Abstract
The goal of the study was to determine baseline protective titers of antibodies to Streptococcus pneumoniae surface protein A (PspA) and capsular polysaccharide in individuals with and individuals without type 2 diabetes mellitus. A total of 561 individuals (131 individuals with diabetes and 491 without) were screened for antibodies to PspA using a standard enzyme-linked immunosorbent assay (ELISA). A subset of participants with antibodies to PspA were retested using a WHO ELISA to determine titers of antibodies to capsular polysaccharide (CPS) (serotypes 4, 6B, 9V, 14, 18C, 19A, 19F, and 23F). Functional activity of antibodies was measured by assessing their ability to enhance complement (C3) deposition on pneumococci and promote killing of opsonized pneumococci. Titers of antibodies to protein antigens (PspA) were significantly lower in individuals with diabetes than controls without diabetes (P = 0.01), and antibodies showed a significantly reduced complement deposition ability (P = 0.02). Both antibody titers and complement deposition were negatively associated with hyperglycemia. Conversely, titers of antibodies to capsular polysaccharides were either comparable between the two groups or were significantly higher in individuals with diabetes, as was observed for CPS 14 (P = 0.05). The plasma specimens from individuals with diabetes also demonstrated a higher opsonophagocytic index against CPS serotype 14. Although we demonstrate comparable protective titers of antibodies to CPS in individuals with and individuals without diabetes, those with diabetes had lower PspA titers and poor opsonic activity strongly associated with hyperglycemia. These results suggest a link between diabetes and impairment of antibody response.
INTRODUCTION
Streptococcus pneumoniae is an important human pathogen, causing each year an estimated 40,000 deaths in the United States alone and 1 million globally. Infections caused by S. pneumoniae can range from mild infections, such as otitis media and sinusitis, to severe and fatal infections, such as pneumonia and meningitis (19). S. pneumoniae mainly causes infections in children, the elderly, and immunocompromised individuals (6, 11). However, elderly individuals with comorbidities such as cardiovascular disease and diabetes are at greater risk of developing severe invasive infections, which are often fatal (23).
The prevalence and incidence of type 2 diabetes mellitus have increased at an alarming rate, and currently, more than 20 million people in the United States have been diagnosed with diabetes. This number is expected to increase to 39 million by 2050. Diabetes has been implicated as the single greatest risk factor for pneumococcal bacteremia in individuals under the age of 40 (odds ratio [OR], 4.2; 95% CI confidence interval [CI], 1.1 to 16.7) (21, 22) and among those with no other documented comorbidities (OR, 2.3; 95% CI, 1.3 to 3.9) (46). According to one study, the risk of community-acquired pneumococcal pneumonia was 1.5-fold higher in individuals with diabetes than nondiabetes controls (45). Infections in individuals with diabetes occurs with greater severity and are associated with an increased risk of complications (4, 38). The increased susceptibility to infections in individuals with diabetes has been reported to be due in part to defects in both adaptive and cell-mediated immunity. Several immune defects have been noted in individuals with diabetes, particularly poorly controlled diabetes. Poor glucose control impairs a range of neutrophil and macrophage functions, such as chemotaxis, adherence, phagocytosis, and intracellular killing of microorganisms (8, 37). Additionally, decreases in mitogen-stimulated lymphocyte proliferation and defects in T-cell, B-cell, and dendritic cell functions have also been described in individuals with diabetes (12).
Protection against pneumococcal carriage and invasive infections is complex and multifactorial and has been shown to involve both antibody-dependent and independent mechanisms. Antibody-mediated protection is mainly dependent on capsular type-specific and anti-S. pneumoniae surface protein A (anti-PspA) antibodies, which develop as a result of either asymptomatic carriage or infection, resulting in protection against future infections (17, 30). The mechanism of protection is characterized by antibody-mediated enhancement of complement deposition followed by clearance of pneumococci via opsonophagocytosis by neutrophils (41). A critical role of CD4+ T cells in antibody-independent immunity to carriage has recently been described, where colonization of the lungs and nasopharyngeal cavity resulted in activation and infiltration of CD4+ T cells, in particular, Th17 cells. Activation of Th17 resulted in synthesis and release of the effector cytokine interleukin-17, recruitment of neutrophils, and phagocytic killing of pneumococci (2, 29, 31).
Several studies have evaluated immune responses to natural exposure and vaccination in individuals with and individuals without diabetes (3, 27). Results of studies evaluating immune responses to vaccines and vaccine efficacy are highly variable. Measurement of antibody titers after pneumococcal vaccination indicated that antibody titers in individuals with and individuals without diabetes were comparable in magnitude (27); however, patients with diabetes showed a delayed response to immunization (44) and impairments in production of circulating B cells and specific IgM in response to vaccination. These impairments were attributed to abnormalities in T-cell function. Contrary to these immune impairments, the overall efficacy of vaccine was reported to be between 56 and 70% (5, 15, 44), which is similar to what has been reported (65 to 81%) for those without diabetes (20, 33, 34, 43). These discrepancies can be explained by the fact that studies determining vaccine efficacy were largely performed using a heterogeneous group of at-risk participants, including those with diabetes. As a result, these trials provide limited information on vaccine efficacy specific for individuals with diabetes. Furthermore, owing to the short-term efficacy of unconjugated polysaccharide (PS) vaccine, revaccination is recommended in both healthy controls and high-risk groups. Measurement of efficacy within the first 5 years of administration suggested that unconjugated polysaccharide vaccine was 75% effective in preventing invasive pneumococcal disease in immunocompetent individuals aged 65 years and above. The efficacy was reduced to 37% the first year, 18% every 5 years for 10 years, and 0% thereafter. However, revaccination in immunocompetent adults resulted in a significant increase in serotype-specific anticapsular IgG antibody and subsequent long-lasting protection against pneumonia and invasive pneumococcal disease (18). Given these findings, it is reasonable to hypothesize that antibodies generated in individuals with diabetes, even though they are similar in titer to those in nondiabetic individuals, are functionally impaired. We therefore designed this study to (i) determine the baseline titer of antibodies to pneumococcal surface protein A and capsular polysaccharide in individuals with and individuals without diabetes and (ii) determine if antibodies in these individuals are functionally comparable to those in individuals without diabetes.
MATERIALS AND METHODS
Subjects.
The study was approved by the University of Texas Health Science Center at Houston Institutional Review Board (IRB) and the Committee for Protection of Human Subjects (CPHS). This study was conducted using stored plasma from 561 recently recruited participants from the Cameron County Hispanic Cohort (CCHC). The CCHC is a community-based cohort in Cameron County, TX, comprised of 2,500 randomly selected Hispanic participants from the U.S.-Mexico border region (20). The rate of diabetes among CCHC participants is 17.9%, using the 2006 American Diabetes Association (ADA) diagnostic criteria for diabetes (1a). However, if we use the 2010 ADA revised diagnostic criteria, which adds a level of glycated hemoglobin (A1c) of over 6.5% to the original criteria, the prevalence rises to 30.7%, twice the reported national rates of diabetes among all Americans and nearly twice as high as that previously established rates among Mexican Americans (1, 14). Plasma was obtained from consenting participants on enrollment into the CCHC and were kept frozen as described previously (14). Variables of interest for this study that were collected during enrollment in the CCHC include age, body mass index (BMI), fasting blood glucose (FBG) levels, and A1c levels. Clinical chemistries were performed in a Clinical Laboratory Improvement Amendments-approved laboratory as described previously (3).
For the purposes of this study, individuals with diabetes were defined on the basis of the original 2006 American Diabetes Association criteria (1a). This includes participants with a diagnosis of diabetes who were also on medication for diabetes or those with fasting blood glucose levels of >126 mg/dl. Those with fasting blood glucose values of ≤126 mg/dl and no history of diabetes or receipt of diabetes medication were classified as not having diabetes. From our study samples, 132 individuals were identified to have diabetes and 429 were identified to not have diabetes. Among the 132 participants with diabetes, only 89 were on medication, whereas 43 were not on any hypoglycemic medication. To determine the overall health status of participants, we collected data on antibiotic usage and hospitalization at the time of enrollment and blood draw. Of the 561 participants, only 5 were on antibiotics and none reported hospitalization 3 months prior to the enrollment. We also compared the values of albumin between those with and those without diabetes and found no significant difference between the groups. Only one participant in the diabetes group (n = 132) reported being on dialysis, whereas the rest did not report any renal impairment.
Measurement of serum concentration of antibodies to PspA.
Antibodies to PspA were measured using standard enzyme-linked immunosorbent assay (ELISA) methods (39, 47). We used stored plasma samples instead of serum samples. Serum is the most desirable specimen for measuring antibody titer; however, both plasma and serum specimens have been used interchangeably for measurement of antibodies in pneumococcal infections, and reported titers were observed to be comparable in both specimens (2, 15a) (Elizen kits; ZenTech). Ninety-six-well ELISA plates were coated with 1 μg/ml of either a recombinant 30-kDa N-terminal fragment of family 1 PspA, 10 μg/ml of a smaller (13-kDa) internal fragment of PspA containing proline repeats (proline-rich region [PRR]) (9), or the recombinant Staphylococcus aureus Efb (28) protein in bicarbonate buffer (50 ml 0.06 M Na2HCO3, 40 ml 0.06 M Na2CO3, 10 ml deionized H2O, pH 9.6). The proline-rich region was used since it is highly conserved in all PspA isoforms and is known to be immunogenic (9). Plates were coated at 4°C overnight, followed by blocking for 1 h at room temperature using phosphate-buffered saline (PBS; pH 7.4; Gibco Invitrogen, MO) containing 1% bovine serum albumin (BSA; Sigma-Aldrich, CA). Plates coated with 1 μg/ml of BSA only were used as a negative control to account for nonspecific binding of serum proteins to BSA. Pooled human serum with a known titer of total IgG to PspA (1.85 mg/ml; a kind gift from David Briles, University of Alabama at Birmingham) was used as the standard for determining the concentration of total IgG to PspA in unknown serum samples. Pooled serum was used at a starting dilution of 1:1,000 in PBS–1% BSA (which corresponds to 1.8 μg/ml of IgG to PspA) and titrated 1:3, in duplicate. Controls were included in each plate to account for nonspecific binding. Plasma samples were diluted 1:30 in PBS–1% BSA. Plates were incubated at 37°C for 1.5 h and washed 3 times with PBS containing 0.05% Tween 20 (PBST), followed by addition of 100 μl of goat anti-human alkaline phosphatase (AP)-conjugated secondary antibody (Southern Biotech). Plates were incubated for an additional hour at room temperature, before they were washed 3 times with PBST and developed using 100 μl of p-nitrophenylphosphate (pNpp; Sigma-Aldrich, CA). The absorbance was read at 450 nm with a preread setting (SpectraMax M5 microplate reader [Molecular Devices] with SoftMax Pro software [version 4.8]). Analysis was repeated to ensure accuracy. A positive IgG outcome was set at an optical density (OD) reading of ≥0.25, corresponding to an IgG titer of ≥150 ng/ml.
Antibody-mediated complement deposition on pneumococcal surface.
To determine if antibodies measured by ELISA are functionally active, we measured their ability to deposit complement on the surface of pneumococci. Plasma specimens with an antibody titer of >150 ng/ml were selected from both diabetic and nondiabetic participants for use in a complement deposition assay. A capsule type 2 strain of S. pneumoniae (strain D39) was used for measurement of complement factor C3 deposition. An isogenic mutant of capsule type 2 strains (mutant Tre 121.13) was used as a positive control. This strain is deficient in surface expression of both PspA and PspC, the two proteins known to prevent complement activation and deposition on pneumococci (10, 40). This mutant is therefore susceptible to complement deposition by the classical and alternative pathways, and complement becomes deposited at significantly elevated levels even in the absence of antibodies. Both strains were cultured to an OD at 600 nm of 0.4 (corresponding to approximately 4 × 108 CFU/ml) in Todd-Hewitt–5% yeast extract (THY) broth at 37°C. An aliquot of culture corresponding to 1 × 106 CFU/ml was spun to pellet bacteria, followed by resuspension of the pellet into either 100 μl of Hanks balanced salt solution with 0.1% gelatin (HBSSG; negative controls) or 10% heat-inactivated human plasma diluted in HBSSG. Samples were incubated for 30 min at 37°C, then washed with HBSSG, and centrifuged; and the supernatants were discarded. Pellets were resuspended in 100 μl of HBSSG containing 25 μg/ml of baby rabbit complement (Pel-Freez Biologicals, Rogers, AK). Specimens were incubated for 30 min at 37°C, followed by washing and resuspension into 100 μl of fluorescein-conjugated goat IgG fraction to rabbit complement C3 (MP Biomedical) diluted to a final concentration of 1:100 in PBS–1% BSA. Samples were incubated for 30 min at 37°C, washed once to remove unbound antibody, and fixed by resuspension in a 1:1 mixture of 2% para-formaldehyde and PBS–1% BSA. Samples were analyzed using a BD FACS CANTO II flow cytometer and FACSDiva software (version 6.1.1).
Measurement of serum concentration of anticapsular antibodies using WHO ELISA.
Measurement of the anticapsular IgG was performed on a total of 64 plasma specimens that previously tested positive for anti-PspA. Titers were measured using a 3rd-generation sandwich ELISA as described previously (48). Briefly, plates were coated with purified capsular polysaccharides from the most common serotypes (serotypes 4, 6B, 9V, 14, 18C, 23F, and 9V) and less common serotypes (serotypes 19F and 19A). Plates were coated for 4 to 5 h at 37°C and then transferred to 4°C. Plasma specimens to be used in the study were absorbed with 5 μg/ml of cell wall PS (Statens Serum Institute, Copenhagen, Denmark) and 10 μg/ml of 22-F PS in a final volume of 1 ml of PBST for 30 min at room temperature. Serum pool 89-SF was absorbed only with cell wall PS and used as the standard. The preabsorbed plasma specimen and the 89-SF serum pool were serially diluted and added to the wells of a PS-coated plate. Plates were incubated for 2 h at room temperature and washed, followed by addition of AP-conjugated goat anti-human IgG. Incubation was continued at room temperature for 2 h, followed by another wash and addition of substrate. The reaction was stopped using 3 N NaOH, and the optical density was measured at 405 nm and 690 nm using a microplate ELISA reader. The amount of antibody was calculated from the standard curve made from sample 89-SF.
Multiplexed OPA.
A total of 52 serum specimen were selected on the basis of their anticapsular IgG titers and were tested against pneumococci of eight serotypes (9V, 23F, 4, 18C, 6B, 19F, 14, and 19A) using a multiplexed opsonophagocytic killing assay (OPA) as described previously (7). For selection purposes, each target bacterium is made resistant to one of the four antibiotics (optochin, streptomycin, spectinomycin, and trimethoprim) and was left sensitive to the other three. Target strains were thawed, washed in opsonization buffer B (Hanks balanced salt solution with Mg2+ and Ca2+ containing 0.1% gelatin and 5% fetal bovine serum), and reconstituted to a final concentration of 2 × 105 CFU/ml. Two pools were made by mixing equal volumes of four serotypes in each pool. To inactivate human complement proteins, plasma specimens were heat inactivated at 56°C for 30 min, followed by serial dilution of each sample. Twenty microliters of diluted serum specimens was added to the wells of 96-well round-bottom plates (Corning Inc., Corning, NY) and mixed with 10 μl of the bacterial suspension, and the mixture was incubated at room temperature on a shaker (mini-orbital shaker; Bellco Biotechnology, Vineland, NJ) at 700 rpm for 30 min. Following incubation, 10 μl of baby rabbit complement (Pel-Freez Biologicals, Rogers, AK) and 40 μl of HL-60 cells (approximately 1 × 107 cells/ml) which were differentiated into granulocytes were added and the incubation was continued in a tissue culture incubator at 37°C (5% CO2) for 45 min with constant shaking at 700 rpm. On completion of the incubation, plates were cooled on ice for 15 min and a 10-μl aliquot was spotted onto four different THY agar plates (Todd-Hewitt broth with 0.5% yeast extract and 1.5% agar). An equal volume of overlay agar (Todd-Hewitt broth with 0.5% yeast extract and 0.75% agar) containing one of the four antibiotics was added to each agar plate. After an overnight incubation at 37°C, the number of bacterial colonies in the agar plates was enumerated. Opsonization titers were defined as the plasma dilution that killed 50% of bacteria.
Statistical analysis.
Univariate analyses of baseline variables found the distributions to be nonnormal and skewed. As a result, nonparametric alternatives were used for data analysis. Differences in median values of baseline characteristics between individuals with and individuals without diabetes and between those with positive IgG titers and those with negative IgG titers were compared using nonparametric Wilcoxon two-sample tests or chi-square tests. Stratified analyses and multivariable logistic regression were used to assess potential confounders or interactions. Unadjusted and adjusted odds ratios comparing outcomes of positive or negative IgG titers were calculated using multivariable logistic regression. Correlations were calculated using nonparametric alternatives. All analyses were considered significant when P values were <0.05. All analyses were run using SAS software (version 9.2; SAS Institute Inc., Cary, NC).
RESULTS
Participant characteristics.
The analysis was performed on a total of 561 participants (132 with diabetes and 429 without diabetes). Participants were not specifically asked if they had previously received a pneumococcal vaccine. This is a population in which only 11.9% has private insurance capable of financing the expensive pneumococcal vaccine (14). It is unlikely that individuals in this study had received the S. pneumoniae vaccine. Less than half of all participants self-reported that they had diabetes, and only half of the self-reported (89/132) individuals were on medication of any kind, further reducing the probability of pneumococcal vaccination (even among the self-reported diabetes participants) (14). Furthermore, only 5 participants out of the total of 561 in this study were on antibiotics when the specimens were obtained. The majority of the participants in this study were overweight or obese (80.9%), and nearly a third of the participants met the Adult Treatment Panel (ATP) III definition of metabolic syndrome (16). A total of 13.6% in this subset had diabetes (14).
Median values (interquartile [IQ] ranges) were compared for gender, age, BMI, FBG level, and glycated hemoglobin (A1c) level. Results of comparisons are presented in Table 1. There were no significant differences between genders; however, participants with diabetes were significantly older than those without diabetes (median age, 59 versus 45 years [P < 0.001]). Significant differences were also observed in BMI (32.2 versus 29.9 [P < 0.001]), FBG concentration (144 versus 96 mmol/liter [P < 0.001]), A1c level (8% versus 5.97% [P < 0.001]), C-reactive protein (CRP) concentration (P = 0.01), high-density lipoprotein (HDL) concentration, and triglyceride concentration (P = 0.001).
Table 1.
Characteristics of participants with and participants without diabetes
| Characteristic | Diabetes participants (n = 132) | Nondiabetes participants (n = 429) | P valuee |
|---|---|---|---|
| No. (%) male | 34.9 (46) | 34.0 (146) | 0.86 |
| Age (yr) | 59.5 (49.0–67.0) | 45.0 (32.0–58.0) | <0.001*** |
| BMI (kg/m2) | 32.2 (28.8–36.1) | 29.9 (26.0–33.1) | <0.001*** |
| FBG concn (mmol/liter) | 144.0 (115.0–202.0) | 96.0 (91.0–103.0) | <0.001*** |
| % A1c | 8.0 (6.7–10.3) | 5.9 (5.3–6.5) | <0.001*** |
| CRP concn (mg/liter)a | 4.6 (2.2–7.7) | 3.3 (1.9–6.0) | 0.01* |
| Albumin concn (g/dl)b | 3.7 (3.6–4.0) | 3.8 (3.7–4.0) | 0.06 |
| HDL concn (mg/dl) | 44.0 (36.5–50.5) | 48.0 (42.0–55.0) | <0.001*** |
| LDL concn (mg/dl)c | 98.0 (77.0–121.0) | 100.5 (80.4–121.0) | 0.56 |
| Triglyceride concn (mg/dl)d | 167.5 (111.0–251.0) | 123.5 (82.0–186.5) | <0.001*** |
n = 557.
n = 159.
LDL, low-density lipoprotein (n = 555).
n = 554.
*, P = 0.01; **, P = 0.001; ***, P < 0.001. P values represent the difference in the median values, presented as medians (IQ range) for continuous variables and median percentages (IQ range) for categorical variables.
Diabetes status is associated with lower titers of IgG to PspA independent of age.
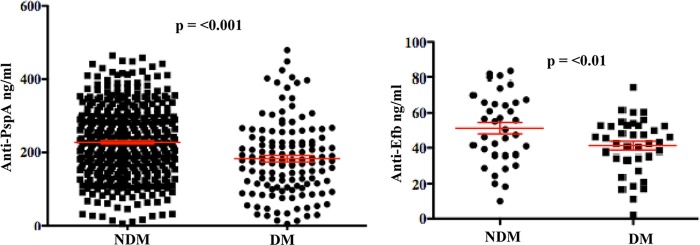
Carriage has been shown to be a prerequisite for invasive pneumococcal infections, and preexisting antibodies to PspA confer protection against carriage (39). We therefore measured anti-PspA titers in the plasma of participants with and individuals without diabetes. Levels of antibody to the full-length 30-kDa N-terminal region of family 1 PspA were measured using a standard ELISA method. A significantly higher titer of antibodies was observed in individuals with diabetes than in diabetic controls (P < 0.001) (Fig. 1). Three hundred twenty-five (57.9%) of 561 participants had a positive baseline IgG titer. A total of 43.2% of plasma specimens from those with diabetes and 63.5% from those without diabetes had a positive IgG titer (P < 0.0001) (Table 2). The mean concentration of IgG in plasma samples of those with diabetes was 183 ng/ml, whereas it was 227 ng/ml in those without diabetes (P < 0.01). Specimens positive for the 30-kDa PspA antigen were screened for reactivity against the smaller internal fragment of PspA. Specimens positive for the full-length 30-kDa fragment were also positive for the smaller internal fragment of PspA; however, no significant difference between titers of individuals with diabetes and those of individuals without diabetes was observed. These results suggested that the responses to the full-length PspA protein observed were the result of specific anti-PspA antibodies in the plasma of participants rather than cross-reacting molecules.
Fig 1.
Comparison of anti-PspA titers between individuals with and individuals without diabetes. Titers of PspA (A) and Efb (B) were measured in a standard ELISA as described in Materials and Methods. Significantly higher titers of antibodies to both PspA and Efb were observed in no-diabetes control participants than participants with diabetes. Each point represents a single plasma sample tested in duplicate. A P value of <0.05 was considered significant. NDM, participants without diabetes mellitus; DM, participants with diabetes mellitus.
Table 2.
Participants positive for IgG antibodies to PspA
| Antibodya | % (no.) of participants |
Chi-square test P value | |
|---|---|---|---|
| Diabetes (n = 132) | Nondiabetes (n = 429) | ||
| PspA | 43.2 (57) | 63.5 (268) | <0.0001b |
| PRRc | 47.37 (9) | 48.51 (65) | 0.93 |
Titers of antibodies to the full-length 30-kDa fragment of PspA were measured.
P = 0.01, representing differences in percent positive.
For PRR analysis, a subset of 134 samples was utilized to determine the specificity of IgG to the PRR of PspA.
To further explore the immune response to protein antigens, we also measured the concentration of antibody to the S. aureus virulence factor Efb. Our results indicated that the response to this antigen was also significantly lower in individuals with diabetes (mean IgG concentration, 41 ng/ml) than controls without diabetes (mean concentration, 51.23 ng/ml) (P = 0.01) (Fig. 1B).
Age and BMI are known to affect antibody response. We therefore determined the association of antibody titers with age and BMI (Table 3). When diabetes was stratified by age, a significant difference was observed between individuals with and individuals without diabetes, indicating that the observed differences in titers were the result of diabetes rather than age. Additionally, the differences in IgG titers remained significantly different between individuals with and individuals without diabetes even after controlling for BMI (P < 0.001 for BMI of >30, P = 0.01 for BMI of <30), suggesting that the differences observed were the result of diabetes status rather than an effect of obesity. When age was stratified by diabetes status, no differences in antibody responses were observed among those diabetic patients older than 40 years of age and those younger than 40 years of age. However, in those without diabetes, we observed a significant association between age and low antibody titers in individuals older than 40 years of age. These results suggested that the lower titers observed in participants with diabetes were not affected by older age. When BMI was stratified by diabetes status, there was no significant difference in IgG titer among diabetics with BMI values of greater than or less than 30 (P = 0.91 and 0.08, respectively).
Table 3.
Association of diabetes with anti-PspA titer with adjustment for age and BMIa
| Stratification group and characteristic | Median (IQ range) anti-PspA IgG concn (ng/ml) | Wilcoxon P valueb |
|---|---|---|
| Stratification of diabetes status by age and BMI | ||
| Participants aged ≥40 yr | ||
| Diabetes | 174.5 (105.0–227.6) | 0.02* |
| Nondiabetes | 197.6 (134.3–260.6) | |
| Participants ages <40 yr | ||
| Diabetes | 259.5 (172.5–263.3) | 0.12 |
| Nondiabetes | 276.0 (192.0–329.5) | |
| Participants with BMIs of ≥30 kg/m2 | ||
| Diabetes | 175.9 (112.5–228.8) | 0.003** |
| Nondiabetes | 218.3 (147.0–287.3) | |
| Participants with BMIs of <30 kg/m2 | ||
| Diabetes | 182.3 (102.4–255.8) | 0.002** |
| Nondiabetes | 227.5 (165.8–298.1) | |
| Stratification of age and BMI by diabetes status | ||
| Participants with diabetes | ||
| Age ≥ 40 yr | 174.5 (105.0–277.6) | 0.09 |
| Age < 40 yr | 259.5 (172.5–263.3) | |
| Participants without diabetes | ||
| Age ≥ 40 yr | 197.6 (134.3–260.6) | <0.001*** |
| Age < 40 yr | 276.0 (192.0–329.3) | |
| Participants with diabetes | ||
| BMI ≥ 30 kg/m2 | 175.9 (112.5–228.8) | 0.85 |
| BMI < 30 kg/m2 | 182.3 (102.4–255.8) | |
| Participants without diabetes | ||
| BMI ≥ 30 kg/m2 | 218.3 (147.0–287.3) | 0.10 |
| BMI < 30 kg/m2 | 227.3 (165.8–298.1) |
Comparisons were made between participants with and participants without diabetes after controlling for age and BMI.
*, P = 0.01; **, P = 0.001; ***, P < 0.001. P values represent differences in the medians.
The unadjusted odds ratio for diabetes status showed that participants without diabetes had 2.19 (95% CI, 1.47 to 3.25) times the odds of having a positive IgG response compared to participants with diabetes (Table 4). However, adjusting for age and BMI attenuated this association, rendering it statistically nonsignificant (OR, 1.40; 95% CI, 0.91 to 2.15). For each year of increase in age, the odds of having a positive IgG response decreased by 0.96 (95% CI, 0.95 to 0.97), and this association remained after adjusting for diabetes status and BMI (OR, 0.96; 95% CI, 0.95 to 0.97). For each kg/m2 of increase in BMI, the odds of having a positive IgG response decreased by 0.98 (95% CI, 0.96 to 1.010); however, this association was not statistically significant for either adjusted or unadjusted odds ratios.
Table 4.
ORs and adjusted ORs comparing participants with positive and negative IgG titers
| Variable | OR (95% CI) | Adjusted ORa (95% CI) |
|---|---|---|
| Diabetes status | 2.19 (1.47, 3.25)b | 1.40 (0.91, 2.15) |
| Age (yr) | 0.96 (0.95, 0.97)b | 0.96 (0.95, 0.97)b |
| BMI (kg/m2) | 0.98 (0.96, 1.01) | 0.99 (0.97, 1.02) |
For adjusted OR, all variables were included in the logistic model.
P = 0.01.
To determine if participants with diabetes on hypoglycemia medication have a better response to PspA than diabetics on no medication, we compared the median titers between those that were taking medication and those that were not taking any medication. Our results indicated that participants on medication for diabetes had significantly lower antibody titers than individuals not on medication (P < 0.01) (Table 5). However, there was a significant difference in the trend of the median anti-PspA IgG titers when comparing all three categories (individuals on one medication, individuals on more than one medication, and ones taking no medication) (P = 0.01). No significant difference in median anti-PspA IgG titers was observed when individuals taking 1 diabetes medication and individuals taking 2+ medications were compared (Table 5).
Table 5.
Association of diabetes medication with anti-PspA titer for all participants and participants with diabetes
| Comparison and group | Median (IQ range) anti-PspA IgG concn (ng/ml) | P value |
|---|---|---|
| Comparison of participants with and participants without diabetes medication | ||
| All participants (n = 561) | ||
| No diabetes medication | 223.5 (156.9–292.1) | <0.001a |
| Diabetes medication | 164.3 (94.5–219.8) | |
| Participants with diabetes (n = 132) | ||
| No diabetes medication | 207.0 (149.3–282.6) | 0.004b |
| Diabetes medication | 164.3 (94.5–219.8) | |
| Comparison of participants with diabetes on and not on diabetes medication | ||
| Participants with diabetes | ||
| No diabetes medication (n = 43) | 207.0 (149.3–282.6) | 0.01c |
| 1 diabetes medication (n = 69) | 164.3 (99.5–221.3) | |
| 2+ diabetes medications (n = 20) | 174.4 (70.8–218.3) | |
| Participants with diabetes on medication | ||
| 1 Diabetes medication (n = 43) | 164.3 (94.5–219.8) | 0.58 |
| 2+ Diabetes medications (n = 20) | 174.4 (70.8–218.3) |
P < 0.001, representing the difference in the medians.
P = 0.001, representing the difference in the medians.
P = 0.01, by analysis of variance, representing the difference in the medians.
Poorly controlled diabetes was negatively associated with antibody titers.
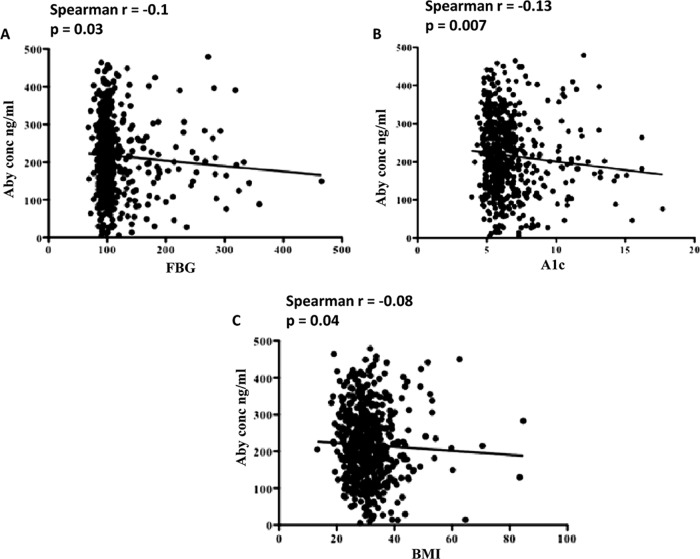
It has been shown that individuals with poorly controlled diabetes are at a higher risk of invasive pneumococcal infections than individuals with well-controlled diabetes (42, 45). To determine if this difference might be associated with any of the measures of diabetes control, we evaluated the association of FBG and A1c with titers of antibodies in our total sample. We observed a moderate negative association of both FBG (r = −0.13, P = 0.01; Fig. 1A and 2A) and HbA1c (r = −0.1, P = 0.1; Fig. 1B and 2B) with IgG titers. The optimum antibody response was observed at FBG values of 100 to 150 mg/dl and A1c values of 5 to 7 mg/dl. The association of BMI with IgG titers was moderately statistically significant (Fig. 2C).
Fig 2.
Association of antibody (Aby) titer with FBG concentration, A1c concentration, and BMI. The association between variables was calculated using the Spearmen rank test. FBG and A1c values were found to be negatively associated with antibody titers. A P value of <0.05 was considered significant.
Plasma specimens from participants with diabetes deposited less complement on pneumococci than samples from nondiabetic participants.
Pneumococcal surface proteins such as PspA and PspC and pneumococcal capsular polysaccharide prevent deposition and activation of complement on the surface of pneumococci. An important function of antibodies during pneumococcal infections is therefore the enhancement of complement deposition and subsequent phagocytosis of the bacterium. We therefore measured the ability of our plasma specimens to deposit complement. We incubated pneumococci in the presence and absence of heat-inactivated human plasma and measured the deposition of baby rabbit complement in a fluorescence-activated cell sorter (FACS) assay. A significant difference in antibody-mediated enhancement of complement deposition was observed between individuals with and individuals without diabetes (Fig. 3) (P = 0.04). Plasma specimens from nondiabetic individuals showed a measurable increase in fluorescence intensity (2-fold), indicating a strong opsonic activity of antibodies. However, only a marginal increase (1- to 1.5-fold) in complement deposition was observed when plasma specimens from diabetic participants were used to measure complement deposition.
Fig 3.
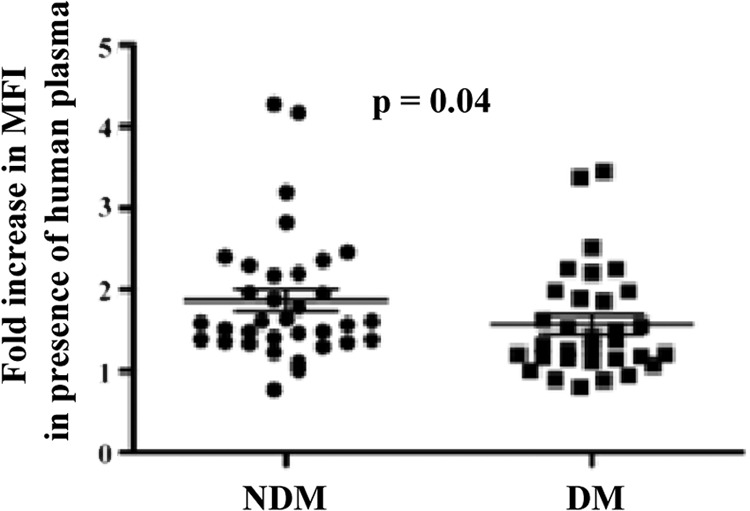
Complement deposition using plasma samples from diabetes and no-diabetes controls. Capsule type 2 strain D39 was incubated in the presence or absence of plasma from participants. Enhancement in deposition of baby rabbit complement was measured as the fold increase in mean fluorescent intensity (MFI) of bacteria in the presence of plasma from participants. Each point represents a single plasma sample run in duplicate. Fold increase was calculated by dividing the mean fluorescent intensity of pneumococci incubated with plasma by the mean fluorescent intensity of pneumococci incubated without plasma. The two groups were compared using a nonparametric t test for comparison of means. A P value of <0.05 was considered significant.
To determine the avidity of antibodies, we measured deposition of complement using 1% and 10% human plasma. A significant increase in deposition of complement was observed at both concentrations when plasma samples from the control group without diabetes were used, indicating a high avidity of the antibodies. Comparable complement deposition occurred at 10% plasma from diabetes participants, which was decreased by 5-fold when plasma was used at a final concentration of 1% (data not shown).
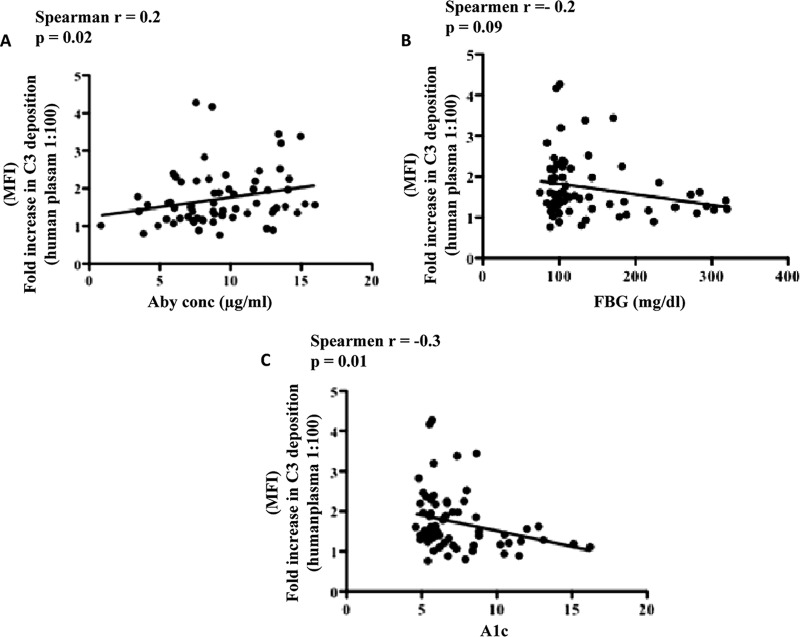
We investigated the association of diabetes-related variables, particularly antibody titer, FBG concentration, and A1c concentration, with complement deposition. A moderately positive association was observed between complement deposition and anti-PspA titer (Fig. 4A). A moderate negative association between fold increases in complement deposition and FBG concentration was observed (r = −0.2, P = 0.09); however, the association was not statistically significant (Fig. 4B). A statistically significant association between fold increases in complement deposition and A1c concentration was observed (r = −0.3, P = 0.02) (Fig. 4C). These results suggested that the opsonic ability of antibodies was altered in individuals with poorly controlled diabetes.
Fig 4.
Correlation of complement deposition with anti-PspA, FBG, and A1c concentrations. The fold increase in complement deposition was correlated with variables associated with diabetes. A strong positive association of antibody concentration with an increase in the mean fluorescent intensity was observed, whereas moderate negative associations between an increase in mean fluorescent intensity and the AIc concentration and an increase in the mean fluorescent intensity and the FBG concentration were observed. Each point represents a plasma sample run in duplicate. Correlations were calculated using a nonparametric Spearman correlation test. A P value of <0.05 was considered significant.
Higher titers of antibodies and respective titers of opsonic activity to capsular type 14 in participants with diabetes than those without diabetes.
Titers of antibodies to eight different capsular polysaccharides were measured in selected plasma samples as described in the Materials and Methods section (Fig. 5). Among all serotypes tested, titers to serotype 19F were highest among the eight serotypes tested. When geometric means for titers were compared between those with and those without diabetes, we observed a significantly higher titer against serotype 14 among participants with diabetes (P < 0.01) than controls without diabetes. Although not statistically significant, titers of antibodies to capsular types 19V and 18C were also higher in participants with diabetes. Additionally, titers against the most commonly occurring serotype (6B) were relatively higher in participants without diabetes than the diabetes group.
Fig 5.
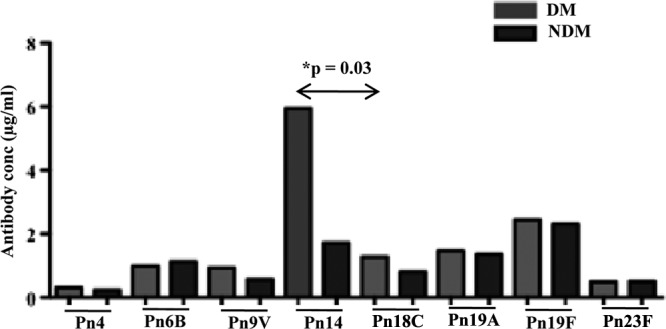
Titers of antibodies to capsular polysaccharide in individuals with diabetes and no-diabetes controls. Concentrations of antibodies to eight different capsular polysaccharides were measured using a WHO ELISA. Each bar represents the geometric mean concentration (in μg/ml). Comparison between individuals with diabetes and no-diabetes controls was performed using a nonparametric test. A P value of <0.05 was considered significant.
To further analyze the functionality of antibodies, we performed opsonophagocytosis assays using the neutrophil-like cell line HL-60 and baby rabbit complement. Heat-inactivated plasma specimens were incubated with target strains of pneumococci, followed by addition of baby rabbit complement, and then incubated with HL-60 cells. The higher titers of antibodies against capsule type 14 strains observed also corresponded with demonstration of higher opsonic activity (Table 6). That is, the opsonic titer (the highest titer of plasma at which 50% killing is observed) of plasma from diabetic participants was 2,016, whereas it was 649 for participants without diabetes (P = 0.05). We observed a strong negative association of titers with A1c and FBG levels.
Table 6.
Geometric means for antibody concentration, opsonization titers, and antibody potency by diabetes status
| Serotype | Diabetes status | IgG concn (μg/ml) |
Opsonic titer |
Antibody potencyd |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GMCa | 95% CI | P value | GMTb | 95% CI | P value | GMPc | 95% CI | P value | ||
| 4 | Diabetes | 0.36 | 0.19–0.66 | 0.42 | 95.6 | 18.6–493.0 | 0.60 | 195.4 | 38.9–982.0 | 0.14 |
| No diabetes | 0.25 | 0.18–0.35 | 240.3 | 67.3–858.5 | 971.3 | 315.0–2,995.0 | ||||
| 6B | Diabetes | 1.29 | 0.68–2.45 | 0.57 | 847.1 | 250.1–2,868.8 | 0.93 | 371.4 | 90.6–1,523.0 | 0.82 |
| No diabetes | 1.06 | 0.73–1.53 | 941.1 | 253.9–3,489.1 | 363.6 | 83.5–1,584.6 | ||||
| 9V | Diabetes | 0.88 | 0.49–1.58 | 0.16 | 764.5 | 149.0–3,923.6 | 0.28 | 419.6 | 82.1–2,144.2 | 0.54 |
| No diabetes | 0.60 | 0.45–0.80 | 570.6 | 225.2–1,445.8 | 955.4 | 469.0–1,946.1 | ||||
| 14 | Diabetes | 3.60 | 1.71–7.61 | 0.03e | 2,016.1 | 840.1–4,838.1 | 0.05e | 487.1 | 165.8–1,431.1 | 0.84 |
| No diabetes | 1.46 | 0.81–2.63 | 649.5 | 224.4–1,879.9 | 385.5 | 101.6–1,462.2 | ||||
| 18C | Diabetes | 1.13 | 0.51–2.53 | 0.57 | 168.6 | 40.4–704.3 | 0.32 | 116.4 | 35.4–382.6 | 0.61 |
| No diabetes | 0.95 | 0.63–1.45 | 43.6 | 6.8–280.6 | 52.1 | 11.6–234.8 | ||||
| 19A | Diabetes | 1.47 | 0.85–2.56 | 0.99 | 543.8 | 142.8–2,071.1 | 0.37 | 195.3 | 46.8–815.4 | 0.71 |
| No diabetes | 1.38 | 0.95–2.02 | 193.9 | 42.5–884.7 | 136.0 | 33.4–553.5 | ||||
| 19F | Diabetes | 2.26 | 1.36–3.78 | 0.40 | 68.9 | 13.6–349.7 | 0.88 | 27.9 | 5.3–145.7 | 0.32 |
| No diabetes | 2.63 | 1.91–3.62 | 55.1 | 16.3–186.3 | 13.4 | 5.8–31.1 | ||||
| 23F | Diabetes | 0.84 | 0.32–2.17 | 0.70 | 134.3 | 22.1–816.6 | 0.66 | 168.2 | 15.2–1,864.2 | 0.60 |
| No diabetes | 0.53 | 0.36–0.78 | 235.5 | 45.1–1,230.7 | 450.0 | 55.3–3,664.3 | ||||
GMC, geometric mean concentration.
GMT, geometric mean titer.
GMP, geometric mean potency.
Antibody potency was calculated as opsonic titer/IgG concentration.
P = 0.01, representing the difference in the geometric mean values.
DISCUSSION
It is well-known that protection against carriage and invasive pneumococcal infection depends on the generation of protective titers of antibodies against both pneumococcal capsular polysaccharide and protein antigens (29, 32, 39). Protective antibodies are defined as antibodies that can efficiently opsonize pneumococci and induce phagocytic killing by resident macrophages or neutrophils (41). To determine if the susceptibility of individuals with diabetes is the result of lower titers or a poor protective ability of generated antibodies to capsular polysaccharide and/or protein antigens, we measured both titers and protective antibody functions. Our observation that diabetes was associated with poor antibody responses to PspA was consistent with observations made by Nam et al. indicating that baseline positive rates of cross-reactive antibodies against pandemic influenza virus in patients with diabetes were lower than those in age- and sex-matched nondiabetes controls (35). Additionally, Fabrizi et al. reported a lower seroprotection rate elicited by the hepatitis B vaccine in diabetics (both influenza virus and hepatitis B virus are protein antigens) (13).
Several factors can contribute to the observed poor antibody responses to protein antigens in individuals with diabetes, such as (i) alterations in antigen presentation, (ii) impairment in antibody generation, and (iii) antibody modification as a result of hyperglycemia. There is strong evidence for the perturbation of functions of sentinel cells such as monocytes/macrophages, neutrophils, antigen-presenting B cells, dendritic cells, and natural killer cells. Any or all of these defects can compromise the antibody responses to protein antigens. Additionally, B-cell immunoglobulin production is also altered in diabetes. This impairment in antibody generation is attributed to diabetes-mediated hyperglycemia. It has been shown that even in the absence of other components of the metabolic syndrome, hyperglycemia can temporarily alter the B-cell response. Using a mouse model, it was demonstrated that stimulation of total spleen cells from Akita mice (a model for hyperglycemia due to insulin misfolding) resulted in delayed immunoglobulin μ production (36). Additionally, a variety of abnormalities in T-cell functions have been reported in individuals with diabetes (with or without poor metabolic control). These abnormalities have been shown to be associated with impairment in the ability to produce circulating B cells and specific IgM and IgG antibody in response to vaccines.
Consistent with these observations, we also observed a negative association of antibody titer with FBG and A1c, indicating a role for hyperglycemia in the B-cell-mediated antibody response. Moreover, the low opsonic titers observed in our studies could also be explained by hyperglycemia. Accumulation of glucose in individuals with poorly controlled diabetes results in nonenzymatic glycation and subsequent loss of antibody function. Mass spectrometric analysis of immunoglobulins isolated from the plasma of diabetics showed an increase in the molecular weight of immunoglobulins. The weight increase corresponded with the number of glucose molecules deposited on the protein. The nonenzymatic glycation of immunoglobulins as a result of hyperglycemia could result in low antigen and receptor binding capacities of these antibodies. It is therefore likely that despite comparable titers, the functionality of the antibody is compromised in diabetics compared to nondiabetics (25, 26).
Contrary to our findings with PspA, we observed comparable titers of antibodies to all eight capsular polysaccharides tested in individuals with and individuals without diabetes. These findings were consistent with previous studies where comparable pre- and postimmunization titers of antibodies to capsular polysaccharides were observed in individuals with and individuals without diabetes. Additionally, diabetes patients also developed a similar magnitude of responses to a pneumococcal vaccine as those without diabetes. Similarly, patients with cystic fibrosis have similar titers of anticapsular antibodies (24), suggesting that responses to capsular polysaccharide are least affected by disorders that affect the immune system in general. Although titers to all serotypes were comparable between individuals with and individuals without diabetes, we observed a significantly higher titer to capsule type 14 in individuals with diabetes. Serotype 14 is mainly associated with bacteremia in elderly individuals. Older age (59 versus 45 years; P = 0.01), and the higher susceptibility of diabetes individuals to invasive bacteremic pneumonia might explain the higher titer to serotype 14 in diabetics. In a study conducted by Schenkein et al., higher titers of capsular type 14 as a result of vaccinations were observed in older individuals than younger adults. Despite higher titers, a low opsonic activity was observed in these individuals (41), which is contrary to our observations demonstrating that higher antibody titers corresponded to higher opsonic activity. A simple explanation for these results is that even though the antibodies used in these assays were from individuals with or individuals without diabetes, the complement and granulocytes were not. Knowing that protection is the cumulative effect of the three components, namely, neutrophils, complement, and antibodies, working in a synergy, it is difficult to interpret this response as a protective response. It is very likely that even though the antibodies had a high opsonic activity, complement or the neutrophils from individuals with diabetes have impaired function, resulting in failure to protect against infections.
Results from our studies suggested that the response to protein antigens was compromised in individuals with diabetes. The T cells play a significant role in response to protein antigens by activating B cells and initiating antibody class switching. We have therefore focused our future studies on understanding the effect of diabetes on T-cell function and differentiation. Our working hypothesis is that diabetes creates an environment that modulates the differentiation and functions of T-cell subsets. Current studies are devoted to measuring the kinetics and strength of the T-cell response to heat-killed S. pneumoniae in whole blood of participants with and participants without diabetes. More specifically, we are measuring the differentiation of T cells into their subsets after antigenic stimuli. Future studies will involve vaccinating our participants and measuring the T-cell response to pneumococcal vaccines (polysaccharide and conjugate) in whole blood of individuals with and individuals without diabetes. These studies will provide us with an in-depth understanding of mechanisms that lead to vaccine failure in individuals with diabetes.
Limitations to this study are that it was performed in a predominantly Hispanic population, making it difficult to generalize our results to individuals of other ethnicities. Elucidating the mechanism resulting in low antibody titers with opsonic potential will also be difficult since these studies were carried out in vitro. A mouse model of diabetes or obesity will be important to the replication of these studies for understanding the mechanisms associated with failure to respond to protein antigens.
ACKNOWLEDGMENTS
We thank David Briles, Department of Microbiology, University of Alabama at Birmingham, for providing us with IgG standards and recombinant PspA protein. We thank our cohort recruitment team, particularly Rocio Uribe, Ariana Garza, Elizabeth Braunstein, and Julie Ramirez. We also thank Marcela Montemayor and other laboratory staff for their contribution, Gloria Sanchez for our database management, and Christina Villarreal for administrative support. We thank Valley Baptist Medical Center, Brownsville, TX, for providing us space for our Center for Clinical and Translational Science Clinical Research Unit. We finally thank the community of Brownsville and the participants in that city who so willingly participated in this study.
The study was directly supported by funding from the K12 award from our CCTS award 1U54RR023417-01. This work was supported by MD000170 P20 funded from the National Center on Minority Health and Health Disparities (NCMHD) and the Centers for Clinical and Translational Science from the National Center for Research Resources (NCRR).
We have no disclosures.
Footnotes
Published ahead of print 3 July 2012.
REFERENCES
- 1. American Diabetes Association 2010. Standards of medical care in diabetes—2010. Diabetes Care 33(Suppl 1): S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1a. American Diabetes Association 2006. Standard of medical care in diabetes—2006. Diabetes Care 29(Suppl 1): S4–S42 [PubMed] [Google Scholar]
- 2. Basset A, et al. 2007. Antibody-independent, CD4+ T-cell-dependent protection against pneumococcal colonization elicited by intranasal immunization with purified pneumococcal proteins. Infect. Immun. 75:5460–5464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beam TR, Jr, Crigler ED, Goldman JK, Schiffman G. 1980. Antibody response to polyvalent pneumococcal polysaccharide vaccine in diabetics. JAMA 244:2621–2624 [PubMed] [Google Scholar]
- 4. Bertoni AG, Saydah S, Brancati FL. 2001. Diabetes and the risk of infection-related mortality in the U.S. Diabetes Care 24:1044–1049 [DOI] [PubMed] [Google Scholar]
- 5. Bolan G, et al. 1986. Pneumococcal vaccine efficacy in selected populations in the United States. Ann. Intern. Med. 104:1–6 [DOI] [PubMed] [Google Scholar]
- 6. Briles DE, Swiatlo E, Edwards K. 2000. Vaccine strategies for Streptococcus pneumoniae, p 419–433 In Stevens DL, Kaplan EL. (ed), Streptococcal infections. Oxford, New York, NY [Google Scholar]
- 7. Burton RL, Nahm MH. 2006. Development and validation of a fourfold multiplexed opsonization assay (MOPA4) for pneumococcal antibodies. Clin. Vaccine Immunol. 13:1004–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chavakis T, Bierhaus A, Nawroth PP. 2004. RAGE (receptor for advanced glycation end products): a central player in the inflammatory response. Microbes Infect. 6:1219–1225 [DOI] [PubMed] [Google Scholar]
- 9. Daniels CC, et al. 2010. The proline-rich region of pneumococcal surface proteins A and C contains surface-accessible epitopes common to all pneumococci and elicits antibody-mediated protection against sepsis. Infect. Immun. 78:2163–2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dave S, Brooks-Walter A, Pangburn MK, McDaniel LS. 2001. PspC, a pneumococcal surface protein, binds human factor H. Infect. Immun. 69:3435–3437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Douglas RM, Miles HB. 1984. Vaccination against Streptococcus pneumoniae in childhood: lack of demonstrable benefit in young Australian children. J. Infect. Dis. 149:861–869 [DOI] [PubMed] [Google Scholar]
- 12. Ekdahl K, Braconier JH, Svanborg C. 1997. Immunoglobulin deficiencies and impaired immune response to polysaccharide antigens in adult patients with recurrent community-acquired pneumonia. Scand. J. Infect. Dis. 29:401–407 [DOI] [PubMed] [Google Scholar]
- 13. Fabrizi F, V Dixit, P Martin, P Messa. 2011. Meta-analysis: the impact of diabetes mellitus on the immunological response to hepatitis B virus vaccine in dialysis patients. Aliment. Pharmacol. Ther. 33:815–821 [DOI] [PubMed] [Google Scholar]
- 14. Fisher-Hoch SP, et al. 2010. Socioeconomic status and prevalence of obesity and diabetes in a Mexican American community, Cameron County, Texas, 2004-2007. Prev. Chronic Dis. 7:A53. [PMC free article] [PubMed] [Google Scholar]
- 15. Forrester HL, Jahnigen DW, LaForce FM. 1987. Inefficacy of pneumococcal vaccine in a high-risk population. Am. J. Med. 83:425–430 [DOI] [PubMed] [Google Scholar]
- 15a.(a). Freijd A, Hammarström L, Persson MA, Smith CI. 1984. Plasma anti-pneumococcal antibody activity of the IgG class and subclasses in otitis prone children. Clin. Exp. Immunol. 56:233–238 [PMC free article] [PubMed] [Google Scholar]
- 16. Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr., Lenfant C. 2004. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association Conference on Scientific Issues Related to Definition. Circulation 109:433–438 [DOI] [PubMed] [Google Scholar]
- 17. Holmlund E, Quiambao B, Ollgren J, Nohynek H, Kayhty H. 2006. Development of natural antibodies to pneumococcal surface protein A, pneumococcal surface adhesin A and pneumolysin in Filipino pregnant women and their infants in relation to pneumococcal carriage. Vaccine 24:57–65 [DOI] [PubMed] [Google Scholar]
- 18. Jackson LA, Janoff EN. 2008. Pneumococcal vaccination of elderly adults: new paradigms for protection. Clin. Infect. Dis. 47:1328–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kadioglu A, Weiser JN, Paton JC, Andrew PW. 2008. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat. Rev. Microbiol. 6:288–301 [DOI] [PubMed] [Google Scholar]
- 20. Koivula I, Sten M, Leinonen M, Makela PH. 1997. Clinical efficacy of pneumococcal vaccine in the elderly: a randomized, single-blind population-based trial. Am. J. Med. 103:281–290 [DOI] [PubMed] [Google Scholar]
- 21. Kornum JB, et al. 2008. Diabetes, glycemic control, and risk of hospitalization with pneumonia: a population-based case-control study. Diabetes Care 31:1541–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kornum JB, et al. 2007. Type 2 diabetes and pneumonia outcomes: a population-based cohort study. Diabetes Care 30:2251–2257 [DOI] [PubMed] [Google Scholar]
- 23. Koziel H, Koziel MJ. 1995. Pulmonary complications of diabetes mellitus. Pneumonia. Infect. Dis. Clin. North Am. 9:65–96 [PubMed] [Google Scholar]
- 24. Lahiri T, Waltz DA. 2001. Preimmunization anti-pneumococcal antibody levels are protective in a majority of patients with cystic fibrosis. Pediatrics 108:E62 doi:10.1542/peds.108.4.e62 [DOI] [PubMed] [Google Scholar]
- 25. Lapolla A, et al. 2000. Matrix-assisted laser desorption/ionization mass spectrometry, enzymatic digestion, and molecular modeling in the study of nonenzymatic glycation of IgG. J. Am. Soc. Mass Spectrom. 11:153–159 [DOI] [PubMed] [Google Scholar]
- 26. Lapolla A, et al. 1999. Evaluation of glycated globins by matrix-assisted laser desorption/ionization mass spectrometry. Clin. Chem. 45:288–290 [PubMed] [Google Scholar]
- 27. Lederman MM, Rodman HM, Schacter BZ, Jones PK, Schiffman G. 1982. Antibody response to pneumococcal polysaccharides in insulin-dependent diabetes mellitus. Diabetes Care 5:36–39 [DOI] [PubMed] [Google Scholar]
- 28. Lee LY, et al. 2004. Inhibition of complement activation by a secreted Staphylococcus aureus protein. J. Infect. Dis. 190:571–579 [DOI] [PubMed] [Google Scholar]
- 29. Malley R. 2010. Antibody and cell-mediated immunity to Streptococcus pneumoniae: implications for vaccine development. J. Mol. Med. (Berl.) 88:135–142 [DOI] [PubMed] [Google Scholar]
- 30. Malley R, et al. 2007. Serum antipneumococcal antibodies and pneumococcal colonization in adults with chronic obstructive pulmonary disease. J. Infect. Dis. 196:928–935 [DOI] [PubMed] [Google Scholar]
- 31. Malley R, et al. 2005. CD4+ T cells mediate antibody-independent acquired immunity to pneumococcal colonization. Proc. Natl. Acad. Sci. U. S. A. 102:4848–4853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McCool TL, Cate TR, Moy G, Weiser JN. 2002. The immune response to pneumococcal proteins during experimental human carriage. J. Exp. Med. 195:359–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Melegaro A, Edmunds WJ. 2004. The 23-valent pneumococcal polysaccharide vaccine. Part I. Efficacy of PPV in the elderly: a comparison of meta-analyses. Eur. J. Epidemiol. 19:353–363 [DOI] [PubMed] [Google Scholar]
- 34. Melegaro A, Edmunds WJ. 2004. The 23-valent pneumococcal polysaccharide vaccine. Part II. A cost-effectiveness analysis for invasive disease in the elderly in England and Wales. Eur. J. Epidemiol. 19:365–375 [DOI] [PubMed] [Google Scholar]
- 35. Nam JS, et al. 2011. The humoral immune response to the inactivated influenza A (H1N1) monovalent vaccine in patients with type 2 diabetes mellitus in Korea. Diabet. Med. 28:815–817 [DOI] [PubMed] [Google Scholar]
- 36. Nikolajczyk BS, Jagannathan-Bogdan M, Shin H, Gyurko R. 2011. State of the union between metabolism and the immune system in type 2 diabetes. Genes Immun. 12:239–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Omori K, et al. 2008. Priming of neutrophil oxidative burst in diabetes requires preassembly of the NADPH oxidase. J. Leukoc. Biol. 84:292–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Peleg AY, Weerarathna T, McCarthy JS, Davis TM. 2007. Common infections in diabetes: pathogenesis, management and relationship to glycaemic control. Diabetes Metab. Res. Rev. 23:3–13 [DOI] [PubMed] [Google Scholar]
- 39. Rapola S, et al. 2000. Natural development of antibodies to pneumococcal surface protein A, pneumococcal surface adhesin A, and pneumolysin in relation to pneumococcal carriage and acute otitis media. J. Infect. Dis. 182:1146–1152 [DOI] [PubMed] [Google Scholar]
- 40. Ren B, et al. 2004. The virulence function of Streptococcus pneumoniae surface protein A involves inhibition of complement activation and impairment of complement receptor-mediated protection. J. Immunol. 173:7506–7512 [DOI] [PubMed] [Google Scholar]
- 41. Schenkein JG, Park S, Nahm MH. 2008. Pneumococcal vaccination in older adults induces antibodies with low opsonic capacity and reduced antibody potency. Vaccine 26:5521–5526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shah BR, Hux JE. 2003. Quantifying the risk of infectious diseases for people with diabetes. Diabetes Care 26:510–513 [DOI] [PubMed] [Google Scholar]
- 43. Shapiro ED, et al. 1991. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N. Engl. J. Med. 325:1453–1460 [DOI] [PubMed] [Google Scholar]
- 44. Smith SA, Poland GA. 2000. Use of influenza and pneumococcal vaccines in people with diabetes. Diabetes Care 23:95–108 [DOI] [PubMed] [Google Scholar]
- 45. Thomsen RW, et al. 2004. Risk of community-acquired pneumococcal bacteremia in patients with diabetes: a population-based case-control study. Diabetes Care 27:1143–1147 [DOI] [PubMed] [Google Scholar]
- 46. Thomsen RW, et al. 2004. Diabetes and outcome of community-acquired pneumococcal bacteremia: a 10-year population-based cohort study. Diabetes Care 27:70–76 [DOI] [PubMed] [Google Scholar]
- 47. Virolainen A, Russell W, Rapola S, Briles DE, Käythy H. 1996. Human antibodies to pneumococcal surface protein A (PspA), abstr. 150. Abstr. 36th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC [Google Scholar]
- 48. Wernette CM, et al. 2003. Enzyme-linked immunosorbent assay for quantitation of human antibodies to pneumococcal polysaccharides. Clin. Diagn. Lab. Immunol. 10:514–519 [DOI] [PMC free article] [PubMed] [Google Scholar]