Abstract
Streptococcus pneumoniae is a pathogen of great importance worldwide. We have previously described the efficacy of a nasal vaccine composed of the pneumococcal surface protein A and the whole-cell pertussis vaccine as an adjuvant against a pneumococcal invasive challenge in mice. Spread of bacteria to the bloodstream was probably prevented by the high levels of systemic antibodies induced by the vaccine, but bacteria were only cleared from the lungs 3 weeks later, indicating that local immune responses may contribute to survival. Here we show that a strict control of inflammatory responses in lungs of vaccinated mice occurs even in the presence of high numbers of pneumococci. This response was characterized by a sharp peak of neutrophils and lymphocytes with a simultaneous decrease in macrophages in the respiratory mucosa at 12 h postchallenge. Secretion of interleukin-6 (IL-6) and gamma interferon (IFN-γ) was reduced at 24 h postchallenge, and the induction of tumor necrosis factor alpha (TNF-α) secretion, observed in the first hours postchallenge, was completely abolished at 24 h. Before challenge and at 12 h postchallenge, vaccinated mice displayed higher numbers of CD4+ T, CD8+ T, and B lymphocytes in the lungs. However, protection still occurs in the absence of each of these cells during the challenge, indicating that other effectors may be related to the prevention of lung injuries in this model. High levels of mucosal anti-PspA antibodies were maintained in vaccinated mice during the challenge, suggesting an important role in protection.
INTRODUCTION
Streptococcus pneumoniae is the main etiological agent of bacterial pneumonia, meningitis, and sepsis and can be of great importance, especially in children from developing countries. Every year, 1 million deaths of children under 5 years occur due to pneumococcal diseases (39). Current vaccines are composed of polysaccharides (PS) from different serotypes conjugated to protein carriers. Acquired immunity is achieved through the induction of anti-PS antibodies, protecting vaccinated children against colonization and invasive pneumococcal diseases caused by the serotypes included in the formulations (8, 46). The induction of serotype-specific protection is an important issue to be considered, since variation in the prevalent serotypes (from more than 90 serotypes described) among different regions of the world is observed (13, 23, 49). Several proposals of new vaccines are based on conserved antigens that could confer protection against virtually all pneumococcal serotypes (10, 30, 32, 37, 40–42). These vaccines also have emerged as a way to avoid a possible consequence related to the massive use of the conjugated vaccines, that is, the replacement of the prevalent serotypes by others not included in the formulations (17, 56). Depending on the composition, the new vaccines can confer protection in mouse models of pneumococcal infection by the induction of specific antibodies and/or cellular immune responses (31).
In an experimental pneumococcal carriage study in humans, McCool and collaborators reported a significant rise in serum IgG against the pneumococcal surface protein A (PspA) (35). Different immunization strategies and animal models were used to confirm PspA as a good vaccine candidate (4, 12, 36, 57). This antigen has already undergone a phase I clinical trial and was shown to be immunogenic in humans (38). Sera from immunized subjects were able to passively protect mice against pneumococcal lethal challenges with different serotypes (11). PspA is expressed by all pneumococcal isolates, but the N-terminal region of the molecule, which contains protective epitopes, is highly variable. Sequencing analyses led to the classification of PspAs in 6 clades that can be grouped into three families (26). Cross-reactivity was reported for molecules that belong to the same family or for specific molecules that induce antibodies that recognize PspAs from different clades (18). Broad-coverage vaccines based on PspA would thus depend on the use of more than one molecule or on the choice of specific PspA molecules (37). In animal models, protection elicited by vaccines composed of PspA is often accompanied by the induction of high levels of specific antibodies (10, 22, 24, 44) which, upon binding to pneumococcal surface, promote the deposition of complement (9, 12, 47, 57) and enhance killing by lactoferrin (9, 48). In addition, the use of adjuvants that elicit Th1 immune responses against PspA seems to optimize protection (4, 19, 20). We have previously used the whole-cell pertussis vaccine (wP) as an adjuvant to a nasal vaccine formulated with an N-terminal fragment of PspA from clade 5 (PspA5-wP) (43). As it is composed of whole bacteria, wP can modulate the immune response, inducing a Th1 and/or a Th17 character (7, 25) that may improve responses against combined antigens (6, 51, 52). Mice immunized with PspA5-wP were protected against an invasive respiratory challenge with a serotype 3 strain (43). Spread of the bacteria to the bloodstream was prevented, probably by the high levels of anti-PspA antibodies observed in vaccinated mice. However, despite the presence of antibodies, complete clearance of bacteria from the lungs occurred only 3 weeks after the challenge, suggesting that immune responses in the respiratory mucosa may contribute to protection (43). The control of inflammatory responses in lungs seems to be an important feature for protection against pneumococcal respiratory invasive challenges in mice (20, 21). Here we have evaluated the mucosal immune responses elicited in lungs of mice vaccinated with PspA5-wP. Infiltration of cells in the respiratory mucosa as well as the secretion of cytokines in the lungs of vaccinated mice after the challenge was evaluated. The roles of CD4+ T, CD8+ T, and B lymphocytes in the protective responses induced by the PspA5-wP vaccine were also addressed.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
S. pneumoniae was grown in Todd-Hewitt broth (Difco) supplemented with 0.5% yeast extract (THY) at 37°C without shaking. Bacteria were always plated in blood agar and grown overnight at 37°C before inoculation in THY. Stocks were maintained at −80°C in THY containing 20% glycerol. The pneumococcal ATCC 6303 strain (serotype 3, PspA clade 5) was a kind gift from Maria Cristina Brandileone (Instituto Adolpho Lutz, São Paulo, Brazil) and was used for the lethal respiratory challenge. ATCC 6303-derived ΔpspA or ΔpspC mutants (6303PspA− and 6303PspC−) were constructed by insertion-deletion using erythromycin and spectinomycin resistance cassettes, respectively (1, 50). In both cases the entire sequences of the genes were replaced. The ATCC 6303 Δpsp ΔpspC double mutant (6303PspA−/PspC−) was constructed by the same procedure using the construct to delete pspC to transform the 6303 ΔpspA strain. Mutant strains were grown in the presence of 1 μg/ml of erythromycin, 300 μg/ml of spectinomycin, or both.
Recombinant proteins and vaccine formulations.
The N-terminal fragment of PspA from clade 5 (from strain 122/02; Instituto Adolpho Lutz, São Paulo, Brazil), was expressed in Escherichia coli BL21 SI (Invitrogen) and purified by chromatography as previously described (18). The whole-cell pertussis vaccine (wP) is composed of the whole bacteria inactivated with 0.2% formalin and is currently produced by Instituto Butantan (São Paulo, Brazil) for the formulation of the DTPw vaccine [composed of diphtheria and tetanus toxoids and inactivated whole-cell pertussis in the presence of Al(OH)3], which is administered to Brazilian children.
Immunization of mice and antibody responses.
BALB/c mice were produced by the animal facility from the Medical School of University of São Paulo. Animals were supplied with food and water ad libitum, and experimental protocols were approved by the Ethics Committee for Animal Use, Instituto Butantan. Immunization was performed in groups of 4 to 6 female mice. Each vaccine dose contained 5 μg of PspA5 alone or in the presence of 1/8 of the human wP dose. Before formulation, the excess lipopolysaccharide (LPS) from E. coli present in PspA5 preparations was removed by Triton X-114 extraction as previously described (2). Nasal immunization was conducted in mice previously anesthetized through the intraperitoneal (i.p.) route with 200 μl of a mixture of 0.2% xilazine and 0.5% ketamine. Vaccines were administered in a volume of 10 μl on days 0, 3, 14, 17, 28, and 31 (total of 6 doses). This protocol was chosen because it induces high levels of circulating anti-PspA5 antibodies in mice immunized with PspA5-wP (43). Groups that received only wP or PspA5, as well as nonimmunized mice, were used as controls. Bronchoalveolar lavage fluids (BALF) collected 21 days after the last immunization or at different time points after the challenge, as described below, were evaluated for antibody levels by enzyme-linked immunosorbent assay (ELISA) in plates coated with PspA5. The assay was performed using goat anti-mouse IgG or IgA and rabbit anti-goat serum conjugated with horseradish peroxidase (HRP) (Southern Biotech, Birmingham, AL). Standard curves were generated using mouse IgG or IgA (Southern Biotech).
Intranasal pneumococcal challenge.
Immunized mice were anesthetized through the intraperitoneal route with 200 μl of a mixture of 0.2% xilazine and 1.0% ketamine. The ATCC 6303 strain was grown in THY until mid-log phase (optical density at 600 nm [OD600] = 0.4), and aliquots were maintained at −80°C until use. Infections were performed with thawed bacteria 21 days after the last immunization. Animals received 3 × 105 CFU in 50 μl of saline, which was inoculated into one nostril with the help of a micropipette. Animals were observed for 10 days or for 21 days, depending on the experiment, for survival records.
Recovery of bacteria from blood and lungs.
Lungs from each mouse were collected and disrupted in 1 ml of half-saline with the use of a cell strainer. Serial dilutions of lung homogenates or blood (collected through the retroorbital plexus) were plated on blood agar. Plates were incubated overnight at 37°C, and the number of CFU was determined. Detection of 0 CFU was considered 1 CFU. The limit of detection was 100 CFU/ml in blood or lung samples.
BALF collection and cytospin and cytokine analysis.
Mice were sacrificed before or at different time points after the challenge through injection of a lethal dose of urethane (15 mg per 10 g of body weight). A catheter was inserted into the tracheae of mice, and lungs were rinsed with 0.5 ml of sterile phosphate-buffered saline (PBS), followed by an additional rinse with 1 ml of PBS. The fluids from both rinses were pooled to compose the BALF. Cells obtained from the fluid of each animal were counted, and 4 × 104 cells were spun onto glass slides (4 min at 1,300 rpm) by the use of a cytocentrifuge (StatSpin Cytofuge; Iris International, Inc., Chatsworth, CA). Cytospin slides were stained, and 100 cells were differentially counted to determine the infiltration of each cell type. Cell-free fluid samples were stored at −80°C for cytokine analysis. BALF samples were analyzed through sandwich ELISA for the presence of interleukin-6 (IL-6), gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), and IL-10 (Peprotech Inc., Rocky Hill, NJ) and IL-17 (monoclonal antibodies from BD Biosciences, Franklin Lakes, NJ, and recombinant protein from R&D Systems, Minneapolis, MN) according to the instructions of the manufacturers.
Detection of antigen-specific IL-17.
BALB/c mice (5 per group) were vaccinated through the nasal route and challenged with the ATCC 6303 pneumococcal strain as described above. Animals were sacrificed at 12 h after challenge for lung and spleen removal. Both lungs were dissected into small pieces and digested with a collagenase-DNase solution (collagenase type IV and DNase at 150 and 50 units/ml, respectively; Sigma-Aldrich, St. Louis, MO). Single-cell suspensions of spleen from each mouse were obtained as described before (19). Viable cell counts in lungs and spleens were determined by trypan blue exclusion. Cells (5 × 106/ml) were incubated in 5% CO2 at 37°C in the absence or presence of 5 μg/ml of PspA5. Detection of IL-17 was performed by sandwich ELISA of culture supernatants (using monoclonal IL-17 antibodies from BD Biosciences and recombinant IL-17 from R&D Systems) 72 h after stimulation with the recombinant protein.
Analysis of lymphocyte infiltration in the respiratory tracts of immunized mice.
BALF samples were collected as described above from immunized mice before or at different time points after the challenge (3 to 5 mice per group). Cells were collected by centrifugation at 100 × g for 10 min. Suspensions of 106 cells in 100 μl of PBS were incubated for 30 min on ice with one of the following antibodies: allophycocyanin (APC)-conjugated anti-mouse CD4 (RM4-5), peridinin chlorophyll protein (PerCP)-conjugated anti-mouse CD8 (53-67), or phycoerythrin (PE)-conjugated anti-B220 (clone RA3-6B2) (BD Biosciences). Samples were washed twice with PBS, suspended in 200 μl of Cytofix (BD Biosciences), and stored at 4°C until analysis. Flow cytometry was performed using FACS Canto II (BD Biosciences) with 10,000 gated lymphocytes. Samples were analyzed using the Flow Jo 7.6.1 software (Tree Star, Ashland, OR).
Depletion of lymphocytes in immunized mice.
Groups of 6 mice were immunized with the combination of PspA5 and wP (PspA5-wP) as described above. Immunized mice received 250 μg of purified anti-CD4 (GK1.5), anti-CD8 (2.43), anti-B220 (RA3-6B2), IgG from rat serum (Sigma-Aldrich), or saline, by the intraperitoneal route, at 48 h and 12 h before the challenge as well as 48 h after the challenge, for a total of three injections. An additional control group was immunized with wP alone. Flow cytometry analysis of splenocytes before and 12 h after the challenge confirmed the depletion of more than 98% of each cell type in all cases.
Antibody binding and complement deposition assays.
S. pneumoniae strains were grown on blood agar overnight. Bacteria were diluted in THY, grown to an OD600 of 0.4 to 0.5 (∼108 CFU/ml), and harvested by centrifugation at 3,200 × g for 10 min. Bacteria were washed, suspended in PBS, and incubated with 10% or 50% of BALF samples for 30 min at 37°C. Samples were washed once with PBS before incubation with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG, IgA, IgG1, or IgG2a (MP Biomedicals, Solon, OH), diluted 1:100 in PBS, for 30 min on ice. For complement deposition assays, BALFs were previously heated at 56°C for 30 min and incubated with bacteria at a concentration of 50% at 37°C for 30 min. Samples were washed once with PBS and incubated with 10% normal mouse serum as source of complement in gelatin-Veronal buffer (Sigma-Aldrich) at 37°C for 30 min. After washing, samples were incubated with FITC-conjugated anti-mouse C3 IgG (MP Biomedicals) in PBS for 30 min on ice. Samples were fixed with 200 μl of Cytofix (BD Biosciences) after two washing steps and stored at 4°C. Flow cytometry analysis was conducted using FACS Canto II (BD Biosciences), and 10,000 gated events were recorded. Fluorescence was analyzed in histograms using the Flow Jo 7.6.1 software, and medians of the curves were used to compare the groups.
Statistical analysis.
Differences in CFU, antibody and cytokine concentrations, and number of infiltrated cells in BALF were analyzed with the Mann-Whitney U test. Differences in CD4+, CD8+, or B+ lymphocytes in BALF samples were analyzed by one-way analysis of variance (ANOVA) (with Tukey's posttest). Survival was analyzed with the Fisher exact test. Statistical analyses were performed using Prism 5.03 software, and a P value of ≤0.05 was considered significantly different.
RESULTS
Pneumococcal loads in the lungs of mice vaccinated with PspA5-wP remain steady in the first hours after challenge.
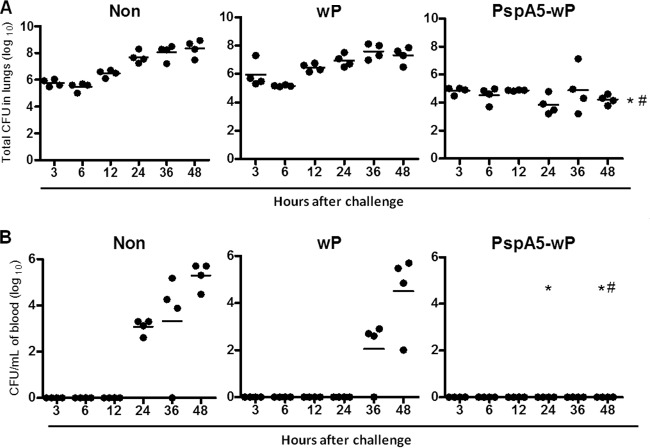
We have previously shown that the PspA5-wP nasal vaccine induces high levels of anti-PspA5 antibodies and confers protection to mice (100% survival) against a respiratory lethal challenge with the ATCC 6303 strain. Although we have not detected bacteria in the bloodstream of immunized mice, complete clearance from the lungs takes around 3 weeks to occur (43). Here we have analyzed the responses elicited in the lungs at the first hours after challenge. Groups of 4 BALB/c mice were immunized with each formulation through the nasal route and given the respiratory challenge with the ATCC 6303 pneumococcal strain (3 × 105 CFU/mouse). Pneumococcal loads in lung homogenates and blood were determined at different time points after the challenge. As observed in Fig. 1A, at 3 h after challenge, pneumococcal counts were around 106 bacteria per lung homogenate in nonimmunized mice or mice immunized with wP. These numbers progressively increased, reaching around 107 to 109 bacteria per lung homogenate at 48 h. On the other hand, pneumococcal loads in the lungs of mice immunized with PspA5-wP were already significantly lower at 3 h after the challenge than those recovered from each control group at the same time point. The numbers remained significantly lower in this group at all time points tested. Although we have detected significant differences between vaccinated mice and mice from control groups, the levels of bacteria detected in the lungs of mice immunized with PspA5-wP remained stable during the entire experiment (P > 0.05 when comparing bacteria recovered at 3 h with bacteria recovered at the different time points) (Fig. 1A). Pneumococci could be recovered from the blood of nonimmunized mice or mice immunized with wP, starting from 24 or 36 h after the challenge (Fig. 1B), and the levels increased at 48 h. Mice from parallel groups were observed for longer periods and started to die at around 72 h after the challenge. As we have previously reported, immunization with PspA5-wP prevented pneumococcal invasion to the bloodstream (Fig. 1B) and death (43).
Fig 1.
Pneumococcal loads in the lungs and blood of mice after challenge with the ATCC 6303 strain. Lungs (A) or blood (B) from nonimmunized BALB/c mice (Non) or mice immunized with wP or PspA5-wP were collected at different time points after the intranasal challenge. CFU were determined in four mice per group after plating the samples in blood agar. Circles represent each animal, and lines represent the mean for each group. # and *, significantly lower numbers of cells compared with those at the same time point in nonimmunized mice or mice immunized with wP, respectively. In panel A, P = 0.03 for all time points except at 36 h, when no significant difference was observed when mice immunized with PspA5-wP were compared with mice inoculated with wP. In panel B, P = 0.02 for the time points shown in the graph.
Protection induced by the PspA5-wP vaccine correlates with controlled cell infiltration in BALF.
BALF samples were collected at different periods after the challenge, and cell infiltrates were evaluated (Fig. 2). A peak in total cell infiltrate at 12 h after challenge was observed in groups inoculated with wP and PspA5-wP (P < 0.05 compared with nonimmunized mice). However, the number of total cells recovered from BALF tended to decrease in mice immunized with PspA5-wP, achieving preinfection levels in 36 h (Fig. 2A). In contrast, nonimmunized mice or mice immunized with wP showed progressively increasing numbers of BALF cells at 36 and 48 h postchallenge (Fig. 2A).
Fig 2.
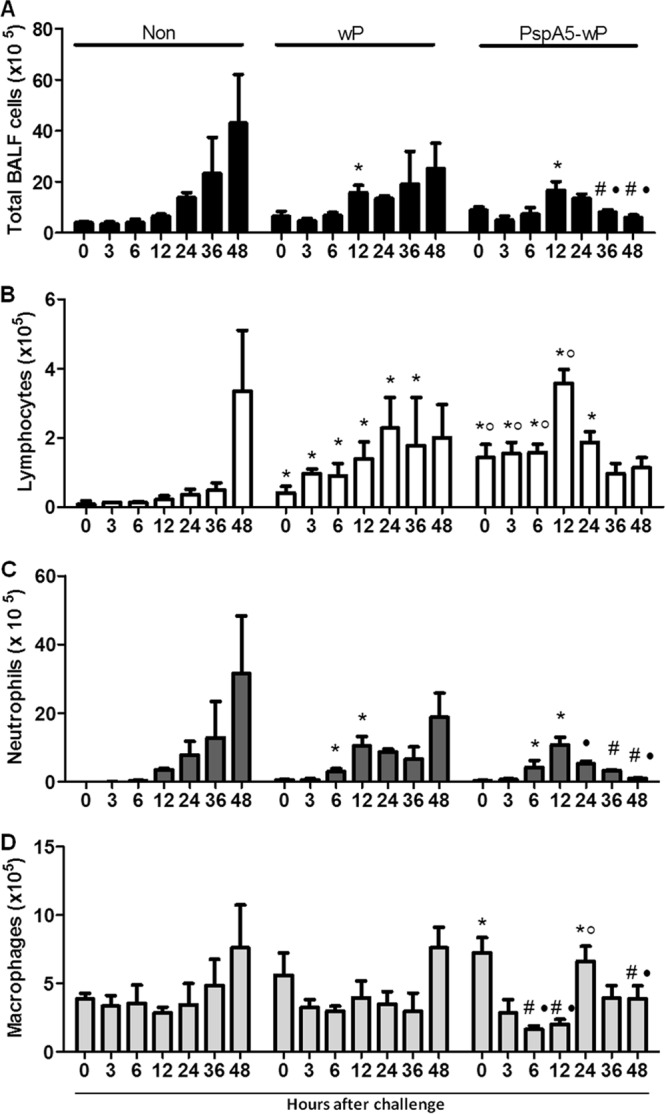
Infiltration of cells in the respiratory tracts of mice after challenge with the ATCC 6303 strain. BALF samples were collected from nonimmunized BALB/c mice (Non) or mice immunized with wP or PspA5-wP at different time points after the intranasal challenge, and slides were prepared with 4 × 104 cells. Numbers of total infiltrated cells (A), lymphocytes (B), neutrophils (C), or macrophages (D) are expressed as means for 4 mice per group with standard deviations. *, significantly higher numbers of cells compared with those at the same time point in nonimmunized mice; ○, significantly higher numbers of cells compared with those at the same time point in mice immunized with wP; #, significantly lower numbers of cells compared with those at the same time point in nonimmunized mice; ●, significantly lower numbers of cells compared with those at the same time point in mice immunized with wP. Statistical analyses were performed with the Mann-Whiney U test, and P ≤ 0.05 for all the comparisons marked.
Differential cell counting revealed that the number of lymphocytes in PspA5-wP-vaccinated mice were significantly higher than that observed in nonimmunized mice or mice immunized with wP (P < 0.05) even before challenge (time 0). These levels remained higher than those observed in both control groups until 12 h, when they started to reduce, reaching preinfection levels at 24 h (Fig. 2B). Preinfection levels of lymphocytes in mice inoculated with wP were also higher than those observed in nonimmunized mice (P < 0.05), but the increase observed after the pneumococcal challenge was not controlled even after 48 h (Fig. 2B). An increase in lymphocytes in nonimmunized mice was detected only at 48 h after the challenge (Fig. 2B), when mice from this group already displayed significant evidence of disease.
Neutrophil levels in all groups were very low before the challenge (Fig. 2C). A narrow peak in neutrophil infiltration was observed in mice vaccinated with PspA5-wP, beginning at 6 h and remaining until 12 h after the challenge (P < 0.05 compared with nonimmunized mice). Basal levels were restored after that (Fig. 2C). An increase in neutrophils was also observed for mice immunized with wP, at 6 h after the challenge, but these cell numbers remained high after that. For nonimmunized mice, infiltration of neutrophils in lungs started only at 12 h after the challenge and was not controlled from then on (Fig. 2C).
Before challenge, macrophage numbers were higher in the lungs of vaccinated mice than those of nonimmunized animals (P < 0.05). A decrease in infiltrated macrophages was observed at 6 h and 12 h after the challenge in mice vaccinated with PspA5-wP (P < 0.05 compared with control groups), corresponding to the same time points for lymphocyte and neutrophil peaks. However, the levels of macrophages increased again at 24 h postchallenge (P = 0.05 or P < 0.05 compared with nonimmunized mice or mice immunized with wP, respectively) and were restored to the basal levels at later time points (Fig. 2D). An increase in total macrophages was observed in both control groups at 48 h after the challenge (Fig. 2D). We have also analyzed cell infiltration in the lungs of mice immunized with PspA5-wP, at longer periods after the pneumococcal challenge. Although effective clearance of pneumococci takes around 21 days to occur (43), no significant changes in cell infiltrates were observed at time points later than 72 h (data not shown).
Protection induced by the PspA5-wP vaccine correlates with controlled secretion of inflammatory cytokines in BALF.
Mice immunized with PspA5-wP displayed higher levels of IL-6 in BALF, before (time 0) and 3 h and 6 h after the challenge, than nonimmunized mice (P = 0.05) (Fig. 3A). This was also observed for mice inoculated with wP (P = 0.05 at 0 and 3 h; P = 0.02 at 6 h). However, a decrease in IL-6 concentration was observed in mice vaccinated with PspA5-wP at 12 h after the challenge (P = 0.02) but not in mice inoculated with wP (Fig. 3A). Low levels of IL-6 were maintained in BALF from mice vaccinated with PspA5-wP until the end of the experiment. In the case of nonimmunized mice, IL-6 levels were low before the challenge and achieved the basal levels observed in the other groups only at 6 h after challenge. For both control groups, the levels of IL-6 remained high at the end of the experiment (Fig. 3A). A marked decrease in IFN-γ levels was observed in BALF from mice vaccinated with PspA5-wP, at 24 h after the challenge (P = 0.02 compared with nonimmunized mice at 24 h and 36 h and compared with mice inoculated with wP at 36 h; P = 0.05 compared with mice inoculated with wP at 24 h and with both groups at 48 h) (Fig. 3B). IFN-γ levels tended to increase at late time points after pneumococcal challenge in control groups (Fig. 3B). Pneumococcal challenge induced an increase in TNF-α in BALF in all groups tested; still, low levels of TNF-α were restored in mice vaccinated with PspA5-wP, at 24 h after challenge, and remained low until the end of the experiment (Fig. 3C). In fact, at 12 h after the challenge, the levels of TNF-α were already significantly lower in mice vaccinated with PspA5-wP than in nonimmunized mice or mice immunized with wP (P = 0.02 for both comparisons). These significant differences were maintained until 48 h after the challenge (Fig. 3C). The profiles of IL-17 secretion in BALF from the three groups of mice were very similar, with decreasing levels as time passed after challenge (Fig. 3D). However, slightly larger amounts of this cytokine were observed before (time 0; P = 0.05) and at 12 h after (P = 0.02) the challenge in the group vaccinated with PspA5-wP than in nonvaccinated mice (Fig. 3D). Significant differences in IL-10 secretion were detected only before and 3 h after the challenge, when higher levels of this cytokine were present in BALF from mice vaccinated with wP or PspA5-wP than in those from nonimmunized mice (see Fig. S1 in the supplemental material).
Fig 3.
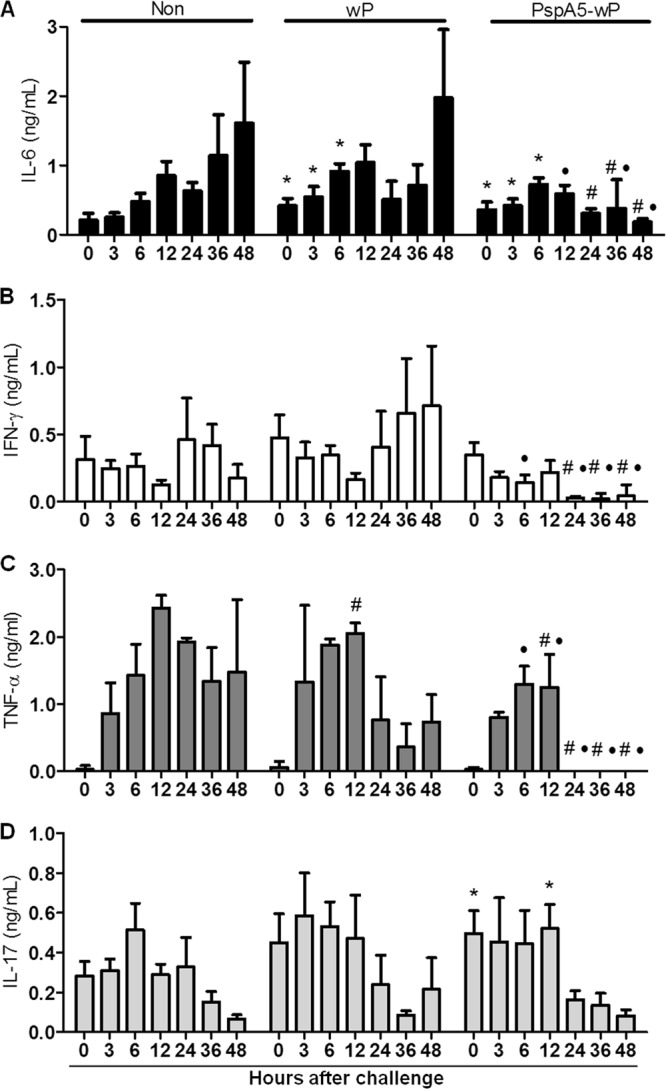
Cytokine secretion in the respiratory tracts of mice after challenge with the ATCC 6303 strain. BALF samples were collected from nonimmunized BALB/c mice (Non) or mice immunized with wP or PspA5-wP at different time points after the intranasal challenge. Cells were removed by centrifugation, and cytokines were measured in the supernatants by sandwich ELISA against IL-6 (A), IFN-γ (B), TNF-α (C), and IL-17 (D). Results are represented as means for 4 animals per group with the standard deviations. *, significantly higher cytokine levels compared with those at the same time point in nonimmunized mice; #, significantly lower cytokine levels compared with those at the same time point in nonimmunized mice; ●, significantly lower cytokine levels compared with those at the same time point in mice immunized with wP. Statistical analyses were performed with the Mann-Whiney U test, and P ≤ 0.05 for all the comparisons marked.
Since pneumococcus levels in the lungs of mice vaccinated with PspA5-wP were still high at 48 h after the challenge (Fig. 1), we analyzed cytokine secretion at later time points. Nevertheless, no changes in BALF levels of IL-6, IFN-γ, TNF-α, IL-17, or IL-10 were observed from 24 h until 21 days after the challenge (data not shown).
Total protein in BALF at different time points was evaluated as an indirect measure of lung injury (27, 45) (see Fig. S2A in the supplemental material). We observed a significant increase in BALF proteins starting from 36 h postchallenge in nonimmunized mice or mice immunized with wP (P < 0.05 compared with the levels observed before the challenge for both groups). Conversely, mice immunized with PspA5-wP showed no increase in total proteins in BALF. Loss of body weight after the challenge was observed in all animals, but a marked decrease in body weight was observed only in nonimmunized mice or mice immunized with wP at later time points. A trend for body weight control was observed in mice immunized with PspA5-wP (see Fig. S2B in the supplemental material).
Protection induced by the PspA5-wP vaccine correlates with antigen-specific induction of IL-17 secretion by spleen cells.
IL-17 has been implicated in innate and acquired immunity against pneumococcal nasal colonization or invasive infection in animal models (29, 33, 58). Since our time course experiments did not support any conclusion on the role of IL-17 in the protection elicited by the PspA5-wP vaccine (Fig. 3D), we decided to analyze antigen-specific secretion of this cytokine by spleen and lung cells of vaccinated mice. For these experiments, mice were sacrificed at 12 h after challenge, a point that seems to be critical for the control of inflammation in our model (Fig. 2 and 3). Basal levels of IL-17 secretion by spleen cells were very low in all groups tested. Significant IL-17 production by PspA5-stimulated spleen cells was observed only for the group immunized with PspA5-wP, correlating with protection (Fig. 4A) (P = 0.01 compared with nonimmunized mice or mice immunized with wP; P = 0.02, compared with mice immunized with PspA5). Interestingly, cultures of lung cells derived from mice immunized with wP or PspA5-wP secreted very high levels of IL-17 into the medium, even in the absence of antigen stimulus (P = 0.01 when comparing mice inoculated with wP with nonimmunized mice; P = 0.007 and P = 0.004 when comparing mice immunized with PspA5-wP with nonimmunized mice and mice immunized with PspA5, respectively). These levels could not be further increased by stimulation with PspA5 (Fig. 4B).
Fig 4.
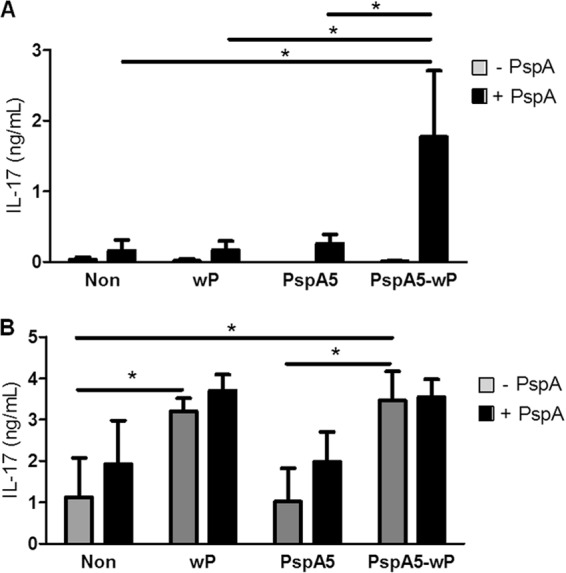
Antigen-specific secretion of IL-17 by spleen and lung cells. Spleen (A) and lung (B) cells were obtained from immunized BALB/c mice at 12 h after challenge with the ATCC 6303 strain, and 5 × 106 cells were incubated in the absence or presence of PspA5 for 72 h. Detection of IL-17 was performed by sandwich ELISA. Results are represented as the means from 5 animals per group with standard deviations. *, significantly higher levels of IL-17 compared with those in the indicated groups. Statistical analyses were performed with the Mann-Whiney U test; P < 0.05 in panel A and P ≤ 0.01 in panel B.
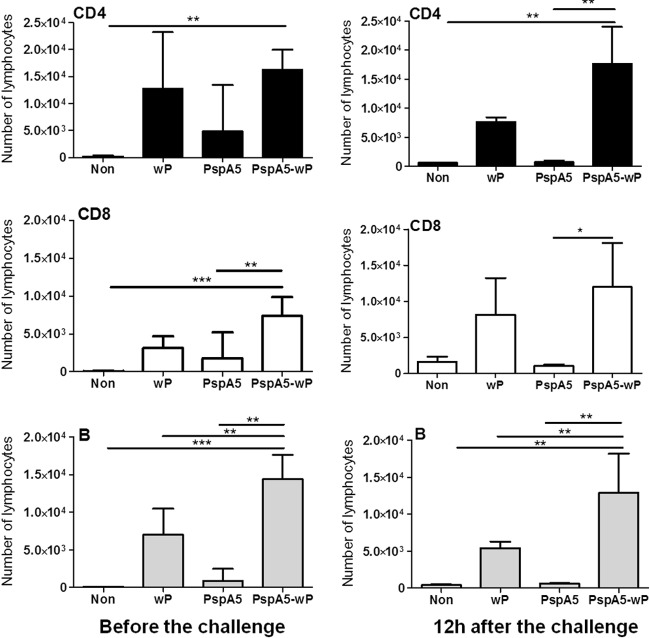
Immunization with PspA5-wP induces an influx of CD4+ T, CD8+ T, and B lymphocytes in mice lungs.
The lymphocytes present in the lungs of immunized mice were characterized. The results showed that nasal inoculation of wP alone already induced an increase of CD4+ T, CD8+ T, and B cells in the respiratory tracts of mice, compared with those in nonimmunized mice, although the results were not significantly different (Fig. 5, left panels). The immunization with PspA5-wP, however, induced significantly higher numbers of CD4+ T cells (P < 0.01 compared with nonimmunized mice), CD8+ T cells (P < 0.001 and P < 0.01 compared with nonimmunized mice or mice immunized with PspA5, respectively), and B cells (P < 0.001 compared with nonimmunized mice and P < 0.01 compared with mice immunized with wP or PspA5). The results were very similar at 12 h after the challenge (Fig. 5, right panels), when the immunized mice displayed higher numbers of CD4+ T cells (P < 0.01 compared with nonimmunized mice or mice immunized with PspA5), CD8+ T cells (P < 0.05 compared with mice immunized with PspA5), and B cells (P < 0.01 compared with all groups). It is interesting to note that, at both time points, only B cells were significantly higher in mice immunized with PspA5-wP than in those inoculated of wP alone (Fig. 5). In mice that survived the challenge, increased numbers of B cells were also observed in the respiratory tracts of animals that were immunized with PspA5-wP compared with animals immunized with PspA5 alone, at 10 days after the challenge (see Fig. S3 in the supplemental material).
Fig 5.
Characterization of the lymphocytes present in the respiratory tracts of mice before and after challenge with the ATCC 6303 strain. BALF samples were collected before or 12 h after the challenge, and 1 × 106 cells were incubated with APC-conjugated anti-mouse CD4 (upper panels), PerCP-conjugated anti-mouse CD8 (middle panels), or PE-conjugated anti-B220 (lower panels). Samples were analyzed by flow cytometry, and results for 10,000 gated lymphocytes are represented as the means of total cells recovered (from groups of 3 to 5 mice) with standard deviations. Asterisks represent significantly different numbers of lymphocytes compared with those in the indicated groups. Statistical analyses were performed by one-way ANOVA with Tukey's posttest. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Results are representative of two independent experiments.
Depletion of CD4+ T, CD8+ T, or B lymphocytes in mice immunized with PspA5-wP does not impair survival against the invasive challenge with the ATCC 6303 strain.
BALB/c mice were immunized with PspA5-wP, using the six dose-protocol which induces high levels of anti-PspA antibodies in serum and in the respiratory tract (43). Depletion of CD4+ T, CD8+ T, or B lymphocytes was achieved by the inoculation of monoclonal antibodies at 48 h and 12 h before the challenge. These cells were maintained at low levels in the first days after the challenge by an additional inoculation of antibodies, at 48 h postchallenge. Mice were monitored for up to 20 days after the challenge, but no signs of disease were observed and 100% of mice immunized with PspA5-wP survived the challenge in all cases (Fig. 6). Mice were sacrificed at day 21 postchallenge, and the lungs were excised for the analysis of pneumococcal CFU. Most of the mice showed no bacteria in the lungs at this time point, independently of the antibody treatment (data not shown).
Fig 6.
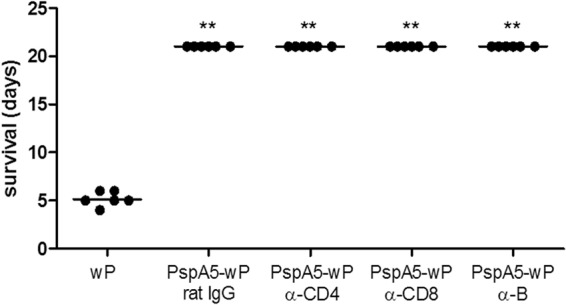
Survival of mice subjected to depletion of specific lymphocytes after challenge with the ATCC 6303 pneumococcal strain. BALB/c mice immunized with PspA5-wP were subjected to the depletion of CD4+ T, CD8+ T, or B lymphocytes by the administration of specific monoclonal antibodies. One control group was immunized with the same formulation and received nonspecific rat IgG, whereas the other group was immunized only with wP. Mice were challenged, and survival was followed for 21 days. Circles represent each animal and, lines represent the mean of the group. **, significantly different survival compared with that of the group immunized with wP, using the Fisher exact test (P = 0.002). Results are representative of two independent experiments.
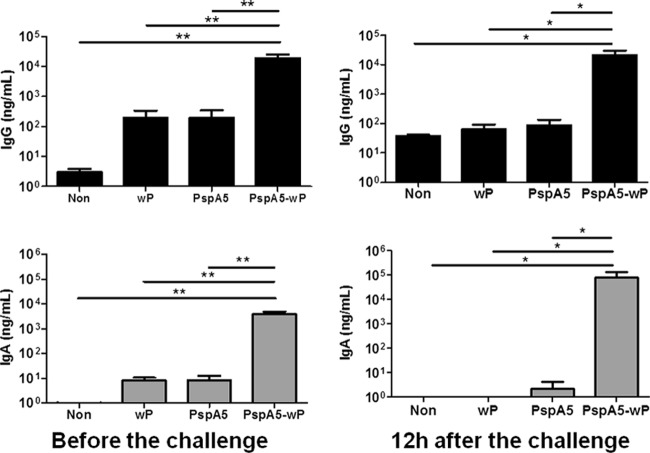
High levels of mucosal anti-PspA antibodies are maintained in the respiratory tracts of mice after challenge.
The levels of anti-PspA antibodies in BALF samples were evaluated before and 12 h after the pneumococcal challenge. Immunization of mice with PspA5-wP induced significantly higher mucosal anti-PspA5 IgG (P = 0.005 compared with nonimmunized mice and P = 0.002 compared with mice immunized with wP or PspA5) and IgA (P = 0.004 compared with nonimmunized mice or mice immunized with PspA5 and P = 0.002 compared with mice immunized with wP), confirming our previous results (43). In addition, immunization with PspA5 alone did not elicit specific antibodies in the respiratory mucosa (Fig. 7, left panels). Evaluation of anti-PspA5 antibodies at 12 h after the challenge with the ATCC 6303 strain revealed no further increase in IgG levels compared with the levels before the challenge (Fig. 7, upper right panel), and once again, the group immunized with PspA5-wP was the only one with significantly higher anti-PspA5 IgG (P = 0.05 compared with nonimmunized mice or mice immunized with PspA5 and P = 0.02 compared with mice immunized with wP). However, more than a 1-log-unit increase in the levels of anti-PspA IgA in BALF from mice immunized with PspA5-wP was observed, compared with the animals immunized with the same formulation before the challenge (Fig. 7, lower right panel). This was the only group that displayed large amounts of antibodies at this time point. The levels of anti-PspA5 antibodies in the respiratory mucosae from mice immunized with PspA5-wP remained high even 10 days after the challenge (see Fig. S4 in the supplemental material).
Fig 7.
Evaluation of anti-PspA5 antibodies in the respiratory tracts of mice immunized with the different vaccine formulations. BALF samples were collected 21 days after the last immunization (left panels) or 12 h after the challenge (right panels). Anti-PspA5 IgG (upper panels) or IgA (lower panels) were detected through ELISA. Concentrations of antibodies (means for 6 animals) with standard deviations are shown. *, significant differences from the indicated groups. Statistical analyses were performed using the Mann-Whitney U test (*, P ≤ 0.05; **, P < 0.01). Results are representative of two independent experiments.
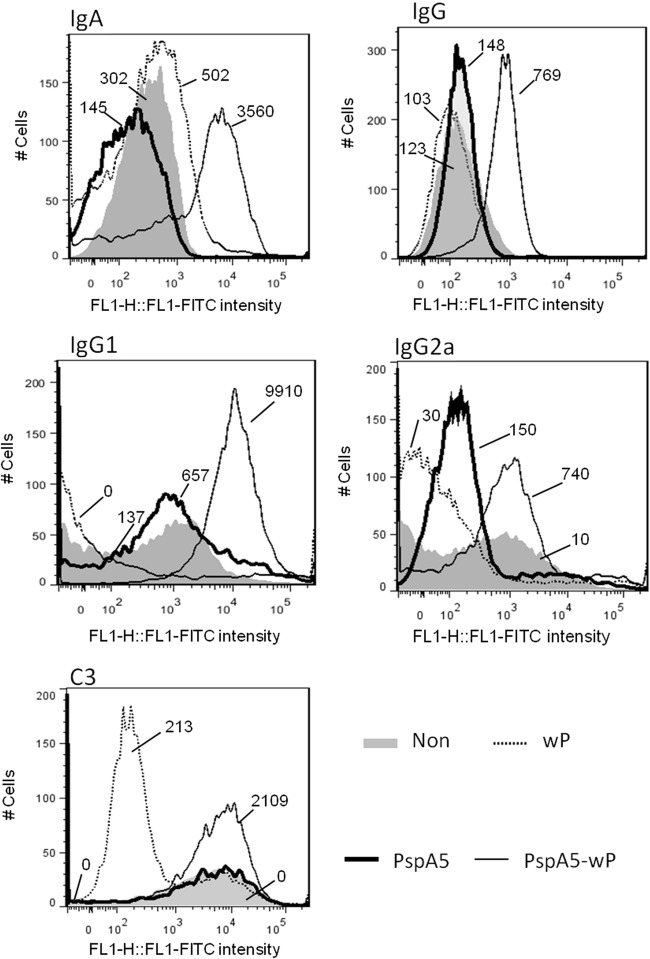
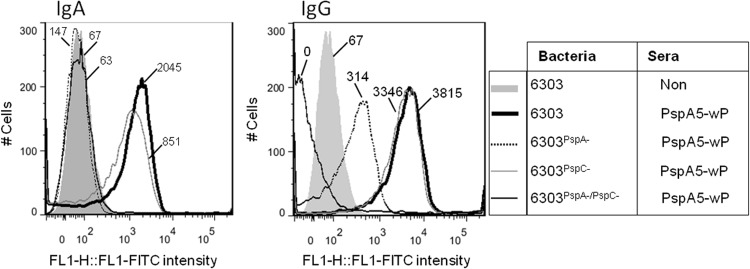
Flow cytometry analyses were performed with the ATCC 6303 bacteria incubated with pools of BALF samples from the different mouse groups collected before the challenge. Higher binding of IgA and IgG to the pneumococcal surface was observed when the samples from mice immunized with PspA5-wP were tested (Fig. 8, upper panels). The same samples also showed higher capacity for binding of IgG1 and IgG2a to the pneumococcal surface (Fig. 8, middle panels). In addition, incubation of the ATCC 6303 strain with BALF samples from mice immunized with PspA5-wP induced the deposition of complement C3 component on the bacterial surface in vitro. (Fig. 8, lower panel). We have also confirmed that these antibodies preferentially bound to PspA exposed on the ATCC 6303 surface, since binding of IgA was completely abolished in the 6303PspA− and 6303PspA/PspC− mutant strains (Fig. 9, left panel) and binding of IgG was significantly lower and completely abolished in the 6303PspA− and 6303PspA/PspC− mutant strains, respectively (Fig. 9, right panel).
Fig 8.
Binding of antibodies recovered from the respiratory mucosae of mice to pneumococcal surface and complement deposition. BALF samples were collected 21 days after the last immunization, and pools (from 3 mice) were incubated with the ATCC 6303 strain. Samples were incubated with FITC-conjugated anti-mouse IgA, IgG, IgG1, or IgG2a (upper and middle panels). For complement deposition, normal mouse sera were added to the samples, followed by the incubation with FITC-conjugated anti-mouse C3 (lower panel). Results were analyzed by flow cytometry with 10,000 events gated. BALF from nonimmunized animals (gray areas) or from mice immunized with wP (dotted black lines), PspA5 (solid heavy black lines), or PspA5-wP (solid black lines) were tested. The median fluorescence intensity is indicated for each sample. Data are representative of two experiments using different pools.
Fig 9.
Absence of antibody binding to the pneumococcal surface of 6303PspA− mutants. BALF samples from mice immunized with PspA5-wP were collected 21 days after the last immunization, and pools (from 3 mice) were incubated with different mutants derived from the ATCC 6303 strain or the wild-type strain. BALF from nonimmunized mice was used as control. Samples were incubated with FITC-conjugated anti-mouse IgA or IgG, and the results were analyzed by flow cytometry with 10,000 events gated. The median fluorescence intensity is indicated for each sample. Data are representative of two experiments using different pools.
DISCUSSION
In the recent years, Streptococcus pneumoniae conjugated vaccines have been becoming widely adopted in different regions of the world, including developing countries. Although the benefits to public health strongly support mass vaccination with pneumococcal conjugated vaccines, careful analyses of the impact of these vaccines in the different regions are necessary (56). Protein-based vaccines are among the alternatives for the induction of serotype-independent protection. Our group has been studying formulations composed of pneumococcal surface proteins such as PspA. Although variability in the PspA amino acid sequence has been well reported (26) and the gene has been recently described as a hot spot for mutations among pneumococcal variants, cross-reactivity between PspA molecules is observed (16). Moreover, cross-protection in animal models can be achieved by the use of formulations containing PspA molecules that can induce antibodies that cross-react with PspAs from both families, such as the PspA5 used in this work (18, 37).
The PspA5-wP vaccine seems to prevent the invasion of the bacteria into the bloodstream of mice, conferring protection. Another possibility is that bacteria are rapidly phagocytosed in the blood, impairing their detection under our experimental conditions. Pneumococcal loads were already lower in the lungs of vaccinated mice at 3 h after infection. Nevertheless, pneumococcus numbers remained steady in the lungs of these mice, with no significant changes from 3 h to 48 h postchallenge. In fact, resolution of infection in vaccinated mice occurs only 3 weeks later (43). These observations indicate that mucosal immune responses may be important to hinder bacterial growth in the lungs and to prevent tissue injuries in this model. An orchestrated cellular response occurred in the lungs of mice vaccinated with PspA5-wP after the challenge with the ATCC 6303 strain. This response was characterized by peaks in the number of lymphocytes and neutrophils infiltrated in the lungs at 12 h postchallenge and a reduction of macrophages numbers at the same time. Infiltration of cells occurred later in control mice, especially in nonimmunized mice, and reached higher counts when mice succumbed to the infection. A review of the role of neutrophils during bacterial pneumonia discusses that the strength of the response determines the outcome of infection (15). While playing a critical antibacterial role, their potential for harm must be controlled to avoid tissue damage (15). The lower levels of bacteria observed in the lungs of mice vaccinated with PspA5-wP from the first hours of infection may have had an impact in the strength of the inflammatory response. In spite of this, it is remarkable that there seems to be a signal to control neutrophil infiltration, even in the presence of pneumococci in the lungs. The control of lung inflammation in vaccinated mice was also supported by a decrease in the secretion of proinflammatory cytokines, such as IL-6, IFN-γ, and, particularly, TNF-α, at 24 h postchallenge. The capacity to rapidly secrete TNF-α to the airways was previously related to innate host responses against pneumococci. This capacity seems to be reduced in the CBA/Ca mouse strain, which is highly susceptible to pneumococcal infection (27). In our results, mice from all groups responded quickly with TNF-α secretion in the respiratory mucosa, but strong control of this cytokine was observed only in protected mice. This is in agreement with previous results from our group that correlated increased protection against pneumococcal invasive challenge in mice with low levels of TNF-α secretion in the airways at 13 h after challenge (20). Secretion of IL-6, IFN-γ, and TNF-α in the respiratory mucosa was also followed for several days until complete pneumococcal clearance (21 days later), but the levels remained low in mice vaccinated with PspA5-wP at all time points tested (data not shown). In contrast to what was observed in control mice, the levels of total protein in BALF remained unchanged in mice immunized with PspA5-wP after the challenge, which indirectly suggests a preservation of the alveolus-capillary barrier (27, 45).
Studies with a killed whole-cell pneumococcal vaccine have shown that protection against colonization induced by this vaccine depends on the production of the IL-17 cytokine by CD4+ T cells and that in the absence of these components, protection no longer occurs (29, 34). This mechanism was also related to the protection elicited by formulations composed of pneumococcal subunits such as the cell wall polysaccharide or protein antigens in animal models (5, 30). Previous colonization of naïve mice with S. pneumoniae also activates CD4+ T cells to produce IL-17, which leads to cell recruitment and clearance of the bacteria after a secondary colonization challenge (34, 58). On the other hand, protection against invasive pneumococcal challenge in animal models may not always rely on Th17 cell-mediated immune responses. Using a mouse model of prior pneumococcal colonization followed by an invasive challenge in which bacteria were inoculated in higher volumes to reach the lungs, Cohen and collaborators have shown that protection against bacteremia was not dependent on IL-17-producing CD4+ T cells but rather was conferred by the presence of antibodies in the bloodstream (14). Secretion of IL-17 in mice vaccinated with PspA5-wP was slightly higher than that in nonimmunized mice before the challenge with the ATCC 6303 strain. This can be explained by the ability of wP to induce an IL-17 responses in mice (3, 25). However, we could not observe a clear pattern of IL-17 secretion in the airways that could be related to the protection elicited by the PspA5-wP vaccine. On the other hand, high levels of PspA5-specific IL-17 secretion by spleen cells of mice vaccinated with PspA5-wP were observed, indicating that systemic IL-17 responses can be activated during the challenge. In vitro results obtained with lung cells collected from mice vaccinated with PspA5-wP confirmed the stimulation of IL-17-producing cells by vaccination with wP or PspA5-wP, which was independent of PspA5 stimulation.
Despite the presence of increased numbers of lymphocytes in the respiratory mucosae of vaccinated mice before and after the challenge, depletion of either CD4+ T, CD8+ T, or B cells did not abrogate protection conferred by the PspA5-wP vaccine. The lack of a requirement for CD4+ T cells for protection in our model resembles the case for the protection observed against invasive pneumococcal challenge in mice by previous nasal colonization (14). CD8+ T cells were described as the key mediator of resistance to a pulmonary serotype 3 pneumococcal infection model in mice. In fact, CD8−/− mice were highly susceptible to infection by the serotype 3 A66.1 strain, and this was related to increased numbers of CD4+ T IL-17-producing cells that could have detrimental inflammatory responses (55). In addition, a serotype 3 polysaccharide (PS3)-conjugated vaccine failed to protect CD8−/− mice against a respiratory invasive challenge with the serotype 3 WU2 strain, although the animals presented high levels of anti-PS3 antibodies (53). Our results show that under conditions of high levels of anti-PspA5 antibodies, there is no requirement for CD8+ T cells during challenge with the ATCC 6303 strain for survival. Regarding B lymphocytes, besides their role in humoral responses, increasing data on their importance in the modulation of immune responses are being published. One important mechanism related to the control of inflammation by B cells is the secretion of the anti-inflammatory cytokine IL-10 (28). However, we could not correlate protection to the presence of B cells in the lungs during the challenge. In addition, no correlation of protection elicited by the PspA5-wP vaccine and the secretion of the anti-inflammatory cytokine IL-10 in the airways could be observed.
We were able to detect high levels of mucosal antibodies in mice vaccinated with PspA5-wP (IgG, IgA, IgG1, and IgG2a), even at 12 h after the challenge, when the pneumococcal levels in the lungs are also high. The majority of these antibodies react with PspA, but some level of cross-reactivity with PspC was observed. Binding of IgG2a and complement deposition on the surface of the ATCC 6303 strain were induced in vitro after incubation of the bacteria with BALF samples. However, the highest signal was observed for IgG1 binding to bacterial surface. Recently, a study showed that a monoclonal antibody to serotype 3 PS (1E2, IgG1) mediated protection against pneumococcal pneumonia in mice, through an antibody-mediated control of inflammation. Rather than rapid bacterial clearance in the lungs, the authors observed increased uptake of bacteria by macrophages followed by apoptosis (54). Here we show that a strong control of the inflammatory response in the lungs correlated with protection elicited by the PspA5-wP vaccine against the challenge with the ATCC 6303 strain. Moreover, the control of inflammation occurred in the presence of relatively high numbers of bacteria in the lungs (around 104 CFU). The PspA5-wP vaccine induces a balanced IgG1/IgG2a response (43), and the importance of this balance in complement-mediated protection was previously demonstrated by us and others (4, 19). Nevertheless, the role of anti-PspA5 antibodies (possibly IgG1) in the control of inflammation in this model needs further investigation.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to the Seção de Vacinas Aeróbicas from Instituto Butantan for providing the wP vaccine. We also thank Jeffrey Weiser, Aoife Roche, and Mikhail Shchepetov, from the University of Pennsylvania, for help with the construction of S. pneumoniae mutants.
This work was supported by FAPESP, CNPq, and Fundação Butantan.
Footnotes
Published ahead of print 3 July 2012
Supplemental material for this article may be found at http://cvi.asm.org/.
REFERENCES
- 1. Achen MG, Davidson BE, Hillier AJ. 1986. Construction of plasmid vectors for the detection of streptococcal promoters. Gene 45:45–49 [DOI] [PubMed] [Google Scholar]
- 2. Aida Y, Pabst MJ. 1990. Removal of endotoxin from protein solutions by phase separation using Triton X-114. J. Immunol. Methods 132:191–195 [DOI] [PubMed] [Google Scholar]
- 3. Andreasen C, Powell DA, Carbonetti NH. 2009. Pertussis toxin stimulates IL-17 production in response to Bordetella pertussis infection in mice. PLoS One 4:e7079 doi:10.1371/journal.pone.0007079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arulanandam BP, Lynch JM, Briles DE, Hollingshead S, Metzger DW. 2001. Intranasal vaccination with pneumococcal surface protein A and interleukin-12 augments antibody-mediated opsonization and protective immunity against Streptococcus pneumoniae infection. Infect. Immun. 69:6718–6724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Basset A, et al. 2007. Antibody-independent, CD4+ T-cell-dependent protection against pneumococcal colonization elicited by intranasal immunization with purified pneumococcal proteins. Infect. Immun. 75:5460–5464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Berstad AK, et al. 2000. Inactivated meningococci and pertussis bacteria are immunogenic and act as mucosal adjuvants for a nasal inactivated influenza virus vaccine. Vaccine 18:1910–1919 [DOI] [PubMed] [Google Scholar]
- 7. Berstad AK, et al. 2000. Induction of antigen-specific T cell responses in human volunteers after intranasal immunization with a whole-cell pertussis vaccine. Vaccine 18:2323–2330 [DOI] [PubMed] [Google Scholar]
- 8. Bettinger JA, et al. 2010. The effect of routine vaccination on invasive pneumococcal infections in Canadian children, Immunization Monitoring Program, Active 2000-2007. Vaccine 28:2130–2136 [DOI] [PubMed] [Google Scholar]
- 9. Bitsaktsis C, et al. 2012. Mucosal immunization with an unadjuvanted vaccine that targets S. pneumoniae PspA to human FcgammaRI protects against pneumococcal infection through complement- and lactoferrin-mediated bactericidal activity. Infect. Immun. 80:1166–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Briles DE, et al. 2000. The potential to use PspA and other pneumococcal proteins to elicit protection against pneumococcal infection. Vaccine 18:1707–1711 [DOI] [PubMed] [Google Scholar]
- 11. Briles DE, et al. 2000. Immunization of humans with recombinant pneumococcal surface protein A (rPspA) elicits antibodies that passively protect mice from fatal infection with Streptococcus pneumoniae bearing heterologous PspA. J. Infect. Dis. 182:1694–1701 [DOI] [PubMed] [Google Scholar]
- 12. Campos IB, et al. 2008. Nasal immunization of mice with Lactobacillus casei expressing the pneumococcal surface protein A: induction of antibodies, complement deposition and partial protection against Streptococcus pneumoniae challenge. Microbes Infect. 10:481–488 [DOI] [PubMed] [Google Scholar]
- 13. Chiba N, et al. 2010. Serotype and antibiotic resistance of isolates from patients with invasive pneumococcal disease in Japan. Epidemiol. Infect. 138:61–68 [DOI] [PubMed] [Google Scholar]
- 14. Cohen JM, et al. 2011. Protective contributions against invasive Streptococcus pneumoniae pneumonia of antibody and Th17-cell responses to nasopharyngeal colonisation. PLoS One 6:e25558 doi:10.1371/journal.pone.0025558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Craig A, Mai J, Cai S, Jeyaseelan S. 2009. Neutrophil recruitment to the lungs during bacterial pneumonia. Infect. Immun. 77:568–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Croucher NJ, et al. 2011. Rapid pneumococcal evolution in response to clinical interventions. Science 331:430–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dagan R. 2009. Serotype replacement in perspective. Vaccine 27(Suppl. 3):C22–C24 [DOI] [PubMed] [Google Scholar]
- 18. Darrieux M, et al. 2008. Recognition of pneumococcal isolates by antisera raised against PspA fragments from different clades. J. Med. Microbiol. 57:273–278 [DOI] [PubMed] [Google Scholar]
- 19. Ferreira DM, Darrieux M, Oliveira ML, Leite LC, Miyaji EN. 2008. Optimized immune response elicited by a DNA vaccine expressing pneumococcal surface protein a is characterized by a balanced immunoglobulin G1 (IgG1)/IgG2a ratio and proinflammatory cytokine production. Clin. Vaccine Immunol. 15:499–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ferreira DM, et al. 2009. Characterization of protective mucosal and systemic immune responses elicited by pneumococcal surface protein PspA and PspC nasal vaccines against a respiratory pneumococcal challenge in mice. Clin. Vaccine Immunol. 16:636–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ferreira DM, et al. 2009. Comparison of the pulmonary response against lethal and non-lethal intranasal challenges with two different pneumococcal strains. Microb. Pathog. 47:157–163 [DOI] [PubMed] [Google Scholar]
- 22. Ferreira DM, et al. 2010. Protection against nasal colonization with Streptococcus pneumoniae by parenteral immunization with a DNA vaccine encoding PspA (pneumococcal surface protein A). Microb. Pathog. 48:205–213 [DOI] [PubMed] [Google Scholar]
- 23. Franco CM, et al. 2010. Survey of nonsusceptible nasopharyngeal Streptococcus pneumoniae isolates in children attending day-care centers in Brazil. Pediatr. Infect. Dis. J. 29:77–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fukuyama Y, et al. 2010. Secretory-IgA antibodies play an important role in the immunity to Streptococcus pneumoniae. J. Immunol. 185:1755–1762 [DOI] [PubMed] [Google Scholar]
- 25. Higgins SC, Jarnicki AG, Lavelle EC, Mills KH. 2006. TLR4 mediates vaccine-induced protective cellular immunity to Bordetella pertussis: role of IL-17-producing T cells. J. Immunol. 177:7980–7989 [DOI] [PubMed] [Google Scholar]
- 26. Hollingshead SK, Becker R, Briles DE. 2000. Diversity of PspA: mosaic genes and evidence for past recombination in Streptococcus pneumoniae. Infect. Immun. 68:5889–5900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kerr AR, et al. 2002. Role of inflammatory mediators in resistance and susceptibility to pneumococcal infection. Infect. Immun. 70:1547–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Klinker MW, Lundy SK. 2012. Multiple mechanisms of immune suppression by B lymphocytes. Mol. Med. 18:123–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lu YJ, et al. 2008. Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathog. 4:e1000159 doi:10.1371/journal.ppat.1000159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lu YJ, Skovsted IC, Thompson CM, Anderson PW, Malley R. 2009. Mechanisms in the serotype-independent pneumococcal immunity induced in mice by intranasal vaccination with the cell wall polysaccharide. Microb. Pathog. 47:177–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Malley R, Anderson PW. 2012. Serotype-independent pneumococcal experimental vaccines that induce cellular as well as humoral immunity. Proc. Natl. Acad. Sci. U. S. A. 109:3623–3627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Malley R, et al. 2004. Multiserotype protection of mice against pneumococcal colonization of the nasopharynx and middle ear by killed nonencapsulated cells given intranasally with a nontoxic adjuvant. Infect. Immun. 72:4290–4292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Malley R, et al. 2006. Antibody-independent, interleukin-17A-mediated, cross-serotype immunity to pneumococci in mice immunized intranasally with the cell wall polysaccharide. Infect. Immun. 74:2187–2195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Malley R, et al. 2005. CD4+ T cells mediate antibody-independent acquired immunity to pneumococcal colonization. Proc. Natl. Acad. Sci. U. S. A. 102:4848–4853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McCool TL, et al. 2003. Serum immunoglobulin G response to candidate vaccine antigens during experimental human pneumococcal colonization. Infect. Immun. 71:5724–5732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Miyaji EN, Dias WO, Tanizaki MM, Leite LC. 2003. Protective efficacy of PspA (pneumococcal surface protein A)-based DNA vaccines: contribution of both humoral and cellular immune responses. FEMS Immunol. Med. Microbiol. 37:53–57 [DOI] [PubMed] [Google Scholar]
- 37. Moreno AT, et al. 2010. Immunization of mice with single PspA fragments induces antibodies capable of mediating complement deposition on different pneumococcal strains and cross-protection. Clin. Vaccine Immunol. 17:439–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nabors GS, et al. 2000. Immunization of healthy adults with a single recombinant pneumococcal surface protein A (PspA) variant stimulates broadly cross-reactive antibodies to heterologous PspA molecules. Vaccine 18:1743–1754 [DOI] [PubMed] [Google Scholar]
- 39. O'Brien KL, et al. 2009. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 374:893–902 [DOI] [PubMed] [Google Scholar]
- 40. Ogunniyi AD, Grabowicz M, Briles DE, Cook J, Paton JC. 2007. Development of a vaccine against invasive pneumococcal disease based on combinations of virulence proteins of Streptococcus pneumoniae. Infect. Immun. 75:350–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Olafsdottir TA, Lingnau K, Nagy E, Jonsdottir I. 2012. Novel protein-based pneumococcal vaccines administered with the Th1-promoting adjuvant IC31 induce protective immunity against pneumococcal disease in neonatal mice. Infect. Immun. 80:461–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Oliveira ML, et al. 2006. Induction of systemic and mucosal immune response and decrease in Streptococcus pneumoniae colonization by nasal inoculation of mice with recombinant lactic acid bacteria expressing pneumococcal surface antigen A. Microbes Infect. 8:1016–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Oliveira ML, et al. 2010. Combination of pneumococcal surface protein A (PspA) with whole cell pertussis vaccine increases protection against pneumococcal challenge in mice. PLoS One 5:e10863 doi:10.1371/journal.pone.0010863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Oma K, et al. 2009. Intranasal immunization with a mixture of PspA and a Toll-like receptor agonist induces specific antibodies and enhances bacterial clearance in the airways of mice. Vaccine 27:3181–3188 [DOI] [PubMed] [Google Scholar]
- 45. Parker JC, Townsley MI. 2004. Evaluation of lung injury in rats and mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 286:L231–L246 [DOI] [PubMed] [Google Scholar]
- 46. Pilishvili T, et al. 2010. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J. Infect. Dis. 201:32–41 [DOI] [PubMed] [Google Scholar]
- 47. Ren B, Szalai AJ, Hollingshead SK, Briles DE. 2004. Effects of PspA and antibodies to PspA on activation and deposition of complement on the pneumococcal surface. Infect. Immun. 72:114–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shaper M, Hollingshead SK, Benjamin WH, Jr, Briles DE. 2004. PspA protects Streptococcus pneumoniae from killing by apolactoferrin, and antibody to PspA enhances killing of pneumococci by apolactoferrin. Infect. Immun. 72:5031–5040 (Erratum, 72:7379.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shouval DS, Greenberg D, Givon-Lavi N, Porat N, Dagan R. 2009. Serotype coverage of invasive and mucosal pneumococcal disease in Israeli children younger than 3 years by various pneumococcal conjugate vaccines. Pediatr. Infect. Dis. J. 28:277–282 [DOI] [PubMed] [Google Scholar]
- 50. Smith CJ, Rollins LA, Parker AC. 1995. Nucleotide sequence determination and genetic analysis of the Bacteroides plasmid, pBI143. Plasmid 34:211–222 [DOI] [PubMed] [Google Scholar]
- 51. Tamizifar H, Jennings R, Ali SA, Potter CW. 1997. Induction of IL-2 and IFN-gamma in BALB/c mice immunised with subunit influenza A vaccine in combination with whole cell or acellular DTP vaccine. J. Med. Microbiol. 46:61–66 [DOI] [PubMed] [Google Scholar]
- 52. Tamizifar H, Robinson A, Jennings R, Potter CW. 1995. Immune response and protection against influenza A infection in mice immunised with subunit influenza A vaccine in combination with whole cell or acellular DTP vaccine. Vaccine 13:1539–1546 [DOI] [PubMed] [Google Scholar]
- 53. Tian H, Groner A, Boes M, Pirofski LA. 2007. Pneumococcal capsular polysaccharide vaccine-mediated protection against serotype 3 Streptococcus pneumoniae in immunodeficient mice. Infect. Immun. 75:1643–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Weber S, Tian H, van Rooijen N, Pirofski LA. 2012. A serotype 3 pneumococcal capsular polysaccharide-specific monoclonal antibody requires FcgammaRIII and macrophages to mediate protection against pneumococcal pneumonia in mice. Infect. Immun. 80:1314–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Weber SE, Tian H, Pirofski LA. 2011. CD8+ cells enhance resistance to pulmonary serotype 3 Streptococcus pneumoniae infection in mice. J. Immunol. 186:432–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Weinberger DM, Malley R, Lipsitch M. 2011. Serotype replacement in disease after pneumococcal vaccination. Lancet 378:1962–1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Xin W, Li Y, Mo H, Roland KL, Curtiss R., III 2009. PspA family fusion proteins delivered by attenuated Salmonella enterica serovar Typhimurium extend and enhance protection against Streptococcus pneumoniae. Infect. Immun. 77:4518–4528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhang Z, Clarke TB, Weiser JN. 2009. Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. J. Clin. Invest. 119:1899–1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.