Abstract
The influence of adjunctive corticosteroids on the cytokine response in community-acquired pneumonia (CAP) is largely unknown. In this study, we analyzed the effect of dexamethasone on the cytokine response in patients with CAP and evaluated whether this effect is dependent on the causative microorganism. We hypothesized that dexamethasone has a larger effect on the cytokine response in patients with pneumococcal pneumonia than in patients with pneumonia caused by an atypical bacterium. A total of 304 hospitalized, nonimmunocompromised patients with CAP were randomized to an adjunctive 4-day course of 5 mg dexamethasone once a day (n = 151) or a placebo (n = 153). Serum concentrations of interleukin-1 receptor antagonist (IL-1Ra), IL-6, IL-8, IL-10, IL-17, tumor necrosis factor alpha (TNF-α), gamma interferon (IFN-γ), macrophage inflammatory protein-1 alpha (MIP-1α), and monocyte chemotactic protein-1 (MCP-1) were measured on days 0, 1, 2, and 4 and at a control visit. Overall, the concentrations of IL-6 (P < 0.01), IL-8 (P < 0.01), MCP-1 (P < 0.01), and TNF-α (P < 0.01) were significantly lower on day 2 in the dexamethasone group than in the placebo group. In patients with pneumococcal pneumonia (n = 72), both treatment groups showed a rapid decrease of cytokine concentrations; only the concentration of TNF-α (P = 0.05) was significantly lower in the dexamethasone group on day 2. In patients with CAP caused by an atypical pathogen (Legionella pneumophila, Chlamydophila species, Coxiella burnetii, or Mycoplasma pneumoniae; n = 58), IL-1Ra (P < 0.01), IL-6 (P < 0.01), and MCP-1 (P = 0.03) decreased more rapidly in the dexamethasone group than in the placebo group. In conclusion, dexamethasone downregulates the cytokine response during CAP. This effect seems to be dependent on the causative microorganism. This study provides insight into which patients with CAP might benefit most from adjunctive dexamethasone.
INTRODUCTION
During a pulmonary infection, invading pathogens shed microbial components into the local environment. As a result, inflammatory cells become activated and will secrete a spectrum of cytokines and chemokines (4). These cytokines and chemokines serve to control and eliminate the infection by leukocyte recruitment and inflammation. If not regulated tightly, the inflammatory response can become excessive and may progress into sepsis and, ultimately, multiple organ dysfunction syndrome (MODS). The nature and magnitude of the inflammatory response are determined by host characteristics, the nature of the causative microorganisms, and antibiotic treatment (10).
Glucocorticoids are potent physiological inhibitors of the inflammatory response. Currently, they are widely used as adjunctive treatment in various infectious diseases, such as meningitis and sepsis (1–3, 17). Recently, we have shown that adjunctive corticosteroids can also be beneficial in the treatment of community-acquired pneumonia (CAP): a 4-day course of dexamethasone reduced the length of the hospital stay by 1 day when added to antibiotic treatment in nonimmunocompromised CAP patients (9). Adjunctive therapy with corticosteroids might, hypothetically, downregulate excessive, potentially detrimental cytokine responses and hereby accelerate clinical recovery.
Over the last decades, major advances have been made in the understanding of the molecular mechanisms by which glucocorticoids suppress inflammation. However, the influence of corticosteroids on the cytokine response in CAP is largely unknown. Up to now, only 2 studies have addressed this issue, and they showed that corticosteroids can reduce the concentrations of interleukin-1β (IL-1β), IL-6, and tumor necrosis factor alpha (TNF-α) (8, 11). Furthermore, whether the effect of dexamethasone on cytokines in CAP is dependent on the causative microorganism has never been investigated.
In this study, we analyzed the effect of dexamethasone on the cytokine response in patients with CAP. Next, we evaluated whether the effect of dexamethasone on the cytokine kinetics depends on the causative microorganism of CAP. We hypothesized that dexamethasone has a larger effect on the cytokine response in patients with a pneumococcal pneumonia than in patients with pneumonia caused by an atypical bacterium, because pneumococci generally elicit a higher proinflammatory response in cases of pneumonia (4).
MATERIALS AND METHODS
Patients and study design.
This was a preplanned subanalysis of patients with CAP who were prospectively enrolled in a study on the effect of dexamethasone on the length of hospital stay. The details of the study population and design have been described previously (9). In short, from November 2007 until September 2010, adult patients with confirmed pneumonia at the emergency department of the St. Antonius Hospital in Nieuwegein or at the Gelderse Vallei Hospital in Ede, both teaching hospitals in the Netherlands, were included. Patients who were immunocompromised or on immunosuppressive therapy (including oral corticosteroids) or who required immediate admission to the intensive care unit (ICU) were excluded. All patients were randomized to a 4-day course of either 5 mg (1 ml) of dexamethasone (5 mg dexamethasone disodium phosphate; Centrafarm BV, Etten-Leur, the Netherlands) intravenously (i.v.) or 1 ml of sterile water (water for injection; Centrafarm BV, Etten-Leur, the Netherlands) i.v. Randomization was based on a one-to-one allocation by means of prenumbered boxes containing four ampoules for i.v. administration. Patients, investigators, and those assessing the data were masked to allocation. We calculated the pneumonia severity index (PSI) score for all patients (5). The local ethics committee approved the study, and informed consent was obtained from all participants (ClinicalTrials.gov number NCT 00471640).
Analysis of the cytokine response.
Serum was obtained on the day of presentation (before the first administration of dexamethasone), and subsequent samples were drawn at 8 a.m. on days 1, 2, and 4 and at a control visit at least 30 days after admission (convalescent phase). Serum samples were frozen at −80°C until analysis. Circulating concentrations of interleukin-1 receptor antagonist (IL-1Ra), IL-6, IL-8, IL-10, IL-17, TNF-α, gamma interferon (IFN-γ), macrophage inflammatory protein-1 alpha (MIP-1α), and monocyte chemotactic protein-1 (MCP-1) were measured by Milliplex multianalyte profiling (Millipore, Billerica, MA) according to the manufacturer's instructions. Data acquisition and analysis were performed on a Luminex 100 instrument (Luminex, Austin, TX).
Pathogen identification.
At least two sets of separate blood and sputum samples (if available) were cultured from each patient. Urine antigen tests were performed for the detection of Legionella pneumophila serogroup 1 (Binax-Now; Binax, Portland, ME) and Streptococcus pneumoniae (Binax-Now; Binax, Portland, ME). In-house-developed PCRs were performed on the sputum to detect L. pneumophila, Mycoplasma pneumoniae, Coxiella burnetii, and Chlamydophila psittaci. Paired serological testing was performed for the presence of antibodies to M. pneumoniae, C. burnetii, C. pneumoniae/psittaci, or respiratory viruses (adenovirus, influenza A and B virus, parainfluenza virus 1, 2, and 3, and the respiratory syncytial virus) (Serodia, Bipharma; Fujirebio Inc., Tokyo, Japan). A 4-fold increase in antibody titer was considered positive. Pharyngeal samples were taken for viral cultures (for [para]influenza virus, adenovirus, and respiratory syncytial virus).
Statistical analysis.
All statistical analyses were performed using statistics software (SPSS version 18.0 for Windows; Chicago, IL). A two-tailed P value of <0.05 was considered significant.
Differences in categorical variables were analyzed with the chi-square test or Fisher's exact test, and differences in continuous data were analyzed with Student's t test. To investigate the influence of dexamethasone on the cytokine dynamics, linear regression analysis was performed. For the analyses, cytokine concentrations were transformed into a natural log scale because of a nonnormal distribution. We chose to analyze the decrease of the cytokines from day 0 to day 2, because all patients randomized to dexamethasone were on dexamethasone for at least 24 h on day 2 and because cytokine concentrations decreased most during the first days. To correct for the magnitude of the cytokine response on day 0, we included the cytokine concentration on day 0 as an independent variable in the linear regression.
To evaluate whether the effect of dexamethasone on the cytokine kinetics is dependent on the causative microorganism of CAP, we selected 3 etiological subgroups: (i) patients with CAP caused by S. pneumoniae, (ii) patients with CAP caused by an atypical pathogen, and (iii) patients with CAP of unknown etiology. Atypical pathogens include L. pneumophila, M. pneumoniae, Chlamydophila species, and C. burnetii. Cytokine concentrations were compared between the three subgroups by linear regression analysis in a manner similar to that described above.
To examine the possible influence of antibiotic treatment on the cytokine response, a linear regression analysis, in which the variable “appropriate antibiotic treatment” was added to the other variables (“randomization” and the various cytokine concentrations on day 0) was performed.
RESULTS
A total of 304 patients were enrolled in the study. The baseline characteristics of the patients are shown in Table 1. In 175 (58%) patients, an etiological diagnosis could be established. In 24% of the patients, S. pneumoniae was detected; in 19%, an atypical bacterium (L. pneumophila, 3.9%; M. pneumoniae, 1.6%; C. burnetii, 8.9%; Chlamydophila species, 4.6%) was detected; in 6%, a Gram-negative bacterium other than L. pneumophila was detected; in 6%, a viral pathogen was detected; in 2%, another Gram-positive bacterium was detected. There were no significant differences in etiological diagnoses between the dexamethasone and placebo groups.
Table 1.
Baseline characteristics of 304 patients with community-acquired pneumonia who were randomized to dexamethasone or a placebo
| Characteristica | Value for patients in each groupb |
|
|---|---|---|
| Dexamethasone (n = 151) | Placebo (n = 153) | |
| No. of males (% of total) | 84 (56) | 87 (57) |
| Age (yr) (SD) | 64.5 (18.7) | 62.8 (18.2) |
| Ethnicity (no. [%])c | ||
| Caucasian | 149 (99) | 150 (98) |
| Other | 2 (1.3) | 3 (2.0) |
| Nursing home resident (no. [%]) | 9 (6.0) | 7 (4.6) |
| Current smoker (no. [%]) | 38 (25) | 38 (25) |
| Antibiotic treatment before admission (no. [%]) | 42 (28) | 39 (25) |
| Comorbidities (no. [%]) | ||
| Neoplastic disease | 9 (6.0) | 10 (6.5) |
| Liver disease | 2 (1.3) | 0 (0.0) |
| Congestive heart failure | 24 (16) | 24 (16) |
| Renal disease | 20 (13) | 10 (7) |
| Diabetes mellitus | 22 (15) | 21 (14) |
| COPD | 20 (13) | 14 (9) |
| PSI score (SD) | 100.2 (33.4) | 95.8 (32.5) |
| PSI risk class (no. of points) (no. [%]) | ||
| I | 18 (12) | 22 (14) |
| II (≤70) | 30 (20) | 34 (22) |
| III (71–90) | 24 (16) | 33 (22) |
| IV (91–130) | 54 (36) | 43 (28) |
| V (>130) | 25 (17) | 21 (14) |
| IV and V | 79 (52) | 64 (42) |
SD, standard deviation; COPD, chronic obstructive lung disease; PSI, pneumonia severity index.
Data are presented as number (%) or mean (SD).
Self-reported.
Dexamethasone reduces the magnitude of the cytokine response.
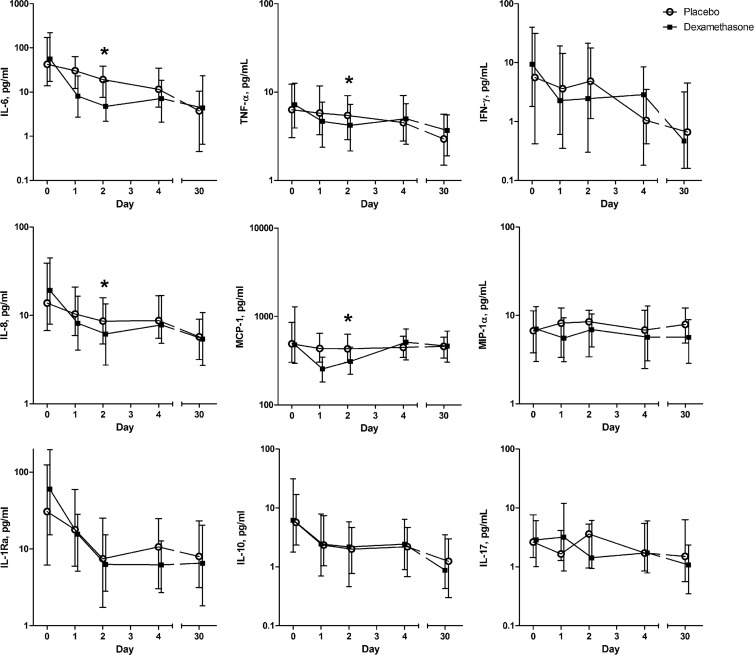
During their hospital stay, 151 patients received a 4-day course of dexamethasone and 153 patients received a placebo. Administration of dexamethasone changed the dynamics of the cytokine response (Fig. 1). Cytokine concentrations were similar in the treatment groups on the day of hospital admission. The concentrations of IL-6 (P < 0.01; β, −1.319 [percent decrease of the indicated cytokine concentration from day 0 to day 2 for the patients treated with dexamethasone, −73%]), IL-8 (P < 0.01; β, −0.423 [−35%]), MCP-1 (P < 0.01; β, −0.385 [−32%]), and TNF-α (P < 0.01; β, −0.484 [−38%]) were significantly lower on day 2 in the dexamethasone-treated patients than in the placebo-treated patients. IL-10 showed a rapid decrease in both treatment groups. Low systemic concentrations of IL-17 were found on admission and remained present during the study period (Fig. 1).
Fig 1.
Median serum cytokine concentrations (with interquartile ranges) in patients with community-acquired pneumonia treated with either dexamethasone or placebo, from hospital admission to day 30. The asterisks indicate significant differences in the cytokine concentrations on day 2 between the placebo and dexamethasone groups (corrected for the magnitude of the cytokine response on day 0).
Relation between the causative microorganism and the decrease in cytokine concentrations.
To determine whether the influence of dexamethasone on the cytokine response is dependent on the causative microorganism, we compared the three selected etiological subgroups of patients: patients with a pneumococcal pneumonia (n = 72), patients with CAP due to an atypical microorganism (n = 58), and patients with CAP of unknown etiology (n = 129).
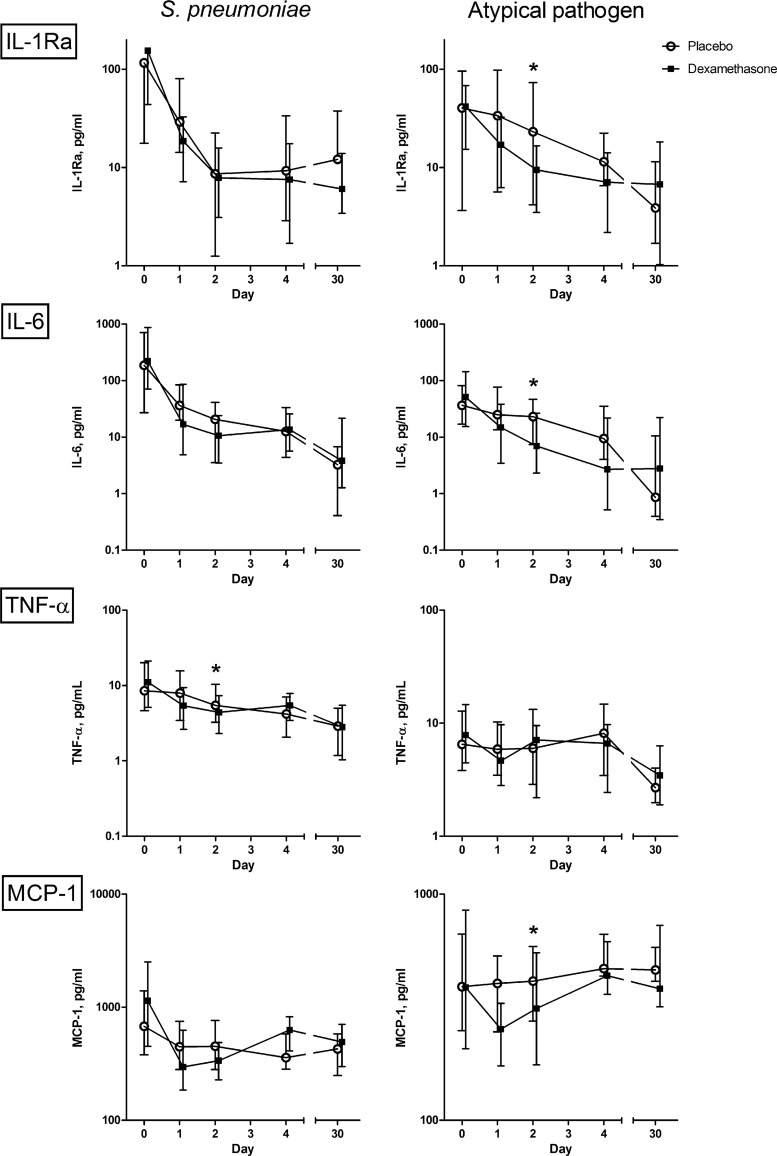
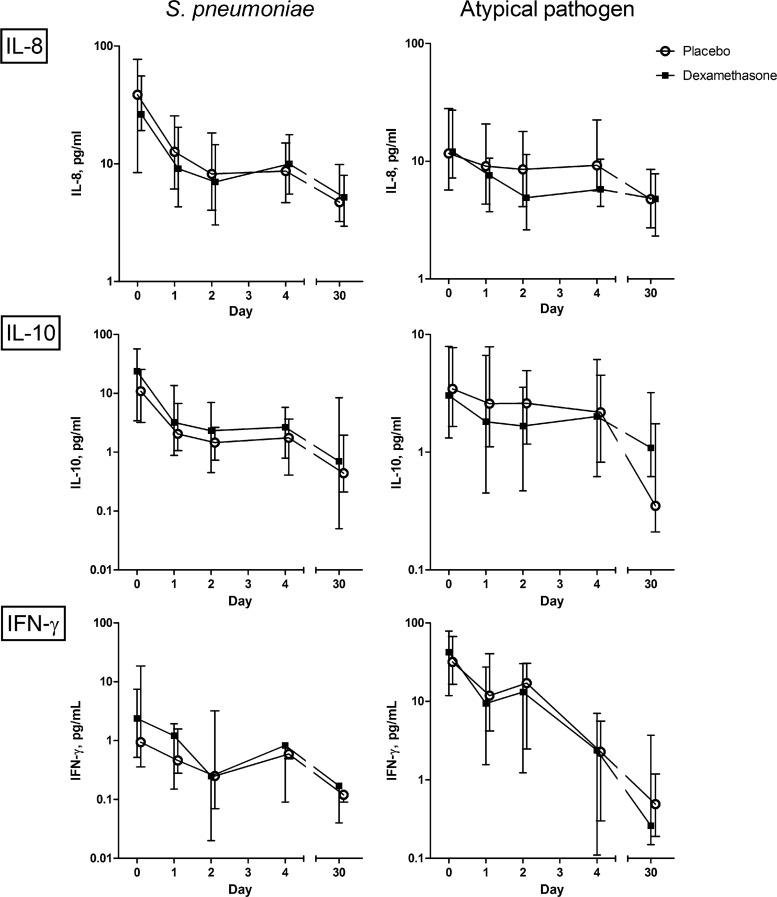
In pneumococcal pneumonia, both treatment groups showed a rapid decrease of cytokine concentrations. Only the concentration of TNF-α (P = 0.05; β, −0.227) was significantly lower in the dexamethasone group than in the placebo group on day 2 (Fig. 2). In patients with CAP caused by an atypical pathogen, IL-1Ra (P < 0.01; β, −0.324 [−28%]), IL-6 (P < 0.01; β, −0.400 [−33%]), and MCP-1 (P = 0.03; β, −0.234 [−21%]) decreased more rapidly in the dexamethasone group (Fig. 2). In patients with CAP of unknown etiology, this effect of dexamethasone was seen for IL-6 (P < 0.01; β, −0.485 [−38%]), IL-8 (P < 0.01; β, −0.251 (−22%), TNF-α (P < 0.01; β, −0.325 [−28%]), and MCP-1 (P < 0.01; β, −0.307 (−26%). The cytokines and chemokines that did not show a significant influence of dexamethasone in CAP caused by either S. pneumoniae or an atypical pathogen are shown in Fig. 3.
Fig 2.
Influence of dexamethasone on median serum cytokine concentrations (with interquartile ranges) in patients with community-acquired pneumonia caused by either Streptococcus pneumoniae or an atypical bacterium, from hospital admission to day 30. This figure represents those cytokines that showed significantly different patterns in the two treatment groups, as indicated by an asterisk. Atypical bacteria include L. pneumophila, M. pneumoniae, Chlamydophila species, and C. burnetii.
Fig 3.
Influence of dexamethasone on median serum cytokine concentrations (with interquartile ranges) in patients with community-acquired pneumonia caused by either Streptococcus pneumoniae or an atypical bacterium, from hospital admission to day 30. This figure represents the cytokines that did not show different patterns in the two treatment groups. Atypical bacteria include L. pneumophila, M. pneumoniae, Chlamydophila species, and C. burnetii. The concentrations of IL-17 and MIP-1α in most cases fell out of range, below 3.2 pg/ml, which precluded reporting of the courses of these cytokines for the separate etiological groups.
We next analyzed whether the differences in effects on cytokine responses between the dexamethasone and placebo groups may be biased by the appropriateness of the empirical antibiotic treatment. This analysis was performed solely in patients with pneumonia caused by an atypical bacterium, because all patients with a pneumococcal pneumonia received the appropriate antibiotics upon admission. Analysis of the antibiotic prescriptions in patients with an atypical pathogen revealed that in the placebo group, 14 patients (44%) received the appropriate antibiotics on admission while 18 patients (56%) were initially treated with an antibiotic that did not cover the causative microorganism. In the dexamethasone group, these figures were 13 patients (50%) for both groups (P = 0.64). Linear regression revealed that only IL-6 concentrations were independently influenced by both randomization (P < 0.01; β, −0.330) and antibiotic treatment (P < 0.01; β, −0.354). Thus, appropriateness of empirical antibiotic therapy had some impact on cytokine concentrations but could not fully explain the differences between the dexamethasone and placebo groups. This confirmed that dexamethasone is capable of causing a considerable reduction of the cytokine concentrations.
DISCUSSION
In this study, we showed that, in general, adjunctive dexamethasone therapy reduces the concentrations of IL-6, IL-8, TNF-α, and MCP-1 in patients with CAP. Interestingly, a clear difference in dexamethasone effects was found between different microbial etiologies of CAP. Dexamethasone appeared to have little additional influence on the cytokine concentrations in patients with a pneumococcal pneumonia, while in patients with CAP caused by an atypical pathogen, dexamethasone gave a significantly faster decrease of cytokine concentrations than the placebo.
The overall dampening effect of dexamethasone on cytokine and chemokine concentrations is in concordance with the findings in other studies (6–8, 11–13). The majority of the former studies on corticosteroids in relation to cytokine responses have been performed in patients with septic shock (6, 7, 12, 13). To the best of our knowledge, only two studies have investigated this subject in patients with pneumonia (8, 11). However, the generalizability of their results is limited, since in one study, all patients were mechanically ventilated, and in the study of Marik et al., only patients with severe pneumonia who were admitted to the ICU were evaluated. We are the first to report the influence of dexamethasone on a broader range of cytokines and in patients with CAP of all severities.
Prior CAP studies have not addressed the effect of dexamethasone on the cytokine kinetics in relation to the causative microorganism. In contrast with our hypothesis, we found little additional influence of dexamethasone in patients with pneumococcal pneumonia. The cytokine concentrations decreased rapidly during the first days of hospital admission in both the dexamethasone and placebo groups. This lack of an effect can possibly be explained by the high sensitivity to β-lactam antibiotics of S. pneumoniae strains in the Netherlands, which might have had an overriding effect over any dexamethasone effect (15).
Interestingly, in the patients with CAP caused by an atypical bacterium, proinflammatory cytokines decreased more rapidly in the dexamethasone-treated patients. An additional analysis confirmed that the more prominent cytokine decrease in dexamethasone-treated patients was a true dexamethasone effect. Next to this dexamethasone effect, appropriateness of antibiotic treatment also played an independent role in the dynamics of IL-6.
A possible explanation for the variation in dexamethasone effects between various types of microorganisms is the difference in the cellular inflammatory responses required for elimination of the pathogen. Atypical pathogens (most of them intracellular) require a mononuclear cell inflammatory response, compared to a more neutrophil-mediated response in typical (extracellular) bacterial microorganisms. This mononuclear cell inflammatory response stimulates a cytokine- and cell-mediated immune response. Corticosteroids particularly downregulate the cell-mediated immune response, and this might explain the more rapid decrease of cytokines in dexamethasone-treated patients with CAP caused by an atypical microorganism. In M. pneumoniae pneumonia, corticosteroid therapy in addition to antibiotics has already been advocated (14, 16).
This study has some limitations. First, in the etiological subgroup analysis, due to a lack of power, we were able to analyze only patients with pneumococcal pneumoniae and a combined group of atypical microorganisms rather than analyzing these pathogens separately. It is possible that there are even differences in cytokine response patterns among the atypical microorganisms, but larger studies are needed to allow statistical analysis of all pathogens separately. Second, only systemic cytokine measurements were performed in this study. The systemic cytokine response during CAP might differ from the local cytokine response in the lung. However, due to medical ethical restrictions, it was impossible to obtain bronchoalveolar lavage fluids of these patients during or after the active phase of the disease.
In conclusion, this study shows that cytokines are downregulated by adjunctive dexamethasone treatment. Our results suggest that the effect of dexamethasone on the cytokine response in CAP is dependent on the causative microorganism. This study provides insight into which patients with CAP might benefit most from adjunctive dexamethasone.
Larger studies are needed to further explore the exact role of the causative pathogen in the response to corticosteroids.
ACKNOWLEDGMENTS
There are no conflicts of interest to declare.
There was no external funding.
Footnotes
Published ahead of print 1 August 2012
REFERENCES
- 1. Annane D, et al. 2002. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA 288:862–871 [DOI] [PubMed] [Google Scholar]
- 2. Bollaert PE, et al. 1998. Reversal of late septic shock with supraphysiologic doses of hydrocortisone. Crit. Care Med. 26:645–650 [DOI] [PubMed] [Google Scholar]
- 3. Briegel J, et al. 1999. Stress doses of hydrocortisone reverse hyperdynamic septic shock: a prospective, randomized, double-blind, single-center study. Crit. Care Med. 27:723–732 [DOI] [PubMed] [Google Scholar]
- 4. Endeman H, et al. 2011. Systemic cytokine response in patients with community-acquired pneumonia. Eur. Respir. J. 37:1431–1438 [DOI] [PubMed] [Google Scholar]
- 5. Fine MJ, et al. 1997. A prediction rule to identify low-risk patients with community-acquired pneumonia. N. Engl. J. Med. 336:243–250 [DOI] [PubMed] [Google Scholar]
- 6. Kaufmann I, et al. 2008. Stress doses of hydrocortisone in septic shock: beneficial effects on opsonization-dependent neutrophil functions. Intensive Care Med. 34:344–349 [DOI] [PubMed] [Google Scholar]
- 7. Keh D, et al. 2003. Immunologic and hemodynamic effects of “low-dose” hydrocortisone in septic shock: a double-blind, randomized, placebo-controlled, crossover study. Am. J. Respir. Crit. Care Med. 167:512–520 [DOI] [PubMed] [Google Scholar]
- 8. Marik P, et al. 1993. Hydrocortisone and tumor necrosis factor in severe community-acquired pneumonia. A randomized controlled study. Chest 104:389–392 [DOI] [PubMed] [Google Scholar]
- 9. Meijvis SC, et al. 2011. Dexamethasone and length of hospital stay in patients with community-acquired pneumonia: a randomised, double-blind, placebo-controlled trial. Lancet 377:2023–2030 [DOI] [PubMed] [Google Scholar]
- 10. Menendez R, et al. 2012. Cytokine activation patterns and biomarkers are influenced by microorganisms in community-acquired pneumonia. Chest 141:1537–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Monton C, et al. 1999. Role of glucocorticoids on inflammatory response in nonimmunosuppressed patients with pneumonia: a pilot study. Eur. Respir. J. 14:218–220 [DOI] [PubMed] [Google Scholar]
- 12. Mussack T, Briegel J, Schelling G, Biberthaler P, Jochum M. 2005. Effect of stress doses of hydrocortisone on S-100B vs. interleukin-8 and polymorphonuclear elastase levels in human septic shock. Clin. Chem. Lab. Med. 43:259–268 [DOI] [PubMed] [Google Scholar]
- 13. Oppert M, et al. 2005. Low-dose hydrocortisone improves shock reversal and reduces cytokine levels in early hyperdynamic septic shock. Crit. Care Med. 33:2457–2464 [DOI] [PubMed] [Google Scholar]
- 14. Radisic M, Torn A, Gutierrez P, Defranchi HA, Pardo P. 2000. Severe acute lung injury caused by Mycoplasma pneumoniae: potential role for steroid pulses in treatment. Clin. Infect. Dis. 31:1507–1511 [DOI] [PubMed] [Google Scholar]
- 15. Schouten JA, et al. 2005. Optimizing the antibiotics policy in The Netherlands. VIII. Revised SWAB guidelines for antimicrobial therapy in adults with community-acquired pneumonia. Ned. Tijdschr. Geneeskd. 149:2495–2500 (In Dutch.) [PubMed] [Google Scholar]
- 16. Tagliabue C, et al. 2008. The impact of steroids given with macrolide therapy on experimental Mycoplasma pneumoniae respiratory infection. J. Infect. Dis. 198:1180–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van de Beek D, de Gans J, McIntyre P, Prasad K. 2007. Corticosteroids for acute bacterial meningitis. Cochrane Database Syst. Rev. 1:CD004405. [DOI] [PubMed] [Google Scholar]