Abstract
In this work, we investigated the Campylobacter jejuni dps (DNA binding protein from starved cells) gene for a role in biofilm formation and cecal colonization in poultry. In vitro biofilm formation assays were conducted with stationary-phase cells in cell culture plates under microaerophilic conditions. These studies demonstrated a significant (>50%) reduction in biofilm formation by the C. jejuni dps mutant compared to that by the wild-type strain. Studies in poultry also demonstrated the importance of the dps gene in host colonization by C. jejuni. Real-time PCR analysis of mRNA extracted from the cecal contents of poultry infected with wild-type C. jejuni indicated that the dps gene is upregulated 20-fold during poultry colonization. Cecal colonization was greater than 5 log CFU lower in chicks infected with the dps mutant than chicks infected with the wild-type C. jejuni strain. Moreover, the dps mutant failed to colonize 75% of the chicks following challenge with 105 CFU. Preliminary studies were conducted in chicks by parenteral vaccination with a recombinant Dps protein or through oral vaccination with a recombinant attenuated Salmonella enterica strain synthesizing the C. jejuni Dps protein. No reduction in C. jejuni was noted in chicks vaccinated with the parenteral recombinant protein, whereas, a 2.5-log-unit reduction of C. jejuni was achieved in chicks vaccinated with the attenuated Salmonella vector after homologous challenge. Taken together, this work demonstrated the importance of Dps for biofilm formation and poultry colonization, and the study also provides a basis for continued work using the Dps protein as a vaccine antigen when delivered through a Salmonella vaccine vector.
INTRODUCTION
Campylobacter jejuni is one of the most common causes of food-borne bacterial gastroenteritis, causing an estimated 846,000 cases in the United States annually (1, 9, 31). Infection results in the rapid onset of severe watery diarrhea, with variable myalgia, fever, and headache. The disease is typically self-limiting, with resolution of symptoms within 1 week, although severe conditions such as reactive arthritis and Guillain-Barré syndrome have been associated with the disease (4).
Campylobacteriosis is primarily food borne in nature, with improper handling or consumption of poultry being the most significant risk factor (6, 35). However, the epidemiology of poultry colonization with C. jejuni is complex. Colonization typically begins at about 14 days of age, and the organism spreads throughout the flock during the grow-out period (9, 13, 19). Although some flocks remain negative, in most flocks, 50 to 100% of the birds are colonized by the end of the grow-out period (9, 33). A universal initial source of infection for poultry remains to be elucidated, but contaminated water, food, and litter, as well as contact with rodents and wild birds, has been implicated (3, 9, 10, 23, 25). Regardless of the source of colonization, infection quickly disseminates throughout the flock, with infected birds typically shedding in excess of 109 CFU of C. jejuni per gram of fecal material (23). Once harvested, these poultry carcasses then serve as the primary vehicle of transmission of C. jejuni to humans.
How Campylobacter survives outside the host is a question that has puzzled researchers for some time. One possible reason for surviving environmental conditions that has received research interest is the formation of biofilms. Biofilms are complex communities of bacteria existing in a viscous exopolysaccharide matrix (7). Campylobacter can create biofilms, albeit under limited conditions, as well as incorporate into preexisting biofilms (12). In a mixed-culture biofilm, viable Campylobacter could be isolated 7 days after introduction into the biofilm (17).
While colonizing the host, bacteria encounter many challenges, including a limited supply of iron. To circumvent this problem, bacteria employ a variety of ferritins to meet their iron needs. In Campylobacter, this includes the genes dps, feoB, cfrA, ceuE, and Cj0178, which are involved with various aspects of iron acquisition (2, 11, 22, 24, 34).
In addition to functioning as a ferritin, the Dps family of proteins has been found to play a role in many other cellular processes. Previous studies on the Dps protein in Campylobacter have provided evidence for a role in attachment and invasion of cultured cells, survival in macrophages, and peroxide resistance. Deletion of the dps gene significantly decreases virulence in the piglet model of infection (11, 32). In this work, we expand on the study of Dps in Campylobacter. In vitro studies using a Dps-deficient mutant indicate a role for the protein in biofilm formation. We demonstrate transcriptional upregulation of dps during colonization of chickens and a significant decrease in colonization by a Δdps mutant. Additionally, we provide preliminary evidence that C. jejuni Dps can be valuable as a vaccine target for poultry.
MATERIALS AND METHODS
Culture of bacterial strains.
All strains used are listed in Table 1. C. jejuni was routinely cultured on Mueller-Hinton agar (BD, Sparks, MD) supplemented with 5% citrated bovine blood (Cleveland Scientific, Bath, OH) (MHB) in an environment supplemented with 10% CO2 at 42°C. Escherichia coli DH5α was routinely cultured on Luria-Bertani (LB) agar (BD, Sparks, MD) at 37°C. Salmonella enterica serovar Typhimurium was routinely cultured on LB agar at 37°C.
Table 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| Strains | ||
| E. coli DH5α | endA1 hsdR17(rK− mK−) supE44 thi-1 recA1 gyrA relA1 Δ(lacZYA-argF)U169 deoR [ϕ 80dlacΔ(lacZΔM15)] | Invitrogen |
| C. jejuni | ||
| NCTC11168 | Sequenced clinical isolate | NCTC |
| JRT10 | dps::cam | L. A. Joens |
| JRT101 | dps::cam (pJRT101) | L. A. Joens |
| S. enterica χ9088 | ΔPfur33::TT araC PBAD fur Δpmi-2426 Δ(gmd-fcl)-26 ΔasdA33 | R. Curtiss III |
| Plasmids | ||
| pYA3493 | Salmonella expression plasmid | R. Curtiss III |
| pHSS19 | Nonreplicating suicide vector | Nickoloff and Reynolds (23a) |
| pYA4495 | pYA3493 carrying C. jejuni dps | R. Curtiss III |
| pRY107 | Complementation vector; Kmr | Yao et al. (36) |
| pJRT101 | pRY107 carrying dps | This study |
| pTRC-HISB | Commercial recombinant protein expression vector | Invitrogen |
Biofilm assay.
The biofilm assay was performed as described previously (27). Briefly, Mueller-Hinton broth was inoculated with C. jejuni to a final optical density at 600 nm (OD600) of 0.05, and 1-ml aliquots were added to a 24-well polystyrene plate. Samples were incubated statically at 42°C in 10% CO2. At desired time points, nonadherent cells and medium were aspirated and the biofilm was dried at 55°C for 10 min. Dried biofilm was stained with 0.1% crystal violet for 5 min and then rinsed twice with distilled water. Sample wells containing the biofilm stain were decolorized with 80% ethanol, 20% acetone for 5 min. One hundred microliters of the decolorizer was removed, and the absorbance (A570) was measured. Each sample was run in triplicate.
RNA isolation.
In vitro, C. jejuni was harvested from plates grown for 72 h under microaerophilic conditions using phosphate-buffered saline (PBS) diluted in an equal volume of RNA Protect reagent (Qiagen, Valencia, CA). For in vivo harvesting of C. jejuni, chicks were challenged with 109 CFU of C. jejuni at 15 days of age. At 10 days postinfection, chicks (n = 3) were euthanized, and the cecal contents were harvested, pooled, and diluted 1:1 (wt/vol) with RNA Protect. The cecal suspension was subjected to low-speed centrifugation (500 × g) to remove debris and then subjected to serial filtration through a final membrane with a pore size of 0.8 μm. Filtered suspensions were then lysed in a French pressure cell at 1,000 lb/in2. Total RNA was isolated from bacterial lysates via an RNeasy minikit (Qiagen, Valencia, CA) and an E.Z.N.A. RNase-free DNase I kit (Omega, Norcross, GA) as per the manufacturers' instructions. Total RNA concentration was determined spectrophotometrically, and each RNA sample was analyzed using PCR to ensure that there was no DNA contamination.
Real-time PCR quantification.
Reverse transcription (RT) of 250 ng of the total isolated RNA was carried out in 10-μl reaction mixtures using qScript cDNA SuperMix (Quanta, Gaithersburg, MD) as per the manufacturer's instructions. Reaction conditions were as follows: 5 min at 25°C, 30 min at 42°C, and 5 min at 85°C. All cDNA samples were stored at 4°C until processed.
Gene amplification and real-time analysis were performed using a Bio-Rad iCycler thermocycler (Bio-Rad, Hercules, CA). One microliter of cDNA and 800 nM concentrations of primers Cj1534RTP1 (5′-AAAAAGAAAGTGATACTACAACAGCT-3′) and Cj1534RTP2 (5′-AAGCACCTTGTAAAGTAGCGCCTATC-3′) were used in 20-μl reaction mixtures using PerfeCta SYBR green FastMix (Quanta, Gaithersburg, MD). Reaction conditions were as follows: 5 min at 95°C, followed by 40 cycles of 30 s at 95°C and 1 min at 57.5°C. PCR efficiencies were determined by generating standard curves using 10-fold serial dilutions of genomic DNA and their respective threshold cycles. To account for differences in total RNA used and RT efficiency, primers Cj402RTP1 (5′-CGATGGAACGGATAATCACC-3′) and Cj402RTP2 (5′-AATACCTGCATTTCCAAGAGC-3′), which target the housekeeping gene Cj0402, were used as an internal control. All results were analyzed using the Pfaffl method (26). Each sample was analyzed in triplicate, with the averages presented.
Genetic manipulation of dps in C. jejuni.
A C. jejuni Δdps mutant was generated as described elsewhere (32). Briefly, genomic DNA was isolated from C. jejuni strain NCTC11168 and used as the template for amplification of the DNA upstream and downstream of the dps gene. These fragments were directionally cloned into a suicide vector (pHSS19) flanking a chloramphenicol resistance gene and introduced into C. jejuni by electroporation. The mutation was then complemented in trans by cloning the entire gene into the replicative plasmid pRY107 and introducing the construct into C. jejuni NCTC11168. Successful genetic manipulation was confirmed using PCR and Western blotting.
Recombinant synthesis of C. jejuni Dps.
C. jejuni Dps was recombinantly synthesized using a pTRC-HIS B recombinant expression system (Invitrogen, Carlsbad, CA), as per the manufacturer's instructions. Briefly, the entire dps gene was PCR amplified from C. jejuni strain NCTC11168 genomic DNA using primers Cj1534RE1F (5′-AAAAAAAGGAGGATCCCATGTCAGTTAC-3′) and Cj1534RER2R (5′-CATAAAGCCCGAATTCTTACATTTTG-3′) and cloned into plasmid pTrcHisB in the proper reading frame. The expression plasmid was then introduced into E. coli DH5α via electroporation. PCR and DNA sequencing were performed to ensure proper construction. To express the protein, LB broth was inoculated with the transformed E. coli isolate and incubated at 37°C with aeration until the OD600 was 0.5. Protein expression was induced by addition of isopropyl-β-d-thiogalactopyranoside to a final concentration of 2.5 mM, and incubation was continued for 3 h. After incubation, cells were harvested by centrifugation and lysed in a French pressure cell at 1,000 lb/in2. The recombinant protein was then extracted from the lysate using Talon resin (Clontech, Mountain View, CA) as per the manufacturer's instructions.
Generation of anti-Dps serum.
To generate antiserum to the Dps protein, recombinant Dps protein was produced and purified as described above and desalted by dialysis against deionized water overnight. The Dps protein (0.5 mg) was combined 1:1 (vol/vol) with adjuvant (Freund's complete adjuvant for the primary immunization and Freund's incomplete adjuvant for all booster injections). Two New Zealand White rabbits were immunized at 10-day intervals and rested for 14 days after the final injection. Rabbits were then bled, and serum was harvested and tested against both native and recombinant Dps protein via Western blotting.
Western blotting.
Samples to be tested were denatured and separated on sodium dodecyl sulfate–12% polyacrylamide gels using the method of Laemmli (16). Separated proteins were then electrophoretically transferred to a polyvinylidene difluoride membrane (Millipore, Bedford, MA) and blocked with 5% skim milk in PBS (PBSM). After blocking, anti-Dps serum diluted 1:5,000 in PBSM was added and the mixture was incubated (1 h). Blots were rinsed and then incubated (1 h) with a peroxidase-labeled goat anti-rabbit IgG (KPL, Gaithersburg, MD) diluted 1:6,000 in PBSM. After incubation, blots were rinsed and tetramethylbenzidine 1-component peroxidase (KPL, Gaithersburg, MD) was used to visualize bands, as per the manufacturer's recommendations.
Chick colonization model.
One-day-old Cornish × Rock chicks were obtained from a commercial hatchery (Ideal Poultry, Cameron, TX) and examined by bacteriologic culture on arrival to ensure that the chicks were free of Campylobacter. Chicks were housed in strict isolation and given a commercial starter diet (Eagle Milling, Casa Grande, AZ) and water ad libitum throughout the study. Challenge was performed by oral gavage of 14-day-old birds with either the wild type (strain NCTC11168), an isogenic dps mutant, or a transcomplemented mutant. To prepare the inoculum, strains were cultivated for 18 h on MHB agar and were then suspended in PBS and diluted to a final concentration of 1 × 105 CFU per ml. Serial dilutions were plated to determine the number of CFU per ml. Each chick was challenged with 1 ml of the bacterial suspension. Fourteen days after challenge, the chicks were sacrificed, the ceca were harvested, and C. jejuni was enumerated from the feces by direct plating of serial dilutions on Campy Cefex agar (BD, Sparks, MD) in an environment supplemented with 10% CO2 at 42°C. All studies were performed in triplicate.
Subcutaneous vaccination with Dps protein.
Recombinant protein was generated as described above and desalted by dialysis against deionized water overnight. Desalted protein was combined 1:1 with Freund's complete adjuvant and administered subcutaneously at 10 days posthatching, with each bird receiving 0.2 mg recombinant Dps protein. A booster immunization with 0.2 mg recombinant Dps protein was administered 14 days later. Ten days after the boost vaccination, chicks were challenged with 1 × 105 CFU of C. jejuni strain NCTC11168. Ten days after challenge, the chicks were sacrificed, the ceca were harvested, and C. jejuni was enumerated by serial dilution and plating on Campy Cefex agar.
Construction of the Salmonella enterica serovar Typhimurium vaccine strain.
S. Typhimurium strain χ9088 was used as the vaccine strain and is described elsewhere (5, 18). S. enterica expression plasmid pYA3493 (15) was used as the backbone for gene expression. This plasmid encodes type 2 secretion signals that, when fused in frame to an antigen gene of interest, directs antigen secretion to the periplasm and outside the cell (15). To construct the expression vector, the dps gene was PCR amplified using primers Cj1534SEF (5′-GCCGAATTCGCTCATCATTTATGGGTTAAATTT-3′) and Cj1534SER (5′-CCGTCGACGCCTATCATCCAAAGACTTTTTTC-3′). The resulting gene fragment was then inserted into the pYA3493 expression plasmid in the proper reading frame to fuse dps with the β-lactamase type 2 secretion signal sequence, enabling Dps secretion. The resulting plasmid, pYA4495, was introduced into S. Typhimurium strain χ9088. Nucleotide sequencing was used to confirm proper construction of the expression plasmid, and Western blotting was used to verify Dps synthesis by the vaccine strain, as shown in Fig. 1. The parent plasmid, pYA3493, was introduced into strain χ9088 to serve as a control.
Fig 1.
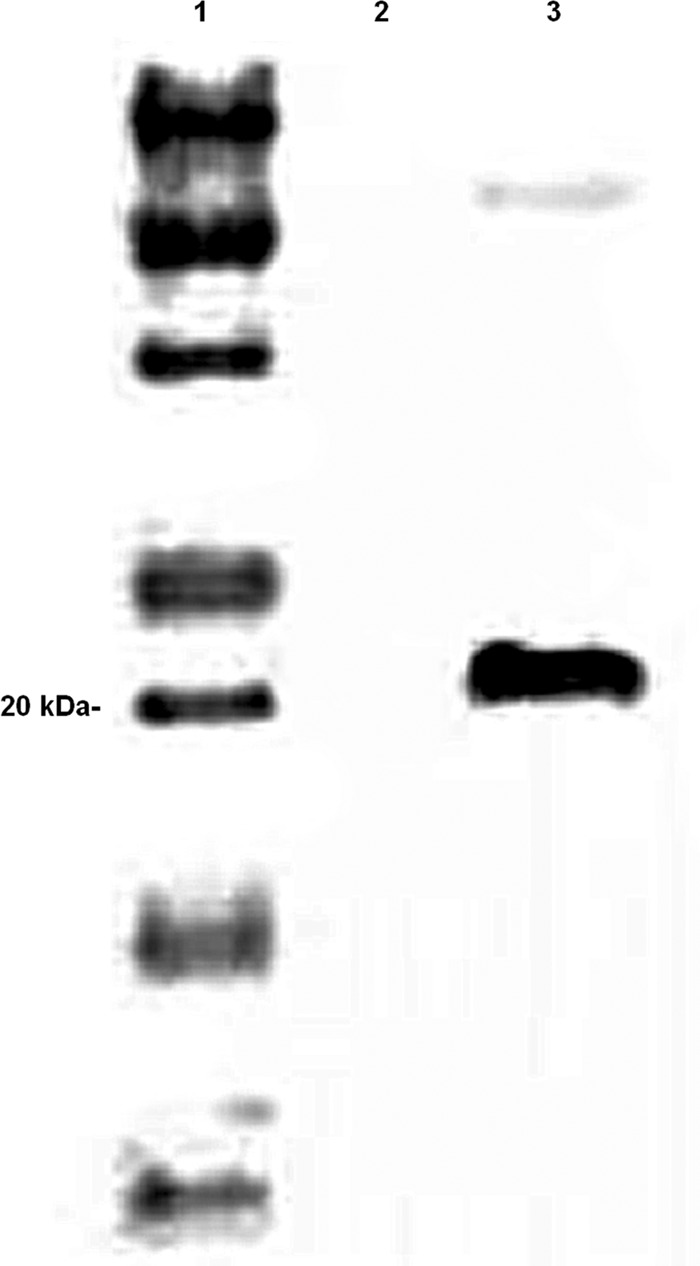
Western blot of the Salmonella vector. Lane 1, marker; lane 2, empty vector [χ9088(pYA3493)]; lane 3, vector encoding the Dps protein [(χ9088(pYA4495)] identified by the recombinant antisera at 21 kDa.
Vaccination of chickens with recombinant vector Salmonella Typhimurium strain χ9088 synthesizing C. jejuni Dps.
Chicks were divided into four groups and orally administered the vaccine construct or empty vector at 3, 10, and 16 days of age. Negative controls and healthy controls were not vaccinated. To prepare the vaccine, Salmonella strains χ9088(pYA3493) and χ9088(pYA4495) were grown statically in LB broth supplemented with 0.1% glucose, 0.2% mannose, and 0.05% arabinose overnight at 37°C. Following incubation, the overnight cultures were diluted 1:10 with fresh LB broth supplemented with 0.1% glucose, 0.2% mannose, and 0.05% arabinose and grown at 37°C with aeration (200 rpm) until the OD600 was 1.0. The cultures were centrifuged (5,000 × g for 10 min at room temperature), and the cells were resuspended in PBS to a final OD600 of 10.0. Serial dilutions were performed to determine the exact titer of the vaccine. On the day of vaccination, feed was removed from the chicks at 8 h prior to vaccination. Each chick was administered 0.5 ml of the appropriate vaccine via oral gavage. Feed was returned at 1 h postvaccination.
Chickens were challenged with C. jejuni at 10 days after the final vaccination. Briefly, C. jejuni was grown for 18 h as described above. Cells were harvested in PBS and diluted to a final titer of ≈1 × 105 CFU/ml. Serial dilutions were performed to determine the exact titer. Each chicken was challenged with 1.0 ml of the C. jejuni suspension via oral gavage. Ar 10 days postchallenge, chickens were euthanized and cecal contents were serially diluted and plated for enumeration of C. jejuni.
Statistical analysis.
A one-way analysis of variance (ANOVA) was used to determine differences in biofilm formation, and an independent two-sample t test was used to determine differences in C. jejuni recovery from chicks in the colonization and vaccination studies, using OpenEpi statistical software, version 2.3.1.
Animal care and use.
All animal work was approved and overseen by the Institutional Animal Care and Use Committee (IACUC) at the University of Arizona under protocol number 06-037.
RESULTS
Effects of C. jejuni dps on biofilm formation.
The wild-type strain (NCTC11168), dps mutant (NCTC11168 Δdps), and complemented mutant strain [NCTC11168 Δdps(pJRT101)] were assayed for biofilm-forming potential. Results of this assay showed significant differences between strains across all time points (P < 0.0001). However, at 48 and 72 h, wild-type cells showed a significant increase in biofilm production compared to cells of the dps mutant (Fig. 2). Although the complemented mutant failed to restore biofilm formation to wild-type levels, the level of biofilm formation was significantly higher than that of the dps mutant (Fig. 2).
Fig 2.
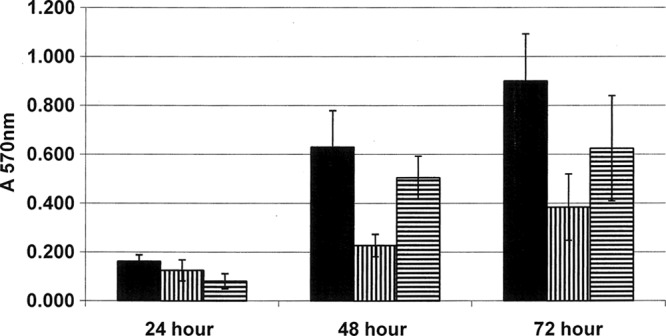
Effects of Dps on biofilm formation. Wild-type strain NCTC11168 (solid bars), the NCTC11168 Δdps mutant (bars with vertical lines), and complemented NCTC11168 Δdps(pJRT101) (bars with horizontal lines) were assayed for ability to form biofilms, as described in Materials and Methods. Each sample was run in triplicate. Error bars indicate 1 standard deviation. A one-way ANOVA was done using OpenEpi software (version 2.3.1) to determine differences in biofilm formation. A significant difference in biofilm formation between the wild-type NCTC11168 strain and dps mutants was observed at all time points (P < 0.0001).
Upregulation of C. jejuni dps during cecal colonization of broiler chickens.
To determine if C. jejuni dps is upregulated during cecal colonization, chicks were challenged with 109 CFU at 15 days of age and the cecal contents were harvested at 10 days postchallenge. Reverse transcription real-time PCR was performed on RNA harvested from the cecal contents of infected broiler chickens, and RNA levels were compared to those of in vitro-cultured C. jejuni. Averaged results of this assay, performed in triplicate, indicate 20-fold greater expression of dps by C. jejuni isolated from chicken ceca than cells grown in vitro.
Effects of dps gene on chick colonization.
In order to determine the role of C. jejuni Dps on the colonization of poultry by C. jejuni, broiler chicks were orally challenged with a 1-ml preparation of ∼1 × 105 CFU of the dps mutant, complemented mutant, or wild-type strain. At 14 days after challenge, the chicks were euthanized, the ceca were harvested, and C. jejuni was enumerated by serial dilution and plating on Campy Cefex agar. Overall, there was a greater than 5-log-unit reduction in the number of CFU in cecal colonization of chicks inoculated with the Δdps mutant compared to the level of cecal colonization of chicks infected with the wild-type strain (Table 2) (P = 0.0042). Moreover, 75% of all chicks challenged with the Δdps mutant showed no cecal colonization. Control birds (n = 8) were negative for C. jejuni at necropsy. Although partial function was restored by plasmid complementation, as shown in the in vitro biofilm assay, the in vivo colonization assay demonstrated a lack of function with the complemented mutant. PCR analysis of recovered colonies indicated that the complemented plasmid was lost after colonization (data not shown).
Table 2.
Effects of a Δdps mutation on cecal colonization by C. jejunia
| Trial and strain | Log of geometric mean no. of CFU | % colonized (no. colonized/total no.) |
|---|---|---|
| Trial 1 | ||
| NCTC11168 | 7.46 | 93.3 (14/15) |
| NCTC11168 Δdps | 1.44 | 33.3 (5/15) |
| Trial 2 | ||
| NCTC11168 | 5.49 | 75.0 (9/12) |
| NCTC11168 Δdps | Not detected | 0.0 (0/12) |
| Trial 3 | ||
| NCTC11168 | 6.11 | 100 (13/13) |
| NCTC11168 Δdps | 1.58 | 38.5 (5/13) |
| Total | ||
| NCTC11168 | 6.43 | 90.0 (36/40) |
| NCTC11168 Δdps | 1.05 | 25.0 (10/40) |
Colonization of the chick cecum by C. jejuni NCTC11168 wild-type and Δdps mutant strains. Chicks were challenged with 1 × 105 CFU of C. jejuni strain NCTC11168 at 14 days of age. Ceca were harvested at 10 days postchallenge, and C. jejuni enumeration was performed by serial dilution and plating of cecal contents on selective medium. An independent, two-sample t test using OpenEpi statistical software (version 3.2.1) was used to determine if there was a difference in colonization between wild-type NCTC11168 and the Δdps mutant. Over all three trials (n = 40 per strain), a significant reduction in colonization was observed with the mutant compared to the wild type (P = 0.0042).
Vaccination of poultry with a Dps subunit or recombinant Salmonella vector encoding Dps.
On the basis of the reduction in colonization by the Δdps mutant strain, subcutaneous and vectored oral vaccination studies were conducted in broiler chickens using the Dps protein as antigen. The subunit vaccine was subcutaneously administered to birds at 10 and 24 days of age, and the Salmonella vector vaccine was administered at 3, 10, and 16 days of age. Chickens were challenged 10 days after the last vaccination and necropsied 10 days later. Cecal contents were harvested, and C. jejuni was enumerated by serial dilution and direct plating on Campy Cefex agar. At necropsy, there were no differences in cecal numbers between the subunit vaccinates and control birds (Table 3). Nonvaccinated control birds (n = 8) remained negative for C. jejuni through the study. However, in the birds vaccinated with the Salmonella vector χ9088(pYA4495), there was a 2.48-log-unit reduction in the numbers of CFU in cecal colonization compared to the numbers of CFU of C. jejuni in birds receiving the empty-vector vaccine χ9088(pYA3493) (P = 0.04) and a 2.92-log-unit reduction in the numbers of CFU in cecal colonization compared to the numbers of CFU of C. jejuni in nonvaccinated healthy control birds (Table 3). Nonvaccinated control birds (n = 11) remained negative for Salmonella and C. jejuni through the study.
Table 3.
Vaccination of broiler chickens with Dps to prevent cecal colonization by C. jejuni NCTC11168a
| Vaccine | Log of geometric mean no. of CFU | % colonized (no. colonized/total no.) |
|---|---|---|
| Subcutaneous vaccination | ||
| Dps + Freund's adjuvant | 8.12 | 100 (13/13) |
| Freund's adjuvant only | 7.96 | 100 (12/12) |
| Oral Salmonella vaccination | ||
| Positive control | 6.64 | 100 (14/14) |
| χ9088(pYA3493) | 6.20 | 100 (10/10) |
| χ9088(pYA4495) | 3.72 | 100 (14/14) |
Chicks were challenged with 1 × 105 CFU of C. jejuni strain NCTC11168 10 days after vaccination. Ceca were harvested at 10 days postchallenge, and C. jejuni enumeration was performed by serial dilution and plating of cecal contents on selective medium. χ9088(pYA3493) was the empty vector; χ9088(pYA4495) was the vector encoding Dps. Negative-control groups (not shown) were negative for C. jejuni for both studies. An independent two-sample t test was used with OpenEpi statistical software (version 2.3.1). There was no significant difference between subcutaneous vaccination with Dps and Freund's adjuvant only (P = 0.469). There was no significant difference in the log number of CFU of C. jejuni recovered between the positive-control and empty-vector vaccinates (pYA3493) (P = 0.317). A marginally significant differencewas seen between vaccination with the empty vector (pYA3493) and the vector expressing Dps (pYA4495) (P = 0.04).
DISCUSSION
The ability of Campylobacter jejuni strains to survive outside the host and effectively colonize the poultry host is paramount to its success, yet the mechanisms by which these are accomplished are still poorly defined. In this work, we tested the role of one C. jejuni gene, dps, in both of these processes. One strategy for extended Campylobacter survival outside the host is through biofilm formation. To address a potential role for Dps in this process, a Δdps mutant was compared to its wild-type parent strain for in vitro biofilm formation ability. A significant difference in biofilm formation was seen at all time points, including 24 h (P = 0.000147), and total biofilm mass was reduced by over 50% at later stages of development (48 and 72 h). Plasmid complementation of the mutation was able to partially restore biofilm production. The inability to completely restore wild-type function by complementation could be due to differences in the regulation and levels of dps expression between the wild-type and complemented mutant strains. Strains carrying plasmid pRY107 constitutively express dps. Based on these findings, we conclude that C. jejuni dps plays a major role in biofilm formation. This work also supports previous findings that identified C. jejuni Dps as a protein whose synthesis is increased during biofilm formation (14).
To reveal a potential role in poultry colonization, we performed real-time PCR to compare dps expression in C. jejuni cells grown in chicken ceca and broth cultures. Results of the assay demonstrated a 20-fold increase in dps expression during poultry colonization compared to that during in vitro growth. To investigate the role of dps in poultry colonization further, we constructed a Δdps mutant. Our experimental results demonstrated a greater than 5-log-unit decrease in the numbers of CFU in cecal colonization by the Δdps mutant compared to that by the wild-type parent strain. Moreover, the Δdps mutant failed to colonize 30 of the 40 challenged chicks. Complementation of the mutant had no effect on colonization. However, analysis of the colonies recovered at harvest revealed that complementing plasmid pJRT101 was not maintained by C. jejuni within the host. This is most likely a result of the long (14-day) period between challenge and harvest, during which the strain was not under selective pressure to maintain the plasmid. Despite the lack of complementation, the differences in colonization rates observed are not believed to be a result of polar effects or growth rates, as these were unaffected in the mutant (32). The lack of polar effects was confirmed by performing the following analysis: (i) cotranscription of dps with its flanking genes was examined, and no evidence which indicated polar effects was found, and (ii) the expression of the dps gene downstream was examined, and no difference in transcription between the mutant and wild-type strain was found.
Although contaminated poultry are believed to be the primary source of Campylobacter for humans, no effective intervention strategy to control colonization of broiler chickens exists. Given the colonization reduction observed in the dps mutant, the surface localization of the protein, and the success obtained with a Dps homologue as a vaccine to prevent peptic ulcers in humans (20, 28, 30), preliminary testing of Dps as a potential vaccine antigen for the reduction of C. jejuni in broiler chickens was performed. Although subcutaneous vaccination of poultry with recombinantly expressed Dps generated good serum responses compared to those for unvaccinated controls (data not shown), there was no impact on cecal colonization. These findings are not unexpected in chickens, especially as Campylobacter colonization does not cause inflammation in the intestinal tract to facilitate the movement of antibodies from the circulation to the intestinal lumen.
In addition to testing Dps purified protein as a subcutaneous vaccine, it was also tested as an oral vaccine delivered by a recombinant S. enterica strain. Chicks receiving the oral vaccination prior to exposure to Campylobacter showed a greater than 2-log-unit decrease in total cecal colonization compared to that by the wild type. No significant difference in serum antibody responses between vaccinated and unvaccinated chicks was observed. Considering the oral vaccine delivery, protection may be occurring through a highly localized response in the mucosa. A recent paper characterizing the protein content of Brucella melitensis outer membrane vesicles demonstrated the presence of a Dps protein in the vesicles (2). Since the protein is in outer membrane vesicles, this is probably the reason that protection against C. jejuni was partially generated following expression of the protein to the intestinal mucosa by the Salmonella vector. Alternatively, a similar Salmonella vaccine strain induced cellular responses in chickens (37), consistent with the observation that Salmonella vaccines elicit cellular responses in mice and humans (8). Regardless of the mechanism, more work is needed to confirm the potential role of Dps as a usable vaccination antigen for poultry.
In summation, while continuing the characterization of the Dps protein, we have demonstrated a potential role in environmental survival, through the formation of biofilms, and in poultry colonization. Additionally, the dps gene is conserved in all of the current C. jejuni strains sequenced, and we have preliminary evidence showing that the Dps molecule may be a good antigen to reduce Campylobacter colonization of the broiler chicken, when delivered orally through a Salmonella vaccine vector.
ACKNOWLEDGMENTS
We thank Rita Mild for her help with the statistical analysis.
This research was supported by the Danish Council for Strategic Research project CamVac (contract 09-067131), Ellison Medical Foundation grant IDSS-0520-03, NIH grant U01 AI60557, and the Food Safety Research Response Network, a Coordinated Agricultural Project, funded through the National Research Initiative (now the National Institute for Food and Agriculture, Agricultural and Food Research Initiative) of the USDA Cooperative State Research, Education and Extension Service, grant number 2005-35212-15287.
Footnotes
Published ahead of print 11 July 2012
REFERENCES
- 1. Altekruse SF, Stern NJ, Fields PI, Swerdlow DL. 1999. Campylobacter jejuni—an emerging foodborne pathogen. Emerg. Infect. Dis. 5:28–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Avila-Calderon ED, et al. 2011. Characterization of outer membrane vesicles from Brucella melitensis and protection induced in mice. Clin. Dev. Immunol. 2012:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bull SA, et al. 2006. Sources of Campylobacter spp. colonizing housed broiler flocks during rearing. Appl. Environ. Microbiol. 72:645–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coker AO, Isokpehi RD, Thomas BN, Amisu KO, Obi CL. 2002. Human campylobacteriosis in developing countries. Emerg. Infect. Dis. 8:237–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Curtiss R, III, et al. 2007. Induction of host immune responses using Salmonella-vectored vaccines, p 297–313 In Brogden KA, et al. (ed.), Virulence mechanisms of bacterial pathogens, 4th ed. ASM Press, Washington, DC [Google Scholar]
- 6. Deming MS, et al. 1987. Campylobacter enteritis at a university: transmission from eating chicken and from cats. Am. J. Epidemiol. 126:526–534 [DOI] [PubMed] [Google Scholar]
- 7. Donlan RM, Costerton JW. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15:167–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Galen JE, Levine MM. 2001. Can a ‘flawless’ live vector vaccine strain be engineered? Trends Microbiol. 9:372–376 [DOI] [PubMed] [Google Scholar]
- 9. Gregory E, Barnhart H, Dreesen DW, Stern NJ, Corn JL. 1997. Epidemiological study of Campylobacter spp. in broilers: source, time of colonization, and prevalence. Avian Dis. 41:890–898 [PubMed] [Google Scholar]
- 10. Humphrey TJ, Henley A, Lanning DG. 1993. The colonization of broiler chickens with Campylobacter jejuni: some epidemiological investigations. Epidemiol. Infect. 110:601–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ishikawa T, et al. 2003. The iron-binding protein Dps confers hydrogen peroxide stress resistance to Campylobacter jejuni. J. Bacteriol. 185:1010–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Joshua GW, Guthrie-Irons C, Karlyshev AV, Wren BW. 2006. Biofilm formation in Campylobacter jejuni. Microbiology 152:387–396 [DOI] [PubMed] [Google Scholar]
- 13. Kaino K, Hayashidani H, Kaneko K, Ogawa M. 1988. Intestinal colonization of Campylobacter jejuni in chickens. Nippon Juigaku Zasshi 50:489–494 [DOI] [PubMed] [Google Scholar]
- 14. Kalmokoff M, et al. 2006. Proteomic analysis of Campylobacter jejuni 11168 biofilms reveals a role for the motility complex in biofilm formation. J. Bacteriol. 188:4312–4320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kang HY, Srinivasan J, Curtiss R., III 2002. Immune responses to recombinant pneumococcal PspA antigen delivered by live attenuated Salmonella enterica serovar Typhimurium vaccine. Infect. Immun. 70:1739–1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- 17. Lehtola MJ, Pitkanen T, Miebach L, Miettinen IT. 2006. Survival of Campylobacter jejuni in potable water biofilms: a comparative study with different detection methods. Water Sci. Technol. 54:57–61 [DOI] [PubMed] [Google Scholar]
- 18. Li Y, et al. 2009. Evaluation of new generation Salmonella enterica serovar Typhimurium vaccines with regulated delayed attenuation to induce immune responses against PspA. Proc. Natl. Acad. Sci. U. S. A. 106:593–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lindblom GB, Sjorgren E, Kaijser B. 1986. Natural campylobacter colonization in chickens raised under different environmental conditions. J. Hyg. (Lond.) 96:385–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Malfertheiner P, et al. 2008. Safety and immunogenicity of an intramuscular Helicobacter pylori vaccine in noninfected volunteers: a phase I study. Gastroenterology 135:787–795 [DOI] [PubMed] [Google Scholar]
- 21.Reference deleted. [Google Scholar]
- 22. Naikare H, Palyada K, Panciera R, Marlow D, Stintzi A. 2006. Major role for FeoB in Campylobacter jejuni ferrous iron acquisition, gut colonization, and intracellular survival. Infect. Immun. 74:5433–5444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Newell DG, Fearnley C. 2003. Sources of Campylobacter colonization in broiler chickens. Appl. Environ. Microbiol. 69:4343–4351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23a. Nickoloff JA, Reynolds RJ. 1991. Subcloning with new ampicillin- and kanamycin-resistant analogs of pUC19. Biotechniques 10:469–470, 472 [PubMed] [Google Scholar]
- 24. Palyada K, Threadgill D, Stintzi A. 2004. Iron acquisition and regulation in Campylobacter jejuni. J. Bacteriol. 186:4714–4729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pearson AD, et al. 1993. Colonization of broiler chickens by waterborne Campylobacter jejuni. Appl. Environ. Microbiol. 59:987–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:e45 doi:10.1093/nar/29.9.e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reeser RJ, Medler RT, Billington SJ, Jost BH, Joens LA. 2007. Characterization of Campylobacter jejuni biofilms under defined growth conditions. Appl. Environ. Microbiol. 73:1908–1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rossi G, et al. 2004. Therapeutic vaccination against Helicobacter pylori in the beagle dog experimental model: safety, immunogenicity, and efficacy. Infect. Immun. 72:3252–3259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reference deleted. [Google Scholar]
- 30. Satin B, et al. 2000. The neutrophil-activating protein (HP-NAP) of Helicobacter pylori is a protective antigen and a major virulence factor. J. Exp. Med. 191:1467–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Scallan E, et al. 2011. Foodborne illness acquired in the United States—major pathogens. Emerg. Infect. Dis. 17:7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Theoret JR, Cooper KK, Glock RD, Joens LA. 2011. A Campylobacter jejuni Dps homolog has a role in intracellular survival and in the development of campylobacteriosis in neonate piglets. Foodborne Pathog. Dis. 8:1263–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thomas LM, Long KA, Good RT, Panaccio M, Widders PR. 1997. Genotypic diversity among Campylobacter jejuni isolates in a commercial broiler flock. Appl. Environ. Microbiol. 63:1874–1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van Vliet AH, Ketley JM, Park SF, Penn CW. 2002. The role of iron in Campylobacter gene regulation, metabolism and oxidative stress defense. FEMS Microbiol. Rev. 26:173–186 [DOI] [PubMed] [Google Scholar]
- 35. Wilson DJ, et al. 2008. Tracing the source of campylobacteriosis. PLoS Genet. 4:e1000203 doi:10.1371/journal.pgen.1000203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yao R, Alm RA, Trust TJ, Guerry P. 1993. Construction of new Campylobacter cloning vectors and a new mutational cat cassette. Gene 130:127–130 [DOI] [PubMed] [Google Scholar]
- 37. Zekarias B, Mo H, Curtiss R., III 2008. Recombinant attenuated Salmonella enterica serovar Typhimurium expressing the carboxy-terminal domain of alpha toxin from Clostridium perfringens induces protective responses against necrotic enteritis in chickens. Clin. Vaccine Immunol. 15:805–816 [DOI] [PMC free article] [PubMed] [Google Scholar]


