Abstract
IgG antibodies against Clostridium difficile toxins A and B were followed in controls and in patients with an initial C. difficile infection (CDI). Of the 50 CDI patients, 38 were cured and 12 developed recurrence. Compared to controls, patients had significantly lower anti-toxin A and B IgGs at inclusion, but the subsequent levels rose slightly regardless of clinical outcome. The results imply that the general serum reactivity against toxins A and B in the population reduces the risk of CDI, which suggests implications for vaccine strategies.
TEXT
The role of serum antibodies against Clostridium difficile toxins A and B in disease outcome is still debated (4, 9). Here, we prospectively followed the immunological response to serum anti-toxin A and B levels to determine whether they could predict the risk of initial or recurrent C. difficile infection (CDI).
This study was performed within a treatment trial (13), and patients with three consecutive serum samples available (n = 50) were enrolled. These subjects (36 females, 14 males; median age, 61 [range 20 to 85] years) had had diarrhea for a mean (median) of 7 (5) days before diagnosis of CDI by a positive C. difficile toxin test, and they were included within 2 days (mean/median) of the positive test. The first serum sample was taken on the day of inclusion, the second between days 8 and 13, and the third between days 35 and 40 or on the day of recurrence (days 9 to 36; median, day 13).
Control sera were collected from healthy blood donors (group 1: n = 59 donors; median age, 51 [range, 23 to 65] years) and on hospital admission day from patients without a reported CDI (group 2: n = 27 donors; median age, 63 [range, 50 to 79] years).
Levels of serum antibodies against toxins A and B were measured in doublets by enzyme-linked immunosorbent assay (ELISA) using microtiter plates coated with TcdA or TcdB (tgcBIOMICS, Mainz, Germany) according to the manufacturer's instructions and standard procedures. Secondary antibodies were horseradish peroxidase (HRP)-conjugated polyclonal rabbit anti-human IgG (Dako P0214) and, for control antibodies (TTC8, anti-TcdA antibody; 2CV, anti-TcdB antibody), rabbit-anti-mouse IgG (Dako P0260). Experimental errors were reduced by using interplate calibration, by including sera from each patient on the same plate, and by including sera from both patients and control subjects on each plate.
The Wilcoxon-Mann-Whitney test was conducted to compare serum antibody levels, and, where indicated, the Bonferroni post hoc test was performed to compensate for multiple comparisons. The Wilcoxon matched-pair test was used to compare serum antibody levels in individual patients at different time points. The Medical Ethics Committee of Lund University approved the study.
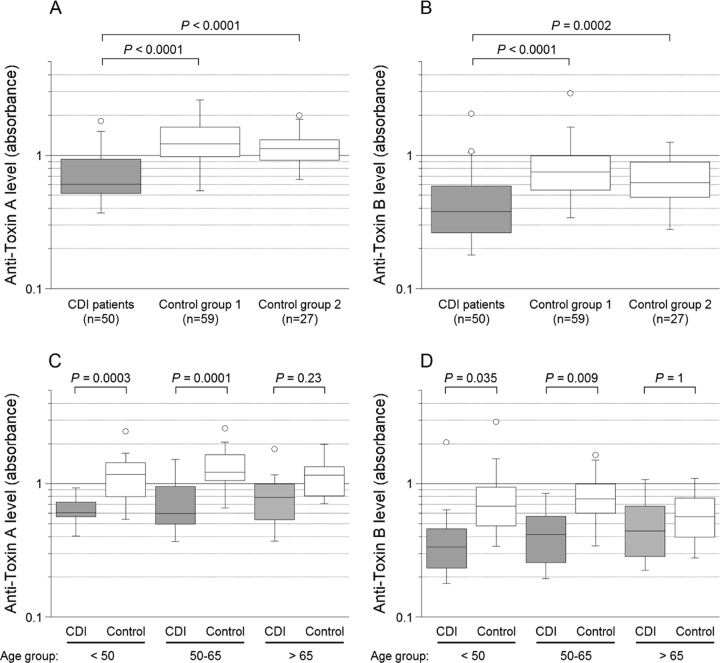
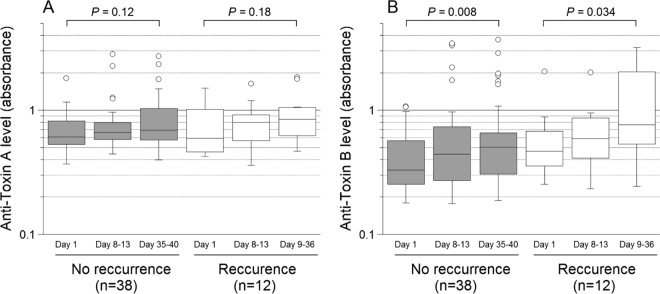
Levels of IgG against toxin A in inclusion sera were significantly lower in CDI patients than in the two control groups, and the same applied to toxin B (Fig. 1A and B). Strikingly, compared to the controls, the younger CDI patients had significantly lower serum levels of the anti-toxin antibodies, whereas that was not noted for those aged >65 years (Fig. 1C and D). There was no gender-related difference in IgG levels in the CDI group. Levels of serum IgG against toxins A and B were slightly higher in blood donors (control group 1) than in patients with no history of CDI (control group 2), although that difference was not statistically significant (Fig. 1A and B) and may have been related to age (median, 51 versus 63 years). Of the 50 CDI patients, 38 were cured and 12 (24%) developed a recurrence. The patients in the groups with and without recurrence had the same median age (both 61 years, ranges of 20 to 85 and 22 to 83 years, respectively). Regardless of outcome, both groups responded with a weak and nonsignificant increase in anti-toxin A IgG antibodies (Fig. 2A) and a modest rise in anti-toxin B antibodies (Fig. 2B).
FIG 1.
Levels of IgG against C. difficile toxins A and B in inclusion sera from CDI patients and sera from controls. Panel A shows serum levels of anti-toxin A in the CDI patients, control group 1 (blood donors), and control group 2 (patients without a history of CDI), and panel B shows the corresponding values for anti-toxin B. Panel C shows serum levels of anti-toxin A in CDI patients and the merged controls divided into three age groups (<50, 50 to 65, and >65 years), and panel D shows the corresponding values for anti-toxin B. Each box represents 50% of the values, and the horizontal lines denote the medians. Error bars include values that extend to the upper quartile value plus 1.5 times the interquartile distance and to the lower quartile value minus 1.5 times the interquartile distance, and open circles represent values outside these ranges (outliers). Bonferroni post hoc test was applied in the analyses illustrated in panels C and D (all groups were tested against each other, thus 15 tests in each panel).
FIG 2.
Levels of IgG against C. difficile toxins A and B in CDI patients with and without a recurrence. The y axis represents absorbance values for anti-toxin A (A) and anti-toxin B (B), and the x axis represents the time points at which follow-up sera were obtained as the number of days after the first serum sample was collected at inclusion. For the recurrence group, the time point of the third follow-up was the day of recurrence. For an explanation of the box plots, see the legend to Fig. 1.
Our study was somewhat limited, because the carriage rate of C. difficile in the control subjects was not known. However, it is reasonable to assume that that rate was low at the time of sampling, since there was no history of recent CDI or hospitalization among the control subjects and the carriage rate in the community is low (10). Carriage status in relation to disease outcome is important in hospitalized patients. This is exemplified by a prospective study in which patients who became colonized with C. difficile showed increasing anti-toxin A IgG levels but remained asymptomatic, whereas colonized nonresponders subsequently developed CDI (6). Serum antibodies against toxins A and B are acquired early in life, and the serum reactivity increases not only with age (11) but also in the general population over time (1). Thus, the apparently constant antibody levels in our control groups may reflect natural exposure to C. difficile, whereas the low levels in the CDI group may indicate an age-related waning of systemic immunity. The latter assumption is supported by the observation that CDI patients had slightly elevated antibody levels in follow-up samples, but despite ongoing infection, those levels were lower than noted in samples from controls. Results reported by other investigators have varied, ranging from no disparities to higher serum anti-toxin A IgG levels in CDI patients than in healthy subjects (3, 11, 12). The conflicting findings are difficult to explain but may be related to differences in study methods or design. For example, we did not test the neutralizing activity of the anti-toxin IgGs, a feature that is probably clinically important and may affect interpretation of the results (3).
Several investigations have examined the dynamic IgG response to C. difficile toxins in relation to the risk of recurrent CDI (5, 7, 8). These studies have indicated that, compared to nonrecurrent cases, recurrent cases have the same or lower anti-toxin A IgG titers and also lower anti-toxin B IgG titers. Kyne et al. (5) found that serum IgG against toxin A increased sharply from day 9 to day 12 in seven patients with a single episode of CDI but remained low in nine patients with subsequent recurrence. However, validation of a method using immunological data for recurrence prediction is complex (2), and our results support the general assumption that immunological data alone, particularly IgG response, cannot reliably predict recurrence. Indeed, other risk factors must be considered, such as comorbidity, severity of CDI, and age, as well as mucosal IgA secretory status and the C. difficile strain/toxinotype (7, 8).
In conclusion, we found that low levels of IgG against toxins A and B were associated with a risk of acquiring CDI and that the anti-toxin A and B IgG responses during acute infection were poor. The results also suggest that low circulating levels of C. difficile toxin A and B antibodies represent a risk factor for CDI, particularly in younger patients. Finally, the antibody levels detected by ELISA did not differ between patients who were cured and those with recurrence.
ACKNOWLEDGMENTS
The authors are grateful to Birgitta Andersson for technical assistance.
This work did not entail a conflict of interest for any of the authors.
The project was supported by research grants from Region Skåne, the Royal Physiographic Society, the Swedish Society of Medicine, and local donations and foundations at Skåne University Hospital, Malmö, Sweden.
Footnotes
Published ahead of print 11 July 2012
REFERENCES
- 1. Fenger RV, Linneberg A, Tvede M, Ostergaard C. 2009. Increasing seroprevalence of Clostridium difficile in an adult Danish general population. Epidemiol. Infect. 137:278–283 [DOI] [PubMed] [Google Scholar]
- 2. Hu MY, et al. 2009. Prospective derivation and validation of a clinical prediction rule for recurrent Clostridium difficile infection. Gastroenterology 136:1206–1214 [DOI] [PubMed] [Google Scholar]
- 3. Johnson S, Gerding DN, Janoff EN. 1992. Systemic and mucosal antibody responses to toxin A in patients infected with Clostridium difficile. J. Infect. Dis. 166:1287–1294 [DOI] [PubMed] [Google Scholar]
- 4. Kuehne SA, et al. 2010. The role of toxin A and toxin B in Clostridium difficile infection. Nature 467:711–713 [DOI] [PubMed] [Google Scholar]
- 5. Kyne L, Warny M, Qamar A, Kelly CP. 2001. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet 357:189–193 [DOI] [PubMed] [Google Scholar]
- 6. Kyne L, Warny M, Qamar A, Kelly CP. 2000. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N. Engl. J. Med. 342:390–397 [DOI] [PubMed] [Google Scholar]
- 7. Leav BA, et al. 2010. Serum anti-toxin B antibody correlates with protection from recurrent Clostridium difficile infection (CDI). Vaccine 28:965–969 [DOI] [PubMed] [Google Scholar]
- 8. Lowy I, et al. Treatment with monoclonal antibodies against Clostridium difficile toxins. N. Engl. J. Med. 362:197–205 [DOI] [PubMed] [Google Scholar]
- 9. Lyras D, et al. 2009. Toxin B is essential for virulence of Clostridium difficile. Nature 458:1176–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miyajima F, et al. 2011. Characterisation and carriage ratio of Clostridium difficile strains isolated from a community-dwelling elderly population in the United Kingdom. PLoS One 6:e22804 doi:10.1371/journal.pone.0022804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Viscidi R, et al. 1983. Serum antibody response to toxins A and B of Clostridium difficile. J. Infect. Dis. 148:93–100 [DOI] [PubMed] [Google Scholar]
- 12. Warny M, Vaerman JP, Avesani V, Delmee M. 1994. Human antibody response to Clostridium difficile toxin A in relation to clinical course of infection. Infect. Immun. 62:384–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wullt M, Odenholt I. 2004. A double-blind randomized controlled trial of fusidic acid and metronidazole for treatment of an initial episode of Clostridium difficile-associated diarrhoea. J. Antimicrob. Chemother. 54:211–216 [DOI] [PubMed] [Google Scholar]