Abstract
Many syndromes have a large number of differential diagnoses, a situation which calls for multiplex diagnostic systems. Myalgic encephalomyelitis (ME), also named chronic fatigue syndrome (CFS), is a common disease of unknown etiology. A mouse retrovirus, xenotropic murine leukemia-related virus (XMRV), was found in ME/CFS patients and blood donors, but this was not corroborated. However, the paucity of serological investigations on XMRV in humans prompted us to develop a serological assay which cover many aspects of XMRV antigenicity. It is a novel suspension array method, using a multiplex IgG assay with nine recombinant proteins from the env and gag genes of XMRV and 38 peptides based on known epitopes of vertebrate gammaretroviruses. IgG antibodies were sought in 520 blood donors and 85 ME/CFS patients and in positive- and negative-control sera from animals. We found no differences in seroreactivity between blood donors and ME/CFS patients for any of the antigens. This did not support an association between ME/CFS and XMRV infection. The multiplex serological system had several advantages: (i) biotinylated protein G allowed us to run both human and animal sera, which is essential because of a lack of XMRV-positive humans; (ii) a novel quality control was a pan-peptide positive-control rabbit serum; and (iii) synthetic XMRV Gag peptides with degenerate positions covering most of the variation of murine leukemia-like viruses did not give higher background than nondegenerate analogs. The principle may be used for creation of variant tolerant peptide serologies. Thus, our system allows rational large-scale serological assays with built-in quality control.
INTRODUCTION
The detection of antibodies to a microbe, also referred to as “serology,” is a basic and clinically useful method for demonstrating recent or previous infections with the microbe. The method is often the only means of diagnosing a past infection and immunity. Serology is often more sensitive than culture of microbes or detection of microbial nucleic acid, because it utilizes the immune response as an amplified microbe-specific signal. Despite these definite advantages, serology also has drawbacks, for example, that the signal is indirect (i.e., it reflects only how the immune system perceived the microbial antigen). The antibody response may be narrow or broad. In the latter case, cross-reactions to similar antigens may occur. Therefore, an optimal serological test for detection of an infection often relies on the use of several microbial antigens. If several epitopes of a microbe are used, both sensitivity and specificity can increase. Immunoblotting is one way to detect antibodies to several antigens in the same test. However, it is possible to simultaneously measure antibodies to many microbial epitopes in a more rational and rather inexpensive way, namely, with suspension microarrays. We have thus created a multiepitope serological assay based on this method.
The presence of xenotropic murine leukemia-related virus (XMRV) infection, demonstrated by PCR, virus isolation, and some antibody tests, in patients suffering from myalgic encephalomyelitis (ME), also called the chronic fatigue syndrome (CFS), was recently reported (38, 42). XMRV is a novel gammaretrovirus belonging to the murine leukemia viruses (MuLVs) (11). It was found in prostate cancer tissue (20, 68). This was later attributed to contamination with XMRV-containing cell culture DNA (58), and the claims for its presence in both prostate cancer and ME/CFS were retracted (2, 64, 65). XMRV has a typical type C gammaretrovirus structure (11). The most prominent serological responses to a retrovirus are to its env- and gag-encoded proteins. MA (p15), p12, CA (p30), and NC (p10) are the major gag-encoded proteins, while SU (gp70) and TM (p15E) are the major env-encoded proteins (17). The retractions possibly made a corroboration of XMRV infection from our serological study less important. However, gammaretroviruses are well-known pathogens causing leukemia, neurological disease, and immunodeficiency in mice, cats, and some nonhuman primates (17). From a broader perspective, it is still important to verify the possible presence of such retroviruses in humans. The negative outcome of a broadly targeted serological test (23) not only disproves that humans were XMRV infected but also disproves the occurrence of human infections with the broader group of murine leukemia virus-like retroviruses (MLLVs) (11, 56, 57).
The serological system reported here contains XMRV proteins and peptides homologous to peptides known to react with antibodies to a number of MLLVs, i.e., MuLV, feline leukemia virus (FeLV), and gammaretroviral porcine endogenous retrovirus group C. Moreover, we tried to widen the detection range by the use of degenerate peptides covering known MLLV epitopes. The value of this effort is in (i) the disproving of the occurrence of antibodies to MLLV in humans and (ii) the creation of a quality-controlled multiepitope serological system which can be adapted to many serological testing situations.
ME/CFS occurs in around 0.2% of the population, predominantly affecting previously healthy, 40- to 60-year-old women. The symptoms include fatigability, cognitive dysfunction with loss of memory and/or concentration, sore throat, painful lymphadenopathy, muscle pain, headache, unrefreshing sleep, and extreme exhaustion after exercise. There is a comorbidity of ME/CFS with fibromyalgia and irritable bowel syndrome (IBS) (53). There are at least four case definitions for ME/CFS: the CDC definition (also known as the Fukuda criteria) (25), the Oxford criteria (see, e.g., reference 48), the 2003 Canadian clinical working definition (13), and the 2011 consensus criteria (14). The first three require a disease duration of at least 6 months. Little is known about the etiology of this disease. However, ME/CFS is often characterized by an unexplained long-term chronic inflammation and immune dysfunction and often appears after a severe viral infection (14, 53). Hence, the involvement of several human viruses, including human herpesvirus 6 (HHV-6) (1), Epstein-Barr virus (EBV) (12, 24), enteroviruses (15, 33, 76), and even human T-lymphotropic virus type 2 (HTLV-2) (18), has been examined, but none of them has been proven to be the cause of ME/CFS (11). We tried to corroborate the serological findings with a suspension array of 47 XMRV antigens, both recombinant proteins and synthetic peptides. A novel rational internal control system was implemented. Applying this quality-controlled system to sera and plasma specimens from patients suffering from ME/CFS and blood donors gave a negative result, giving no evidence for the existence of XMRV infections in the Swedish population.
MATERIALS AND METHODS
Synthetic peptides.
The amino acid sequences of our peptides were chosen to match those of known antibody epitopes on the Env and Gag proteins of Friend mouse leukemia virus (a variant of MuLV), a virus similar to XMRV, and of feline leukemia virus (FeLV), a gammaretrovirus somewhat less similar to MuLV.
The peptide sequences are shown in Fig. 1. The Gag peptides (MA and CA) were selected from the XMRV VP62 genome to match those of known cross-reacting epitopes in other MuLVs ((16, 43). Additional peptides were chosen from the beginning of the amino acid sequence of the MuLV glyco-Gag protein, a known epitope of murine sarcoma virus (MSV)-induced tumors (3, 19, 27, 29, 46, 47). The Env peptides (SU and TM) were chosen from (i) known epitopes of the FeLV gp70 (52, 70, 71), (ii) the epitope of the broadly neutralizing XMRV Env monoclonal antibody 83A25 (39, 45, 50, 51), (iii) epitopes of narrowly reacting nonneutralizing monoclonal antibodies, (iv) a major cytotoxic T lymphocyte (CTL) epitope in p15E (21, 63), (v) studies on FeLV TM epitopes (37, 44), and (vi) critical portions of the immunosuppressive domain as defined in MuLV (7, 35, 41) and analogous to antigenic peptides from HIV.
Fig 1.
Synthetic peptides used in the study. The design of many of the peptides was based on the literature (see references in Materials and Methods).
Variable amino acid positions were identified by comparing differences in genomes of XMRV strains. Degenerate peptides containing most variants of the variable regions were also produced (ma0deg, g1deg, su12bdeg ca0a, and ca0bdeg) (Fig. 1). This was an attempt to make the test more tolerant for antigen sequence variation. The peptides were synthesized by R. Pipkorn at the DKFZ (Deutsches Krebsforschungszentrum, Heidelberg, Germany).
All peptides were 30-mers and had a three-carbon polyethylene glycol spacer coupled at the amino end. The spacer started with a primary amino group. Lyophilized peptides were dissolved in sterile phosphate-buffered saline (PBS), pH 7. The dissolution sometimes had to be facilitated by warming the solution to 37°C overnight on a shaker or by sonication for 5 min. Highly hydrophobic peptides were dissolved in 10 to 50% (vol/vol) dimethyl sulfoxide (DMSO) (Sigma D2650).
Recombinant proteins.
Nine XMRV proteins, covering the gag and env genes, were designed (Table 1). Their design, expression, and purification and the verification of their antigenicity were reported previously (61).
Table 1.
Recombinant proteins used in the studya
| Portion of XMRV provirus | Length (amino acids) | Protein construct (abbreviation) |
|---|---|---|
| Gag (MA) | 92 | p15 short TrxA His tag (p15) |
| 129 | p15 long TrxA His tag (p15L) | |
| Gag (p12) | 85 | p12 TrxA His tag (p12) |
| 207 | p30 TrxA His tag (p30) | |
| Gag (NC) | 13 | p10 short TrxA His tag (p10) |
| 105 | p10 long TrxA His tag (p10L) | |
| Env (SU) | 442 | gp70 TrxA His tag (gp70) |
| Env | 645 | gp85 TrxA His tag (gp85) |
| Env (TM) | 149 | p15E TrxA His tag (p15E) |
| 109 | TrxA | |
| E. coli (Origami B) extract |
A further description of the proteins is presented elsewhere (61).
Control antigens.
One bead was coupled with Haemophilus influenzae type b (Hib) vaccine (Act-HIB; Sanofi Pasteur). It served as a commonly positive control for human sera, since most Swedes have been vaccinated against, or have been infected with, Hib. It is therefore suitable as a control for the presence of functional antibodies in the samples.
Human sera.
The 85 Swedish patients included 78 patients with the diagnosis of ME/CFS according to the Canadian criteria (13) and 30 patients with both ME/CFS and fibromyalgia diagnoses. Seven patients who fulfilled only the criteria for fibromyalgia were also included. The fibromyalgia diagnosis was made according to the ACR classification (74). Irritable bowel syndrome (IBS) was diagnosed in 40% of the total group of 85 patients, with no significant difference in the subgroups. All patients were rated by the FibroFatigue scale (75). The mean score was 41 ± 9 points, indicating a moderate to severe degree of disorder. The total variance of the scale is 0 to 72. Diagnosis was made by three individuals, all holding M.D. and Ph.D. degrees, who were well trained in the use of the rating scale and in the diagnosis of the disorders. The study was approved by the Ethical Committee of the University of Gothenburg (Dnr 680-09). As controls, a total of 520 blood donor sera from the Uppsala Academic Hospital blood bank were used. They were tested according to a general permission in Uppsala to test for blood-borne viruses obtained at blood donation.
Animal sera.
Control animal sera were goat anti-MuLV p30, gp70, and p15E. They were kindly provided by William Switzer, Centers for Disease Control, Atlanta, GA, and Sandra Ruscetti, Laboratory of Cancer Prevention, National Cancer Institute, Bethesda, MD. Sera from XMRV-infected Mus pahari were a kind gift from Yasuhiro Ikeda, Mayo Clinic, Rochester, MN (60).
Sera from three rabbits immunized with a mixture of peptides (rabbit anti-pan-peptide sera) were obtained from Genscript Inc. (Newark, NJ). Thirty-eight XMRV peptides (Table 1), with an amino group attached to the peptide via a polyethylene glycol spacer (see “Synthetic peptides” above), were sent to Genscript, where they were conjugated as a mixture to keyhole limpet hemocyanin and used for immunization of three rabbits. Pre- and postimmunization sera were obtained.
Coupling of antigens.
The recombinant proteins and peptides were coupled to Luminex carboxylated beads essentially as described in the “Sample Protocol for Two Step Carbodimide Coupling of Protein to Carboxylated Microspheres” provided by the Luminex Corporation (Austin, TX). In order to disperse bead aggregates, the bead stocks (xMAP Technology, Austin, TX) were sonicated for 20 s, followed by vortex mixing for 20 s. Two hundred microliters of the stock microspheres, containing 1.25 × 107 beads per ml, was transferred to a 1.5-ml Eppendorf tube. The beads were then centrifuged at 13,000 × g for 3 min and the supernatants removed. The beads were washed with 100 μl of distilled water, followed by vortexing and sonication for 20 s, and then centrifuged at 13,000 × g for 3 min. Supernatants were subsequently removed. Eighty microliters of 100 mM monobasic sodium phosphate (MSP) (pH 6.2) (Sigma, catalog no. S3139) was added to the bead pellet, after which the suspension was vortexed and sonicated for 20 s. Ten microliters of freshly made N-hydroxysuccinimide (NHS) (Pierce) and 10 μl of 50-mg/ml 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) (water-soluble carbodiimide) (Pierce; sold by Nordic Biolabs AB, Sweden) in H2O were added to the beads, and the suspension was incubated in the dark for 20 min at room temperature. The beads were then centrifuged as described above. The supernatants were carefully removed, and the pellet was washed twice in 250 μl of 50 mM 2-(N-morpholino)ethanesulfonic acid (MES) sodium salt (CAS no. 71119-23-8; Sigma), pH 5. One hundred microliters of MES buffer was added to the bead pellet, which was vortexed and sonicated. Fifty micrograms of the protein or peptide was added, and more MES buffer was added up to 500 μl. Beads were mixed by carefully vortexing and then incubated with shaking for 2 h in the dark. The coupled microspheres were pelleted by centrifugation at 13,000 × g for 3 min and resuspended in 500 μl of StabilGuard (catalog no. SG01-1000; SurModics) buffer. The coupled microspheres were again pelleted by centrifugation as described above and resuspended in 1 ml of StabilGuard buffer twice. The final pellet was resuspended in 400 μl StabilGuard. This created a bead mixture consisting of 6,250 beads/μl. The coupled beads were stored at 4°C in the dark.
Serological procedure.
The serological procedure was performed as described by Sheikholvaezin et al. (61), with the exception that 38 synthetic peptides were also included. Briefly, the multiplex assay was carried out in a 96-well MV Multiscreen 200-μm filter plate (Millipore, Hertfordshire, United Kingdom), which allows washing and retention of the Luminex beads. Wells were prewetted twice, using 100 μl of PBS. The PBS was removed from the wells by aspiration through the Millipore filter.
A bead mixture consisting of 100 beads/μl was made using StabilGuard as a diluent. All the beads with coupled proteins as well as the naked bead were both sonicated and vortexed for 20 s before being added to the bead mixture. The mixture was sonicated and vortexed for 20 s. Fifty microliters of the bead mix was then added to each well. Fifty microliters of serum diluted as described below in StabilGuard buffer was then, after a brief vortexing, added to all wells except two, the negative control, also referred to as a nontemplate control (NTC) (StabilGuard), and a blank (PBS). After this, the wells were incubated in the dark with gentle rotation for 30 min. During this incubation period, 0.5-mg/ml biotinylated protein G (Pierce catalog no. 29988; Thermo Science) was diluted in an Eppendorf tube to a final concentration of 4 μg/ml using StabilGuard as the diluent. The wells were then washed by adding and aspirating 100 μl of PBS. This washing procedure was repeated 3 times. The beads were then resuspended 3 times in 50 μl of StabilGuard. The diluted protein G was first vortexed, and then 50 μl was added to each well. After 30 min of incubation in the dark with rotation, the wells were washed three more times in PBS as described above. The beads were then resuspended again in 50 μl of StabilGuard. Fifty microliters of streptavidin-phycoerythrin (SA-PE) (Molecular Probes, Leiden, The Netherlands), diluted in blocking/storage buffer to 4 μg/ml, was added to the microplate wells. The plate was then incubated for 15 min in the dark with gentle agitation. The filter plate was washed twice as previously before the contents of the wells were resuspended in 100 μl of PBS. The beads were analyzed in a Luminex-200 instrument following the manufacturer's instructions.
Data reduction and automated quality control.
A program which orders the results for the antigens according to the gene order in the XMRV genome, subtracts the nontemplate control and naked bead values, and checks the results of the rabbit anti-pan-peptide sera for each peptide and protein was written in Visual Foxpro by J. Blomberg. The results were then stored in a database.
Statistical evaluation and multivariate analysis.
A search for significant differences in antigen reactivity between ME/CFS patient sera and blood donor sera was done using the nonparametric Wilcoxon rank sum test. Multivariate analysis (principal-component analysis) (data not shown) and descriptive statistics were performed using the Unscrambler program (CAMO AS, Norway).
RESULTS
Development of an internal control system.
An overview of the internal controls of the serology system is shown in Fig. 2. The antipeptide sera obtained from Genscript were tested for reactivity by running them in the Luminex multiplex assay using the method as described above. Three pre- and three postimmune sera were diluted to concentrations of 1:100 and 1:1,000 and then added to one well each. The median fluorescence intensity (MFI) was calculated to evaluate antipeptide serum reactivity (Fig. 3). This was intended to serve as a control for the presence and antigenicity of the synthetic peptide and recombinant protein on the bead.
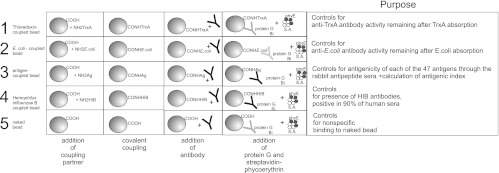
Fig 2.
Overview of internal controls of the serology system. (1) A bead containing thioredoxin A (TrxA) is used to control for anti-TrxA antibodies remaining after absorption with free TrxA. (2) An Escherichia coli lysate-coupled bead controls for anti-E. coli antibodies remaining after absorption with E. coli lysate. Both TrxA- and E. coli-containing beads are necessary for controlling the results with recombinant TrxA fusion proteins prepared in E. coli. (3) Antigen-coated beads are used for surveillance of the antigenicity of individual antigens via rabbit antipeptide hyperimmune serum, of overall antigenic performance via the antigenic index (Fig. 4), and of background binding without serum (nontemplate control [NTC]). (4) The presence of antibodies capable of binding to a common antigen was ensured with the Haemophilus influenzae B (Hib)-containing bead. (5) A “naked” bead, without any bound antigen, was used to control for nonspecific binding to the Luminex beads. Weak nonspecific binding is symbolized with a light gray antibody symbol.
Fig 3.
Comparison of the pre- and postimmunization antipeptide sera (dilution, 1:100) received from Genscript. The postimmunization sera reacted more strongly to all peptides than the preimmunization sera. Sera 19 and 22, 20 and 23, and 21 and 24 came from rabbits 1, 2, and 3, respectively. The difference between pre- and postimmunization sera was greatest for rabbit 3, which yielded sera 21 and 24. MFI, median fluorescence intensity.
The antipeptide rabbit sera received from Genscript reacted to all peptides and recombinant proteins, but not to the control beads, when diluted 1:100 and 1:1,000. We found that rabbit serum 21 was the most reactive serum and that 1:100 was the optimal dilution,. The absence of reactivity in the preimmunization rabbit sera and strong reactivity in the postimmunization sera provided evidence that the beads contained XMRV peptides and recombinant proteins. As expected, the three rabbit postimmunization antipeptide sera also gave strong reactions with recombinant XMRV proteins, proving that epitopes recognized by these sera also were present on whole recombinant XMRV proteins.
To detect any antibody reaction to the microspheres themselves, a naked non-protein/peptide-containing bead was added to the bead mixture in each experiment. A known problem for suspension microarrays is that occasional sera give significant binding to the underivatized (“naked”) beads (49, 69). In our experience, from this and from further work with thousands of sera (unpublished data), mostly from humans, it is a relatively small problem. Using the protocol described in this paper, the average naked bead MFI was 27 (standard deviation [SD], 11), with occasional high-binding sera. Among 321 human sera tested consecutively, we found three sera with MFIs of 71, 88, and 140, a frequency of 0.9%. After subtraction of the naked-bead value, it is our general experience (from this study and other unpublished work of ours) that a cutoff MFI of at least 100 or higher gives a sufficient discrimination of a positive from a negative result. However, each antigen may have its own problems in this regard. One negative-control well, where PBS (nontemplate control [NTC]) instead of serum was added, was also used in all the experiments. The results from the rabbit antisera were used to follow the coupling quality and activity of all reagents between experiments. A bead containing Hib vaccine was also added to the bead mixture in each experiment to evaluate the average reactivity of human sera, which was a further means of controlling for methodological variation and ensuring antibody presence.
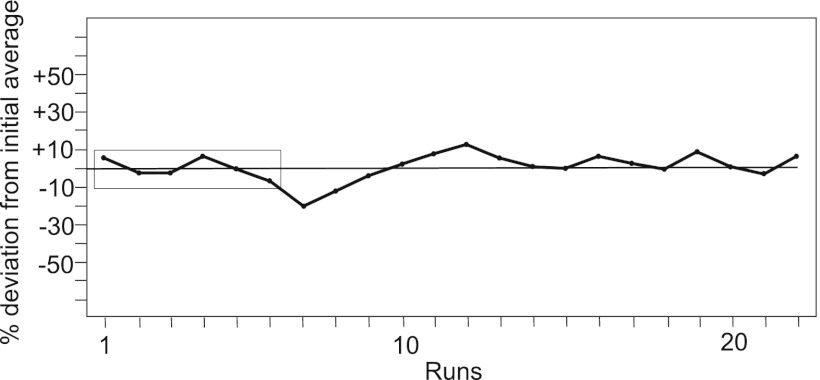
An antigenic index was then constructed from the reactivities (MFIs) of 16 synthetic peptides (ma0, ca0a, ca1, ca2, su2, su3b, su4, su6, su7, su8, su9, su9b, tm0a, tm0c, tm3, and tm4) with the anti-pan-peptide serum 21 of rabbit 3. An expected value was defined for each of the peptides, being the average of 6 initial runs. The antigenic index was then calculated as the average of the 16 ratios of observed versus expected MFI for each peptide. The antigenic index for a run would be 1 if the run essentially gave the same values for all 16 peptides as the average of the initial 6 runs. It turned out to be stable over 22 successive runs during a time period of 3 months, with an interassay variation (SD) of 11% (Fig. 4).
Fig 4.
Successive average antigenicities of 16 indicator peptides with rabbit serum 21, plotted as the percentage of the average median fluorescence intensity in relation to an initial average (of the first six values).
Sera from animals hyperimmunized with MuLV proteins and from animals infected with XMRV.
High-level reactions with several recombinant proteins were obtained with sera from XMRV-infected mice (Fig. 5). The most intense reactions were obtained at 5 weeks postinfection. Uninfected mice did not develop reactivity. The recombinant proteins p15, p10 (but not p10L), p15E, and gp85 gave clear reactions (Fig. 6). No reactions with synthetic peptides were observed, illustrating the importance of conformational epitopes.
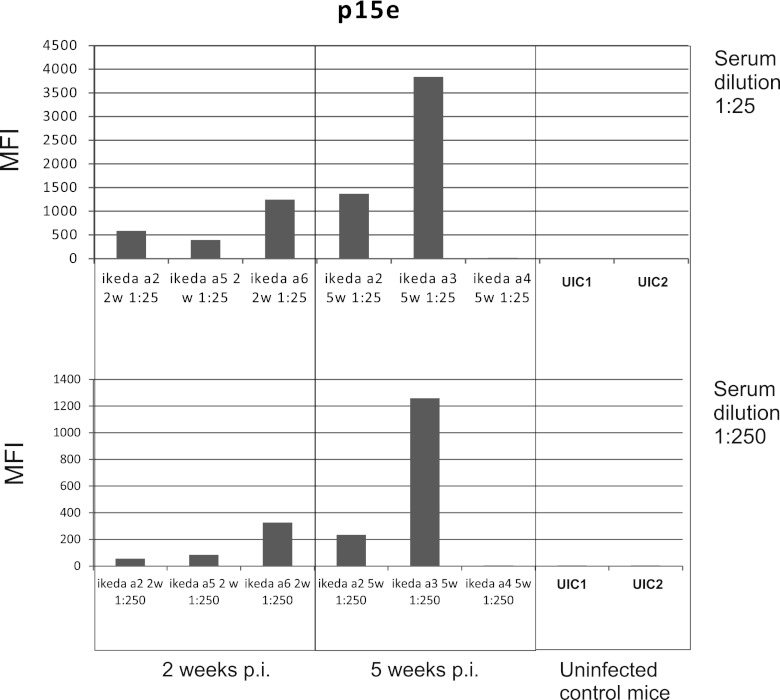
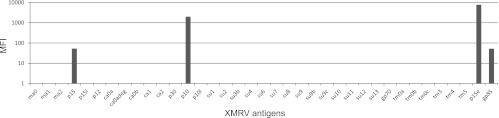
Fig 5.
Reactivities (median fluorescence intensity [MFI]) of XMRV-infected and uninfected Mus pahari mice against recombinant p15E protein. Sera were obtained at 2, 5, 8, and 12 weeks postinfection (p.i.). Sera (a kind gift from Yasuhiru Ikeda) were obtained from mice a2, a3, a4, a5, and a6, which were XMRV infected, at 2 and 5 weeks postinfection. All except mouse a4 developed an antibody response. Results with control sera from two other, noninfected, mice (UIC1 and UIC2) are also shown. The top panel shows results with a 1:25 serum dilution, while the bottom panel shows results with a 1:250 serum dilution.
Fig 6.
Reactivities (median fluorescence intensity [MFI]) of a serum (diluted 1/25) from XMRV-infected Mus pahari mouse a3 obtained at 5 weeks postinfection. A suspension array of 37 XMRV synthetic peptides and recombinant proteins was used. The ordinate has a log scale. The absence of reactions with the synthetic peptides and presence of reactions with the recombinant p15 (but not the longer version p15L), p10 (but not the longer version p10L), p15E, and gp85 (but not gp70) are illustrated.
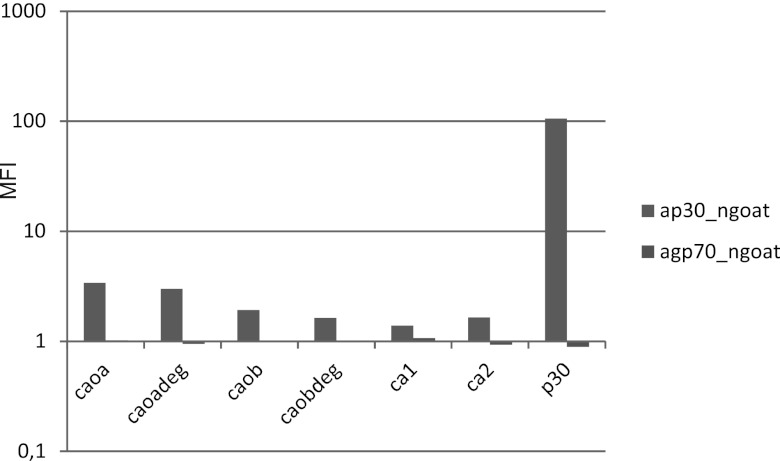
Sera from goats hyperimmunized with purified MuLV proteins gave strong reactions with the respective immunogen but only weak reactions with the synthetic peptides derived from the immunogen. Goat anti-p30 gave a strong anti-p30 reaction but only moderate to weak reactions with capsid protein (CA)-derived MuLV peptides (Fig. 7). The reactions of goat anti-gp70 with gp70 were also strong, but the reactions with gp70-derived synthetic peptides were weak (not shown).
Fig 7.
Ratio of reactivity (median fluorescence intensity [MFI], shown with a log scale) of a goat anti-p30 hyperimmune serum versus reactivity of a normal goat serum (“ap30_ngoat”) and of a goat anti-gp70 hyperimmune serum versus reactivity of the same normal goat serum (“agp70_ngoat”). Results with beads with synthetic peptides from the capsid protein and recombinant capsid protein (p30) are shown. The sequences of the peptides are shown in Fig. 1. Despite the rather high degeneracy of the two peptides ca0adeg (432 variants) and ca0bdeg (144 variants), they did not give a stronger binding than the nondegenerate counterparts.
Development of data handling and quality control software.
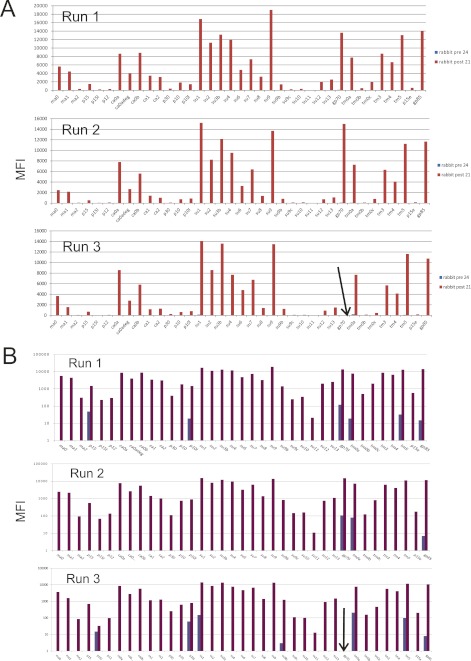
The MFI values obtained from the negative control and the naked bead was subtracted from the MFI values obtained from patient sera and specimens from blood donors. By comparing MFIs of immunized-rabbit sera between runs, we calculated an average of the rabbit antipeptide serum MFI. This was used to monitor the antigenicity of peptide- and recombinant protein-containing beads. Figure 8 illustrates an incident of insufficient antigenicity of the gp70 bead. It was restored after recoupling of the gp70 protein.
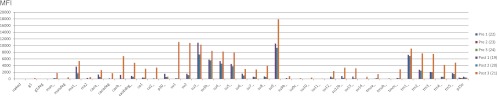
Fig 8.
Antibody reactivities (median fluorescence intensities [MFI]) to 38 XMRV antigens of sera taken before (pre 24) and after (post 21) immunization of rabbit 3 with an XMRV peptide mixture. Results obtained on three separate runs (1 to 3) on three days are shown. The arrow indicates that in run 3 the gp70 bead did not contain enough of recombinant gp70 protein, an illustration of the utility of this quality control feature. The reactivities of some peptides were rather weak but are evident in the logarithmic presentation. Blue bars are from preimmunization serum 24. Red/magenta bars are from postimmunization serum 21. (A) Linear ordinate scale. (B) Log10 ordinate scale.
Comparison of samples from blood donors and ME patients.
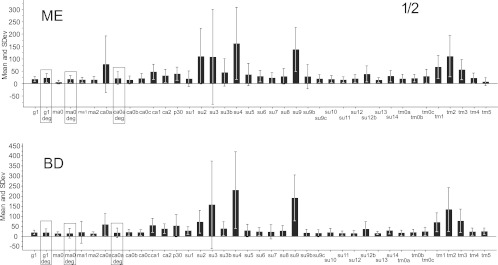
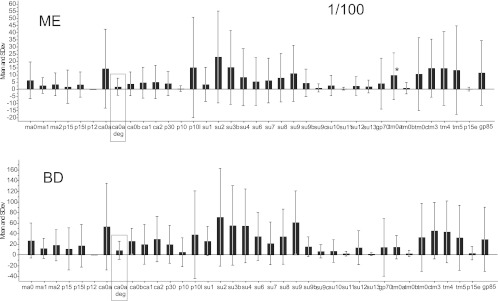
Samples from patients and blood donors were tested at dilutions of 1/2 (Fig. 9, 1/10 and 1/25 (data not shown), and 1/100 (Fig. 10). The panel of antigens was not identical between the experiments with dilutions of 1/2 (Fig. 9) and 1/100 (Fig. 10). Although all reactions became stronger at higher sample concentrations, in no case was there a stronger reaction in the ME samples (Wilcoxon rank sum test). At 1/100, most beads gave an MFI of 0 to 100 for both ME and blood donor sera. At 1/25, most beads gave an MFI of 0 to 150 for both categories. At a dilution of 1/2, both ME and blood donor sera gave an MFI of 10 to 300. In contrast, hyperimmune rabbit and goat sera often gave MFIs of over 1,000 at a dilution of 1/100 or 1/1,000. Several antigens gave lower reactions with ME samples than with blood donor samples. Most of the ME and blood donor sera yielded strong reactions with the Hib bead (Table 2). The SU (surface unit) peptides and the TM (transmembrane) peptides reacted most strongly. The SU3 bead tended to react more than the other peptides with both ME and blood donor sera. Hence, we found no evidence that ME patient sera reacted more strongly than blood donor sera at any of the dilutions tested. The weak reactions with XMRV recombinant proteins and peptides in human sera contrasted with the strong reactions of animal hyperimmune sera and sera from XMRV-infected mice to some of the same antigens. Comparing with the strong reactivity obtained from our vaccine-loaded beads, we did not find any convincing sign of an immune response elicited toward XMRV infection in any of our human sera.
Fig 9.
Average reactivities (MFI) and standard deviations of 39 XMRV antigens run against 64 ME/CFS sera and 90 blood donor (BD) sera at a dilution of 1/2. Note that even at the low dilution of 1/2, which should favor nonspecific binding, the g1deg (24 variants) and ma0deg (108 variants) degenerate peptides (boxed) did not have significantly higher binding activity than the nondegenerate variants.
Fig 10.
Mean reactivities (MFI) of 38 XMRV antigens (synthetic peptides and recombinant proteins). The upper section (ME) shows the reactivities of 85 sera from ME/CFS and fibromyalgia patients, diluted 1/100. The lower section (BD) shows the reactivities of 254 blood donor sera diluted 1/100. The asterisk indicates that two outlying values were removed. The panel of antigens was somewhat different from the ones shown in Fig. 9; this was due to the successive production of new recombinant proteins and limited availability of xMAP beads. The result with the one degenerate peptide in this experiment is boxed.
Table 2.
Outcome for quality controls over time, using human blood donor sera diluted 1/100 and rabbit anti-pan-peptide serum 21
| Control | Mean MFI ± SDa (n) |
|---|---|
| TrxA-containing bead | 42 ± 34 (104) |
| E. coli-containing bead | 35 ± 44 (106) |
| NTC for sera diluted 1/100 | 34 ± 10 (211)b |
| HIB (avg) | 2101 ± 420 (240) |
| Naked bead | 27 ± 5 (130) |
Without background subtraction.
Antigenicity index, 110 ± 11 (n = 22).
Attempts to detect IgM.
We also evaluated whether 30 Swedish ME patient sera and sera from 64 blood donors elicited an IgM immune response to our peptides and proteins (data not shown). Instead of using protein G as a secondary antibody, we used biotinylated anti-human IgM (Sigma) at a dilution of 1/1,000. No reactions with XMRV proteins or peptides were seen. Serum dilutions of 1/25 and 1/100 were tested. It was found that the Hib-coated bead also bound IgM from the human sera and gave an MFI of 300 to 1,000 with both blood donor and ME/CFS sera. The Hib vaccine is a protein-polysaccharide conjugate. Many polysaccharide antibodies belong to the IgM class. Thus, the absence of IgM reactions to all of the XMRV antigens of the ME/CFS and blood donor sera could be contrasted with the ready detection of IgM binding to the Hib beads (data not shown). However, this cannot be considered a critical test for the presence of anti-XMRV IgM.
DISCUSSION
Because serology is indirect and measures the adaptive immune response to a microbe rather than the presence of the microbe itself, no serological test can be 100% sensitive and specific. One way of increasing the accuracy of serology is to simultaneously measure the immune responses to several antigens from a microbe. An especially demanding situation is to declare a sample “negative,” because of the many methodological reasons for a false-negative reaction. We therefore opted for a rather comprehensive combination of XMRV antigens, using a multiplex suspension array. Such arrays are increasingly being used for infectious disease serology. A few recent examples are described in references 5, 22, 30, 34, and 62. Their main advantages versus enzyme-linked immunosorbent assay (ELISA) are multiplexity, sample economy, and speed.
The proof of the presence of a virus in a population rests on virus isolation, nucleic acid detection, and serology (demonstration of a specific antibody response). Serology is often the most sensitive technique for demonstrating a present or past virus infection, because nucleic acid detection depends on a measureable concentration in easily obtained samples such as blood, which often is not present. Virus isolation is often difficult and more laborious to perform. A reliable serology is needed to establish the seroprevalence and seroepidemiology of the virus (54, 55). Rational multiantigen ELISA systems for viral antibodies have been implemented (9). However, the degree of multiplexity can be much greater in suspension microarrays, as demonstrated here. Suspension microarray serology is also quicker than ELISAs.
So far, the testing for presence of XMRV antibodies in humans has been limited to a few methods (6, 26, 31, 32, 38, 42, 66, 67). There is room for a serological study where multiple antigens are tested in the same reaction. This increases the comparability of results for individual antigens. A few previous studies have examined the antibody immune response to XMRV infection in animals (54, 55). Serological assays for detection of antibodies against XMRV include flow cytometry, Western blotting, chemiluminescent immunoassays, and enzyme-linked immunoassay techniques. Virus neutralization in cell culture has also been used (28, 40, 59, 77). The first report of antibodies to a retrovirus related to XMRV came from Lombardi et al., who found that 9 out of 18 ME/CFS patients infected with XMRV reacted with antibodies to a mouse B-cell line expressing spleen focus-forming virus (SFFV) Env, a protein closely related to XMRV Env. These antibodies were detected neither with SFFV-negative cell lines nor in 7 healthy blood donors (38). Eleven of 40 prostate cancer patients were reported to be XMRV antibody positive (6). In contrast, a Western blot study of sera from 104 ME/CFS patients and healthy control sera was negative (67). In a study of 565 non-ME/CFS and ME/CFS sera, 4.6% of the samples contained neutralizing antibodies. Only one of these was from an ME/CFS patient. Most of these antibodies were able to neutralize other similar viruses, indicating significant cross-reactivity (28). Hohn et al. searched for antibody activity of 173 sera from ME/CFS patients, multiple sclerosis patients, and healthy controls using recombinant Env in an ELISA (31); none were considered to be true positives. To determine the dynamics of the antibody response elicited by XMRV, three macaques were infected with XMRV. Using recombinant gp70, p15E, and P30 in Western blotting and chemiluminescent immunoassay (CMIA), Qiu et al. found evidence of antibodies at 2 weeks postinfection (54). They persisted for at least 158 days. Although all three proteins elicited an immune response, antibodies to recombinant gp70 and p15E showed a higher sensitivity for detection of infection than did antibodies to p30 (54, 55). In another study, Mus pahari was infected with XMRV. Antibodies reactive with XMRV Env and Gag proteins were observed in neutralization assays and Western blots (60). Since the report of XMRV in humans, many questions have been raised about how it is transmitted, its epidemiology, and its capability of causing human disease. The differences in the reported findings underscore the importance of a comprehensive and standardized diagnostic test. The absence of “true” positive human sera mandated the development of a series of computer-aided quality control features in our multiepitope serology. It was based on several types of controls (Fig. 2). The most important was based on sera from rabbits hyperimmunized with a mixture of 38 synthetic XMRV peptides. Most of the peptides gave rise to an immune reaction. Thus, the anti-pan-peptide sera could be used to assess the antigenicity of most XMRV peptides in each run. An index of peptide antigenicity was devised, which allowed the control of the general performance of the test. It depends both on the average performance of peptides and on general aspects such as protein G and phycoerythrin activities. The plot of peptide antigenicity is similar to Levey-Jennings control charts (72, 73) and can be used to approve or reject serological rounds. Interestingly, the antipeptide sera could also be used to assess the antigenicity of the 10 recombinant XMRV proteins, proving that antibodies elicited by peptides also reacted with the proteins from which the peptides were derived. Rules for issuing a warning regarding the antigenicity of a peptide or recombinant protein in a run can be programmed into the result calculation program. The variables in the control system can be followed between runs and report the stability over time (Table 2).
In this project we used a novel serological method for detecting XMRV antibodies. In addition to using nine recombinant proteins (p15, p12, p10, p30, gp70, p15E, and gp85, with some as short and long variants), we also used 38 synthetic peptides from the gag and env genes of XMRV. The peptides were designed to match those of known epitopes of feline leukemia virus (FeLV) and MuLV, both of which are thoroughly studied gammaretroviruses. FeLV causes cat leukemia. It has a high amino acid sequence identity to XMRV. The method of using synthetic peptides has been proven to be successful when developing serological assays for detection of other viruses such as HIV and HTLV (8, 10). The low frequency of antipeptide reactions but presence of strong anti-recombinant protein reactions in the sera of XMRV-infected mice and hyperimmunized goats was unexpected. One reason could be that the amino-terminally linked peptides were suboptimally accessible to antibodies through the 3-carbon-atom spacer. However, the strong reactions of the antipeptide sera indicated that the peptides were bound to beads in a form accessible to antibodies. In unpublished work we have tested a longer amino-terminal spacer (a polyethylene glycol spacer with six carbon atoms) (data not shown) with a Borrelia IR6 peptide, which had a marginal effect (an average of 16% increase in MFI with three Borrelia antibody-positive sera), and a carboxy-terminal three-carbon-atom polyethylene glycol (PEG) spacer with an amino group with an HIV peptide known to be highly antigenic with HIV-antibody positive sera, giving an average of a 24% increase in MFI with 10 HIV-positive sera. Moreover, we have now tested over 200 synthetic 30-mer peptides from a variety of human viruses coupled with the amino-terminal 3-carbon PEG spacer, and we found that approximately 20% of them gave high and specific serological reactions (J. Blomberg et al., unpublished data). Although there is more to explore regarding the optimal coupling of synthetic peptides to beads in suspension arrays, the available information indicates that the chosen format is valid for the problem addressed in this paper.
Another possible reason for the absence of antipeptide reactions in the XMRV-infected mice could be that the mice were selectively tolerant to linear XMRV-like epitopes due to endogenous retroviruses related to XMRV. A linear epitope is less susceptible to mutation in a protein than a conformational epitope. Breakage of B-cell tolerance after infection with a related exogenous retrovirus may therefore primarily affect linear epitopes. Further control experiments with FeLV antibody-positive sera could have given more information, but such sera were not available to us. The use of degenerate synthetic peptides is a novelty. We expected that such degenerate peptides would give more cross-reactions than nondegenerate analogs. However, this was not the case. We therefore envisage that degenerate peptides could be used in research studies or diagnostic procedures where antibodies to a number of related microbes are tested, to cover a range of antigenic variation, instead of a large number of separate nondegenerate peptides.
The fact that our study failed to detect a clear immune response to XMRV could have many explanations. It is possible that XMRV elicits a low-grade “stealth” infection in a limited number of cell types and is therefore able to hide from the immune response. Such may be the case for HTLV-2 infection, which sometimes is accompanied by a low-grade immune response (4, 36). Macaques infected with XMRV initially elicit a substantial immune response during the first weeks, but antibody titers and virus particles in sera were barely detectable at several months postinfection, which is a sign of either an abortive or a latent infection. Determining the dynamics of XMRV infection in humans is therefore crucial to set up a good diagnostic method. Geographical differences in seroprevalence could also occur. Other retroviruses, such as HTLV-1, show a great variation in distribution worldwide. Although our peptides were designed to accept some antigen amino acid sequence variability, divergent strains of unknown, potentially pathogenic, MuLV-like viruses could be missed by our method. No test can claim 100% sensitivity for targets at the outskirts of its probable detection range. It would therefore be valuable to further evaluate the range of detection of our test. A problem facing the development of our method was the shortage of positive-control sera from known XMRV-positive individuals. Peptides will not adopt the same tertiary conformation as the human cell-expressed virus proteins and thus may not have the same antigenic properties. This may affect the anti-XMRV antibody affinity to our peptides. Obviously, the 9 recombinant XMRV proteins (61) mimicked the antigenicity of XMRV proteins better than the peptides.
In conclusion, despite using a rather comprehensive multiplex serological analysis, we found no significant increases in seroreactivity to XMRV antigens among blood donors and ME/CFS patients. The absence of reactions of human sera contrasted with the high reactivities of sera from infected mice and hyperimmunized goats.
The conclusion of an absence of XMRV antibodies was aided by the novel internal quality control system. It allowed us to control the antigenicity of each antigen and the performance of most of the components of the assay, and it will be useful in other large-scale serological tests using suspension microarrays.
ACKNOWLEDGMENTS
We gratefully acknowledge financial support from ME Research UK (charity no. SC 036942), the Irish ME Trust, Stiftelsen Lars Hiertas Minne (FO2010-0672), the Olle Engqvist Foundation, and Replico Medical AB.
We thank Yasuhiro Ikeda, Sandra Ruscetti, and William Switzer for providing XMRV antibody-positive animal sera.
Footnotes
Published ahead of print 11 July 2012
REFERENCES
- 1. Ablashi DV, et al. 1991. Human herpesvirus 6 (HHV6) and chronic fatigue syndrome (CFS). Can. Dis. Wkly. Rep. 17(Suppl. 1E):33–40 [PubMed] [Google Scholar]
- 2. Alberts B. 2011. Retraction. Science 334:1636. [DOI] [PubMed] [Google Scholar]
- 3. Albino AP, et al. 1986. Class II histocompatibility antigen expression in human melanocytes transformed by Harvey murine sarcoma virus (Ha-MSV) and Kirsten MSV retroviruses. J. Exp. Med. 164:1710–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Andersson S, et al. 1995. HTLV infections among Swedish intravenous drug users in 1992. Scand. J. Infect. Dis. 27:547–550 [DOI] [PubMed] [Google Scholar]
- 5. Antonsson A, et al. 2010. Prevalence and stability of antibodies to the BK and JC polyomaviruses: a long-term longitudinal study of Australians. J. Gen. Virol. 91:1849–1853 [DOI] [PubMed] [Google Scholar]
- 6. Arnold RS, et al. 2010. XMRV infection in patients with prostate cancer: novel serologic assay and correlation with PCR and FISH. Urology 75:755–761 [DOI] [PubMed] [Google Scholar]
- 7. Blaise S, Mangeney M, Heidmann T. 2001. The envelope of Mason-Pfizer monkey virus has immunosuppressive properties. J. Gen. Virol. 82:1597–1600 [DOI] [PubMed] [Google Scholar]
- 8. Blomberg J, et al. 1993. A survey of synthetic HIV-1 peptides with natural and chimeric sequences for differential reactivity with Zimbabwean, Tanzanian and Swedish HIV-1-positive sera. AIDS 7:759–767 [DOI] [PubMed] [Google Scholar]
- 9. Blomberg J, Nilsson I, Andersson M. 1983. Viral antibody screening system that uses a standardized single dilution immunoglobulin G enzyme immunoassay with multiple antigens. J. Clin. Microbiol. 17:1081–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blomberg J, Robert-Guroff M, Blattner WA, Pipkorn R. 1992. Type- and group-specific continuous antigenic determinants of HTLV. Use of synthetic peptides for serotyping of HTLV-I and -II infection. J. Acquir. Immune Defic. Syndr. 5:294–302 [PubMed] [Google Scholar]
- 11. Blomberg J, et al. 2011. Phylogeny-directed search for murine leukemia virus-like retroviruses in vertebrate genomes and in patients suffering from myalgic encephalomyelitis/chronic fatigue syndrome and prostate cancer. Adv. Virol. 2011:341294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cameron B, et al. 2010. Serological and virological investigation of the role of the herpesviruses EBV, CMV and HHV-6 in post-infective fatigue syndrome. J. Med. Virol. 82:1684–1688 [DOI] [PubMed] [Google Scholar]
- 13. Carruthers BM. 2007. Definitions and aetiology of myalgic encephalomyelitis: how the Canadian consensus clinical definition of myalgic encephalomyelitis works. J. Clin. Pathol. 60:117–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carruthers BM, et al. 2011. Myalgic encephalomyelitis: international consensus criteria. J. Intern. Med. 270:327–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chia J, Chia A, Voeller M, Lee T, Chang R. 2010. Acute enterovirus infection followed by myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and viral persistence. J. Clin. Pathol. 63:165–168 [DOI] [PubMed] [Google Scholar]
- 16. Chiang CY, Chang JT, Lin MS, Wang SR, Chang HY. 2005. Characterization of a monoclonal antibody specific to the Gag protein of porcine endogenous retrovirus and its application in detecting the virus infection. Virus Res. 108:139–148 [DOI] [PubMed] [Google Scholar]
- 17.Coffin JM, Hughes SH, Varmus HE. (ed). 1997. Retroviruses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY: [PubMed] [Google Scholar]
- 18. DeFreitas E, et al. 1991. Retroviral sequences related to human T-lymphotropic virus type II in patients with chronic fatigue immune dysfunction syndrome. Proc. Natl. Acad. Sci. U. S. A. 88:2922–2926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. DeLeo AB, et al. 1982. Possible role of a retrovirus in the expression of tumor-specific antigens of the Meth A sarcoma. Int. J. Cancer 29:687–693 [DOI] [PubMed] [Google Scholar]
- 20. Dong B, et al. 2007. An infectious retrovirus susceptible to an IFN antiviral pathway from human prostate tumors. Proc. Natl. Acad. Sci. U. S. A. 104:1655–1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Earl PL, et al. 1986. T-lymphocyte priming and protection against Friend leukemia by vaccinia-retrovirus env gene recombinant. Science 234:728–731 [DOI] [PubMed] [Google Scholar]
- 22. Elberse KE, Tcherniaeva I, Berbers GA, Schouls LM. 2010. Optimization and application of a multiplex bead-based assay to quantify serotype-specific IgG against Streptococcus pneumoniae polysaccharides: response to the booster vaccine after immunization with the pneumococcal 7-valent conjugate vaccine. Clin. Vaccine Immunol. 17:674–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Elfaitouri A, et al. 2011. Murine gammaretrovirus group g3 was not found in Swedish patients with myalgic encephalomyelitis/chronic fatigue syndrome and fibromyalgia. PLoS One 6:e24602 doi:10.1371/journal.pone.0024602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eligio P, Delia R, Valeria G. 2010. EBV chronic infections. Mediterr. J. Hematol. Infect. Dis. 2:e2010022 doi:10.4084/MJHID.2010.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fukuda K, et al. 1994. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann. Intern. Med. 121:953–959 [DOI] [PubMed] [Google Scholar]
- 26. Furuta RA, et al. 2011. No association of xenotropic murine leukemia virus-related virus with prostate cancer or chronic fatigue syndrome in Japan. Retrovirology 8:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Geering G, Hardy WD, Jr, Old LJ, de Harven E, Brodey RS. 1968. Shared group-specific antigen of murine and feline leukemia viruses. Virology 36:678–680 [DOI] [PubMed] [Google Scholar]
- 28. Groom HC, et al. 2010. Absence of xenotropic murine leukaemia virus-related virus in UK patients with chronic fatigue syndrome. Retrovirology 7:10 doi:10.1186/1742-4690-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hardy WD, Jr, et al. 1969. Feline leukemia virus: occurrence of viral antigen in the tissues of cats with lymphosarcoma and other diseases. Science 166:1019–1021 [DOI] [PubMed] [Google Scholar]
- 30. Heiligenberg M, et al. 2010. Seroprevalence and determinants of eight high-risk human papillomavirus types in homosexual men, heterosexual men, and women: a population-based study in Amsterdam. Sex. Transm. Dis. 37:672–680 [DOI] [PubMed] [Google Scholar]
- 31. Hohn O, et al. 2009. Lack of evidence for xenotropic murine leukemia virus-related virus(XMRV) in German prostate cancer patients. Retrovirology 6:92 doi:10.1186/1742-4690-6-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hohn O, et al. 2010. No evidence for XMRV in German CFS and MS patients with fatigue despite the ability of the virus to infect human blood cells in vitro. PLoS One 5:e15632 doi:10.1371/journal.pone.0015632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kerr JR. 2008. Enterovirus infection of the stomach in chronic fatigue syndrome/myalgic encephalomyelitis. J. Clin. Pathol. 61:1–2 [DOI] [PubMed] [Google Scholar]
- 34. Khan IH, et al. 2010. Microbead arrays for the analysis of ErbB receptor tyrosine kinase activation and dimerization in breast cancer cells. Assay Drug Dev. Technol. 8:27–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Klasse PJ, Pipkorn R, Blomberg J. 1988. Presence of antibodies to a putatively immunosuppressive part of human immunodeficiency virus (HIV) envelope glycoprotein gp41 is strongly associated with health among HIV-positive subjects. Proc. Natl. Acad. Sci. U. S. A. 85:5225–5229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Krook A, Blomberg J. 1994. HTLV-II among injecting drug users in Stockholm. Scand. J. Infect. Dis. 26:129–132 [DOI] [PubMed] [Google Scholar]
- 37. Langhammer S, Fiebig U, Kurth R, Denner J. 2005. Neutralising antibodies against the transmembrane protein of feline leukaemia virus (FeLV). Vaccine 23:3341–3348 [DOI] [PubMed] [Google Scholar]
- 38. Lombardi VC, et al. 2009. Detection of an infectious retrovirus, XMRV, in blood cells of patients with chronic fatigue syndrome. Science 326:585–589 [DOI] [PubMed] [Google Scholar]
- 39. Lostrom ME, et al. 1979. Monoclonal antibodies against murine leukemia viruses: identification of six antigenic determinants on the p 15(E) and gp70 envelope proteins. Virology 98:336–350 [DOI] [PubMed] [Google Scholar]
- 40. Makarova N, et al. 2011. Antibody responses against xenotropic murine leukemia virus-related virus envelope in a murine model. PLoS One 6:e18272 doi:10.1371/journal.pone.0018272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mangeney M, Heidmann T. 1998. Tumor cells expressing a retroviral envelope escape immune rejection in vivo. Proc. Natl. Acad. Sci. U. S. A. 95:14920–14925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mikovits JA, et al. 2010. Distribution of xenotropic murine leukemia virus-related virus (XMRV) infection in chronic fatigue syndrome and prostate cancer. AIDS Rev. 12:149–152 [PubMed] [Google Scholar]
- 43. Miyazawa M, Fujisawa R. 1994. Physiology and pathology of host immune responses to exogenous and endogenous murine retroviruses—from gene fragments to epitopes. Tohoku J. Exp. Med. 173:91–103 [DOI] [PubMed] [Google Scholar]
- 44. Nick S, et al. 1990. Virus neutralizing and enhancing epitopes characterized by synthetic oligopeptides derived from the feline leukaemia virus glycoprotein sequence. J. Gen. Virol. 71:77–83 [DOI] [PubMed] [Google Scholar]
- 45. Nowinski RC, Pickering R, O'Donnell PV, Pinter A, Hammerling U. 1981. Selective neutralization of ecotropic murine leukemia virus by monoclonal antibodies: localization of a site on the gp70 protein associated with ecotropism. Virology 111:84–92 [DOI] [PubMed] [Google Scholar]
- 46. Old LJ, Boyse EA. 1965. Antigens of tumors and leukemias induced by viruses. Fed Proc. 24:1009–1017 [PubMed] [Google Scholar]
- 47. Ono T, et al. 2000. Serological analysis of BALB/C methylcholanthrene sarcoma Meth A by SEREX: identification of a cancer/testis antigen. Int. J. Cancer 88:845–851 [DOI] [PubMed] [Google Scholar]
- 48. Pheby D, et al. 2011. A disease register for ME/CFS: report of a pilot study. BMC Res. Notes 4:139 doi:10.1186/1756-0500-4-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pickering JW, Larson MT, Martins TB, Copple SS, Hill HR. 2010. Elimination of false-positive results in a Luminex assay for pneumococcal antibodies. Clin. Vaccine Immunol. 17:185–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pinter A, Honnen WJ. 1984. Characterization of structural and immunological properties of specific domains of Friend ecotropic and dual-tropic murine leukemia virus gp70s. J. Virol. 49:452–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pinter A, Honnen WJ. 1983. Topography of murine leukemia virus envelope proteins: characterization of transmembrane components. J. Virol. 46:1056–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pinter A, Honnen WJ, Tung JS, O'Donnell PV, Hammerling U. 1982. Structural domains of endogenous murine leukemia virus gp70s containing specific antigenic determinants defined by monoclonal antibodies. Virology 116:499–516 [DOI] [PubMed] [Google Scholar]
- 53. Prins JB, Bleijenberg G, van der Meer JW. 2002. Chronic fatigue syndrome and myalgic encephalomyelitis. Lancet 359:1699. [DOI] [PubMed] [Google Scholar]
- 54. Qiu X, et al. 2010. Characterization of antibodies elicited by XMRV infection and development of immunoassays useful for epidemiologic studies. Retrovirology 7:68 doi:10.1186/1742-4690-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Qiu X, et al. 2012. Seroprevalence of xenotropic murine leukemia virus-related virus in normal and retrovirus-infected blood donors. Transfusion 52:307–316 [DOI] [PubMed] [Google Scholar]
- 56. Rucheton M, et al. 1985. Presence of circulating antibodies against gag-gene MuLV proteins in patients with autoimmune connective tissue disorders. Virology 144:468–480 [DOI] [PubMed] [Google Scholar]
- 57. Rucheton M, Graafland H, Valles H, Larsen CJ. 1987. Human autoimmune serum antibodies against gag gene p30 retroviral protein also react with a U1-SnRNP 68K comigrant protein. Biol. Cell 60:71–72 [DOI] [PubMed] [Google Scholar]
- 58. Rusmevichientong A, Das Gupta J, Elias PS, Silverman RH, Chow SA. 2011. Analysis of single-nucleotide polymorphisms in patient-derived retrovirus integration sites reveals contamination from cell lines acutely infected by xenotropic murine leukemia virus-related virus. J. Virol. 85:12830–12834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sakuma T, et al. 2011. No evidence of XMRV in prostate cancer cohorts in the midwestern United States. Retrovirology 8:23 doi:10.1186/1742-4690-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sakuma T, et al. 2011. Early events in retrovirus XMRV infection of the wild-derived mouse Mus pahari. J. Virol. 85:1205–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sheikholvaezin A, Blomberg F, Ohrmalm C, Sjosten A, Blomberg J. 2011. Rational recombinant XMRV antigen preparation and bead coupling for multiplex serology in a suspension array. Protein Expr. Purif. 80:176–184 [DOI] [PubMed] [Google Scholar]
- 62. Shoma S, et al. 2011. Development of a multiplexed bead-based immunoassay for the simultaneous detection of antibodies to 17 pneumococcal proteins. Eur. J. Clin. Microbiol. Infect. Dis. 30:521–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sijts EJ, et al. 1994. Cloning of the MCF1233 murine leukemia virus and identification of sequences involved in viral tropism, oncogenicity and T cell epitope formation. Virus Res. 34:339–349 [DOI] [PubMed] [Google Scholar]
- 64. Silverman RH, et al. 2011. Partial retraction. Detection of an infectious retrovirus, XMRV, in blood cells of patients with chronic fatigue syndrome. Science 334:176. [DOI] [PubMed] [Google Scholar]
- 65. Simmons G, et al. 2011. Failure to confirm XMRV/MLVs in the blood of patients with chronic fatigue syndrome: a multi-laboratory study. Science 334:814–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Stieler K, et al. 2011. No detection of XMRV in blood samples and tissue sections from prostate cancer patients in Northern Europe. PLoS One 6:e25592 doi:10.1371/journal.pone.0025592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Switzer WM, et al. 2010. Absence of evidence of xenotropic murine leukemia virus-related virus infection in persons with chronic fatigue syndrome and healthy controls in the United States. Retrovirology 7:57 doi:10.1186/1742-4690-7-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Urisman A, et al. 2006. Identification of a novel gammaretrovirus in prostate tumors of patients homozygous for R462Q RNASEL variant. PLoS Pathog. 2:e25 doi:10.1371/journal.ppat.0020025. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 69. Waterboer T, Sehr P, Pawlita M. 2006. Suppression of non-specific binding in serological Luminex assays. J. Immunol. Methods 309:200–204 [DOI] [PubMed] [Google Scholar]
- 70. Weijer K, et al. 1993. Induction of feline leukaemia virus-neutralizing antibodies by immunization with synthetic peptides derived from the FeLV env gene. Vaccine 11:946–956 [DOI] [PubMed] [Google Scholar]
- 71. Weijer K, Uytdehaag FG, Osterhaus AD. 1989. Control of feline leukaemia virus. Vet. Immunol. Immunopathol. 21:69–83 [DOI] [PubMed] [Google Scholar]
- 72. Westgard JO. 2003. Internal quality control: planning and implementation strategies. Ann. Clin. Biochem. 40:593–611 [DOI] [PubMed] [Google Scholar]
- 73. Westgard JO, Barry PL, Hunt MR, Groth T. 1981. A multi-rule Shewhart chart for quality control in clinical chemistry. Clin. Chem. 27:493–501 [PubMed] [Google Scholar]
- 74. Wolfe F, et al. 1990. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 33:160–172 [DOI] [PubMed] [Google Scholar]
- 75. Zachrisson O, Regland B, Jahreskog M, Kron M, Gottfries CG. 2002. A rating scale for fibromyalgia and chronic fatigue syndrome (the FibroFatigue scale). J. Psychosom. Res. 52:501–509 [DOI] [PubMed] [Google Scholar]
- 76. Zhang L, et al. 2010. Microbial infections in eight genomic subtypes of chronic fatigue syndrome/myalgic encephalomyelitis. J. Clin. Pathol. 63:156–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zhou Y, et al. 2012. Development and application of a high-throughput microneutralization assay: lack of xenotropic murine leukemia virus-related virus and/or murine leukemia virus detection in blood donors. Transfusion 52:332–342 [DOI] [PMC free article] [PubMed] [Google Scholar]