Abstract
Recently, novel Brucella strains with phenotypic characteristics that were atypical for strains belonging to the genus Brucella have been reported. Phenotypically many of these strains were initially misidentified as Ochrobactrum spp. Two novel species have been described so far for these strains, i.e., B. microti and B. inopinata, and other strains genetically related to B. inopinata may constitute other novel species as well. In this study, we analyzed the lipopolysaccharides (LPS) (smooth LPS [S-LPS] and rough LPS [R-LPS]) of these atypical strains using different methods and a panel of monoclonal antibodies (MAbs) directed against several epitopes of the Brucella O-polysaccharide (O-PS) and R-LPS. Among the most striking results, Brucella sp. strain BO2, isolated from a patient with chronic destructive pneumonia, showed a completely distinct S-LPS profile in silver stain gels that looked more similar to that of enterobacterial S-LPS. This strain also failed to react with MAbs against Brucella O-PS epitopes and showed weak reactivity with anti-R-LPS MAbs. B. inopinata reference strain BO1 displayed an M-dominant S-LPS type with some heterogeneity relative to the classical M-dominant Brucella S-LPS type. Australian wild rodent strains belonging also to the B. inopinata group showed a classical A-dominant S-LPS but lacked the O-PS common (C) epitopes, as previously reported for B. suis biovar 2 strains. Interestingly, some strains also failed to react with anti-R-LPS MAbs, such as the B. microti reference strain and B. inopinata BO1, suggesting modifications in the core-lipid A moieties of these strains. These results have several implications for serological typing and serological diagnosis and underline the need for novel tools for detection and correct identification of such novel emerging Brucella spp.
INTRODUCTION
Brucellae are Gram-negative, facultative, intracellular bacteria that can infect humans and many species of animals. The genus Brucella has traditionally been classified into six species, i.e., B. melitensis, B. suis, B. abortus, B. neotomae, B. ovis, and B. canis, which are reflective of animal host preference (18, 19, 24, 33, 36). The genus Brucella has been further expanded with a set of recently discovered species. Such species include B. ceti and B. pinnipedialis, which have been isolated from cetaceans and pinnipeds, respectively (15). B. microti was isolated initially from the common vole but later from the red fox and from soil (27–29). The latest validly published species is B. inopinata, which was isolated from a human breast implant infection and represents the most distant Brucella species at the phenotypic and phylogenetic levels relative to the others (11, 30). The animal or environmental reservoir of the last species is not known. New Brucella species will likely be described in the future, including isolates from baboons (26), isolates from wild rodents in Australia (31), and strain BO2, isolated from a patient with chronic destructive pneumonia (32). Strain BO2 and strains from wild Australian rodents have been proposed as novel lineages of the B. inopinata species (31, 32).
Interestingly, this group of divergent strains present phenotypic characteristics that are not characteristic of the classical Brucella species, such as faster growth and higher metabolic activities, and they were therefore often initially misidentified as Ochrobactrum spp., with the risk of compromising treatment by the use of inappropriate antibiotics and treatment duration in human cases. Some of these strains have also been shown to be untypeable with monospecific polyclonal sera commonly used to classify smooth (S) Brucella species as A- or M-dominant strains, suggesting possible modifications at the lipopolysaccharide (LPS) level. This in addition may compromise serological diagnosis of infections caused by these strains, because serological tests are mainly based on detection of antibodies against smooth LPS (S-LPS) and in particular its O-polysaccharide (O-PS) moiety, which is known to be the immunodominant part of Brucella S-LPS (12, 19, 20).
Besides the A and M determinants mentioned above, S Brucella strains share common epitopes on the O-PS with cross-reacting bacteria, of which the most important is Yersinia enterocolitica O:9 (4, 5, 12). By using monoclonal antibodies (MAbs) a number of epitope specificities on the O-PS have been reported: A, M, and epitopes shared by both A- and M-dominant strains, which have been named common (C) epitopes (3, 7, 9, 10, 13, 25, 34, 35). The C epitopes have been further subdivided, according to relative preferential MAb binding in enzyme-linked immunosorbent assays (ELISA) to A- and M-dominant strains of B. abortus or B. melitensis and to cross-reacting Y. enterocolitica O:9, into five epitopic specificities: C (M>A), C (A=M), C/Y (M>A), C/Y (A=M), and C/Y (A>M) (9, 35). MAbs indicated as C are specific for Brucella, while those indicated as C/Y cross-react with Y. enterocolitica O:9. The preferential binding to A- or M-dominant strains and equal binding to both strains are indicated by A>M, M>A, and A=M, respectively. It has been suggested from data from competitions between MAbs that the different O-PS epitopes are probably overlapping structures (35). The structural differences specifying these O-PS epitopes have been previously partly elucidated (3, 23) and are further discussed in Results and Discussion.
Because the LPS antigenic status of strains belonging to the divergent B. inopinata lineage has not clearly been defined, the purpose of the present study was to investigate LPS expression in these strains as well as the distribution of the different Brucella O-PS epitopes identified to date by using MAbs.
MATERIALS AND METHODS
The Brucella strains used in this study are listed in Table 1. The strains were checked for purity, colony phase, and species and biovar characterization by standard procedures (1). Typing of strains with monospecific polyclonal sera was done as described by Alton et al. (1).
Table 1.
Binding in ELISA of the anti-O-PS MAbs to the Brucella strains used in this study
| Species (biovar) | Strain | Host or source | Geographic origin | Agglutination with monospecific serum |
Binding titer (maximal absorbance) by ELISA of MAba: |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | M | 2E11 (M) | 04F03 (M) | 12G12 [C (A=M)] | 07F09 [C (A=M)] | 12B12 [C (M>A)] | 18H08 [C/Y (A=M)] | 04F9 [C/Y (A>M)] | 05D4 [C/Y (A>M)] | 16C10 [C/Y (M>A)] | ||||
| B. melitensis (1) | 16 M | Goat | USA | − | + | 90 (2.506) | 90 (2.572) | 7,290 (2.701) | 2,430 (2.791) | 90 (2.907) | 90 (2.858) | 30 (2.420) | 810 (2.432) | 810 (2.482) |
| B. suis (1) | 1330 | Swine | USA | + | − | <10 | 270 (3.930) | 7,290 (5.272) | 2,430 (4.573) | 30 (3.234) | 270 (4.368) | 7,290 (4.669) | 21,870 (4.317) | 90 (4.250) |
| B. suis (2) | Thomsen | Swine | Denmark | + | − | <10 | <10 | 10 (2.422) | 10 (1.249) | <10 | 90 (3.356) | 7,290 (3.109) | 7,290 (3.211) | 10 (2.533) |
| B. microti | CCM 4915 | Common vole | Czech Republic | − | + | 90 (3.691) | 90 (4.162) | 2,430 (4.782) | 810 (4.481) | 30 (2.700) | 30 (3.288) | 90 (3.293) | 2,430 (4.481) | 270 (4.414) |
| B. inopinata | BO1 | Human | USA | − | + | <10 | 90 (3.170) | 7,290 (3.345) | 2,430 (3.444) | 90 (3.076) | 90 (3.512) | 21,870 (3.192) | 7,290 (3.230) | 810 (3.441) |
| Brucella sp. | BO2 | Human | Australia | − | − | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 |
| 83-211 (83-13) | Wild rodent | Australia | + | − | <10 | <10 | <10 | <10 | <10 | 90 (4.120) | 7,290 (4.857) | 7,290 (3.890) | 10 (1.730) | |
| NF 2627 | Wild rodent | Australia | + | − | <10 | <10 | <10 | <10 | <10 | 30 (3.472) | 7,290 (4.669) | 2,430 (4.617) | 10 (1.748) | |
| NF 2629 | Wild rodent | Australia | + | − | <10 | <10 | <10 | <10 | <10 | 90 (3.896) | 7,290 (4.605) | 7,290 (4.329) | 10 (2.135) | |
| NF 2637 | Wild rodent | Australia | + | − | <10 | <10 | <10 | <10 | <10 | 90 (3.401) | 2.430 (3.960) | 7,290 (3.558) | <10 | |
| NF 2640 | Wild rodent | Australia | + | − | <10 | <10 | 10 (1.511) | <10 | <10 | 90 (4.140) | 7,290 (5.254) | 7,290 (4.953) | 30 (3.596) | |
| NF 2651 | Wild rodent | Australia | + | − | <10 | <10 | 10 (1.234) | <10 | <10 | 90 (4.396) | 7,290 (4.669) | 7,290 (5.572) | 10 (1.660) | |
| NF 2653 | Wild rodent | Australia | + | − | <10 | <10 | 10 (1.543) | <10 | <10 | 90 (4.162) | 7,290 (4.861) | 2,430 (5.083) | 10 (3.302) | |
| NF 2668 | Wild rodent | Australia | + | − | <10 | <10 | 10 (1.255) | <10 | <10 | 30 (4.060) | 7,290 (5.264) | 2,430 (4.786) | 10 (2.528) | |
| NF 2810 | Wild rodent | Australia | + | − | <10 | <10 | <10 | <10 | <10 | 30 (3.098) | 2,430 (3.844) | 7,290 (3.888) | 10 (1.521) | |
| NF 2815 | Wild rodent | Australia | + | − | <10 | <10 | 30 (2.965) | 10 (1.719) | <10 | 90 (4.326) | 7,290 (5.556) | 7,290 (5.556) | 30 (3.832) | |
| NF 2816 | Wild rodent | Australia | + | − | <10 | <10 | 10 (1.583) | <10 | <10 | 90 (4.710) | 7,290 (4.856) | 7,290 (4.953) | 10 (2.546) | |
| NF 2816b | Wild rodent | Australia | + | − | <10 | <10 | <10 | <10 | <10 | 90 (4.233) | 2,430 (4.857) | 7,290 (4.325) | 10 (1.141) | |
Results are expressed as the titers of the MAb, i.e., the highest dilutions of the MAb giving an absorbance value above 1.0. Maximal absorbance was observed mostly at a 1/10 dilution of the MAb. The epitope specificities of the MAbs are identified in parentheses.
The MAbs used were produced and characterized previously (6, 8, 10). The anti-rough LPS (anti-R-LPS) MAbs used were A68/10A06/B11 (IgM), A68/24D08/G09 (IgG1), and A68/24G12/A08 (IgG3). The MAbs specific for the O-PS epitopes were 2E11 (IgG3; M epitope), 0F03 (IgM; M epitope), 12G12 (IgG1; C [A=M] epitope), 07F09 (IgG1; C [A=M] epitope), 12B12 (IgG3; C [M>A] epitope), 18H08 (IgA; C/Y [A=M] epitope), 04F9 (IgG2a; C/Y [A>M] epitope), 05D4 (IgG1; C/Y [A>M] epitope), and 16C10 (IgG3; C/Y [M>A] epitope) (Table 1). All MAbs were used as hybridoma supernatants in ELISA and Western blotting.
ELISA using whole bacteria as the antigen and Western blotting after SDS-PAGE of proteinase K-digested S-LPS preparations were performed as described previously (2, 6, 10, 14, 16). Silver staining of S-LPS gels was performed as described previously (14, 16).
RESULTS AND DISCUSSION
Serotyping performed in our laboratory with anti-A and anti-M monospecific polyclonal sera confirmed the antigenic heterogeneity of strains belonging to the divergent B. inopinata lineage (11, 30–32); i.e., B. inopinata strain BO1 showed weak agglutination with anti-M monospecific polyclonal serum, all wild rodent isolates from Australia were clearly A-dominant, and the human Brucella sp. strain BO2 isolate showed absence of agglutination with both monospecific polyclonal sera (Table 1). B. microti reference strain CCM 4915 was confirmed as being M dominant (29). ELISA data using the anti-O-PS MAbs confirmed the A-dominant status of the 12 wild rodent Brucella sp. strains, as previously shown for classical A-dominant Brucella strains such as B. suis 1330, used as a control in this study (Table 1). The distribution of O-PS epitopes appeared to be homogeneous within this group of Australian wild rodent isolates. However, of particular interest is that all these strains showed an absence of binding or weak binding of MAbs specific for the C epitopes, as previously reported for B. suis biovar 2 strains and some marine mammal Brucella isolates (2, 9). Therefore, the O-PS structure of the wild rodent isolates may be identical or close to that of B. suis biovar 2. The Brucella O-PS structure has been described as being constituted by homopolymers of 4,6-dideoxy-4-formamido-α-d-mannopyranose residues. O-PS from A-dominant strains is a linear α-1,2-linked polymer with about 2% α-1,3 linkages, while O-PS from M-dominant strains is a linear polymer of pentasaccharide repeating units containing one α-1,3-linked and four α-1,2-linked monosaccharide residues (3, 23). MAbs specific for the C/Y epitopes probably recognize α-1,2-linked tri- or tetrasaccharides of the O-PS (3). The α-1,3 linkage should be mainly involved in the structure recognized by MAbs specific for the M epitope, since such MAbs fail to react with Y. enterocolitica O:9, lacking the α-1,3 linkages, and their preferential binding to M-dominant O-PS correlates with an increased number of α-1,3-linked monosaccharide residues. According to a recent study, B. suis biovar 2 also lacks α-1,3-linked monosaccharide residues in its O chain, and therefore the α-1,3 linkage may be involved in C epitope MAb recognition as well (M. V. Zaccheus et al., submitted for publication).
For most anti-O-PS MAbs, B. inopinata BO1 showed a MAb binding pattern in ELISA that was close to that of the M-dominant B. melitensis 16 M strain used as a control (Table 1). However, MAbs specific for the C/Y (A>M) epitopes showed significantly higher binding titers to strain BO1 than to strain 16 M, and in this case these were highly similar to those observed for the A-dominant strain B. suis 1330 (Table 1). To our knowledge, this is a new situation in the distribution of the C/Y (A>M) epitope, since the balance for this epitope between A- and M-dominant strains has always been clear before, with a usually superior binding to A-dominant strains (9). It is worth mentioning that MAbs specific for this epitope were initially classified as specific for the A epitope in early studies in the 1980s and the beginning of the 1990s (7, 14, 16, 21, 22).
Finally, the human Brucella sp. strain BO2 isolate proved to be the most atypical of this study, with a lack of binding in ELISA of all MAbs directed against O-PS epitopes (Table 1).
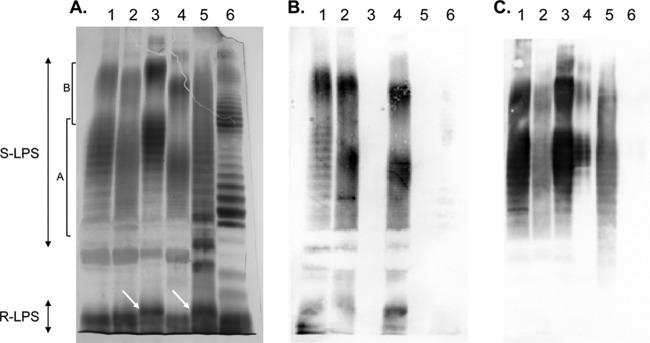
The LPS heterogeneity of strains of this study was also assessed by SDS-PAGE and Western blotting using the MAbs (Fig. 1). Silver staining of the LPS gels revealed the typical A-dominant banding pattern, with a close succession of regularly spaced narrow bands, in the S-LPS parts of all Australian wild rodent isolates (Fig. 1 and data not shown). The bimodal distribution, according to the O-chain length, of S-LPS molecules was similar to that observed for A-dominant control strain B. suis 1330 and as also reported for previously characterized S-LPSs of A-dominant Brucella strains (14, 16). This banding pattern was also confirmed by Western blotting using the MAbs specific for the O-PS epitopes and R-LPS epitopes. The latter MAbs revealed both the R-LPS part in the bottom of the gel and the S-LPS molecules, as previously reported (6). The banding pattern in silver staining of the S-LPS part of B. inopinata BO1 was typically M dominant, with regularly spaced doublets or triplets, as observed for control strain B. melitensis 16 M and for B. microti CCM 4915 (Fig. 1). As revealed by silver staining, there was some heterogeneity in the bimodal distribution of S-LPS molecules between these M-dominant strains. The M-dominant pattern was also revealed in Western blotting using the anti-O-PS MAbs (Fig. 1). Interestingly, in silver-stained gels B. inopinata BO1 displayed an additional regularly spaced banding pattern in the intermediate region between R-LPS and S-LPS that was not seen in any other Brucella species (Fig. 1). However, these bands were not detected in Western blotting using the MAbs of this study (Fig. 1 and data not shown). The additional bands detected by silver staining may thus constitute a distinct structural region in the LPS of B. inopinata BO1. None of the anti-R-LPS MAbs reacted with B. inopinata BO1 and B. microti CCM 4915, although they revealed, as expected, both R-LPS and S-LPS bands in M-dominant control strain 16 M (Fig. 1 and data not shown). Interestingly in silver staining of the LPS gel, an additional band or a shift in size of one of the R-LPS bands was observed for strains B. inopinata BO1 and B. microti CCM 4915 relative to the other strains used in this study (Fig. 1). This observation suggests a structural modification in the core-lipid A moiety of LPS of these strains, which may mask the epitopes recognized by the anti-R-LPS MAbs. To our knowledge, this kind of variation has also not been reported before for any of the classical Brucella species.
Fig 1.
Silver staining (A) and Western blot profiles with MAbs A68/10A06/B11 (anti-R-LPS) and 12G12 (anti-S-LPS; C [A = M] epitope) (B and C, respectively) after SDS-PAGE of proteinase K-digested S-LPS preparations of B. melitensis 16 M (M-dominant reference strain) (lanes 1), B. suis 1330 (A-dominant reference strain) (lanes 2), B. microti CCM 4915 (lanes 3), Brucella sp. strain 83-211 (wild rodent isolate from Australia) (lanes 4), B. inopinata BO1 (lanes 5), and Brucella sp. strain BO2 (lanes 6). The R-LPS part and the S-LPS parts with short and intermediate O-chains (bracket A) and with long O-chains (bracket B) are indicated. The arrows in the R-LPS part indicate the bands of higher molecular mass observed for B. microti CCM 4915 and B. inopinata BO1.
Brucella sp. strain BO2 showed a completely distinct S-LPS profile in silver-stained gels, with a higher regular spacing of the S-LPS bands that looked more similar to that observed for enterobacterial S-LPSs such as that from Escherichia coli or Salmonella (14, 16, 17). However, according to the typical S-LPS banding pattern, indicating in addition a multimodal distribution of S-LPS molecules, there is no doubt that this strain is smooth. As expected from the ELISA data, this strain failed to react in Western blotting with all MAbs against Brucella O-PS epitopes (data not shown) but showed a very weak reactivity with anti-R-LPS MAbs (Fig. 1 and data not shown).
Two genetic regions have been identified in the genomes of classical Brucella species as being essential for O-PS biosynthesis and translocation (20, 37). They are called wbo and wbk and encode several enzymes, such as glycosyltransferases, and proteins involved in O-PS polymerization and translocation. A blastn search (http://www.ncbi.nlm.nih.gov/sutils/genom_table.cgi), using these genetic regions, in the genome sequences of strain BO2 (available in GenBank) failed to detect any of the classical Brucella O-PS biosynthetic genes (data not shown). Although the genome sequences of strain BO2 are not completely assembled, we suspect that these genes are truly absent because in the other novel strains of this study with the same genomic assembly status as BO2, these genes were detected using blastn. On the other hand, since there is clearly S-LPS production in strain BO2 as evidenced by the LPS gels of this study, another genetic region absent from the classical Brucella species must be involved in a new O-PS biosynthetic pathway of this strain. The molecular basis of the novel LPS variations shown in this study therefore merits further investigation.
The results of the present study have several implications for serological typing and serological diagnosis and underline the need for novel tools for detection and correct identification of such novel emerging Brucella spp.
ACKNOWLEDGMENT
We thank B. K. De (CDC, Atlanta, GA) for providing the Australian wild rodent strains and strains BO1 and BO2 used in this study.
Footnotes
Published ahead of print 3 July 2012
REFERENCES
- 1. Alton GG, Jones LM, Angus RD, Verger JM. 1988. Techniques for the brucellosis laboratory. Institut National de la Recherche Agronomique, Paris, France [Google Scholar]
- 2. Baucheron S, Grayon M, Zygmunt MS, Cloeckaert A. 2002. Lipopolysaccharide heterogeneity in Brucella strains isolated from marine mammals. Res. Microbiol. 153:277–280 [DOI] [PubMed] [Google Scholar]
- 3. Bundle DR, et al. 1989. Definition of Brucella A and M epitopes by monoclonal typing reagents and synthetic oligosaccharides. Infect. Immun. 57:2829–2836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bundle DR, Gidney MAJ, Perry MB, Duncan JR, Cherwonogrodzky JW. 1984. Serological confirmation of Brucella abortus and Yersinia enterocolitica O:9 O-antigens by monoclonal antibodies. Infect. Immun. 46:389–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Caroff M, Bundle DR, Perry MB. 1984. Structure of the O-chain of the phenol-phase soluble cellular lipopolysaccharide of Yersinia enterocolitica serotype O:9. Eur. J. Biochem. 139:195–200 [DOI] [PubMed] [Google Scholar]
- 6. Cloeckaert A, Jacques I, Bowden RA, Dubray G, Limet JN. 1993. Monoclonal antibodies to Brucella rough lipopolysaccharide: characterization and evaluation of their protective effect against B. abortus. Res. Microbiol. 144:475–484 [DOI] [PubMed] [Google Scholar]
- 7. Cloeckaert A, Jacques I, de Wergifosse P, Limet JN. 1992. Protection against Brucella melitensis or Brucella abortus in mice with immunoglobulin G (IgG), IgA, and IgM monoclonal antibodies specific for a common epitope shared by the Brucella A and M smooth lipopolysaccharides. Infect. Immun. 60:312–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cloeckaert A, de Wergifosse P, Dubray G, Limet JN. 1990. Identification of seven surface-exposed Brucella outer membrane proteins by use of monoclonal antibodies: immunogold labeling for electron microscopy and enzyme-linked immunosorbent assay. Infect. Immun. 58:3980–3987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cloeckaert A, et al. 1998. O-polysaccharide epitopic heterogeneity at the surface of Brucella spp. studied by enzyme-linked immunosorbent assay and flow cytometry. Clin. Diagn. Lab. Immunol. 5:862–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cloeckaert A, Zygmunt MS, Dubray G, Limet JN. 1993. Characterization of O-polysaccharide specific monoclonal antibodies derived from mice infected with the rough Brucella melitensis strain B115. J. Gen. Microbiol. 139:1551–1556 [DOI] [PubMed] [Google Scholar]
- 11. De BK, et al. 2008. Novel Brucella strain (BO1) associated with a prosthetic breast implant infection. J. Clin. Microbiol. 46:43–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Díaz-Aparicio E, et al. 1993. Comparative analysis of Brucella serotype A and M and Yersinia enterocolitica O:9 polysaccharides for serological diagnosis of brucellosis in cattle, sheep, and goats. J. Clin. Microbiol. 31:3136–3141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Douglas JT, Palmer DA. 1988. Use of monoclonal antibodies to identify the distribution of A and M epitopes on smooth Brucella species. J. Clin. Microbiol. 26:1353–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dubray G, Limet J. 1987. Evidence of heterogeneity of lipopolysaccharides among Brucella biovars in relation to A and M specificities. Ann. Inst. Pasteur Microbiol. 138:27–37 [DOI] [PubMed] [Google Scholar]
- 15. Foster G, Osterman BS, Godfroid J, Jacques I, Cloeckaert A. 2007. Brucella ceti sp. nov. and Brucella pinnipedialis sp. nov. for Brucella strains with cetaceans and seals as their preferred hosts. Int. J. Syst. Evol. Microbiol. 57:2688–2693 [DOI] [PubMed] [Google Scholar]
- 16. Garin-Bastuji B, Bowden RA, Dubray G, Limet JN. 1990. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting analysis of smooth-lipopolysaccharide heterogeneity among Brucella biovars related to A and M specificities. J. Clin. Microbiol. 28:2169–2174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Giraud E, Cloeckaert A, Kerboeuf D, Chaslus-Dancla E. 2000. Evidence for active efflux as the primary mechanism of resistance to ciprofloxacin in Salmonella enterica serovar Typhimurium. Antimicrob. Agents Chemother. 44:1223–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Godfroid J, et al. 2005. From the discovery of the Malta fever's agent to the discovery of a marine mammal reservoir, brucellosis has continuously been a re-emerging zoonosis. Vet. Res. 36:313–326 [DOI] [PubMed] [Google Scholar]
- 19. Godfroid J, et al. 2011. Brucellosis at the animal/ecosystem/human interface at the beginning of the 21st century. Prev. Vet. Med. 102:118–131 [DOI] [PubMed] [Google Scholar]
- 20. Gonzalez D, et al. 2008. Brucellosis vaccines: assessment of Brucella melitensis lipopolysaccharide rough mutants defective in core and O-polysaccharide synthesis and export. PLoS One 3:e2760 doi:10.1371/journal.pone.0002760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Limet JN, Bosseray N, Garin-Bastuji B, Dubray G, Plommet M. 1989. Humoral immunity in mice mediated by monoclonal antibodies against the A and M antigens of Brucella. J. Med. Microbiol. 30:37–43 [DOI] [PubMed] [Google Scholar]
- 22. Limet J, Plommet AM, Dubray G, Plommet M. 1987. Immunity conferred upon mice by anti-LPS monoclonal antibodies in murine brucellosis. Ann. Inst. Pasteur Immunol. 138:417–424 [DOI] [PubMed] [Google Scholar]
- 23. Meikle PJ, Perry MB, Cherwonogrodzky JW, Bundle DR. 1989. Fine structure of A and M antigens from Brucella biovars. Infect. Immun. 57:2820–2828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moreno E, Cloeckaert A, Moriyón I. 2002. Brucella evolution and taxonomy. Vet. Microbiol. 90:209–227 [DOI] [PubMed] [Google Scholar]
- 25. Palmer DA, Douglas JT. 1989. Analysis of Brucella lipopolysaccharide with specific and cross-reacting monoclonal antibodies. J. Clin. Microbiol. 27:2331–2337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schlabritz-Loutsevitch NE, et al. 2009. A novel Brucella isolate in association with two cases of stillbirth in non-human primates—first report. J. Med. Primatol. 38:70–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Scholz HC, et al. 2009. Isolation of Brucella microti from mandibular lymph nodes of red foxes, Vulpes vulpes, in Lower Austria. Vector Borne Zoonotic Dis. 9:153–156 [DOI] [PubMed] [Google Scholar]
- 28. Scholz HC, et al. 2008. Isolation of Brucella microti from soil. Emerg. Infect. Dis. 14:1316–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Scholz HC, et al. 2008. Brucella microti sp. nov., isolated from the common vole Microtus arvalis Int. J. Syst. Evol. Microbiol. 58:375–382 [DOI] [PubMed] [Google Scholar]
- 30. Scholz HC, et al. 2010. Brucella inopinata sp. nov., isolated from a breast implant infection. Int. J. Syst. Evol. Microbiol. 60:801–808 [DOI] [PubMed] [Google Scholar]
- 31. Tiller RV, et al. 2010. Characterization of novel Brucella strains originating from wild native rodent species in North Queensland, Australia. Appl. Environ. Microbiol. 76:5837–5845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tiller RV, et al. 2010. Identification of an unusual Brucella strain (BO2) from a lung biopsy in a 52 year-old patient with chronic destructive pneumonia. BMC Microbiol. 10:23 doi:10.1186/1471-2180-10-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Verger JM, Grimont F, Grimont PAD, Grayon M. 1985. Brucella, a monospecific genus as shown by deoxyribonucleic-acid hybridization. Int. J. Syst. Bacteriol. 35:292–295 [Google Scholar]
- 34. Vizcaino N, Chordi A, Fernández-Lago L. 1991. Characterization of smooth Brucella lipopolysaccharides and polysaccharides by monoclonal antibodies. Res. Microbiol. 142:971–978 [DOI] [PubMed] [Google Scholar]
- 35. Weynants V, et al. 1997. Characterization of smooth lipopolysaccharide and O polysaccharides of Brucella species by competition binding assays with monoclonal antibodies. Infect. Immun. 65:1939–1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Whatmore AM. 2009. Current understanding of the genetic diversity of Brucella, an expanding genus of zoonotic pathogens. Infect. Genet. Evol. 9:1168–1184 [DOI] [PubMed] [Google Scholar]
- 37. Zygmunt MS, Blasco JM, Letesson JJ, Cloeckaert A, Moriyon I. 2009. DNA polymorphism analysis of Brucella lipopolysaccharide genes reveals marked differences in O-polysaccharide biosynthetic genes between smooth and rough Brucella species and novel species-specific markers. BMC Microbiol. 9:92 doi:10.1186/1471-2180-9-92 [DOI] [PMC free article] [PubMed] [Google Scholar]