Abstract
Bacteremia is the second leading cause of death in patients with end-stage renal disease who are on hemodialysis. A vaccine eliciting long-term immune responses against Staphylococcus aureus in patients on chronic hemodialysis may reduce the incidence of bacteremia and its complications in these patients. V710 is a vaccine containing iron surface determinant B (IsdB), a highly conserved S. aureus surface protein, which has been shown to be immunogenic in healthy subjects. In this blinded phase II immunogenicity study, 206 chronic hemodialysis patients between the ages of 18 and 80 years old were randomized to receive 60 μg V710 (with or without adjuvant), 90 μg V710 (with adjuvant), or a placebo in various combinations on days 1, 28, and 180. All 201 vaccinated patients were to be followed through day 360. The primary hypothesis was that at least 1 of the 3 groups receiving 2 V710 doses on days 1 and 28 would have a ≥2.5 geometric mean fold rise (GMFR) in anti-IsdB IgG titers over the baseline 28 days after the second vaccination (day 56). At day 56, all three groups receiving 2 doses of V710 achieved a ≥2.5 GMFR in anti-IsdB antibodies compared to the baseline (P values of <0.001 for all 3 groups), satisfying the primary immunogenicity hypothesis. None of the 33 reported serious adverse experiences were considered vaccine related by the investigators. V710 induced sustained antibody responses for at least 1 year postvaccination in patients on chronic hemodialysis.
INTRODUCTION
Infections account for almost 14% of deaths in patients with end-stage renal disease (ESRD) (2, 4, 12, 15, 19, 21). As a result of the considerable morbidity, mortality, and economic toll of Staphylococcus aureus infections in dialysis patients, a well-tolerated S. aureus vaccine that provides long-term protection in ESRD patients may have a substantial public health impact (2, 3, 4, 12, 15, 17, 19, 21).
Iron surface determinant B (IsdB) is a cell surface protein of S. aureus involved in extracellular binding of iron (14). IsdB is thought to enhance bacterial virulence. This polypeptide was chosen as a vaccine candidate because its cell surface expression makes it accessible to opsonic antibodies and the protein is highly conserved in S. aureus isolates from diverse clinical and taxonomical backgrounds with differing resistance patterns (11, 17).
V710 contains a recombinant IsdB shown to be immunogenic against S. aureus in murine and primate models (11) as well as in healthy human volunteers (8, 9). However, it has not been studied in patients at high risk for S. aureus infections who have suppressed immune systems. Because immune responses to a vaccine may be altered by immunosuppression, it is important to ensure that immunogenicity in immunocompromised patients is similar to that observed in healthy subjects.
We report the first study to demonstrate the immunogenicity and safety of V710 in patients with ESRD who are on chronic hemodialysis, an at-risk population with clinically meaningful immunosuppression.
MATERIALS AND METHODS
Patients.
Men and nonpregnant women aged 18 to 80 years with ESRD who were on chronic hemodialysis via one of a variety of access sites (including native-vessel fistula, synthetic/heterologous graft, or dual-lumen cuffed catheter) and who had a Karnofsky score of ≥50 were eligible. Pertinent exclusion criteria included known or suspected immunologic impairment (beyond ESRD), anticipated renal transplantation within 180 days of study entry, serious S. aureus infection in the previous 12 months, an oral-equivalent temperature of ≥100.4°F (≥38.0°C) in the 48 h before vaccination, previous vaccination with V710, vaccination with a live-virus vaccine within 30 days before the study vaccination or anticipated vaccination with a live-virus vaccine within 60 days following study entry, any other vaccination within 14 days of the study vaccination or anticipated vaccination within 60 days following study entry (except for influenza and hepatitis B vaccines, which were prohibited only from 7 days before to 15 days after each study injection), and systemic corticosteroid (prednisone equivalent dose of ≥20 mg daily) or other immunosuppressive therapy or biological agents within 14 days of the study vaccination or anticipated administration of such medications within 60 days of study entry.
All patients gave written informed consent prior to participation. The study was conducted in accordance with the principles of the Declaration of Helsinki and good clinical practices and was approved by the appropriate institutional review boards and regulatory agencies.
Study design.
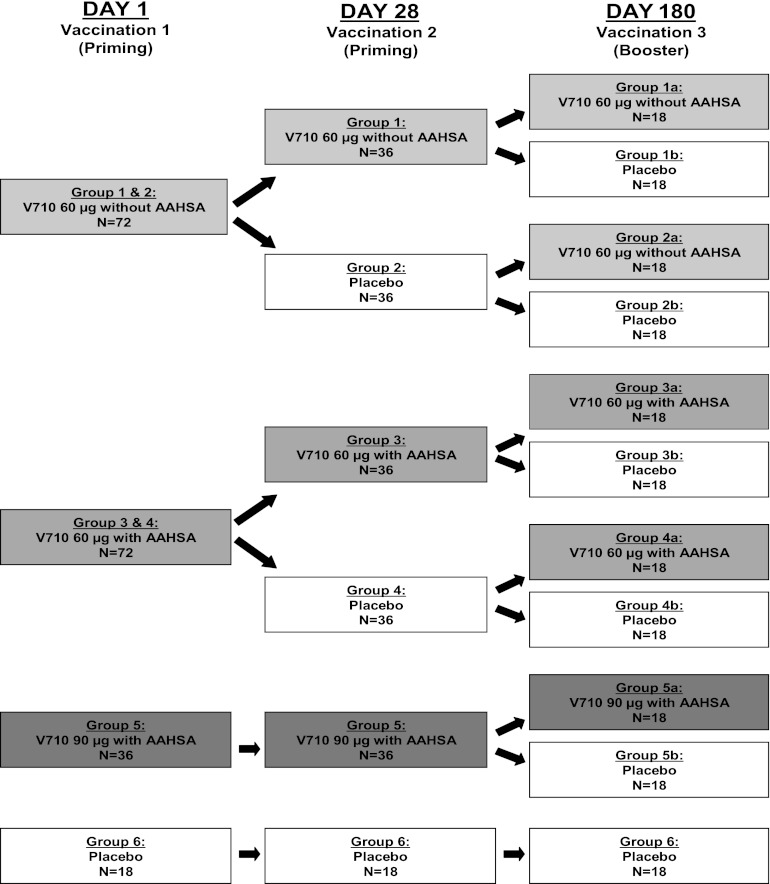
This 1-year, randomized, blinded, placebo-controlled study of adults with ESRD who were on chronic hemodialysis (Merck protocol 005, ClinicalTrials.gov registration number NCT00572910) was conducted at 12 centers in the United States from 25 August 2008 to 28 January 2010. Eligible patients were randomized in a 2:2:2:2:2:1 ratio to 1 of 5 experimental groups to receive at least 1 dose of V710 (groups 1 to 5) or to 1 control group receiving saline placebo only (group 6) (Fig. 1). All patients received their priming doses on day 1 and day 28, with a booster dose (equally divided between V710 and placebo) on day 180. For data presentation in this paper, each group is described by the 3 doses received on days 1, 28, and 180 (for example, 60 μg/60 μg/60 μg represents group 1a, who received 3 60-μg doses of V710).
Fig 1.
Patient groups and dosing regimens. AAHSA, amorphous aluminum hydroxyphosphate sulfate adjuvant.
V710 was supplied as a lyophilized product in a glass vial and was reconstituted with either 0.45% saline or amorphous aluminum hydroxyphosphate sulfate adjuvant (AAHSA), depending on the treatment group. Because of the differences in appearance between the lyophilized V710 solution and the saline placebo, an unblinded member of the study site staff prepared and administered vaccine or placebo but had no further involvement with any subsequent study procedure, including safety follow-up. All other individuals involved with the study, including investigators, participants, and the clinical monitors and statisticians employed by the sponsor, remained blinded to treatment group throughout the study. Randomization and allocation were assigned using an interactive voice response system. Numbered packaging was used to implement allocation.
Immunogenicity.
Sera were assayed for IsdB-specific IgG antibodies using the Luminex multianalyte profiling platform. Samples were collected at day 1 (prevaccination), day 28 (prevaccination), and days 56, 84, 180 (prevaccination), 210, 270, and 360 after the initial vaccination. Specimens were drawn before or within the first hour of hemodialysis when hemodialysis coincided with a study visit.
Immunogenicity was evaluated as the geometric mean fold rise (GMFR) over baseline and the geometric mean concentration (GMC) of anti-IsdB IgG. The primary immunogenicity endpoint was ≥2.5 GMFR over baseline in anti-IsdB IgG titers 28 days after the second vaccination (i.e., day 56) in groups receiving 2 doses of 60 or 90 μg of V710 (i.e., groups 1, 3, and 5). The threshold of 2.5 GMFR represents a value exceeding assay and routine biological variability so that a change of this magnitude or greater is a reliable index of immunogenicity (even if not necessarily a marker for clinical protection). The criterion was empirically derived from the observed within-subject variability and maximum longitudinal fluctuations in titers among control subjects in earlier studies (7, 8).
Secondary endpoints included (i) ≥2.5 GMFR 28 days after the first vaccination of 60 or 90 μg of V710 (groups 1 and 2 combined, groups 3 and 4 combined, and group 5), (ii) ≥2.0 GMFR 180 days after the first vaccination of 60 or 90 μg of V710 in groups receiving 2 active doses (groups 1, 3, and 5), and (iii) ≥2.5 GMFR 56 days after a single vaccination of 60 μg of V710 (groups 2 and 4).
Exploratory endpoints included the kinetics and durability of the immune response assessed by GMFRs and GMCs over time, comparisons between 60- and 90-μg doses of V710, and immunogenicity of 60 μg V710 with and without AAHSA. For a subset of patients in each group, preimmune and 28-day postvaccination sera were tested for opsonophagocytic antibody (16); bactericidal activity was not assessed.
Safety and tolerability.
Patients were monitored for 30 min after each vaccination for immediate reactions. Patients recorded the following on vaccination report cards after each vaccination: oral temperatures and injection site reactions for 5 days, all systemic and local adverse events (AEs) for 14 days, and concomitant medications and non-study vaccinations for 14 days. Report cards were collected and reviewed by study personnel 14 days after each vaccination. Fever was defined as any oral temperature ≥100.4°F (≥38.0°C); documented or subjective fever within 14 days of vaccination was to be reported as an AE.
Safety parameters included all serious AEs and systemic AEs for 14 days following each vaccination, injection site AEs for 5 days following each vaccination, and body temperatures for 5 days following each vaccination. To provide an overall safety assessment, vaccine-related serious AEs, serious AEs leading to death, and serious AEs involving S. aureus infection were assessed for the entire study duration.
An independent safety evaluation committee reviewed all unblinded safety data once at least 50% of patients had received each of the first, second, and third vaccinations to determine whether enrollment should continue in a given treatment group.
Statistical methods.
The primary hypothesis was that at least 1 of the 3 groups receiving 2 doses of V710 (i.e., groups 1, 3, and 5) would have a ≥2.5-fold rise from the baseline in anti-IsdB IgG titers (i.e., the lower bound of the 95% confidence interval [CI] of the GMFR would be ≥2.5 times the baseline titers) 28 days after the second vaccination (i.e., day 56). GMFR was analyzed using a linear mixed longitudinal model that included baseline and postbaseline natural-log-transformed antibody titers as response variables. Repeated measures included terms for treatment, time, the interaction of time and treatment, and age (<50 years and ≥50 years). The primary analysis was based on the per-protocol population, defined as subjects who were followed for 56 days postvaccination and did not develop an S. aureus infection during this time frame. Patients who missed vaccinations were not included in serology analysis at time points after the missed vaccinations. For patients who developed S. aureus infection after day 56, serology data collected at a visit right before or any time subsequent to infection onset were also excluded from immunogenicity analyses.
A step-down procedure was used to account for multiple comparisons among treatment groups and endpoints. Group 5 was tested first (1-sided α = 0.025), and if significant, testing proceeded to groups 1 and 3, which were tested using Hochberg's approach. Both groups were first tested using a 1-sided α of 0.025. If both tests were significant or nonsignificant, testing was complete. If only one test was significant, that group was retested at a 1-sided α of 0.0125.
Each of the secondary immunogenicity analyses were controlled individually for type I errors at 0.025 in the same manner as for the primary immunogenicity analysis. No multiplicity adjustments were made for the safety comparisons.
All patients who were vaccinated and who had follow-up safety data were included in the safety summaries. Only descriptive statistics without formal between-group comparisons were computed.
Assuming GMFRs at day 56 of 5.0 in group 1, 5.0 in group 3, and 6.0 in group 5 and a standard deviation of log (fold rise) of 1.0, 34 evaluable patients (i.e., in the per-protocol population) in each of these groups would provide >99% power to detect a GMFR of ≥2.5 at day 56 (1-sided α = 0.025) for group 5 and ∼99% power for groups 1 and 3, with a global power of ∼97.9%.
RESULTS
Patients.
Of the 206 randomized patients, slightly more than half were men (59.2%) and half were black (51.5%) (Table 1). The mean age was 55.2 years (median, 56.0 years), and 69.4% of subjects were ≥50 years of age. The mean Karnofsky score was 79.6. Patient demographics were generally comparable across the small treatment groups. The mean length of time on hemodialysis was 45.5 months (median, 24.0 months), and most patients (65.5%) had native-vessel fistulae. Compared to other groups, the median length of any type of dialysis at baseline was slightly higher in groups 1a and 1b and slightly lower in groups 3b and 4a.
Table 1.
Patient characteristics
| Characteristica | Value for each group (no. of patients in each group)b |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1a (18) | 1b (18) | 2a (19) | 2b (18) | 3a (19) | 3b (19) | 4a (19) | 4b (18) | 5a (19) | 5b (19) | 6 (18) | |
| Male | 10 (52.6) | 14 (77.8) | 12 (63.2) | 10 (55.6) | 12 (63.2) | 7 (36.8) | 11 (57.9) | 13 (68.4) | 12 (63.2) | 10 (56.2) | 11 (61.1) |
| Age | |||||||||||
| Mean (yr) (SD) | 55.8 (9.4) | 56.4 (12.4) | 52.7 (15.6) | 51.3 (16.8) | 52.6 (13.0) | 60.1 (11.1) | 60.4 (12.0) | 56.6 (8.4) | 56.5 (17.0) | 51.6 (15.7) | 52.3 (14.4) |
| Range (yr) | 35–75 | 33–79 | 30–79 | 21–78 | 33–78 | 39–75 | 33–79 | 41–70 | 27–80 | 28–75 | 22–80 |
| No. aged <50 | 6 (31.6) | 4 (22.2) | 8 (42.1) | 6 (33.3) | 10 (52.6) | 4 (21.1) | 2 (10.5) | 3 (15.8) | 10 (52.6) | 4 (21.1) | 2 (10.5) |
| Race or ethnicity | |||||||||||
| American Indian or Alaska native | 1 (5.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Asian | 0 (0.0) | 2 (11.1) | 0 (0.0) | 1 (5.6) | 0 (0.0) | 1 (5.3) | 1 (5.3) | 0 (0.0) | 1 (5.3) | 0 (0.0) | 0 (0.0) |
| Black or African American | 12 (63.2) | 10 (55.6) | 8 (42.1) | 9 (50) | 12 (63.2) | 10 (52.6) | 11 (57.9) | 9 (47.4) | 7 (36.8) | 11 (57.9) | 7 (38.9) |
| Multiracial | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (5.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (5.3) | 2 (10.5) | 0 (0.0) |
| Native Hawaiian or other Pacific Islander | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (5.3) | 0 (0.0) | 0 (0.0) | 1 (5.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| White | 6 (31.6) | 6 (33.3) | 11 (57.9) | 8 (44.4) | 5 (26.3) | 8 (42.1) | 7 (36.8) | 9 (47.4) | 10 (52.6) | 6 (31.6) | 11 (61.1) |
| Hispanic or Latino | 4 (21.1) | 4 (22.2) | 8 (42.1) | 0 (0.0) | 6 (31.6) | 5 (26.3) | 1 (5.3) | 6 (31.6) | 9 (47.4) | 4 (21.1) | 7 (38.9) |
| Mean Karnofsky score (SD) | 76.1 (9.2) | 82.2 (10.6) | 83.2 (8.2) | 80.0 (12.8) | 79.5 (10.3) | 80.5 (12.2) | 81.1 (4.6) | 79.5 (9.1) | 72.6 (10.5) | 80.5 (12.7) | 80.6 (10.0) |
| Mean time on dialysis (mos) (SD) | 85.3 (78.0) | 58.8 (69.8) | 39.2 (35.0) | 30.9 (25.8) | 44.5 (47.3) | 38.2 (55.4) | 26.8 (23.6) | 44.7 (42.8) | 59.6 (72.3) | 47.3 (49.7) | 50.4 (66.9) |
| Mean time on hemodialysis (mos) (SD) | 85.3 (78.0) | 55.5 (68.1) | 38.6 (34.3) | 30.9 (25.8) | 43.9 (47.2) | 31.1 (43.6) | 26.3 (24.0) | 42.2 (38.6) | 59.6 (72.3) | 42.9 (42.7) | 45.7 (63.6) |
| Type of hemodialysis access | |||||||||||
| Dual-lumen cuffed catheter | 3 (15.8) | 5 (27.8) | 1 (5.3) | 5 (27.8) | 3 (15.8) | 3 (15.8) | 6 (31.6) | 2 (10.5) | 2 (10.5) | 8 (42.1) | 4 (22.2) |
| Native-vessel fistula | 14 (73.7) | 9 (50.0) | 15 (78.9) | 12 (66.7) | 11 (57.9) | 13 (68.4) | 10 (52.6) | 14 (73.7) | 15 (78.9) | 9 (47.4) | 13 (72.2) |
| Synthetic/heterologous graft | 1 (5.3) | 4 (22.2) | 3 (15.8) | 1 (5.6) | 5 (26.3) | 3 (15.8) | 3 (15.8) | 3 (15.8) | 2 (10.5) | 2 (10.5) | 1 (5.6) |
| Mean baseline GMC (μg/ml) (SD) | 22.7 (2.6) | 21.8 (2.9) | 36.3 (2.3) | 24.6 (3.1) | 26.9 (2.7) | 33.7 (2.7) | 35.2 (2.4) | 27.1 (2.4) | 24.4 (2.5) | 25.0 (2.1) | 30.3 (2.0) |
Values are n (% of total) unless indicated otherwise. SD, standard deviation.
Group 1a, 60 μg/60 μg/60 μg; group 1b, 60 μg/60 μg/PBO; group 2a, 60 μg/PBO/60 μg; group 2b, 60 μg/PBO/PBO; group 3a, 60 μg (+)/60 μg (+)/60 μg (+); group 3b, 60 μg (+)/60 μg (+)/PBO; group 4a, 60 μg (+)/PBO/60 μg (+); group 4b, 60 μg/PBO/PBO; group 5a, 90 μg (+)/90 μg (+)/90 μg (+); group 5b, 90 μg (+)/90 μg (+)/PBO; group 6, PBO/PBO/PBO; +, formulation contains amorphous aluminum hydroxyphosphate sulfate adjuvant; PBO, placebo.
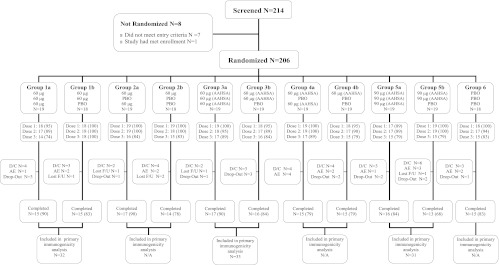
A total of 201 patients (98%) received at least 1 dose of V710 or placebo, 196 (95%) received 2 doses, 173 (84%) received all 3 doses, and 168 (82%) completed the study (Fig. 2). The most common reason for study discontinuation was an AE (8.3%), which occurred at similar rates across treatment groups. A total of 109 patients received V710 for the first 2 vaccinations, 19 of which were excluded from the per-protocol population for the primary immunogenicity analysis. The most common reason for exclusion was corticosteroid use (5 [2.5%]). All 201 patients who received at least 1 vaccination were included in the safety analysis.
Fig 2.
Patient accounting. AAHSA, amorphous aluminum hydroxyphosphate sulfate adjuvant; D/C, discontinued; F/U, follow-up; N/A, not applicable; PBO, placebo.
Immunogenicity.
For the primary efficacy endpoint at 28 days after the second vaccination, the GMFR in anti-IsdB IgG titers was ≥2.5 times the baseline level in all 3 groups receiving 2 doses of V710 (P values of <0.001 for each pairwise comparison), satisfying the primary efficacy hypothesis (Table 2). In contrast, the GMFR for the placebo-only group was 1.1 (95% CI, 0.9 to 1.4), indicating essentially no change from baseline. By 28 days after the first dose, every V710 group had an ∼1-log increase in GMFR from baseline (P values of <0.001 for each pairwise comparison). Response rates were numerically higher in patients ≥50 years of age than in patients <50 years old. However, when age groups were further categorized as <50, 50 to 59, and 60 to 80 years, there was no trend of increased antibody response with increasing age.
Table 2.
Primary and key secondary immunogenicity resultsa
| Time point and dosing regimen (group no.) | N | n | GMFR (95% CI) | P value |
|---|---|---|---|---|
| 28 days after 2nd vaccination (primary endpoint, day 56) | ||||
| 90 μg (+AAHSA)/90 μg (+AAHSA) (group 5) | 36 | 31 | 17.8 (13.0, 24.3) | <0.001 |
| 60 μg (+AAHSA)/60 μg (+AAHSA) (group 3) | 37 | 33 | 15.1 (11.0, 20.8) | <0.001 |
| 60 μg/60 μg (group 1) | 36 | 32 | 18.9 (13.7, 26.1) | <0.001 |
| PBO/PBO (group 6) | 18 | 16 | 1.1 (0.9, 1.4) | N/A |
| 28 days after 1st vaccination (day 28) | ||||
| 90 μg (+AAHSA) (group 5) | 36 | 35 | 12.9 (8.8, 18.8) | <0.001 |
| 60 μg (+AAHSA) (groups 3 and 4) | 74 | 68 | 12.9 (9.8, 16.9) | <0.001 |
| 60 μg (groups 1 and 2) | 73 | 68 | 11.9 (9.0, 15.6) | <0.001 |
| PBO/PBO (group 6) | 18 | 16 | 1.2 (0.9, 1.5) | N/A |
| 56 days after a single vaccination (day 56) | ||||
| 60 μg (+AAHSA)/PBO (group 4) | 37 | 34 | 11.8 (8.6, 16.1) | <0.001 |
| 60 μg/PBO (group 2) | 37 | 32 | 9.6 (7.1, 13.1) | <0.001 |
| PBO/PBO (group 6) | 18 | 16 | 1.1 (0.9, 1.4) | N/A |
| 180 days after first vaccination of a 2-dose regimen (day 180) | ||||
| 90 μg (+AAHSA)/90 μg (+AAHSA) (group 5) | 36 | 28 | 7.6 (5.5, 10.6) | <0.001 |
| 60 μg (+AAHSA)/60 μg (+AAHSA) (group 3) | 37 | 32 | 5.7 (4.2, 7.9) | <0.001 |
| 60 μg/60 μg (group 1) | 36 | 30 | 8.5 (6.2, 11.8) | <0.001 |
| PBO/PBO (group 6) | 18 | 14 | 1.0 (0.8, 1.3) | N/A |
PBO values are observed values; no formal statistical analysis was planned or performed. Values for the V710 treatment groups were based on a repeated-measures model adjusting for age at vaccination and baseline antibody level. N, number vaccinated at baseline; n, number contributing to analysis; GMFR, geometric mean fold rise from baseline; CI, confidence interval; AAHSA, amorphous aluminum hydroxyphosphate sulfate adjuvant; PBO, placebo; N/A, not applicable.
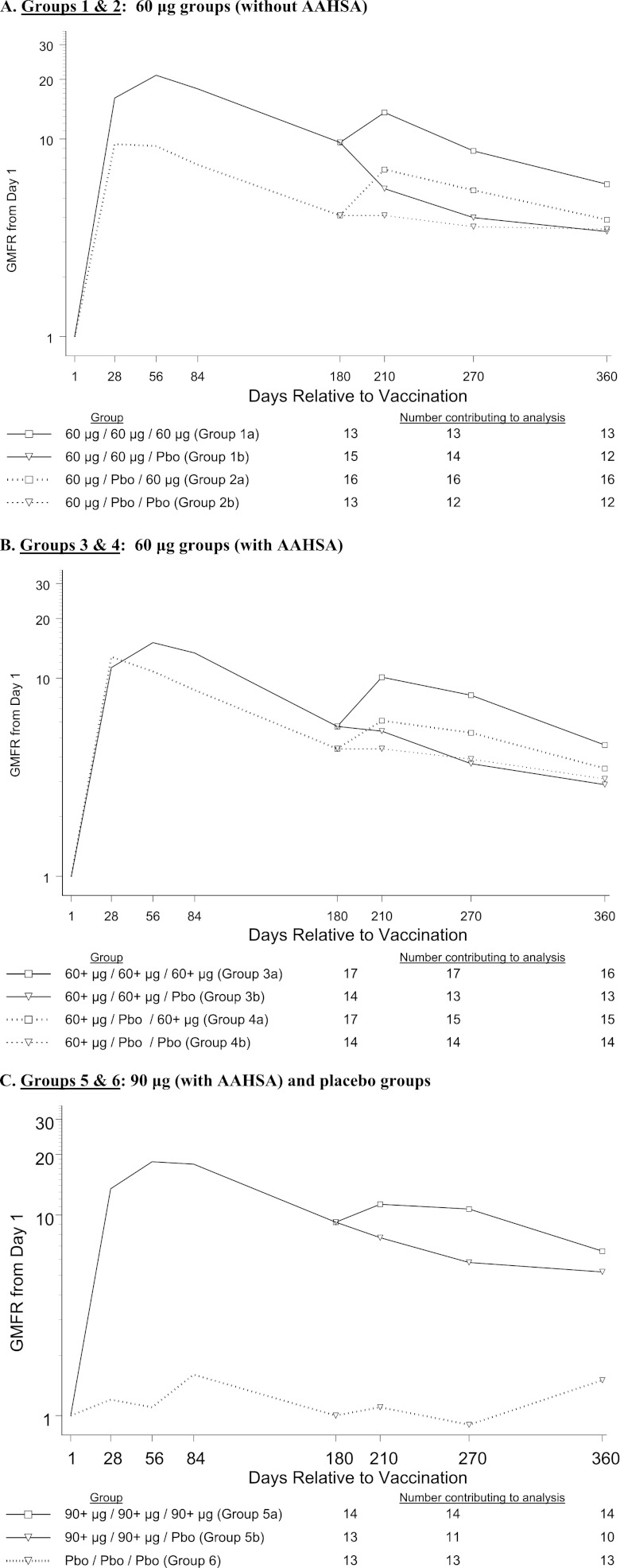
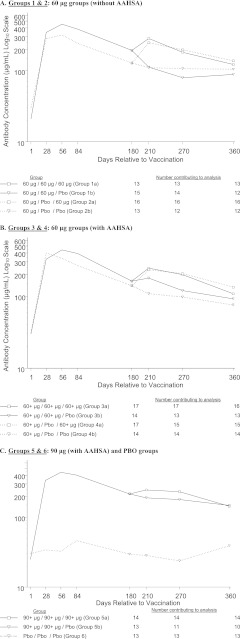
The GMFRs and GMCs over time are displayed in Fig. 3 and 4. At day 56 postvaccination, more than 93% of patients in the five V710 groups (groups 1 to 5) had a ≥2-fold rise in anti-IsdB IgG titers from baseline, with a GMFR of >9. At day 180 postvaccination, the V710 groups had GMFRs ranging from 4.1 to 9.6, whereas the placebo-only group (group 6) had a GMFR of 1.0. Through day 360 postvaccination, the GMFRs for the V710 groups ranged from 2.9 to 6.6, compared with 1.5 in the placebo-only group.
Fig 3.
Geometric mean fold rise (GMFR) by vaccine group through day 180 postvaccination. (A) Groups 1 and 2: 60-μg doses (without AAHSA). (B) Groups 3 and 4: 60-μg doses (with AAHSA). (C) Groups 5 and 6: 90-μg (with AAHSA) and placebo (PBO) doses. AAHSA, amorphous aluminum hydroxyphosphate sulfate adjuvant.
Fig 4.
Geometric mean concentrations (GMC) by vaccine group through day 360 postvaccination. (A) Groups 1 and 2: 60-μg doses (without AAHSA). (B) Groups 3 and 4: 60-μg doses (with AAHSA). (C) Groups 5 and 6: 90-μg (with AAHSA) and PBO doses. AAHSA, amorphous aluminum hydroxyphosphate sulfate adjuvant.
The results of the exploratory analyses demonstrated comparable immune responses at all postvaccination time points for the 60-μg (with and without AAHSA) and 90-μg (with AAHSA) dosages. In comparisons of immune responses following single-dose and multiple-dose regimens without correction for multiple comparisons, only antibody responses to the 60-μg, two-dose regimen (without AAHSA) were statistically higher (P < 0.05) at day 56 through day 180 (GMFR range, 8.5 to 18.9) than those of the single-dose regimen of 60 μg (GMFR range, 4.4 to 9.6). For day 210 through day 360, no significant differences were observed between the 60-μg single-dose and two-dose regimens (administered 28 days apart).
GMFRs at day 210 were significantly (P < 0.05) higher in patients in the 1- and 2-dose 60-μg (without AAHSA) groups (groups 1a and 2a) and the 2-dose 90-μg group (group 5a) who received a dose at day 180 than in those who did not receive a dose at day 180. At day 270, significant differences were still observed only for the 2-dose 60-μg (without AAHSA) (group 1a) and 2-dose 90-μg (group 5a) regimens. At day 360, the groups receiving a dose at day 180 had higher GMFRs than those not receiving a dose at that time point, but these differences were not statistically significant.
Subsets of 12 patients from each group were tested for opsonophagocytic antibody. When immune sera obtained 28 days after the first dose were compared to baseline sera, a ≥2-fold increase in opsonophagocytic activity was observed in 34 (67%) of 60 evaluable V710 recipients and 2 (17%) of 12 evaluable placebo recipients. The corresponding numbers of patients with ≥4-fold increases in baseline opsonophagocytic activity at day 28 were 17 (28%) after 1 dose of V710 with a GMFR ranging from 2.1 to 4.0 across groups 1 to 5 and 1 (9%) after 1 dose of placebo with a 1.1 GMFR.
Safety.
Safety results are summarized in Table 3. Serious AEs during the 14 days immediately following any of the 3 doses were reported in 11 of 183 patients who received ≥1 dose of V710 in groups 1 to 5 (9 of these AEs followed a dose of V710, while 2 cases were temporally related to a placebo dose in the mixed regimen); none of the 18 placebo-only recipients in group 6 developed a serious AE during this 14-day postvaccination period. Over the 360-day course of the entire study, 30 V710 recipients and 3 placebo-only recipients experienced serious adverse events, none of which were considered vaccine related by the investigators. No patients discontinued the study due to a vaccine-related AE. Four V710 recipients and 1 placebo-only recipient developed a serious AE involving an S. aureus infection during the study. Overall, 14 patients in the V710 groups and 2 patients in the placebo-only group died during the study; the causes of death were sepsis and/or cardiovascular events in all instances where known except for azotemia and intestinal ischemia in 1 V710 recipient each. The small group sizes (especially given only 18 patients in the placebo-only group) preclude meaningful comparisons of AE rates.
Table 3.
Adverse events following vaccinations 1, 2, and 3a
| Adverse event | No. (%) of patients with each AE in each group (n) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1a (18) | 1b (18) | 2a (19) | 2b (18) | 3a (19) | 3b (18) | 4a (19) | 4b (18) | 5a (17) | 5b (19) | 6 (18) | |
| AE reported days 1–14 after each vaccination | |||||||||||
| Systemic AE | 6 (33.3) | 6 (33.3) | 4 (21.1) | 4 (22.2) | 5 (26.3) | 6 (33.3) | 8 (42.1) | 5 (27.8) | 6 (35.3) | 6 (31.6) | 7 (38.9) |
| AE with ≥10% incidence in any group | |||||||||||
| Diarrhea | 0 (0.0) | 1 (5.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (10.5) | 0 (0.0) | 0 (0.0) | 2 (10.5) | 0 (0.0) |
| Nausea | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (11.1) | 1 (5.3) | 1 (5.6) | 0 (0.0) | 1 (5.3) | 0 (0.0) |
| Pancreatitis | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (11.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Vomiting | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (10.5) | 1 (5.6) |
| Pyrexia | 0 (0.0) | 2 (11.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (5.3) | 0 (0.0) | 2 (11.8) | 0 (0.0) | 0 (0.0) |
| Headache | 0 (0.0) | 0 (0.0) | 1 (5.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (5.3) | 1 (5.6) | 0 (0.0) | 0 (0.0) | 2 (11.1) |
| Rhinorrhea | 1 (5.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (10.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (5.6) |
| AE reported days 1–5 after each vaccination | |||||||||||
| Injection site AE | 5 (27.8) | 6 (33.3) | 1 (5.3) | 4 (22.2) | 8 (42.1) | 7 (38.9) | 9 (47.4) | 6 (33.3) | 7 (41.2) | 9 (47.4) | 2 (11.1) |
| AE with ≥10% incidence in any group | |||||||||||
| Erythema | 3 (16.7) | 2 (11.1) | 0 (0.0) | 1 (5.6) | 3 (15.8) | 3 (16.7) | 4 (21.1) | 0 (0.0) | 2 (11.8) | 2 (10.5) | 2 (11.1) |
| Pain | 5 (27.8) | 5 (27.8) | 1 (5.3) | 3 (16.7) | 8 (42.1) | 4 (22.2) | 8 (42.1) | 5 (27.8) | 5 (29.4) | 9 (47.4) | 2 (11.1) |
| Swelling | 2 (11.1) | 0 (0.0) | 0 (0.0) | 1 (5.6) | 2 (10.5) | 4 (22.2) | 5 (26.3) | 3 (16.7) | 2 (11.8) | 3 (15.8) | 2 (11.1) |
| AE reported days 1–360 | |||||||||||
| Systemic AE | 7 (38.9) | 7 (38.9) | 7 (36.8) | 8 (44.4) | 6 (31.6) | 8 (44.4) | 11 (57.9) | 7 (38.9) | 8 (47.1) | 10 (52.6) | 10 (55.6) |
| Clinical AEb | 9 (50.0) | 10 (55.6) | 8 (42.1) | 10 (55.6) | 10 (52.6) | 13 (72.2) | 12 (63.2) | 11 (61.1) | 11 (64.7) | 12 (63.2) | 10 (55.6) |
| Serious AE | 2 (11.1) | 3 (16.7) | 2 (10.5) | 5 (27.8) | 0 (0.0) | 5 (27.8) | 4 (21.1) | 2 (11.1) | 2 (11.8) | 5 (26.3) | 3 (16.7) |
| Serious vaccine-related AEc | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Serious AE involving S. aureus infection | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (5.6) | 0 (0.0) | 2 (11.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (5.3) | 1 (5.6) |
| Discontinued study due to a vaccine-related AEc | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0(0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Death | 1 (5.6) | 2 (11.1) | 0 (0.0) | 2 (11.1) | 0 (0.0) | 0 (0.0) | 4 (21.1) | 1 (5.6) | 1 (5.9) | 3 (15.8) | 2 (11.1) |
AE, adverse event.
Patient-reported systemic or injection site AEs during the entire study period.
Determined by the investigator to be related to the vaccine/placebo.
DISCUSSION
Our dose-ranging immunogenicity study demonstrated a robust antibody response to V710 in ESRD patients on hemodialysis. V710 was immunogenic after a single vaccination with all dosages evaluated. The antibody response was evident by 28 days after the first dose and largely sustained for up to 360 days after the initial vaccination especially in patients receiving multiple doses. None of the 33 reported serious AEs were attributed to the vaccine by the site investigators.
Although the immune repertoire of patients was not directly interrogated in this study, production of antigen-specific memory CD4+ T cells after vaccination is frequently impaired in patients with ESRD (13). Only 50 to 75% of dialysis patients receiving 3 doses of hepatitis B virus vaccine develop protective antibody levels, whereas more than 90% of immunocompetent patients with normal renal function achieve adequate titers (1). High vaccination failure rates in hemodialysis patients have also been reported with influenza, tetanus, and diphtheria vaccines (6, 10, 18).
Antibody levels after V710 vaccination in hemodialysis patients in our study were on average higher than the levels previously reported in healthy volunteers from the phase 1 studies (7, 8), perhaps reflecting a high frequency of previous immunizing contact with S. aureus as a result of ESRD and hemodialysis. Baseline antibody levels and the brisk responses to the initial dose may signify that patients with ESRD often mount an anamnestic response to the first dose of V710 because of prior natural exposure to S. aureus. Additional vaccine doses may serve to augment the magnitude and durability of the antibody response. Despite generally increased titers after additional doses, a consistent dose-response relationship was not evident.
Vaccination with V710 induced functional antibodies. While neutrophils are critical to host defenses against S. aureus, vaccine immunogenicity measured solely as antibody titers or opsonophagocytic activity does not always translate into clinical efficacy. T helper cells, especially through the production of interleukin 17 (IL-17), appear to be more critically involved in controlling cutaneous S. aureus infections than previously recognized (3, 16). A key function of the Th-17/IL-17 axis (which may be impaired in ESRD patients [13]) is to recruit neutrophils to the infected site, implying that the cell-mediated and phagocytic arms of the immune system may work cooperatively to eradicate S. aureus.
To date, no immunologic correlate of protection has been established for S. aureus infection. Previous studies of immunogenic S. aureus vaccines have failed to produce efficacious results in later pivotal studies (19) (S. Deresinski, presented at the 12th International Symposium on Staphylococci and Staphylococcal Infections, Cairns, Australia, 7 to 10 September 2008). An event-driven phase IIb/III efficacy trial of V710 in patients undergoing cardiothoracic surgery (ClinicalTrials.gov registration number NCT00518687) was recently terminated after the second planned interim analysis because of potential safety concerns as well as low vaccine efficacy closely approaching the protocol-stipulated futility threshold of 20% (5) in spite of brisk antibody responses in V710 recipients.
Prevention of S. aureus infections remains an important unmet medical need (3, 5, 17, 20) (S. Deresinski, presented at the 12th International Symposium on Staphylococci and Staphylococcal Infections, Cairns, Australia, 7 to 10 September 2008). Dialysis-associated infections are second to cardiovascular complications as a cause of death in patients with ESRD. The most common infectious etiology is S. aureus (21). Patients infected with S. aureus frequently develop a wide range of serious complications, including dialysis site infections (often with resultant loss of access), infective endocarditis, and osteomyelitis, eventuating in substantial morbidity and mortality (4, 12). S. aureus infections in patients on hemodialysis absorb substantial resources (2, 4, 15, 19). The economic burden has been further exacerbated by the nosocomial and community spread of methicillin-resistant S. aureus (MRSA) (2). Effective immunization against S. aureus infection in the many patients undergoing chronic hemodialysis could greatly reduce both the health and health care toll exacted by this virulent and widespread pathogen (2, 3, 4, 15, 19, 21).
In our study, V710 vaccination induced robust antibody responses to S. aureus in a high-risk population. Antibodies appeared rapidly after the first dose and were sustained for at least a year with multidose regimens. However, in the absence of established serologic correlates of protection and given the discouraging results of the prophylactic V710 study in cardiothoracic surgery patients, adequately powered efficacy trials are ultimately required to assess whether promising vaccine candidates can reduce S. aureus infection rates in practice (3, 5, 7, 8, 11, 17).
ACKNOWLEDGMENTS
We thank the Merck protocol 005 patients and investigators who participated in this study. We also are indebted to Tessie McNeely of Merck for her thoughtful suggestions regarding vaccine immunology and Jennifer Pawlowski of Merck for her expert technical assistance in the preparation and submission of the manuscript.
All authors contributed to the conception, data interpretation, and/or drafting or revising of the manuscript for important intellectual content and accept responsibility for the work described in this paper. Each author provided final approval of the version to be submitted. No writing assistance outside the named authors was used.
J. S. Hartzel, S. S. Smugar, N. A. Kartsonis, M. J. DiNubile, D. Guris, and E. Brown are employees of Merck Sharp & Dohme Corp. and may own or have options to own company stock. All the academic authors have been investigators for Merck. M. Moustafa has also been an investigator for Novartis, Amgen, Genzyme, Abbott, and Fibrogen and is on the speaker's bureau for Novartis, Fresenius, and Abbot. G. R. Aronoff is a consultant to Amgen, Affymax, and Fresenius, has received honoraria from Amag, Fresenius, and Novartis, and has received research grants from NxStage and the National Institutes of Health. C. Chandran and L. U. Mailloux report no conflicts.
This study was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Whitehouse Station, NJ.
Footnotes
Published ahead of print 25 July 2012
REFERENCES
- 1. Aronoff GR, Maxwell DR, Batteiger BE, Fineberg NS. 1985. Hepatitis B virus vaccine: a randomized trial of a reduced dose regimen in hemodialysis patients. Am. J. Kidney Dis. 6:170–172 [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control 2007. Invasive methicillin-resistant Staphylococcus aureus infections among dialysis patients-United States, 2005. MMWR Morb. Mortal. Wkly. Rep. 56:197–199 [PubMed] [Google Scholar]
- 3. Daum RS, Spellberg B. 2012. Progress toward a Staphylococcus aureus vaccine. Clin. Infect. Dis. 54:560–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Engemann JJ, et al. 2005. Clinical outcomes and costs due to Staphylococcus aureus bacteremia among patients receiving long-term hemodialysis. Infect. Control Hosp. Epidemiol. 26:534–539 [DOI] [PubMed] [Google Scholar]
- 5. Fowler VG, et al. 2012. Efficacy and safety of V710, a Staphylococcus aureus vaccine, in preventing bacteraemia and/or deep sternal wound infections in patients undergoing cardiac surgery, abstr O164. Abstr. 22nd Eur. Congr. Infect. Dis. Clin. Microbiol., London, United Kingdom; 31 March to 3 April 2012. http://onlinelibrary.wiley.com/doi/10.1111/j.1469-0691.2012.03801.x/pdf [Google Scholar]
- 6. Girndt M, Pietsch M, Kohler H. 1995. Tetanus immunization and its association to hepatitis B vaccination in patients with chronic renal failure. Am. J. Kidney Dis. 26:454–460 [DOI] [PubMed] [Google Scholar]
- 7. Harro C, Betts R, Orenstein W. 2010. Safety and immunogenicity of a novel Staphylococcus aureus vaccine: results from the first study of the vaccine dose range in humans. Clin. Vaccine Immunol. 17:1868–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Harro C, et al. 2012. The immunogenicity and safety of different formulations of a novel Staphylococcus aureus vaccine (V710): results of two phase I studies. Vaccine 30:1729–1736 [DOI] [PubMed] [Google Scholar]
- 9. Kelly-Quintos C, Cavacini LA, Posner MR, Goldmann D, Pier GB. 2006. Characterization of the opsonic and protective activity against Staphylococcus aureus of fully human monoclonal antibodies specific for the bacterial surface polysaccharide poly-N-acetylglucosamine. Infect. Immun. 74:2742–2750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kreft B, Klouche M, Kreft R, Kirchner H, Sack K. 1997. Low efficiency of active immunization against diphtheria in chronic hemodialysis patients. Kidney Int. 52:212–216 [DOI] [PubMed] [Google Scholar]
- 11. Kuklin NA, et al. 2006. A novel Staphylococcus aureus vaccine: iron surface determinant B induces rapid antibody responses in rhesus macaques and specific increased survival in a murine S. aureus sepsis model. Infect. Immun. 74:2215–2223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li Y, et al. 2009. Outcomes of Staphylococcus aureus infection in hemodialysis-dependent patients. Clin. J. Am. Soc. Nephrol. 4:428–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Litjens NH, Huisman M, van den Dorpel M, Betjes MG. 2008. Impaired immune responses and antigen-specific memory CD4+ T cells in hemodialysis patients. J. Am. Soc. Nephrol. 19:1483–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mazmanian SK, Ton-That H, Su K, Schneewind O. 2002. An iron-regulated sortase anchors a class of surface protein during Staphylococcus aureus pathogenesis. Proc. Natl. Acad. Sci. U. S. A. 99:2293–2298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nissenson AR, et al. 2005. Clinical and economic outcomes of Staphylococcus aureus septicemia in ESRD patients receiving hemodialysis. Am. J. Kidney Dis. 46:301–308 [DOI] [PubMed] [Google Scholar]
- 16. Pancari G, et al. 2012. Characterization of the mechanism of protection mediated by CS-D7, a monoclonal antibody to Staphylococcus aureus iron regulated surface determinant B (IsdB). Front. Cell. Infect. Microbiol. 2:36 doi:10.3389/fcimb.2012.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Proctor RA. 2012. Is there a future for a Staphylococcus aureus vaccine? Vaccine 30:2921–2927 [DOI] [PubMed] [Google Scholar]
- 18. Rautenberg P, Teifke I, Schlegelberger T, Ullmann U. 1988. Influenza subtype-specific IgA, IgM and IgG responses in patients on hemodialysis after influenza vaccination. Infection 16:323–328 [DOI] [PubMed] [Google Scholar]
- 19. Reed SD, et al. 2005. Costs and outcomes among hemodialysis-dependent patients with methicillin-resistant or methicillin-susceptible Staphylococcus aureus bacteremia. Infect. Control Hosp. Epidemiol. 26:175–183 [DOI] [PubMed] [Google Scholar]
- 20. Shinefield HS, et al. 2002. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N. Engl. J. Med. 346:491–496 [DOI] [PubMed] [Google Scholar]
- 21.United States Renal Data System 2006. United States Renal Data System 2006 annual data report: atlas of end-stage renal disease in the United States. U.S. Department of Health and Human Services, National Institutes of Health, Bethesda, MD [Google Scholar]