP) to angiotensin I (Ang I) or angiotensin II (Ang II) in ACE1c, ACE2c, or ACE3c mice under basal conditions. Area under curve (AUC) of pressor responses (absolute increases in MBP) vs. log dose Ang I; data are means ± se. *P < 0.05 vs. ACE2c.
Effect of ACE gene titration on IR injury and IPC
There was no difference in body weight, LV weight, AR to LV ratio, or HR before occlusion of the coronary artery or after reperfusion between ACE1c, ACE2c, or ACE3c mice, regardless of whether they had been subjected to IPC (Table 1).
Table 1.
Basal characteristics of groups of mice with 1–3 copies of the ACE gene subsequently subjected to IR without or with IPC
| Genotype | Group | n | BW (g) | LVW/BW | AR (%LV) | HR (bpm) |
|
|---|---|---|---|---|---|---|---|
| Before occlusion | At reperfusion | ||||||
| ACE1c | IR | 8 | 31.3 ± 1.1 | 3.4 ± 0.2 | 26.8 ± 2.0 | 420 ± 18 | 446 ± 22 |
| IPC+IR | 8 | 32.9 ± 1.0 | 3.6 ± 0.1 | 26.2 ± 2.2 | 398 ± 16 | 413 ± 18 | |
| ACE2c | IR | 12 | 30.9 ± 0.7 | 3.6 ± 0.1 | 28.8 ± 1.6 | 340 ± 13 | 440 ± 15 |
| IPC+IR | 14 | 32.4 ± 0.6 | 3.8 ± 0.1 | 29.4 ± 1.2 | 411 ± 14 | 425 ± 17 | |
| ACE3c | IR | 8 | 29.5 ± 0.5 | 3.5 ± 0.1 | 27.1 ± 2.2 | 383 ± 19 | 405 ± 15 |
| IPC+IR | 9 | 29.7 ± 0.6 | 3.8 ± 0.1 | 27.9 ± 1.8 | 386 ± 18 | 437 ± 17 | |
Data are means ± se. BW, body weight; LVW/BW, left ventricle weight to body weight ratio; AR, area at risk; HR, heart rate; bpm, beats per minute.
Effect on IS
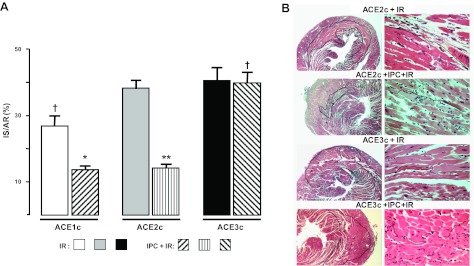
In basal conditions (IR without IPC), IS/AR in ACE1c (27 ± 3%) was significantly lower than IS/AR in ACE2c (38 ± 3%, −29%, P<0.05) but IS/AR in ACE3c (41 ± 4%) did not differ from that in ACE2c (Fig. 3).
Figure 3.
Effect of ACE gene titration on IR after IR injury without or with IPC. A) IR determined by TTC staining expressed as percentage of AR (IS/AR) was measured in ACE1c, ACE2c, or ACE3c mice. Data are means ± se. For n, see Table 1. *P < 0.05, **P < 0.001 vs. corresponding IR; †P < 0.05 vs. corresponding ACE2c. B) Representative H&E staining of cross sections of the hearts from ACE2c or ACE3c mice after ischemia and 24-h reperfusion period. Left panel: original view, ×2.5; black line surrounds infarcted area. Right panel: high-magnification view, ×40; black line shows border between living area (above line) and infarcted area (below line). ACE2c mice after IPC+IR had a smaller infarct compared to ACE2c mice with IR without IPC. ACE3c mice had a similar infarct after IPC+IR compared to ACE3c mice after IR without IPC.
After IPC (IPC+IR), a marked cardioprotective effect of IPC was observed in the IPC-ACE2c and IPC-ACE1c groups. IS/AR fell to 14 ± 1% (−63% vs. ACE2c without IPC, P<0.001) and 13 ± 1% (−52% vs. ACE1c without IPC, P<0.05, NS vs. IPC-ACE2c), respectively. In ACE3c mice, IPC no longer exerted a significant cardioprotective effect (40±3% after IPC vs. 41±4% without IPC, −2%, NS; Fig. 3A). The loss of the cardioprotection afforded by IPC in IPC-ACE3c mice was also documented by histology (Fig. 3B).
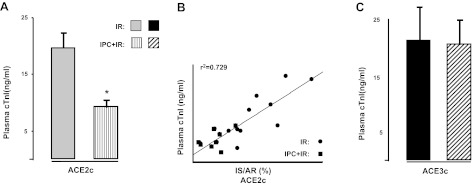
Effect on plasma troponin I levels
The loss of the cardioprotection afforded by IPC in IPC-ACE3c mice was further documented by measuring cTnI plasma levels. The cTnI plasma levels were significantly reduced by IPC in IPC-ACE2c mice (P<0.01), but not in IPC-ACE3c mice (Fig. 4).
Figure 4.
Measurement of cTnI plasma levels in ACE2c or ACE3c mice after ischemia and 24-h reperfusion period without IPC (IR) or with IPC (IPC+IR). A) IPC-ACE2c mice had significantly lower cTnI plasma levels compared to ACE2c mice without IPC. B) Correlation between IR (IS/AR) and cTnI plasma levels in ACE2c mice with or without IPC (r2= 0.729, P<0.01). C) IPC no longer affected cTnI plasma levels in IPC-ACE3c mice as compared to ACE3c mice without IPC. Data are means ± se. *P < 0.01 vs. corresponding IR.
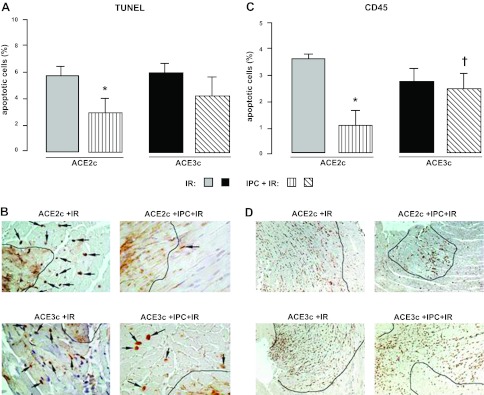
Apoptosis and inflammation
In ACE2c mice, IPC induced a reduction of 49% (P<0.05) in cardiomyocyte apoptosis compared to IR without IPC. No significant effect of IPC was observed in IPC-ACE3c (Fig. 5A, B).The number of CD45-positive cells in the infarcted area of the LV was reduced by IPC in IPC-ACE2c mice (−72% compared to IR-ACE2c, P<0.05), whereas no difference was found in IPC-ACE3c mice as compared to IR-ACE3c mice without IPC (Fig. 5C, D).
Figure 5.
Assessment of myocardial apoptosis and inflammation in ACE2c or ACE3c mice. Mice were studied after ischemia and a 24-h reperfusion period without IPC (IR) or with IPC (IPC+IR). A) Quantification of TUNEL-positive cardiomyocytes (%) in the border zone surrounding the myocardial infarction. Data are means ± se. *P < 0.05 vs. corresponding IR. B) Representative sections of hearts from ACE2c or ACE3c IR and IPC+IR mice showing the variable density of apoptic nuclei (arrows) in myocytes in the border zone. Black line delineates the infarcted area. TUNEL, original view ×40. C) Semiquantification of CD45-positive cells (score) in the infarcted area. Data are means ± se. *P < 0.05 vs. corresponding IR; †P < 0.05 vs. IPC-ACE2c. D) Representative sections of hearts from ACE2c or ACE3c IR and IPC+IR mice showing variable density of the leukocytes: inflammation is mainly limited to infarcted area. Immunohistochemistry with anti-CD45 antibody labeling all the types of leukocytes. Original view ×10.
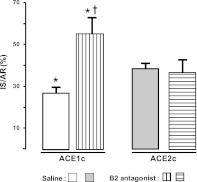
Mechanism of cardioprotection in ACE1c mice; role of kinins: effect of B2 receptor blockade by icatibant
Pretreatment with icatibant, a selective kinin B2 receptor antagonist, did not affect IS/AR in ACE2c mice, but completely suppressed the reduction in IS/AR reduction in pretreated ACE1c mice (Fig. 6).
Figure 6.
Role of kinins in cardioprotection in ACE1c mice after IR injury. IR expressed as percentage of AR (IS/AR) was measured in ACE1c or ACE2c mice receiving saline (ACE1c, n=8; ACE2c, n=12) or the specific kinin B2 receptor antagonist, icatibant (ACE1c, n=4; ACE2c, n=5). Data are means ± se. *P < 0.05 vs. saline-ACE2c; †P < 0.001 vs. corresponding saline.
Mechanism of loss of cardioprotection by IPC in ACE3c mice; role of angiotensins: effect of renin inhibition by alikiren
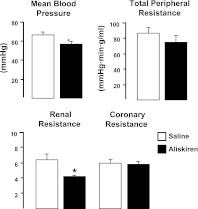
Hemodynamic effects of aliskiren
Preliminary experiments indicated that in wild-type anesthetized mice, aliskiren, a direct renin inhibitor, at the dose used, induced a mild hypotensive response that was maximal between 10 and 20 min after administration and averaged 15% 5 min after administration. Blood pressure and systemic, renal, and coronary vascular resistance were reduced by 15% (P<0.05), 18% (NS), 35% (P<0.001), and 11% (NS), respectively (Fig. 7). PRA was decreased by 60% (from 1304±104 to 516±46 pg/ml/h, P<0.001).
Figure 7.
Systemic and regional vascular effects of aliskiren in wild-type mice. MBP and total peripheral, renal, and coronary resistance were measured in anesthetized wild-type mice (n=6–7/group) using the fluorescent microsphere technique. Animals received saline or aliskiren (ALK, 1 mg/kg, i.v. bolus followed by an infusion 10 μg/kg/min). Data are means ± se. *P < 0.05 vs. saline.
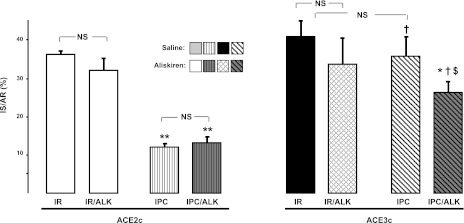
Effect of aliskiren on myocardial IR injury
After IR, pretreatment with aliskiren did not significantly affect IS/AR either in ACE2c or in ACE3c mice. However, after IPC, the loss of cardioprotection observed in IPC-ACE3c mice was partially restored by 38% in aliskiren-pretreated ACE3c mice (P<0.01, Fig. 8). Interestingly, no effect of aliskiren was observed in IPC-ACE2c mice (Fig. 8).
Figure 8.
Role of renin and angiotensins in loss of IPC in ACE3c mice. IR expressed as percentage of AR (IS/AR) was measured in ACE2c or ACE3c mice receiving saline or aliskiren (ALK). Data are means ± se. *P < 0.01, **P < 0.001 vs. corresponding IR + saline; †P < 0.001 vs. ACE2c after IPC + saline; $P < 0.01 vs. ACE2c after IPC + ALK.
DISCUSSION
We report here findings relating genetically determined ACE level to myocardial tolerance to ischemia. To do this, we used an original in vivo experimental model of IR injury in mice having 1, 2, or 3 functional copies of the ACE gene. Through backcrossing of the ACE gene mutations to an inbred mouse strain (C57BL/6), these mice differed only at the ACE locus, providing an opportunity to study changes in the ACE gene as a single experimental variable. Tissue pattern of ACE gene expression, and ACE gene regulation are maintained in the genetically modified mice allowing extrapolation to physiological situations. The mice displayed moderate differences in ACE mRNA level and an ACE activity range in plasma and tissue of the same order of magnitude as that found in humans (Fig. 1) (10, 18, 32, 33). This is the first study assessing the role of ACE level and its physiological variation in cardiac ischemia. This role, and the effect of a genetic increase in ACE activity, could not be inferred from data obtained with ACE inhibitors. We found that a genetic decrease in ACE activity reduced IS after IR, and that a genetic increase in ACE activity level did not worsen IR, but suppressed the cardioprotection afforded by IPC.
There were no preexisting cardiac hemodynamic or anatomical differences among the mice, which could have influenced the results of IR and IPC. Specifically, in agreement with previous observations (10, 18), blood pressure values measured under basal conditions were not affected by ACE gene copy number. We also found that HR, LV mass and morphology, and AR after IR or IPC+IR were not affected by ACE gene copy number. A similar observation has been made for cardiomyocyte size and morphology (33).
A genetically induced 35% decrease in ACE activity in ACE1c mice was sufficient to reduce IS by 29%, supporting the hypothesis that ACE level is an important determinant of myocardial tolerance to ischemia. In the ACE1c mice, reduced angiotensin II formation and increased kinin availability could both participate in this effect. Kinins are induced in the ischemic heart (22) along with the synthesis of kinin B1 and B2 receptor (19, 34, 35). The kinin system may protect against IR injury by eliciting coronary vasodilatation, inhibiting platelet aggregation, promoting fibrinolysis, and decreasing the production of free radicals, while other actions such as leukocyte recruitment and activation may not result in cardioprotection (19, 36, 37). The reduction of IS in ACE1c mice was completely suppressed by pretreatment with icatibant, a specific kinin B2 receptor antagonist. Potentiation of endogenous kinins through B2 receptor activation may thus be the most important pathway by which genetically determined decrease in ACE activity in the ACE1c mice mediates cardiac protection against ischemia. Interestingly, in icatibant-treated ACE1c mice submitted to IR, IS was higher than in ACE2c mice. This effect may be due to enhanced effect of vasoconstrictor agents in absence of kinin action (38). Our current findings in the ACE1c mice are consistent with observations made in wild-type mice treated with ACE inhibitors and submitted to IR (19, 39). However, data obtained after complete pharmacological ACE inhibition cannot be predictive of consequences of low amplitude physiological variation in ACE activity. The present study shows that a modest decrease in ACE activity is sufficient to influence the outcome of cardiac IR injury.
ACE3c mice that have genetically increased ACE levels did not show increased IS after IR compared to wild-type mice but did show that the beneficial consequence of IPC was lost. In ACE2c mice, it is possible that IS may already be maximal. Additionally, in the absence of IPC, kinin concentration and/or B2 receptor density may be insufficient to significantly affect IR. This is supported by previous studies documenting lack of effect of B2 receptor blockade in wild-type mice, an observation confirmed in the present study, or lack of effect of genetic deficiency in tissue kallikrein and kinins, in IR without IPC (19, 21). The reduction in IS afforded by IPC in ACE2c mice was not observed in ACE3c mice. In ACE3c mice, IPC did not reduce plasma troponin levels, myocardial apoptosis, or inflammation. This effect of a genetically increased ACE level on IPC could result from an increase in the conversion of angiotensin I to angiotensin II or from a decrease in the concentration of kinins. Some evidence suggests that kinin depletion may at least partially explain these observations. Kinin production and B2 receptor synthesis are strongly stimulated by IPC (19, 22). Blockade of the B2 receptor reduces the cardioprotective effect of IPC (20). In addition, tissue kallikrein-deficient mice do not produce kinins, and while their response to IR is similar to that of wild-type mice, IPC has a reduced effect to suppress IS in these mice (19, 21). Thus, the effect on IPC of increased ACE gene expression in ACE3c mice is similar to the effect of kallikrein and kinin deficiency in tissue kallikrein gene inactivated mice (19). However, pretreatment of IPC-ACE3c mice with aliskiren, a direct renin inhibitor, partially restored the beneficial effect of IPC, suggesting that angiotensin II is also involved in the loss of IPC in ACE3c mice. A stimulatory effect of an increased ACE level on angiotensin II production is further documented in the present study by monitoring blood pressure during angiotensin I injection in ACE3c mice, and has also been observed in other experimental settings (40, 41). Thus, our present data suggest that kinin depletion and elevated angiotensin II formation are both involved in decreasing the protective effect of IPC in ACE3c mice. Coronary artery occlusion both increases sympathetic tone and activates the renin angiotensin system, resulting in increased angiotensin II formation (42). Angiotensin II causes coronary vasoconstriction by activation of postsynaptic angiotensin type1 (AT1) receptors as well as presynaptic AT1 receptors inducing additional catecholamine release. The cardiac and peripheral effects of angiotensins can result in increased metabolic demand on the heart and decreased blood supply, and hence susceptibility to ischemia. Induction of AT1 receptor synthesis in the heart during IR (21, 26, 43) may also contribute to the increase in coronary vascular resistance and cardiac dysfunction.
To the best of our knowledge, this study is the first one assessing the effect of renin inhibition in the setting of IR injury. Aliskiren had no effect on IR or IPC in wild-type (i.e., ACE2c) mice. The inhibitor was used at a dosage reducing blood pressure and renal vascular resistance to the similar extent as the dosage of ramiprilat previously shown to provide a 30% reduction in IS during IR (19, 21). Our data with aliskiren indicate that the renin angiotensin system plays no or little role in IR injury in wild-type mice. However, the beneficial effect of aliskiren in ACE3c mice suggests that cardiac angiotensin II is increased in these mice, compared to ACE2c mice, and this increase has deleterious consequence. Theoretical and experimental considerations have suggested that variation in ACE level influences kinin rather than angiotensin II concentrations, based in part on retrocontrol of renal renin secretion by angiotensin II (8). The present data do not fully support this hypothesis with regard to cardiac angiotensin II in the setting of ischemia. Tissue-specific regulation of the renin-angiotensin system, and ACE, explains the discrepancy between these studies.
Overall, the data suggest that the cardioprotective effect of low ACE level is mainly mediated by kinins, while the deleterious effect of high ACE level is dependent on both kinin depletion and angiotensin II. However, we cannot exclude a role of other ACE substrates.
In summary, the study shows that moderate variations in ACE gene expression around physiological value influence myocardial damage in the setting of acute ischemia in the mouse. These data support the hypothesis that the physiological level of ACE is rate limiting in angiotensin II production and kinin inactivation, at least in the ischemic mouse heart, and that this effect has physiological consequence. The study documents a deleterious effect of a modest genetic increase in ACE in the ischemic heart. Mouse data should be extrapolated with caution to human disease. IPC is an experimental maneuver performed in animals, but it is believed to have a clinical counterpart in humans and to play a role in the evolution of coronary insufficiency (44). The study supports the hypothesis that ACE levels affects prognosis of ischemic heart disease. Reduced tolerance to ischemia may be involved in the occurrence of myocardial infarction in patients having genetically high ACE levels. Moreover, the data for aliskiren, documenting ACE gene dependency of the cardiac effect of renin inhibition, raise a pharmacogenetic issue which can be addressed in humans.
Acknowledgments
The authors thank Georges Zadigue for his excellent technical assistance.
This study was supported by INSERM, the National Research Agency (ANR 05-PCOD-029), Universities Paris-Descartes, Pierre and Marie Curie, and Paris-Sud. This study was performed in the European Vascular Genomics Network, a Network of Excellence supported by the European Community's Sixth Framework Program for Research Priority 1 “Life sciences, genomics and biotechnology for health” (contract no, LSHM-CT-2003-503254). E.M. and V.G.-C. were supported by fellowships from the Ministère de la Recherche and from Academie of Medecine. The results were presented in part at the High Blood Pressure Research Conference 2008 (Atlanta, GA, USA).
REFERENCES
- 1. Erdos E. G. (1990) Angiotensin I converting enzyme and the changes in our concepts through the years. Lewis, K. Dahl memorial lecture. Hypertension 16, 363–370 [DOI] [PubMed] [Google Scholar]
- 2. Cambien F., Alhenc-Gelas F., Herbeth B., Andre J. L., Rakotovao R., Gonzales M. F., Allegrini J., Bloch C. (1988) Familial resemblance of plasma angiotensin-converting enzyme level: the Nancy study. Am. J. Hum. Genet. 43, 774–780 [PMC free article] [PubMed] [Google Scholar]
- 3. Rigat B., Hubert C., Alhenc-Gelas F., Cambien F., Corvol P., Soubrier F. (1990) An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J. Clin. Invest. 86, 1343–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Danser A. H., Schalekamp M. A., Bax W. A., van den Brink A. M., Saxena P. R., Riegger G. A., Schunkert H. (1995) Angiotensin-converting enzyme in the human heart. Effect of the deletion/insertion polymorphism. Circulation 92, 1387–1388 [DOI] [PubMed] [Google Scholar]
- 5. Costerousse O., Allegrini J., Lopez M., Alhenc-Gelas F. (1993) Angiotensin I-converting enzyme in human circulating mononuclear cells: genetic polymorphism of expression in T-lymphocytes. Biochem. J. 290(Pt. 1), 33–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alhenc-Gelas F., Corvol P. (2000) Molecular and physiological aspects of angiotensinI-converting enzyme. In Handbook of Physiology, Sec. 7, Vol. 3 (Fray J. C. S, Goodman H. eds) pp. 81–103, American Physiological Society and Oxford University Press, New York [Google Scholar]
- 7. Falkenhahn M., Franke F., Bohle R. M., Zhu Y. C., Stauss H. M., Bachmann S., Danilov S., Unger T. (1995) Cellular distribution of angiotensin-converting enzyme after myocardial infarction. Hypertension 25, 219–226 [DOI] [PubMed] [Google Scholar]
- 8. Takahashi N., Hagaman J. R., Kim H. S., Smithies O. (2003) Minireview: computer simulations of blood pressure regulation by the renin-angiotensin system. Endocrinology 144, 2184–2190 [DOI] [PubMed] [Google Scholar]
- 9. Dzau V. J., Bernstein K., Celermajer D., Cohen J., Dahlof B., Deanfield J., Diez J., Drexler H., Ferrari R., van Gilst W., Hansson L., Hornig B., Husain A., Johnston C., Lazar H., Lonn E., Luscher T., Mancini J., Mimran A., Pepine C., Rabelink T., Remme W., Ruilope L., Ruzicka M., Schunkert H., Swedberg K., Unger T., Vaughan D., Weber M. (2001) The relevance of tissue angiotensin-converting enzyme: manifestations in mechanistic and endpoint data. Am J Cardiol. 88, 1L–20L [DOI] [PubMed] [Google Scholar]
- 10. Huang W., Gallois Y., Bouby N., Bruneval P., Heudes D., Belair M. F., Krege J. H., Meneton P., Marre M., Smithies O., Alhenc-Gelas F. (2001) Genetically increased angiotensin I-converting enzyme level and renal complications in the diabetic mouse. Proc. Natl. Acad. Sci. U. S. A. 98, 13330–13334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sayed-Tabatabaei F. A., Oostra B. A., Isaacs A., van Duijn C. M., Witteman J. C. (2006) ACE polymorphisms. Circ. Res. 98, 1123–1133 [DOI] [PubMed] [Google Scholar]
- 12. Cambien F., Poirier O., Lecerf L., Evans A., Cambou J. P., Arveiler D., Luc G., Bard J. M., Bara L., Ricard S., Tiret L., Amouyel P., Alhenc-Gelas F., Soubrier F. (1992) Deletion polymorphism in the gene for angiotensin-converting enzyme is a potent risk factor for myocardial infarction. Nature 359, 641–644 [DOI] [PubMed] [Google Scholar]
- 13. Cambien F., Costerousse O., Tiret L., Poirier O., Lecerf L., Gonzales M. F., Evans A., Arveiler D., Cambou J. P., Luc G., Rakotovao R., Ducimetiere P., Soubrier F., Alhenc-Gelas F. (1994) Plasma level and gene polymorphism of angiotensin-converting enzyme in relation to myocardial infarction. Circulation 90, 669–676 [DOI] [PubMed] [Google Scholar]
- 14. Mattu R. K., Needham E. W., Galton D. J., Frangos E., Clark A. J., Caulfield M. (1995) A DNA variant at the angiotensin-converting enzyme gene locus associates with coronary artery disease in the Caerphilly Heart Study. Circulation 91, 270–274 [DOI] [PubMed] [Google Scholar]
- 15. Samani N. J., Thompson J. R., O'Toole L., Channer K., Woods K. L. (1996) A meta-analysis of the association of the deletion allele of the angiotensin-converting enzyme gene with myocardial infarction. Circulation 94, 708–712 [DOI] [PubMed] [Google Scholar]
- 16. Lindpaintner K., Pfeffer M. A., Kreutz R., Stampfer M. J., Grodstein F., LaMotte F., Buring J., Hennekens C. H. (1995) A prospective evaluation of an angiotensin-converting-enzyme gene polymorphism and the risk of ischemic heart disease. N. Engl. J. Med. 332, 706–711 [DOI] [PubMed] [Google Scholar]
- 17. Zintzaras E., Raman G., Kitsios G., Lau J. (2008) Angiotensin-converting enzyme insertion/deletion gene polymorphic variant as a marker of coronary artery disease: a meta-analysis. Arch. Intern. Med. 168, 1077–1089 [DOI] [PubMed] [Google Scholar]
- 18. Krege J. H., Kim H. S., Moyer J. S., Jennette J. C., Peng L., Hiller S. K., Smithies O. (1997) Angiotensin-converting enzyme gene mutations, blood pressures, and cardiovascular homeostasis. Hypertension 29, 150–157 [DOI] [PubMed] [Google Scholar]
- 19. Griol-Charhbili V., Messadi-Laribi E., Bascands J. L., Heudes D., Meneton P., Giudicelli J. F., Alhenc-Gelas F., Richer C. (2005) Role of tissue kallikrein in the cardioprotective effects of ischemic and pharmacological preconditioning in myocardial ischemia. FASEB J. 19, 1172–1174 [DOI] [PubMed] [Google Scholar]
- 20. Goto M., Liu Y., Yang X. M., Ardell J. L., Cohen M. V., Downey J. M. (1995) Role of bradykinin in protection of ischemic preconditioning in rabbit hearts. Circ. Res. 77, 611–621 [DOI] [PubMed] [Google Scholar]
- 21. Messadi-Laribi E., Griol-Charhbili V., Pizard A., Vincent M. P., Heudes D., Meneton P., Alhenc-Gelas F., Richer C. (2007) Tissue kallikrein is involved in the cardioprotective effect of AT1-receptor blockade in acute myocardial ischemia. J. Pharmacol. Exp. Ther. 323, 210–216 [DOI] [PubMed] [Google Scholar]
- 22. Pan H. L., Chen S. R., Scicli G. M., Carretero O. A. (2000) Cardiac interstitial bradykinin release during ischemia is enhanced by ischemic preconditioning. Am. J. Physiol. Heart Circ. Physiol. 279, H116–H121 [DOI] [PubMed] [Google Scholar]
- 23. Costerousse O., Allegrini J., Huang H., Bounhik J., Alhenc-Gelas F. (1994) Regulation of ACE gene expression and plasma levels during rat postnatal development. Am. J. Physiol. 267, E745–753 [DOI] [PubMed] [Google Scholar]
- 24. Bodin S., Chollet C., Goncalves-Mendes N., Gardes J., Pean F., Heudes D., Bruneval P., Marre M., Alhenc-Gelas F., Bouby N. (2009) Kallikrein protects against microalbuminuria in experimental type I diabetes. Kidney Int. 76, 395–403 [DOI] [PubMed] [Google Scholar]
- 25. Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 26. Yang X. P., Liu Y. H., Scicli G. M., Webb C. R., Carretero O. A. (1997) Role of kinins in the cardioprotective effect of preconditioning: study of myocardial ischemia/reperfusion injury in B2 kinin receptor knockout mice and kininogen-deficient rats. Hypertension 30, 735–740 [DOI] [PubMed] [Google Scholar]
- 27. Eckle T., Grenz A., Kohler D., Redel A., Falk M., Rolauffs B., Osswald H., Kehl F., Eltzschig H. K. (2006) Systematic evaluation of a novel model for cardiac ischemic preconditioning in mice. Am. J. Physiol. Heart Circ. Physiol. 291, H2533–H2540 [DOI] [PubMed] [Google Scholar]
- 28. Aouam K., Tissier R., Bruneval P., Mandet C., Berdeaux A., Ghaleh B. (2005) Preconditioning of salvaged myocardium in conscious rabbits with postinfarction dysfunction. Am. J. Physiol. Heart Circ. Physiol. 288, H2763–H2769 [DOI] [PubMed] [Google Scholar]
- 29. Menard J., Catt K. J. (1972) Measurement of renin activity, concentration and substrate in rat plasma by radioimmunoassay of angiotensin I. Endocrinology 90, 422–430 [DOI] [PubMed] [Google Scholar]
- 30. Pons S., Griol-Charhbili V., Heymes C., Fornes P., Heudes D., Hagege A., Loyer X., Meneton P., Giudicelli J. F., Samuel J. L., Alhenc-Gelas F., Richer C. (2008) Tissue kallikrein deficiency aggravates cardiac remodelling and decreases survival after myocardial infarction in mice. Eur. J. Heart Fail. 10, 343–351 [DOI] [PubMed] [Google Scholar]
- 31. Ludbrook J. (1994) Repeated measurements and multiple comparisons in cardiovascular research. Cardiovasc. Res. 28, 303–311 [DOI] [PubMed] [Google Scholar]
- 32. Tian B., Meng Q. C., Chen Y. F., Krege J. H., Smithies O., Oparil S. (1997) Blood pressures and cardiovascular homeostasis in mice having reduced or absent angiotensin-converting enzyme gene function. Hypertension 30, 128–133 [DOI] [PubMed] [Google Scholar]
- 33. Evangelista F. S., Krieger J. E. (2006) Small gene effect and exercise training-induced cardiac hypertrophy in mice: an Ace gene dosage study. Physiol. Genomics 27, 231–236 [DOI] [PubMed] [Google Scholar]
- 34. Tschope C., Heringer-Walther S., Koch M., Spillmann F., Wendorf M., Leitner E., Schultheiss H. P., Walther T. (2000) Upregulation of bradykinin B1-receptor expression after myocardial infarction. Br. J. Pharmacol. 129, 1537–1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tschope C., Heringer-Walther S., Koch M., Spillmann F., Wendorf M., Hauke D., Bader M., Schultheiss H. P., Walther T. (2000) Myocardial bradykinin B2-receptor expression at different time points after induction of myocardial infarction. J. Hypertens. 18, 223–228 [DOI] [PubMed] [Google Scholar]
- 36. Brown N. J., Gainer J. V., Murphey L. J., Vaughan D. E. (2000) Bradykinin stimulates tissue plasminogen activator release from human forearm vasculature through B(2) receptor-dependent, NO synthase-independent, and cyclooxygenase-independent pathway. Circulation 102, 2190–2196 [DOI] [PubMed] [Google Scholar]
- 37. Oldenburg O., Qin Q., Krieg T., Yang X. M., Philipp S., Critz S. D., Cohen M. V., Downey J. M. (2004) Bradykinin induces mitochondrial ROS generation via NO, cGMP, PKG, and mitoKATP channel opening and leads to cardioprotection. Am. J. Physiol. Heart Circ. Physiol. 286, H468–H476 [DOI] [PubMed] [Google Scholar]
- 38. Bergaya S., Meneton P., Bloch-Faure M., Mathieu E., Alhenc-Gelas F., Levy B. I., Boulanger C. M. (2001) Decreased flow-dependent dilation in carotid arteries of tissue kallikrein-knockout mice. Circ. Res. 88, 593–599 [DOI] [PubMed] [Google Scholar]
- 39. Yang X. P., Liu Y. H., Shesely E. G., Bulagannawar M., Liu F., Carretero O. A. (1999) Endothelial nitric oxide gene knockout mice: cardiac phenotypes and the effect of angiotensin-converting enzyme inhibitor on myocardial ischemia/reperfusion injury. Hypertension 34, 24–30 [DOI] [PubMed] [Google Scholar]
- 40. Xiao H. D., Fuchs S., Bernstein E. A., Li P., Campbell D. J., Bernstein K. E. (2008) Mice expressing ACE only in the heart show that increased cardiac angiotensin II is not associated with cardiac hypertrophy. Am. J. Physiol. Heart Circ. Physiol. 294, H659–H667 [DOI] [PubMed] [Google Scholar]
- 41. Muller D. N., Bohlender J., Hilgers K. F., Dragun D., Costerousse O., Menard J., Luft F. C. (1997) Vascular angiotensin-converting enzyme expression regulates local angiotensin II. Hypertension 29, 98–104 [DOI] [PubMed] [Google Scholar]
- 42. Sato M., Engelman R. M., Otani H., Maulik N., Rousou J. A., Flack J. E., 3rd, Deaton D. W., Das D. K. (2000) Myocardial protection by preconditioning of heart with losartan, an angiotensin II type 1-receptor blocker: implication of bradykinin-dependent and bradykinin-independent mechanisms. Circulation 102, III346-III351 [DOI] [PubMed] [Google Scholar]
- 43. Sun Y., Weber K. T. (1994) Angiotensin II receptor binding following myocardial infarction in the rat. Cardiovasc. Res. 28, 1623–1628 [DOI] [PubMed] [Google Scholar]
- 44. Yellon D. M., Alkhulaifi A. M., Pugsley W. B. (1993) Preconditioning the human myocardium. Lancet 342, 276–277 [DOI] [PubMed] [Google Scholar]