Abstract
Interleukin-18 (IL-18), which was originally called gamma interferon (IFN-γ)-inducing factor, has been shown to play an important role in innate and acquired immune responses. In this study, attenuated Erysipelothrix rhusiopathiae strains were engineered to produce porcine IL-18 (poIL-18) and evaluated for their potential immunostimulatory effect in animals. Recombinant poIL-18 was successfully expressed in the recombinant E. rhusiopathiae strains YS-1/IL-18 and KO/IL-18. The culture supernatant of YS-1/IL-18 was confirmed to induce IFN-γ production in murine splenocytes in vitro, and this production was inhibited by incubation with anti-poIL-18 monoclonal antibodies. Furthermore, more IFN-γ production was induced upon stimulation of splenocytes with concanavalin A for splenocytes from mice that were intraperitoneally inoculated with YS-1/IL-18 than for splenocytes from control mice inoculated with the parent strain YS-1. Peritoneal macrophages from mice preinoculated with YS-1/IL-18 exhibited enhanced phagocytosis of Salmonella enterica subsp. enterica serovar Typhimurium compared with peritoneal macrophages from control mice preinoculated with YS-1. We also confirmed the immunostimulatory effect on humoral immune responses against antigens of E. rhusiopathiae and Mycoplasma hyopneumoniae in gnotobiotic pigs that were orally preinoculated with KO/IL-18. Thus, these results provide evidence that E. rhusiopathiae is a promising vector for the expression of host cytokines and suggest the potential utility of E. rhusiopathiae vector-encoded cytokines in the activation of host innate and acquired immune responses.
INTRODUCTION
The design of efficient delivery systems for vaccine development is an area of intensive research. A large number of innovative strategies for animal vaccines or vaccine formulations are being investigated, including the use of live vectors, microparticles, and liposomes, and significant advances have been achieved (7, 11). In the livestock industry, one of the most challenging strategies is the development of a novel system that is efficient, cost-effective, and deliverable through the oral or nasal route to induce mucosal immune responses (7, 11). Because most infections begin at mucosal surfaces, these routes should be the most effective in blocking pathogens at their entry point (7).
Swine erysipelas is a disease caused by the Gram-positive facultative intracellular pathogen Erysipelothrix rhusiopathiae and is one of the best-known and most serious diseases affecting domestic pigs. Currently, E. rhusiopathiae vaccines are used worldwide. Moreover, these vaccines can be used for oral delivery, the most attractive route for the mucosal immunization of livestock (7, 11). The cost of the production of E. rhusiopathiae-based vaccines or vaccine formulations is low, so there are advantages in using E. rhusiopathiae as a delivery vehicle.
Thus far, we have assessed the potential use of attenuated strains of E. rhusiopathiae as vectors for delivering the P97 adhesin antigen of Mycoplasma hyopneumoniae, the etiological agent of mycoplasmal or enzootic pneumonia of swine (21, 27, 28). We used the YS-1 strain (26), an acapsular attenuated mutant of E. rhusiopathiae, and the Koganei 65-0.15 strain, the live swine erysipelas vaccine in Japan, and engineered these strains to express a recombinant protein containing the carboxyl-terminal portion of the P97 adhesin on the cell surface. We examined the vaccine efficacy of these strains against erysipelas and mycoplasmal pneumonia by immunizing pigs intranasally or orally, and we demonstrated that E. rhusiopathiae is a promising vaccine vector for delivering foreign antigens to the immune system of pigs (21, 27, 28).
Interleukin-18 (IL-18) was initially regarded as a gamma interferon (IFN-γ)-inducing factor because of its ability to induce IFN-γ production by Th1 cells (22). However, it has been reported that depending on the cell type, IL-18 can also act as an inducer of Th2 cytokines such as IL-4 and IL-13 (12, 33). Thus, the biological activity of IL-18 is complex, and IL-18 is a unique cytokine that enhances innate immunity and both Th1- and Th2-driven adaptive immune responses (2, 20). It has been shown that IL-18 is expressed by many types of cells, including macrophages, peripheral blood mononuclear cells (PBMCs), keratinocytes, and dendritic cells (6, 23, 30, 32), and is essential in host defenses against a wide variety of infections caused by bacteria, viruses, fungi, and protozoa (9). Intriguingly, the epithelial cells lining intestinal and respiratory surfaces express this cytokine, suggesting that IL-18 has an important role in the induction of mucosal immunity (1, 31). Thus, the unique immunological properties of IL-18 and its constitutive expression in various immune cells and tissues and at mucosal surfaces indicate that this cytokine may be a promising vaccine component or adjuvant to stimulate a wide variety of local and systemic immune responses to infection.
In this study, we examined whether our E. rhusiopathiae system could be used to express recombinant porcine IL-18 (poIL-18) and to deliver the cytokine in vivo for immunostimulation. We showed that recombinant E. rhusiopathiae expressing poIL-18 has an immunostimulatory effect in vivo in mice and enhances the local and systemic humoral immune responses against bacterial antigens in pigs receiving the vector via the oral route.
MATERIALS AND METHODS
Microorganisms and media.
The E. rhusiopathiae strains used were YS-1 (26), Koganei 65-0.15 (live Japanese vaccine strain), and the recombinant derivatives YS-1/IL-18 and KO/IL-18. These strains were grown in brain heart infusion (BHI; Difco Laboratories, Detroit, MI) medium containing 0.3% Tris and 0.1% Tween 80, pH 8.0 (BHI-T80), or on BHI-T80 agar plates. The wild-type Salmonella enterica subsp. enterica serovar Typhimurium strain L-3543, which was isolated from a pig that was serologically positive for Salmonella but had no clinical signs, was cultivated in LB broth supplemented with ampicillin (200 μg/ml). To determine the number of bacteria in mouse organs, tissue homogenates were plated on desoxycholate-hydrogen sulfide-lactose agar plates. The cultivation of M. hyopneumoniae strain E-1 was performed as previously described (17).
Generation of recombinant E. rhusiopathiae strains.
The plasmid and the recombinant E. rhusiopathiae strains were constructed according to previously described procedures (27). Briefly, a poIL-18 gene (GenBank/EMBL/DDBJ accession no. AB010003) with caspase-1 recognition sequences was amplified from pVL1392-IL-18 (19) by a PCR using KOD FX (Toyobo Co. Ltd., Osaka, Japan) and the primers IL18D (5′-CCCCGAATTCTGGAATCGGATTACTTTGGCA-3′) and IR18R (5′-CCCCGAATTCGAGTTCTTGTTTTGAACAGTGAACA-3′), each of which contains an EcoRI site (underlined). The PCR cycling parameters were 94°C for 2 min and 35 cycles of 94°C for 30 s, 50°C for 30 s, and 68°C for 1 min. The PCR product was digested with EcoRI and then used to replace the EcoRI fragment of pGA14/Mh1, which contained a SpaA.1-P97 chimeric gene (27). The resulting plasmid was introduced into the YS-1 and Koganei 65-0.15 strains through electroporation. The SpaA.1/IL-18 chimeric gene was integrated into the chromosome in the transformants through homologous recombination. Recombination of the chimeric gene was confirmed by PCR.
Detection of recombinant poIL-18 by ELISA and immunoblotting.
Overnight cultures of the E. rhusiopathiae strains were centrifuged, and the supernatants were recovered. The bacterial cell pellets were washed once with phosphate-buffered saline containing 0.1% Tween 20 (PBS-T20) and resuspended in PBS-T20. After ultrasonic treatment, the debris was removed by centrifugation, and the supernatant, which contained cell surface proteins, was recovered. The culture supernatants and the cell surface preparations were assayed using a poIL-18-specific enzyme-linked immunosorbent assay (ELISA) (18). For immunoblotting, the proteins in the culture supernatants were further precipitated with 10% trichloroacetic acid (TCA) for 90 min on ice, analyzed by SDS-PAGE, transferred onto a membrane, and probed with poIL-18-specific monoclonal antibodies (MAbs) (18).
Recombinant poIL-18 biological activity assay.
The in vitro biological activity of the recombinant poIL-18 produced by E. rhusiopathiae was assessed by assaying its IFN-γ-inducing activity after incubation with murine splenocytes. To avoid the inclusion of Tween 80 in the medium, as this compound causes cell damage, the bacteria were grown overnight in the dialyzable components of BHI-T80 medium, not including Tween 80 micelles. The bacterial culture supernatant was collected by centrifugation, concentrated by ultrafiltration, dialyzed against PBS, and then used for the following experiments. Murine splenocytes were collected from 13-week-old female BALB/c mice (Japan SLC, Inc., Hamamatsu, Japan) and suspended at a cell density of 2 × 106/ml in RPMI 1640 (Gibco, Invitrogen, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS), 1 mM sodium pyruvate, 55 μM 2-mercaptoethanol, penicillin (50 U/ml), and streptomycin (50 μg/ml). A 100-μl aliquot of the cell suspension was cultured for 48 h with 100 μl of a 50-fold-concentrated culture supernatant of the E. rhusiopathiae strains. For poIL-18-neutralizing experiments, 100 μl of cell suspension was cultured with 50 μl of a 100-fold-concentrated bacterial culture supernatant and a 50-μl mixture (50 μg of protein) of three poIL-18-neutralizing MAbs (9H6, 11H5, and 12C12) (18) or mouse IgG2a and IgG2b (Sigma-Aldrich Inc., St. Louis, MO), as controls. After 48 h, the IFN-γ concentration in the cell culture supernatant was measured using an ELISA (Biosource International, Inc.) with a limit of detection of <1 pg/ml according to the manufacturer's instructions.
The mouse 50% lethal dose (LD50) of the highly virulent E. rhusiopathiae Fujisawa strain, the parent strain of YS-1, varies with the route of inoculation (subcutaneous LD50, 101.2 CFU; intraperitoneal LD50, 103.5 CFU) (26, 29); however, the strain cannot cause disease via the oral route, even at a dose of 108 CFU. The in vivo biological activity of recombinant poIL-18 was therefore assayed by intraperitoneal inoculation. BALB/c mice were inoculated intraperitoneally with 1.3 × 108 CFU of YS-1 or YS-1/IL-18. Five days after inoculation, the mice were sacrificed and their spleens were collected. Splenocytes were prepared as described above and then cultured in the presence or absence of concanavalin A (5 μg/ml) (Sigma). After 24 h, the IFN-γ concentration in the cell culture supernatants was measured with an ELISA (Biosource International, Inc.).
Phagocytosis assay.
Two groups of 24 female 9-week-old BALB/c mice were inoculated intraperitoneally with either YS-1 (1.0 × 107 CFU) or YS-1/IL-18 (1.3 × 107 CFU), and on days 5, 7, and 14, 8 mice from each group were sacrificed and resident macrophages were obtained by peritoneal lavage with 5.0 ml of PBS containing 1 mM EDTA. The macrophages were washed once and resuspended in RPMI 1640 medium containing 0.25% bovine serum albumin (RPMI-BSA) at a cell concentration of 106/ml. Phagocytosis assays were performed as previously reported (4, 29), with minor modifications. S. Typhimurium (107 CFU/ml) was incubated with macrophages (106 cells/ml) in a total volume of 1.0 ml of RPMI-BSA containing 10% mouse serum for 60 min at 37°C with rotation (8 rpm). At time zero, the bacteria were removed by washing three times with PBS, and the cells were incubated for 1 h at 37°C in medium containing gentamicin (40 μg/ml) (Sigma), which was used to kill the extracellular salmonellae. A 0.1-ml sample was then removed, serially diluted in sterile distilled water containing 0.5% Tween 20 and 5% FBS, and plated on LB agar to determine the number of viable intracellular bacteria.
Pig experiment.
Seventeen germfree piglets were obtained by cesarean section and housed separately in sterile isolators. The piglets were divided into 3 groups: 6 pigs were orally inoculated once with the KO/IL-18 strain by feeding with milk replacer (SPF-LAC; Weyerhaeuser, Eaton, OH) containing KO/IL-18 (3.4 × 107 CFU), and 6 pigs and 5 pigs were orally inoculated with Koganei 65-0.15 (parental strain) (4.2 × 107 CFU) and BHI-T80 medium in the milk replacer, respectively, as controls. Eleven days after inoculation, the piglets were each challenged intranasally with a suspension of 7.24 × 108 color-changing units (CCU) of M. hyopneumoniae strain E-1 on three consecutive days. Fifteen days after the challenge, the pigs were euthanized and necropsied.
To examine the immunostimulatory effect of KO/IL-18, the humoral and cell-mediated immune responses were assayed. Lymphocyte blastogenesis assays using formalin-killed whole E. rhusiopathiae cells or ethanol-killed M. hyopneumoniae E-1 (2 × 107 CCU/ml) as antigen were performed as previously described (28). To assess the humoral immune responses against M. hyopneumoniae, the IgA and IgG antibody titers in serum and bronchoalveolar lavage fluid (BALF), both of which were collected at the time of necropsy, were measured by an ELISA using recombinant P46 as the antigen (5). It has been shown that antibodies against P46 in the sera of pigs experimentally infected with M. hyopneumoniae can be detected most often in the early stages of infection (16). To determine the humoral immune responses against E. rhusiopathiae, the IgG and IgM antibody titers in the serum were assayed with ELISAs using the recombinant SpaA.1 protein of E. rhusiopathiae as the antigen (27).
Ethics in animal experimentation.
All of the animal experiments in this study were performed according to regulations and guidelines approved by the Animal Ethics Committee of the National Institute of Animal Health.
Statistical analyses.
Statistical significance was assessed using the Mann-Whitney U test or Student's unpaired t test.
RESULTS
Expression of poIL-18 on the cell surface of E. rhusiopathiae.
To express poIL-18 on the cell surface of E. rhusiopathiae, poIL-18 was fused to the SpaA.1 molecule, a cell surface protective antigen of E. rhusiopathiae (25); SpaA.1 served as a carrier of the heterologous protein, transporting poIL-18 to the bacterial surface. IL-18 is usually expressed as a precursor, and the precursor protein produced by cells is processed to yield the biologically active form by the IL-1β-converting enzyme (ICE; caspase-1) (8, 10). The SpaA.1/IL-18 chimeric gene was therefore designed to include the ICE recognition sequence at the amino-terminal end of poIL-18, which allowed the recombinant protein to be processed by host caspase-1 and released in its biologically active form. The SpaA.1/IL-18 chimeric gene was introduced into the chromosomes of the E. rhusiopathiae YS-1 and Koganei 65-0.15 strains through homologous recombination, resulting in the construction of the YS-1/IL-18 and KO/IL-18 strains, respectively. We confirmed the integration of the SpaA.1/IL-18 chimeric gene into the chromosome as a consequence of a double-crossover event by use of PCR (data not shown).
The expression of the recombinant poIL-18 protein was confirmed with a poIL-18-specific ELISA. The recombinant poIL-18 protein was detected in the bacterial culture supernatants and cell surface preparations. The concentration of recombinant poIL-18 in the culture supernatants was 593 ± 237 (mean ± standard deviation [SD]) pg/ml for YS-1/IL-18 and 1,620 ± 184 pg/ml for KO/IL-18. The concentration in the cell surface preparations was 572 ± 74 pg/ml for YS-1/IL-18 and 756 ± 207 pg/ml for KO/IL-18 (Table 1).
Table 1.
Concentrations of poIL-18 in culture supernatants and cell surface preparations
| Sample type | IL-18 concn (pg/ml)a |
|||
|---|---|---|---|---|
| YS-1 | YS-1/IL-18 | Koganei 65-0.15 | KO/IL-18 | |
| Culture supernatant | 95.3 ± 75.0 | 592.6 ± 237.4 | 103.7 ± 99.9 | 1,620.0 ± 183.8 |
| Cell surface prepn | 309.5 ± 109.3 | 571.5 ± 73.8 | 2.1 ± 5.1 | 756.1 ± 206.8 |
Data are means ± SD.
The hybrid SpaA.1/IL-18 protein in the supernatants of the strains was further detected by Western blotting (Fig. 1). The size of the visualized protein band was not in agreement with the estimated molecular mass of the hybrid protein (58.7 kDa after signal peptide cleavage), suggesting that the protein had partially degraded or was naturally processed, as observed for the SpaA.1 protein (25). The results showed that the expression of the recombinant protein was higher in KO/IL-18 than in YS-1/IL-18, demonstrating a difference between the strains in efficiency of expression.
Fig 1.
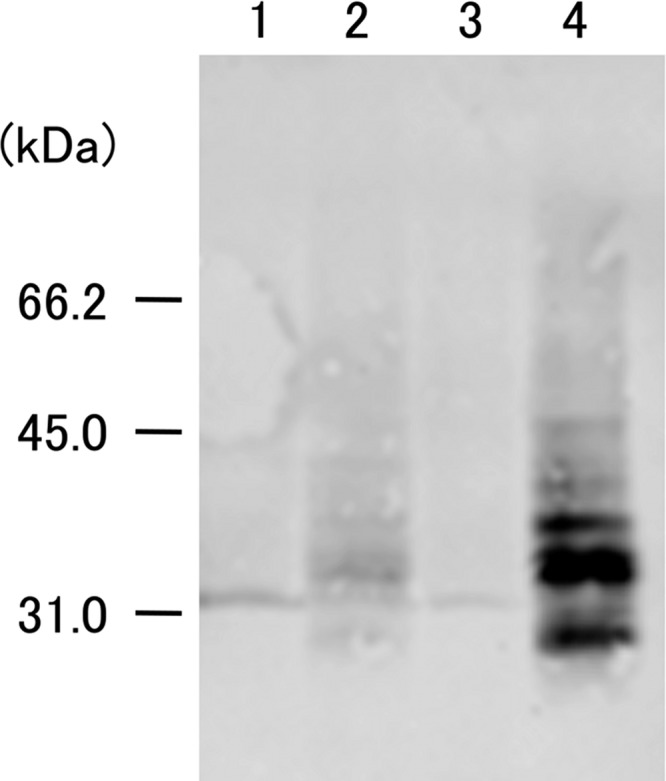
Expression of poIL-18 in E. rhusiopathiae strains. Proteins in culture supernatants were precipitated with TCA, separated by SDS-PAGE, transferred onto a membrane, and probed with an anti-poIL-18 mouse MAb (11H5) (18). Lane 1, YS-1; lane 2, YS-1/IL-18; lane 3, Koganei 65-0.15; lane 4, KO/IL-18. Molecular mass markers (kilodaltons) are shown on the left.
IFN-γ-inducing activity of poIL-18 fused to SpaA.1.
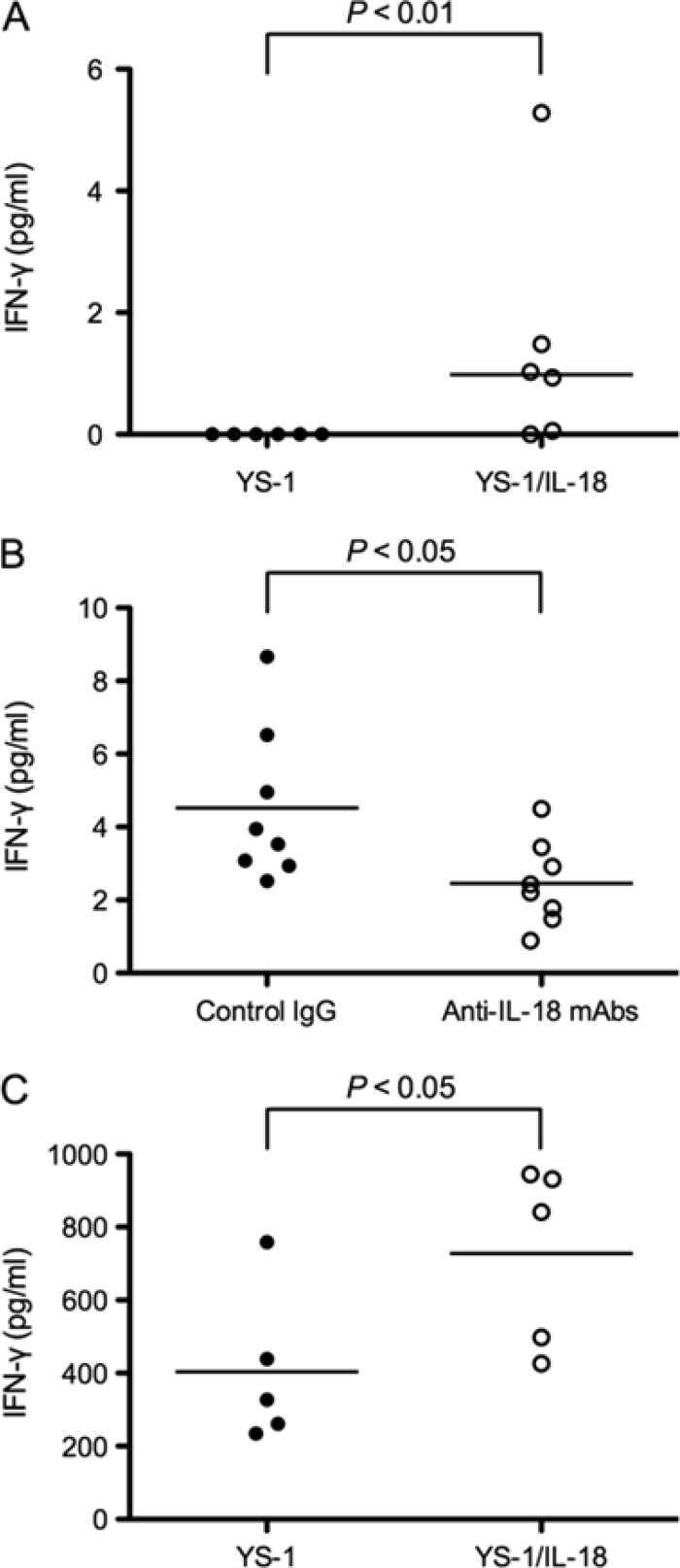
Using concentrated culture supernatants of the E. rhusiopathiae strains, the induction of IFN-γ in murine splenocytes was examined. The concentrated culture supernatant of YS-1/IL-18 significantly induced the production of IFN-γ in murine splenocytes (P < 0.01) (Fig. 2A). In addition, the IFN-γ production was significantly inhibited by the addition of poIL-18-neutralizing MAbs (P < 0.05) (Fig. 2B), showing that recombinant poIL-18 fused to SpaA.1 was biologically active as an IFN-γ inducer.
Fig 2.
IFN-γ-inducing activity of the recombinant YS-1/IL-18 strain. (A) IFN-γ-inducing activity in vitro. Six mice were used in each group. Murine splenocytes were incubated with concentrated culture supernatants of the YS-1 (closed circles) or YS-1/IL-18 (open circles) strain for 48 h. The levels of murine IFN-γ in the culture supernatants were measured using an ELISA. Significance was assessed using the Mann-Whitney U test. The bar represents the median for the group. (B) Neutralization by anti-poIL-18 MAbs. Eight mice were used in each group. Murine splenocytes were incubated with concentrated culture supernatants of the YS-1/IL-18 strain in the presence of IgG2a and IgG2b isotype controls (closed circles) or poIL-18-neutralizing MAbs (open circles). The concentration of murine IFN-γ in the culture supernatants was measured using an IFN-γ ELISA. Significance was assessed using Student's unpaired t test. The bars represent the means for the groups. (C) In vivo biological activity. Five mice were used in each group. Splenocytes from mice preinoculated intraperitoneally with the YS-1 (closed circles) or YS-1/IL-18 (open circles) strain were incubated with concanavalin A. The concentration of murine IFN-γ in the culture supernatants was measured using an IFN-γ ELISA. Significance was assessed using Student's unpaired t test. The bars represent the means for the groups.
The IFN-γ-inducing ability was further examined in vivo. Mice were inoculated intraperitoneally with YS-1 or YS-1/IL-18, and 5 days after inoculation, the splenocytes were prepared and stimulated with concanavalin A. The results showed that the splenocytes from the mice inoculated with YS-1/IL-18 produced significantly more IFN-γ in response to concanavalin A than the splenocytes from mice inoculated with YS-1 (P < 0.05) (Fig. 2C).
Immunostimulatory effect of E. rhusiopathiae YS-1/IL-18 in mice.
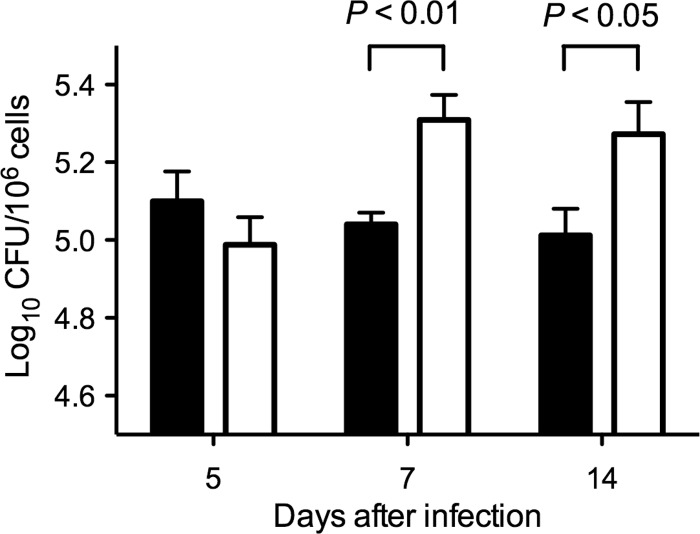
To investigate whether recombinant E. rhusiopathiae had an immunostimulatory effect in vivo, we compared the phagocytic functions of murine peritoneal macrophages from YS-1- and YS-1/IL-18-inoculated mice. Peritoneal macrophages were collected on days 5, 7, and 14 after the inoculation of mice with YS-1 or YS-1/IL-18 and were incubated with S. Typhimurium for 60 min. Lysates of the macrophages were then plated for enumeration of phagocytosed bacteria. As shown in Fig. 3, an enhancement in the phagocytosis of S. Typhimurium was observed in macrophages from the YS-1/IL-18-inoculated mice; the immunostimulatory effect was observed on days 7 and 14 after inoculation (Fig. 3).
Fig 3.
Phagocytosis of S. Typhimurium by peritoneal macrophages. Two groups of 24 mice were inoculated intraperitoneally with either YS-1 (filled bars) or YS-1/IL-18 (open bars), and at days 5, 7, and 14 after inoculation, 8 mice from each group were euthanized and resident peritoneal macrophages were obtained. The phagocytosis assay was performed as described in Materials and Methods. The results are expressed as mean log10 CFU ± SEM. Significance was assessed using Student's unpaired t test.
Immunostimulatory effect of E. rhusiopathiae strain KO/IL-18 in pigs.
We previously showed that unlike an E. rhusiopathiae YS-1-based vaccine, a Koganei 65-0.15-based vaccine could colonize the tonsils of pigs who received the vaccine via the oral route and could induce protective immunity against erysipelas and mycoplasmal pneumonia (21). Therefore, in the pig experiment, we used KO/IL-18, a Koganei 65-0.15 derivative expressing poIL-18.
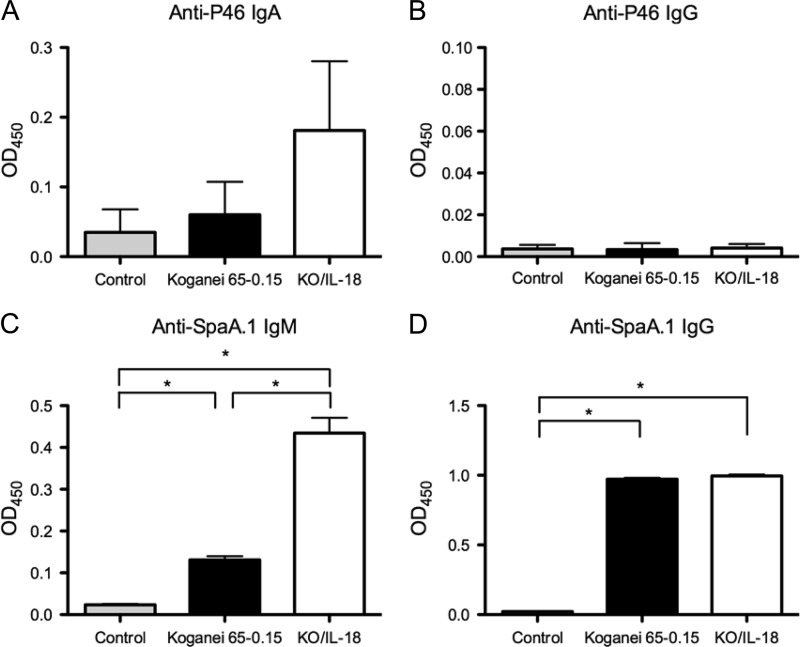
Three groups of gnotobiotic piglets were fed milk replacer containing BHI-T80 medium, Koganei 65-0.15, or KO/IL-18. The piglets were challenged with M. hyopneumoniae 11 days later and were autopsied 15 days after the day of the final challenge. After postmortem examination, we found no significant differences in the severity of lung lesions due to mycoplasmal pneumonia or in the number of M. hyopneumoniae organisms in BALF between the three groups (data not shown). Despite the lack of a significant effect on protective immunity against M. hyopneumoniae infection, an enhancement was observed in the humoral immune responses against M. hyopneumoniae and E. rhusiopathiae antigens. The total IgA levels in the BALF were similar in both E. rhusiopathiae-inoculated groups (means ± standard errors of the means [SEM] for the medium-, Koganei 65-0.15-, and KO/IL-18-inoculated groups were 2.35 ± 0.41 μg/ml, 10.64 ± 1.66 μg/ml, and 11.66 ± 1.71 μg/ml, respectively); the concentration of IgA against the P46 protein of M. hyopneumoniae, but not that of IgG, was higher in the KO/IL-18-inoculated pigs than in the Koganei 65-0.15-inoculated pigs (Fig. 4A and B). In addition, the serum concentration of IgM against the SpaA.1 protein of E. rhusiopathiae, but not that of IgG, was significantly higher in the KO/IL-18-inoculated group than in the Koganei 65-0.15-inoculated group (Fig. 4C and D). Thus, the oral administration of KO/IL-18 to pigs enhanced the local and systemic humoral immune responses against pathogens causing mucosal or systemic infections. There were no differences in the concentrations of P46-specific IgA or IgG in the serum or in the results of the lymphocyte blastogenesis assays using whole cells of killed M. hyopneumoniae or E. rhusiopathiae or concanavalin A as the stimulator (data not shown).
Fig 4.
Immunostimulatory effect of E. rhusiopathiae KO/IL-18 in pigs. The concentrations of P46-specific IgA (A) and IgG (B) in BALF and of SpaA.1-specific IgM (C) and IgG (D) in serum were determined. The pigs were orally inoculated with BHI-T80 medium (shaded bars), Koganei 65-0.15 (filled bars), or KO/IL-18 (open bars). Eleven days after inoculation, the pigs were challenged intranasally with M. hyopneumoniae on three consecutive days. Fifteen days after the challenge exposure, the pigs were euthanized and necropsied, and BALF and sera were obtained and assayed with an ELISA. OD450, optical density at 450 nm. The results are expressed as means ± SEM. The asterisks indicate significant differences (P < 0.01) as determined using Student's unpaired t test.
DISCUSSION
We previously established an expression system for heterologous genes in the attenuated E. rhusiopathiae YS-1 and Koganei 65-0.15 strains by utilizing the SpaA.1 gene, which encodes a surface protective antigen of E. rhusiopathiae (25). In the present study, we also expressed poIL-18 in attenuated E. rhusiopathiae strains and evaluated the immunostimulatory effects of these strains in mice and pigs.
IL-18 is usually produced as a precursor polypeptide. The precursor of IL-18 is inactive and yields a biologically active monomer after cleavage by caspase-1 (8, 10). We confirmed that the splenocytes from mice inoculated with a recombinant E. rhusiopathiae strain significantly induced IFN-γ upon stimulation with concanavalin A, showing that the recombinant poIL-18 produced by E. rhusiopathiae has biological activity in vivo. We do not know whether the ICE recognition sequence included in the SpaA.1/IL-18 hybrid protein was recognized and processed by host caspase-1, leading to the active form. It has been shown that a fusion of mature bovine IL-18 with the VP1 protein of foot-and-mouth disease virus has an adjuvant effect in a murine model (24), so our observation is not surprising. These results also show that IL-18 activity is not species specific, at least between mice and pigs or cows, and that a gene fusion system can be used for the expression of IL-18 with biological activity.
We observed that recombinant poIL-18 produced by E. rhusiopathiae had IFN-γ-inducing activity in vitro, and the inoculation of mice with YS-1/IL-18 enhanced phagocytosis of S. Typhimurium. IFN-γ activates macrophage functions, including phagocytic activity (14), suggesting that the recombinant poIL-18 produced by E. rhusiopathiae enhanced phagocytosis of S. Typhimurium through induction of IFN-γ production. We observed that 19 days after oral infection with 109 CFU of the S. Typhimurium strain, the bacteria were completely cleared from the Peyer's patches (in two of five mice tested) and the mesenteric lymph nodes (in one of five mice tested) of mice that had been inoculated intraperitoneally with YS-1/IL-18 but not from those of mice inoculated with YS-1 (data not shown). The capacity of IL-18 to induce IFN-γ production resulting in host resistance to Salmonella infection has been shown previously (3, 15), implying that poIL-18 produced by E. rhusiopathiae bacteria may enhance the in vivo clearance of S. Typhimurium. However, further experiments are required to support this hypothesis.
Our results showed that the oral administration of recombinant E. rhusiopathiae enhanced the production of specific antibodies against M. hyopneumoniae and E. rhusiopathiae antigens in pigs. It has been shown that IL-18 can drive not only Th1- but also Th2-mediated immune responses (2, 20), suggesting that the poIL-18 produced by the recombinant E. rhusiopathiae bacteria activates humoral immune responses. In our study, increases were seen in the concentrations of BALF IgA (but not IgG) and serum IgM against M. hyopneumoniae and E. rhusiopathiae antigens, respectively. The reason for the low level of local IgG antibodies against M. hyopneumoniae is unclear, but it could be that the IgG response was not induced at this early stage after infection. Because M. hyopneumoniae and E. rhusiopathiae cause local and systemic infections, respectively, our results suggest that poIL-18 may enhance immune responses in both compartments.
In our previous study, we showed that the oral inoculation of pigs with E. rhusiopathiae expressing a recombinant P97 protein of M. hyopneumoniae induced strong, systemic cell-mediated immune responses, but not humoral immune responses, against the P97 antigen (21). In this study, we did not observe a significant effect of poIL-18 on cell-mediated immune responses in comparing the KO/IL-18- and Koganei 65-0.15-inoculated pigs. This result was most likely due to the effect of poIL-18 being overshadowed by the strong cell-mediated immune responses induced by E. rhusiopathiae. However, we observed an enhancement in the IgA response against a mycoplasmal antigen in pig lungs by the oral inoculation of poIL-18-producing E. rhusiopathiae, suggesting the intriguing possibility that the use of poIL-18 in vaccine formulations may help pigs to produce IgA antibodies against antigens with low immunogenicity, including the P97 protein. Several lines of evidence suggest the potential use of IL-18 as a vaccine adjuvant (13, 24). We must further examine whether other routes of inoculation of poIL-18-producing E. rhusiopathiae have significant effects on the immune response and the practical use of the strain as a vaccine for the control of other infections.
ACKNOWLEDGMENTS
We acknowledge Y. Nakamura, T. Harada, and K. Shiraiwa for helpful assistance with the experiments.
This study was supported in part by a grant from the Ministry of Agriculture, Forestry and Fisheries of Japan (to Y.S.).
Footnotes
Published ahead of print 3 July 2012
REFERENCES
- 1. Cameron LA, et al. 1999. Airway epithelium expresses interleukin-18. Eur. Respir. J. 14:553–559 [DOI] [PubMed] [Google Scholar]
- 2. Dinarello CA. 2009. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 27:519–550 [DOI] [PubMed] [Google Scholar]
- 3. Eckmann L, Kagnoff MF. 2001. Cytokines in host defense against Salmonella. Microbes Infect. 3:1191–1200 [DOI] [PubMed] [Google Scholar]
- 4. Eguchi M, Sekiya Y, Suzuki M, Yamamoto T, Matsui H. 2007. An oral Salmonella vaccine promotes the down-regulation of cell surface Toll-like receptor 4 (TLR4) and TLR2 expression in mice. FEMS Immunol. Med. Microbiol. 50:300–308 [DOI] [PubMed] [Google Scholar]
- 5. Futo S, et al. 1995. Recombinant 46-kilodalton surface antigen (P46) of Mycoplasma hyopneumoniae expressed in Escherichia coli can be used for early specific diagnosis of mycoplasmal pneumonia of swine by enzyme-linked immunosorbent assay. J. Clin. Microbiol. 33:680–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gardella S, et al. 1999. Interleukin-18 synthesis and secretion by dendritic cells are modulated by interaction with antigen-specific T cells. J. Leukoc. Biol. 66:237–241 [PubMed] [Google Scholar]
- 7. Gerdts V, Mutwiri GK, Tikoo SK, Babiuk LA. 2006. Mucosal delivery of vaccines in domestic animals. Vet. Res. 37:487–510 [DOI] [PubMed] [Google Scholar]
- 8. Ghayur T, et al. 1997. Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-gamma production. Nature 386:619–623 [DOI] [PubMed] [Google Scholar]
- 9. Gracie JA, Robertson SE, McInnes IB. 2003. Interleukin-18. J. Leukoc. Biol. 73:213–224 [DOI] [PubMed] [Google Scholar]
- 10. Gu Y, et al. 1997. Activation of interferon-gamma inducing factor mediated by interleukin-1beta converting enzyme. Science 275:206–209 [DOI] [PubMed] [Google Scholar]
- 11. Heegaard PM, et al. 2011. Adjuvants and delivery systems in veterinary vaccinology: current state and future developments. Arch. Virol. 156:183–202 [DOI] [PubMed] [Google Scholar]
- 12. Hoshino T, Wiltrout RH, Young HA. 1999. IL-18 is a potent coinducer of IL-13 in NK and T cells: a new potential role for IL-18 in modulating the immune response. J. Immunol. 162:5070–5077 [PubMed] [Google Scholar]
- 13. Kim SB, et al. 2012. Oral administration of Salmonella enterica serovar Typhimurium expressing swine interleukin-18 induces Th1-biased protective immunity against inactivated vaccine of pseudorabies virus. Vet. Microbiol. 155:172–182 [DOI] [PubMed] [Google Scholar]
- 14. Maródi L, et al. 1993. Enhancement of macrophage candidacidal activity by interferon-gamma. Increased phagocytosis, killing, and calcium signal mediated by a decreased number of mannose receptors. J. Clin. Invest. 91:2596–2601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mastroeni P, et al. 1999. Interleukin 18 contributes to host resistance and gamma interferon production in mice infected with virulent Salmonella typhimurium. Infect. Immun. 67:478–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mori Y, Hamaoka T, Sato S, Takeuchi S. 1988. Immunoblotting analysis of antibody response in swine experimentally inoculated with Mycoplasma hyopneumoniae. Vet. Immunol. Immunopathol. 19:239–250 [DOI] [PubMed] [Google Scholar]
- 17. Mori Y, Yoshida Y, Kuniyasu C, Hashimoto K. 1983. Improvement of complement fixation test antigen for the diagnosis of Mycoplasma hyopneumoniae infection. Natl. Inst. Anim. Health Q. (Tokyo) 23:111–116 [PubMed] [Google Scholar]
- 18. Muneta Y, et al. 2000. Detection of porcine interleukin-18 by sandwich ELISA and immunohistochemical staining using its monoclonal antibodies. J. Interferon Cytokine Res. 20:331–336 [DOI] [PubMed] [Google Scholar]
- 19. Muneta Y, Mori Y, Shimoji Y, Yokomizo Y. 2000. Porcine interleukin 18: cloning, characterization of the cDNA and expression with the baculovirus system. Cytokine 12:566–572 [DOI] [PubMed] [Google Scholar]
- 20. Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. 2001. Interleukin-18 regulates both Th1 and Th2 responses. Annu. Rev. Immunol. 19:423–474 [DOI] [PubMed] [Google Scholar]
- 21. Ogawa Y, et al. 2009. Oral vaccination against mycoplasmal pneumonia of swine using a live Erysipelothrix rhusiopathiae vaccine strain as a vector. Vaccine 27:4543–4550 [DOI] [PubMed] [Google Scholar]
- 22. Okamura H, et al. 1995. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature 378:88–91 [DOI] [PubMed] [Google Scholar]
- 23. Puren AJ, Fantuzzi G, Dinarello CA. 1999. Gene expression, synthesis, and secretion of interleukin 18 and interleukin 1beta are differentially regulated in human blood mononuclear cells and mouse spleen cells. Proc. Natl. Acad. Sci. U. S. A. 96:2256–2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shi XJ, Wang B, Wang M. 2007. Immune enhancing effects of recombinant bovine IL-18 on foot-and-mouth disease vaccination in mice model. Vaccine 25:1257–1264 [DOI] [PubMed] [Google Scholar]
- 25. Shimoji Y, Mori Y, Fischetti VA. 1999. Immunological characterization of a protective antigen of Erysipelothrix rhusiopathiae: identification of the region responsible for protective immunity. Infect. Immun. 67:1646–1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shimoji Y, Mori Y, Sekizaki T, Shibahara T, Yokomizo Y. 1998. Construction and vaccine potential of acapsular mutants of Erysipelothrix rhusiopathiae: use of excision of Tn916 to inactivate a target gene. Infect. Immun. 66:3250–3254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shimoji Y, et al. 2002. Erysipelothrix rhusiopathiae YS-1 as a live vaccine vehicle for heterologous protein expression and intranasal immunization of pigs. Infect. Immun. 70:226–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shimoji Y, Oishi E, Muneta Y, Nosaka H, Mori Y. 2003. Vaccine efficacy of the attenuated Erysipelothrix rhusiopathiae YS-19 expressing a recombinant protein of Mycoplasma hyopneumoniae P97 adhesin against mycoplasmal pneumonia of swine. Vaccine 21:532–537 [DOI] [PubMed] [Google Scholar]
- 29. Shimoji Y, Yokomizo Y, Sekizaki T, Mori Y, Kubo M. 1994. Presence of a capsule in Erysipelothrix rhusiopathiae and its relationship to virulence for mice. Infect. Immun. 62:2806–2810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stoll S, et al. 1997. Production of IL-18 (IFN-gamma-inducing factor) messenger RNA and functional protein by murine keratinocytes. J. Immunol. 159:298–302 [PubMed] [Google Scholar]
- 31. Takeuchi M, et al. 1997. Immunohistochemical and immuno-electron-microscopic detection of interferon-gamma-inducing factor (“interleukin-18”) in mouse intestinal epithelial cells. Cell Tissue Res. 289:499–503 [DOI] [PubMed] [Google Scholar]
- 32. Ushio S, et al. 1996. Cloning of the cDNA for human IFN-gamma-inducing factor, expression in Escherichia coli, and studies on the biologic activities of the protein. J. Immunol. 156:4274–4279 [PubMed] [Google Scholar]
- 33. Yoshimoto T, et al. 1999. IL-18, although antiallergic when administered with IL-12, stimulates IL-4 and histamine release by basophils. Proc. Natl. Acad. Sci. U. S. A. 96:13962–13966 [DOI] [PMC free article] [PubMed] [Google Scholar]