Abstract
This is the first efficacy study using the experimental goat model, a natural host of tuberculosis (TB), to evaluate the efficacy of heterologous Mycobacterium bovis bacillus Calmette-Guérin (BCG) prime followed by boosting with a replication-deficient adenovirus expressing the antigen Ag85A (AdAg85A). Three experimental groups of 11 goat kids each were used: BCG vaccinated, BCG vaccinated and AdAg85A boosted, and nonvaccinated. Twenty-two goat kids were vaccinated with ∼5 × 105 CFU of BCG (week 0), and 11 of them were boosted at week 8 with 109 PFU of AdAg85A. At week 14, all goats were challenged by the endobronchial route with ∼1.5 × 103 CFU of Mycobacterium caprae. The animals were euthanized at week 28. Cellular and humoral immunity induced by vaccination and M. caprae infection was measured throughout the study. After challenge BCG-AdAg85A-vaccinated animals exhibited reduced pathology compared to BCG-vaccinated animals in lungs and in pulmonary lymph nodes. There were significant reductions in bacterial load in both groups of vaccinated goats, but the reduction was more pronounced in prime-boosted animals. Antigen-specific gamma interferon (IFN-γ) and humoral responses were identified as prognostic biomarkers of vaccination outcome depending on their correlation with pathological and bacteriological results. As far as we know, this is the first report using multidetector computed tomography (MDCT) to measure vaccine efficacy against pulmonary TB in an animal model. The use in vaccine trials of animals that are natural hosts of TB may improve research into human TB vaccines.
INTRODUCTION
Tuberculosis (TB) in goats can be caused by Mycobacterium caprae or Mycobacterium bovis, which are both members of the Mycobacterium tuberculosis complex (MTBC) (2) and are endemic throughout the Iberian Peninsula (14, 26). Apart from being a reservoir of TB for animals, infected goats present a zoonotic risk for human infections (11, 18, 23, 25).
Eradication of TB infection from domestic animals seems to be unachievable in areas where wildlife is endemically infected with M. bovis (14, 26), and vaccination against M. bovis infection in domestic and wild animals has become a field of intensive research (10, 19, 35). M. tuberculosis bacillus Calmette-Guérin (BCG) is the basis of most new vaccine strategies assayed in animals, and there is a large body of knowledge regarding its use against M. tuberculosis in humans. In this regard, BCG shows protection against severe and disseminated forms of childhood TB, but protection against pulmonary TB in adults is limited (3). BCG has been shown to confer a degree of protection in cattle challenged with Mycobacterium bovis (4, 17, 32). Attempts to increase this protection by boosting with BCG in BCG-primed calves have failed (6). Therefore, in recent years, new vaccination regimens to improve upon BCG vaccination have been developed. Heterologous prime-boost strategies have been established as promising approaches combining BCG priming with virus-vectored subunit vaccine boosting. These strategies have been applied to humans (20, 38) and cattle (34, 35). In this regard, a molecular construct based on a recombinant replication-deficient human type 5 adenovirus expressing the MTBC protein Ag85A (AdAg85A) was developed and assessed as a booster vaccine in BCG-primed mice (36) and cattle (35). In both cases, this approach provided greater protection than BCG alone.
Neither BCG vaccination against TB nor heterologous prime-boost vaccination regimens have yet been assessed as a prophylactic treatment against TB in goats. In a study carried out in goats experimentally infected with M. caprae, we observed that, a short time after infection (14 weeks), the majority of goats had developed typical caseous necrotizing granulomas, often with liquefactive necrosis and cavitary lung lesions (22), features similar to those of active TB in humans (13). In this study, we assessed quantitative methods for measuring pathological changes after M. caprae infection.
Attempts to improve the semiquantitative scoring systems used until now, employing image technologies and quantitative reading of macroscopic lesions, have been carried out recently. Magnetic resonance imaging (MRI) has been used in macaques experimentally infected with M. tuberculosis for scoring pathological changes in a precise and quantitative way (29, 30), and volumetric computed tomography (CT) has been applied for scanning the thoraxes of humans in TB vaccine clinical trials (27). We have also shown that multidetector computed tomography (MDCT) represents a rigorous quantitative method to measure the magnitude of lung TB lesions in goats experimentally infected with M. caprae (22).
The aim of the present work was to compare relative levels of protection of goats vaccinated with BCG, alone or in combination with AdAg85A, against M. caprae. MDCT was used for assessing the magnitude of pulmonary pathological changes, and commonly used immunological tests were performed to determine immunological responses to vaccination and challenge.
MATERIALS AND METHODS
Experimental animals.
A total of 33 3-month-old Murciano-Granadina female goats obtained from an officially certified TB-free flock were used in the study. All of them were reconfirmed negative for TB by the single intradermal comparative cervical tuberculin (SICCT) test and a gamma interferon (IFN-γ) assay (Bovigam, Prionics, Schlieren, Switzerland). Also, the goats were seronegative for paratuberculosis (Paratub.Serum-S; Institut Pourquier, Montpellier, France).
All experimental procedures were approved by the Animal Welfare Committee of the Universitat Autònoma de Barcelona and the Generalitat de Catalunya and were in agreement with European Union laws for protection of experimental animals.
Vaccination and challenge procedures.
Goats were divided randomly in three experimental groups of 11 animals each. The first group (BCG group) was vaccinated with BCG at week 0; a second group (BCG-Ad group) was vaccinated at the same time with BCG and boosted at week 8 with a recombinant adenovirus expressing Ag85A (AdAg85A); a third group of unvaccinated goats was kept as a control group. The BCG Pasteur strain (ATCC accession no. 35734) was subcultured in Middlebrook 7H9 media (BD Diagnostics, Sparks, MD). For immunization, BCG growth was diluted to 5 × 105 CFU by suspension in 0.5 ml of phosphate-buffered saline (PBS) and injected subcutaneously into the right axilla. The AdAg85A inoculum was prepared at 109 PFU in 0.5 ml of PBS without adjuvant, as previously described (36), and injected intramuscularly into the left brachiocephalic muscle. Rectal temperatures were recorded before and at 6, 24, 48, and 72 h after boosting with AdAg85A.
At week 14, goats were anesthetized with 4 to 6 mg/kg of body weight of propofol (Propofol Lipuro) and 0.2 mg/kg of midazolam (Dormicum) administered intravenously and then challenged with an M. caprae field strain from Catalonia (SB0416; www.mbovis.org) by endobronchial inoculation of 1.5 × 103 CFU suspended in 0.5 ml of PBS; the dose of inoculum was confirmed by plating dilutions on Middlebrook 7H11 medium (BD Diagnostics, Sparks, MD) as described previously (22).
Antigens and peptides.
Tuberculins of M. bovis (PPD-B) and M. avium (PPD-A) were obtained from CZ Veterinaria (Porriño, Galicia, Spain). Immunodominant MTBC peptide cocktail ESAT-6/CFP-10 (E/C) and Rv3615c (ESAT-6 system 1 substrate protein C) were received from the Animal Health and Veterinary Laboratories Agency (Weybridge, United Kingdom) and were synthesized as described previously (31, 33). Recombinant MTBC-specific antigens ESAT-6, Ag85A, and MPB83 were obtained from Lionex (Braunschweig, Germany). Phytohemagglutinin (PHA) obtained from Sigma-Aldrich (Steinheim, Germany) was used as a positive control of blood stimulation assays.
Skin test.
The SICCT test was performed in all goats at week 26 (2 weeks before sacrifice) by inoculating 0.1 ml of both PPD-B and PPD-A into the left and the right sides of the neck, respectively. The preinoculation skin fold thickness was recorded before PPD injection, and the skin fold thickness was measured again after 72 h. The goats were considered positive if the increase of skin fold thickness after PPD-B application was greater than 2 mm and greater than the increase at the site of PPD-A application.
Whole-blood IFN-γ assay.
The progression of the infection was followed with the IFN-γ assay by collecting whole-blood samples from the jugular vein in heparinized blood tubes. Whole blood was stimulated in 96-well cell culture plates with the following final concentrations of stimuli: 10 μg/ml of PPD-B and PHA and 5 μg/ml of Ag85A, ESAT-6, E/C, and Rv3615c. PBS was added to cultures used as nonstimulated controls. Plasma supernatants were collected after 24 h of culture at 37°C and 5% CO2 and were stored at −20°C; samples were thawed just before performing the Bovigam IFN-γ enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions. ELISA results were obtained as optical density determined at 450 nm (OD450). The specific reaction was expressed as ΔOD450 (OD450 of antigen-stimulated sample minus OD450 of nonstimulated control). A sample was classified as a positive reactor to any antigen when specific ΔOD450 was higher than 0.05. The assay was performed every 2 weeks throughout the experiment using PPD-B, ESAT-6, and Ag85A as stimuli. Peptide cocktails were also included at weeks 14, 16, 20, 24, and 28.
Serology.
The presence of antibodies to mycobacterial antigens was analyzed in plasma samples using an IgG indirect ELISA as described previously (22). Flat-bottom 96-well plates (Nunc Multisorp; Thermo Fisher Scientific, Roskilde, Denmark) were coated with MPB83 (1 μg/ml) and Ag85A (2 μg/ml) separately. The antigens were diluted in carbonate/bicarbonate buffer and incubated overnight at 4°C. Plates were washed five times with PBS containing 0.05% Tween 20 (PBS-T20) and then incubated for 45 min at 37°C with PBS-T20 with 0.5% casein (blocking solution). After a washing, goat sera were added in duplicate (at 1/200 dilution in PBS-T20 with 1% casein) and incubated for 1 h at 37°C. Plates were washed again, and a mixture of protein A (50 ng/ml) and protein G (100 ng/ml), conjugated with peroxidase (Sigma-Aldrich, Steinheim, Germany) was added to each well. Plates were then read in a spectrophotometer, and ΔOD450 was calculated as sample OD450 minus background OD450 (unspecific absorbance in wells where antigen had not been added). A sample was classified as positive when the ΔOD450 was higher than the cutoff point, calculated as the mean of background OD450 plus 3 standard deviations (SD). Samples were analyzed at weeks 0, 14, and 28 for the MPB83 ELISA and at weeks 0, 4, and 8 and every 2 weeks thereafter for the Ag85A-ELISA.
Postmortem examination.
All goats were euthanized at week 28 (14 weeks postinfection) by intravenous sodium pentobarbital overdose and carefully examined in order evaluate the extension of tuberculous lesions in lungs and respiratory lymph nodes (LN). TB lesions in nonrespiratory organs were also recorded.
(i) Lungs.
Lung gross lesions were first evaluated by palpation and visual examination of each lobe. Then whole lungs were fixed with 10% buffered formalin by pouring the fixative into the trachea while sustaining it in a vertical position. After that, the trachea was tied, and whole lungs were immersed in a container with formalin. After complete fixation, lungs were scanned with a high-resolution 64-slice multidetector computed tomography (MDCT) scan (Brillance CT, 64 channels; Philips Medical Systems, Cleveland, OH), and sequential slices were analyzed on a workstation (Aquarius Station; TeraRecon, Foster City, CA) as described previously (22), allowing calculation of the total volume of granulomatous lesions and the whole lung volume.
(ii) Lymph nodes.
The diameters of gross lesions in pulmonary (caudal and cranial mediastinal, right and left tracheobronchial) and right and left retropharyngeal LN were also recorded by direct observation after slicing. The degree of pathology in LN was determined by adding together the approximated volumes of granulomas in respiratory LN of each animal. The volume was calculated as 4/3 × π × r3 (where r is lesion radius) considering the sphere-like morphology of the lesions found. The measurement of gross lesions was performed by the same pathologist in order to ensure the same criterion for all samples.
Bacterial count.
After pathological evaluation, the whole pulmonary and retropharyngeal LN were homogenized in 10 ml of sterile distilled water in a Masticator (IUL Instruments, Barcelona, Catalonia, Spain). The homogenate was decontaminated with a final concentration of 0.35% (wt/vol) hexadecylpyridinium chloride (HPC) (9) for 15 min in continuous shaking, after which it was centrifuged at 2,471 × g for 30 min. The supernatant was discarded, and the pellet was resuspended in 10 ml of PBS containing 0.05% Tween 80. The viable bacterial enumeration was determined by plating 0.1 ml of serial dilutions of LN homogenates on Middlebrook 7H11 agar plates. The inoculated plates were incubated at 37°C for 28 days. After that, the total count of CFU of each LN was calculated.
Data analysis.
Differences in antigen-specific IFN-γ responses, as well as Ag85A-specific IgG responses, among treatment groups were compared by using the nonparametric Kruskal-Wallis test with the post hoc Mann-Whitney test, whereas one-way analysis of variance (ANOVA) with the Student Newman-Keuls (SNK) multiple comparison test was applied to compare SICCT results and MPB83-specific IgG responses among treatment groups. Rectal temperature changes after AdAg85A vaccination were also compared by one-way ANOVA with the post hoc SNK test. All postmortem data (pathological parameters and log10-transformed bacterial counts) were compared by the Kruskal-Wallis test with the post hoc Mann-Whitney test. Correlations between immune responses and postmortem parameters were assessed by employing the nonparametric Spearman rank test. Data analysis was performed using SPSS for Windows statistical package, version 17.0 (IBM Inc., Chicago, IL).
RESULTS
Effect of different vaccination regimens on cell-mediated antigen-specific responses.
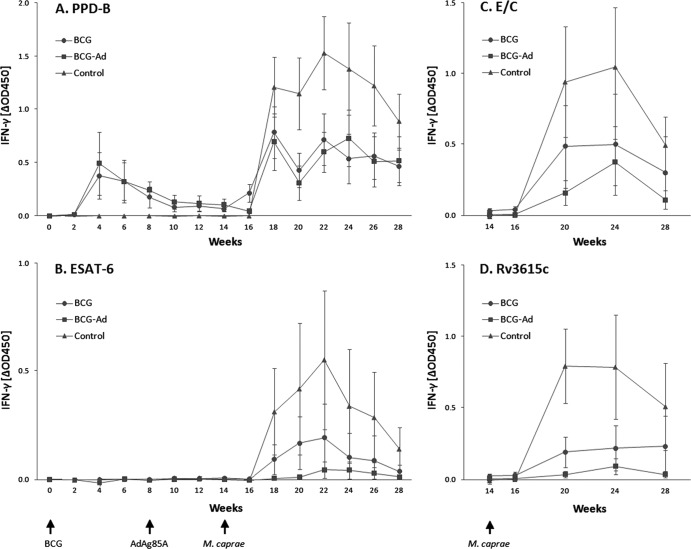
The release of IFN-γ after ex vivo stimulation of whole blood with PPD-B, Ag85A, and ESAT-6 was measured by ELISA every 2 weeks throughout the course of the experiment, whereas responses to ESAT-6/CFP-10 (E/C) or Rv3615c peptide cocktails were measured at weeks 14, 16, 20, 24, and 28 (Fig. 1; data for Ag85A are not shown). Ag85A-specific IFN-γ responses were not detectable in any of the groups and at any time point during the study. BCG vaccination induced an increase in mean IFN-γ responses to PPD-B in vaccinated groups, with a peak at week 4 followed by a progressive decrease until week 12, at which point responses were still significantly higher than unvaccinated control group responses (P < 0.001). After M. caprae challenge (at week 14), all goats showed a considerable increase in the levels of IFN-γ to PPD-B beginning at week 18. The two vaccinated groups responded with similar intensities, which were considerably lower than that for the unvaccinated control group at weeks 18 to 22 and 26 (Fig. 1A). Responses to ESAT-6 after challenge with M. caprae were slightly lower in AdAg85A-boosted goats (BCG-Ad group) than in goats vaccinated with BCG alone (BCG group), although this difference was statistically significant only at week 26 (P < 0.05). As occurred with PPD-B, responses to ESAT-6 in unvaccinated goats were also stronger than those in the vaccinated groups (Fig. 1B); they were significantly higher than those in the BCG-Ad group at weeks 18 to 28 and in the BCG group at weeks 18 to 26 (P < 0.05). The peptide cocktails E/C and Rv3615c induced an ESAT-6-like pattern of IFN-γ responses (Fig. 1C and D, respectively), with higher responses in the control group than in vaccinated groups (P < 0.05 at weeks 20 and 28 for E/C and at weeks 20, 24, and 28 for R3615c). The results of the IFN-γ assay at weeks 14, 16, 20, 24, and 28 (positive or negative) using PPD-B, ESAT-6, E/C, and Rv3615c as diagnostic reagents are shown in Table 1. The highest and the lowest numbers of positive animals were found when using PPD-B and ESAT-6, respectively. However, if results of E/C and Rv3615c are combined, the number of animals scoring positive in the IFN-γ assay was similar to results with PPD-B from weeks 20 to 28. Moreover, 11 out of 22 BCG-vaccinated goats were positive prior to M. caprae challenge (week 14) when PPD-B was used, whereas all of them were negative when ESAT-6 was used.
Fig 1.
Antigen-specific IFN-γ responses in goats after vaccination and M. caprae challenge. Whole blood was stimulated in vitro with PPD-B (A), ESAT-6 (B), E/C (C), and Rv3615c (D). Results are expressed as means of ΔOD450 ± 95% CIs.
Table 1.
Numbers of goats of each treatment group positive in the IFN-γ assay (Bovigam) using different antigens
| Group (n) | Antigen | No. of goats positive at wk: |
||||
|---|---|---|---|---|---|---|
| 14a | 16 | 20 | 24 | 28 | ||
| BCG (11) | PPD-B | 6 | 9 | 8 | 9 | 11 |
| ESAT-6 | 0 | 0 | 4 | 2 | 2 | |
| E/C | 0 | 0 | 7 | 8 | 5 | |
| Rv3615c | 0 | 0 | 5 | 4 | 5 | |
| E/C + Rv3615c | 0 | 0 | 9 | 9 | 9 | |
| BCG-Ad (11) | PPD-B | 5 | 3 | 9 | 11 | 11 |
| ESAT-6 | 0 | 0 | 1 | 2 | 1 | |
| E/C | 0 | 0 | 8 | 10 | 6 | |
| Rv3615c | 0 | 0 | 2 | 5 | 3 | |
| E/C + Rv3615c | 0 | 0 | 10 | 11 | 8 | |
| Control (11) | PPD-B | 0 | 0 | 11 | 11 | 11 |
| ESAT-6 | 0 | 0 | 7 | 4 | 7 | |
| E/C | 0 | 0 | 10 | 9 | 10 | |
| Rv3615c | 0 | 0 | 11 | 9 | 9 | |
| E/C + Rv3615c | 0 | 0 | 11 | 9 | 10 | |
Prior to challenge with M. caprae.
At week 26 all goats were positive by the SICCT test (individual data not shown). There were minor differences among groups in the mean increase in skin fold thickness 72 h after the application of PPD-B and PPD-A: 19.3 mm (17.6 to 21 mm, 95% confidence interval [CI]) and 9.7 mm (8 to 11.3 mm, 95% CI), respectively, for the BCG group, 17.3 mm (15.7 to 19 mm, 95% CI) and 8.7 mm (6.9 to 10.4 mm, 95% CI), respectively, for the BCG-Ad group, and 21.3 mm (19.4 to 23.2 mm, 95% CI) and 10.9 mm (9 to 12.7 mm, 95% CI), respectively, for the control group. The PPD-B-induced increase in skin fold thickness in the BCG-Ad group was significantly lower than that obtained in the control group (P < 0.05).
Humoral responses to MPB83 and Ag85A.
All goats were seronegative by the MPB83 indirect ELISA prior to vaccination (week 0), whereas two goats of the BCG group seroconverted at week 14 (just before challenge). At week 28 (2 weeks after the SICCT test), all goats were seropositive (data not shown). At this time point, the mean ΔOD450 found in vaccinated groups was significantly lower than that in the control group (P < 0.05; individual data not shown), with mean ΔOD450s of 1.614 (0.895 to 2.334, 95% CI) for the BCG group, 1.502 (0.932 to 2.071, 95% CI) for the BCG-Ad group, and 2.644 (2.105 to 3.183, 95% CI) for the control group. Thus, the overall levels of MPB83-specific antibody responses showed a temporal pattern similar to that seen with M. caprae antigen-specific T cell responses (Fig. 1).
The IgG responses to Ag85A were determined at weeks 0, 4, and 8 and then every 2 weeks (Fig. 2). Goats in the control group and BCG group were seronegative at all time points during the experiment. Goats in the BCG-Ad group were also negative prior to AdAg85A inoculation. Two weeks after that, the mean values of ΔOD450 reached a peak, followed by a progressive decrease until week 28, when the boost effect of the SICCT test (applied at week 26) dramatically raised again the mean value of ΔOD450. These values of ΔOD450 were significantly higher than values for the other groups (P < 0.001).
Fig 2.
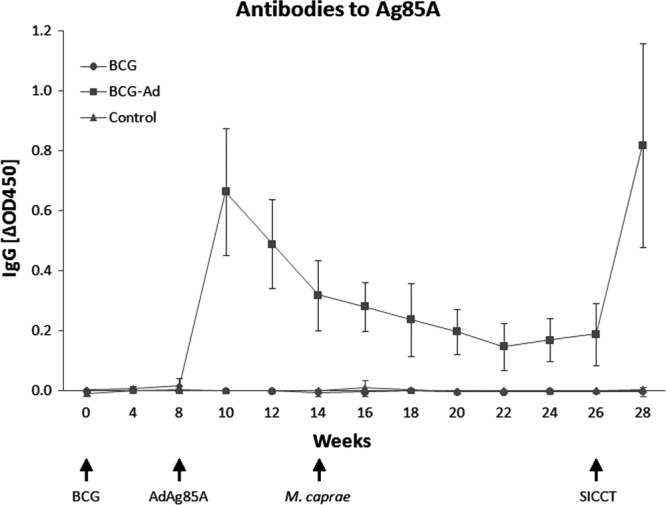
Temporal kinetics of goat IgG responses to Ag85A protein. Results are expressed as means of ΔOD450 ± 95% CIs.
Clinical, pathological, and bacteriological findings.
A mild but not statistically significant increase of mean rectal temperature was detected at 6 h after BCG inoculation (0.31°C [0.13 to 0.49°C, 95% CI]), followed by normalization at 24 h and subsequent time points (data not shown). After AdAg85A inoculation, a significant change in mean rectal temperature was observed at 24 h in comparison to the mean basal temperature (+1.47°C [1.11 to 1.84°C, 95% CI]; P < 0.001), which returned to normal at 48 h. Inoculation with M. caprae produced only minimal clinical signs. Occasional coughing was observed from week 20 (6 weeks after challenge) until the end of the experiment in 8 out of 11 goats of the BCG group, 7 out of 11 goats of BCG-Ad group, and 9 out of 11 unvaccinated goats. The main weight gains from challenge (week 14) to the end of the experiment (week 28) were 6.5 kg (5.4 to 7.7 kg, 95% CI) in the BCG group, 6.5 kg (6.0 to 7.0 kg, 95% CI) in the BCG-Ad group, and 6.4 kg (5.2 to 7.5 kg, 95% CI) in the control group. Therefore, there was no difference in weight gain during the challenge period among the three groups.
At necropsy, lungs and respiratory lymph nodes were processed separately. Pathological findings were restricted to the thoracic cavity in vaccinated animals, whereas the disease was more disseminated in 4 out of 11 control goats, which presented TB extrathoracic lesions in retropharyngeal LN, mesenteric LN, or spleen.
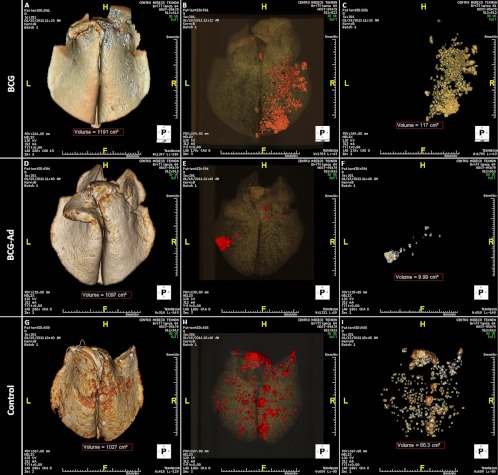
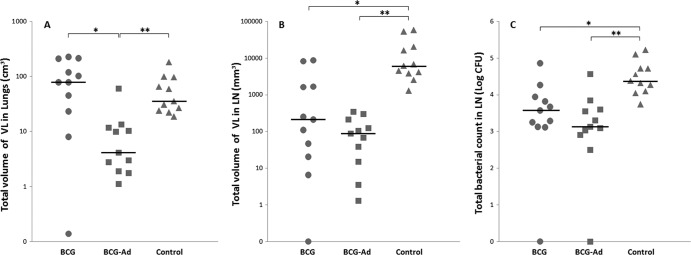
The mean volumes of TB lesions in formalin-fixed lungs were compared among groups by MDCT. All goats showed granulomatous necrotizing lesions in lung parenchyma and in lymph nodes, and although none of the lungs was completely free of TB lesions, AdAg85A-boosted goats showed much less gross lung lesions than goats belonging to the BCG group and unvaccinated group (group-representative MDCT lung images are shown in Fig. 3). As shown in Table 2, the volume of TB lung lesions in the BCG-Ad group was very much lower than in the BCG group (P < 0.005) and in the control group (P < 0.001). Also, the percentage of lung involvement (volume of TB lesions relative to the volume of the whole lung) was much lower in the BCG-Ad group than in the BCG group (P < 0.005) and control group (P < 0.001). Individual data and median values of the volumes of TB lesions are plotted in Fig. 4A. The number of affected lung lobes (of a total of 7 in goats) has been used frequently to assess the spread of the infection within the lung. The extents of scoring for lung lobe involvement were similar for both vaccinated groups and lower than that for the control unvaccinated group, where TB lesions were more widespread (Table 2). Gross lesions in pulmonary and retropharyngeal LN were also analyzed by measuring the volume of TB lesions by direct visual assessment. In the BCG-Ad group there was a strong reduction of mean volume of TB lesions in comparison to the unvaccinated control group (P < 0.001), and the volume of lesions was also smaller in the BCG group than in the control group (P < 0.005), whereas the difference between both vaccinated groups was not statistically significant (Fig. 4B).
Fig 3.
Multidetector computed tomography analysis of gross lung lesions. Representative lungs from goats in treatment groups BCG (A to C), BCG-AdAg85A prime-boost (D to F), and control (G to I) are shown. (A, D, and G) Three-dimensional reconstructions of the whole lung. (B, E, and H) Volume-rendering images of the whole lungs with the different tissue densities discriminated by color: water in gray, air in black, and TB lesions in red. Total volumes of the lungs are shown in red dashed boxes. (C, F, and I) Volume-rendering images of the affected lungs showing only lesions. Total volumes of the affected lungs are shown in red dashed boxes. Lung orientation is indicated as follows: H, head; F, foot, L, left, R, right.
Table 2.
Pathological and bacteriological findings at postmortem in vaccinated and control goatsa
| Group | Lungs |
LN |
|||
|---|---|---|---|---|---|
| Vol of VL (cm3) | Vol of VL/lung vol (%) | No. of lobes with VLb | Vol of VL (cm3) | Bacterial load (log CFU) | |
| BCG | 80 (44–157) | 8.1 (4–13.5) | 2/7 (1–3/7) | 0.2 (0–4.1)* | 3.7 (2.6–4.3)* |
| BCG-Ad | 4 (0–22)** | 0.5 (0.1–1.8)** | 1.9/7 (1.4–2.5/7) | 0.1 (0–0.2)** | 3.2 (2.3–3.9)** |
| Control | 36 (27–94) | 3.8 (2.4–8.1) | 3.4/7 (2.3–4.4/7) | 6.1 (2.3–30.1) | 4.5 (4.3–4.9) |
The volumes of visible lung lesions were calculated using multidetector computed tomography and those for lymph nodes (LN) by direct visual observation. Values are medians; interquartile ranges are in parentheses. Significant differences between groups are shown as follows: ∗, P < 0.005; ∗∗, P < 0.001 (Kruskal-Wallis test with the post hoc Mann-Whitney test).
Values are means.
Fig 4.
Postmortem results for individual goats for the three treatment groups. (A and B) Total volume of VL in lungs and LN. (C) Total bacterial counts in the pulmonary LN expressed as log10-transformed CFU. ●, BCG group; ■, BCG-AdAg85A prime-boosted group; ▲, unvaccinated control group. Horizontal lines indicate median values. Significance was determined by Kruskal-Wallis test with the post hoc Mann-Whitney test (∗, P < 0.005; ∗∗, P < 0.001).
In order to investigate the effect of the different vaccination protocols, the mycobacterial burden in respiratory LN was also assessed. Median values of total burden of mycobacteria (expressed as log CFU/pulmonary LN) of each group are shown in Table 2. The amounts of mycobacteria recovered in respiratory LN were significantly smaller in both BCG and BCG-Ad groups than in the control group (P < 0.005 and P < 0.001, respectively) (Fig. 4C).
Immunological responses as predictors of vaccine efficacy and disease status.
Immunological parameters were correlated with pathological and bacteriological parameters in order to assess their predictive value as biomarkers of vaccine efficacy or disease status of individual goats.
Antigen-specific IFN-γ responses were compared with pathological and bacteriological parameters (volume of visible lesions [VL] and log10 CFU in LN, respectively) at weeks 20, 24, and 28 (Table 3). Significant positive correlations between IFN-γ to all antigens (PPD-B, ESAT-6, E/C, and Rv3615c) and the two postmortem parameters were found only at week 20, whereas inconstant and less-significant correlations were found at subsequent time points.
Table 3.
Correlation of antigen-specific IFN-γ released in whole blood as measured by ELISA (ΔOD450) with postmortem parameters volume of VL and log10-transformed bacterial countsa
| Antigen | Wk 20 |
Wk 24 |
Wk 28 |
|||
|---|---|---|---|---|---|---|
| Vol of VL | Log CFU | Vol of VL | Log CFU | Vol of VL | Log CFU | |
| PPD-B | 0.461** | 0.533** | 0.211 | 0.310* | 0.148 | 0.133 |
| ESAT-6 | 0.388* | 0.363* | 0.181 | 0.212 | 0.380* | 0.361* |
| E/Cb | 0.451** | 0.554*** | 0.303* | 0.368* | 0.077 | 0.014 |
| Rv3615c | 0.446** | 0.561*** | 0.202 | 0.319* | 0.401* | 0.487** |
Tabulated are results of the Spearman rank test. Significance is shown as follows: ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001.
E/C, ESAT-6/CFP-10.
Humoral responses were also compared with postmortem parameters. There was a significant inverse correlation between Ag85A-induced IgG responses at weeks 10 to 28 and the total volume of VL found postmortem (P < 0.005, data not shown), whereas the observed negative correlations between the bacterial counts in LN and Ag85A-specific IgG responses were statistically significant only at weeks 10, 12, 16, 18, 20, 24, and 26 (P < 0.05; data not shown). However, at week 28 (just prior to sacrifice) we found a significant positive correlation between MPB83-induced IgG responses and both total volume of VL (Spearman ρ = 0.312; P < 0.05) and bacterial counts (Spearman ρ = 0.389; P < 0.05).
DISCUSSION
Research in vaccines against human TB also benefits from large-animal models based on natural TB hosts where histopathological features are similar to those in human disease (39). We have reported recently the development of a TB infection model in goats and found the induction of TB lesions in all the infected animals after inoculating M. caprae by the endobronchial route (22). In the present work we have used this model for testing a candidate human and cattle vaccine against TB, based on BCG priming and subsequent boost with an adenoviral vector expressing the immunogenic antigen Ag85A (AdAg85A), followed by challenge with M. caprae. To our knowledge, this is the first attempt to assess the safety and efficacy of a prophylactic vaccination strategy against TB in goats.
The rationale for this strategy is based on previous studies in cattle showing only partial efficacy of BCG or adenovirus-vectored vaccines alone but improved results when a combined prime-boost strategy was used. In cattle, homologous prime-boost vaccination strategies failed to confer protective immunity against M. bovis, either by using BCG-BCG or AdAg85A-AdAg85A (6, 34). In contrast, heterologous BCG-AdAg85A prime-boost procedures showed strong improvement in protection, compared to vaccination with BCG alone (28, 35, 36).
In our goat model, BCG vaccination followed by AdAg85A boosted protection against a challenge with M. caprae, leading to a reduction of lung and lymph node lesions and reduction of mycobacterial burden in respiratory lymph nodes in the vaccinated group in comparison to control nonvaccinated goats.
The strong reduction of pathological and bacteriological parameters observed in the BCG-Ad group is in agreement with the results obtained previously in mice (28) and, particularly, with those reported in a recent study in calves challenged with M. bovis (34, 35). In the latter study, with a design analogous to that of the present study, 4 out of 10 BCG-vaccinated and AdAg85A-boosted calves did not show visible pulmonary lesions at the necropsy. The main difference between the two studies is that we did not find any goat free of VL. This could be due to the use of a highly sensitive, quantitative method for evaluation of pulmonary lesions (MDCT), which allowed us to find TB lesions in all goats, in some cases even smaller than 1 mm3; such lesions would be difficult to observe by direct macroscopic evaluation. However, another possible explanation for this difference in pathological expression of TB between both experiments could be that different species of host and mycobacteria were used and that there was faster progression of TB infection in goats than in cattle, as has been postulated recently (22).
Unexpectedly, the mean volume of lung lesions of the BCG group, measured by MDCT, was slightly higher than that for the control group (without statistically significant differences). This result contrasts with that obtained in calf and badger vaccination studies, where a significant reduction of pulmonary pathology score was reported in BCG (Pasteur strain)-vaccinated and M. bovis-challenged animals (5, 10, 19, 37). Nevertheless, the different scoring system used in our study precludes us from making firm conclusions about a different response to BCG vaccination in goats and other species. In cattle experiments, the size and distribution of gross lesions were integrated in a semiquantitative pathology scoring, whereas we have quantified lung lesion volumes and have evaluated extension to the different lung lobes and extrapulmonary involvement separately.
Considering the intrapulmonary distribution of lesions, measured as number of affected lobes, we observed better containment of internal dissemination of infection within the lung in the two vaccinated groups than in the unvaccinated goats. Also, both gross lesions and bacterial burden in pulmonary drainage lymph nodes were significantly higher in the control group, corroborating the greater disease severity in untreated goats. In addition, extrathoracic TB lesions were found only in unvaccinated goats (4 out of 11), showing that vaccination mitigates hematogenous dissemination of mycobacteria. This finding is consistent with the widely reported capacity of BCG to prevent extrapulmonary TB in children (8, 24).
With regard to the relationship between cell-mediated immunity and vaccination outcome, we found strong positive correlations between postmortem parameters and IFN-γ produced in peripheral blood effector T cells in response to MTBC antigens at week 20 (6 weeks postinfection). However, very weak or no correlations were found at subsequent time points. It is well known that IFN-γ is a necessary Th1 cytokine for host defense against mycobacterial infection (15), although its production alone is insufficient for a protective response to TB (16). Thus, paradoxically, IFN-γ response is also a biomarker of protective immunity failure (reviewed in reference 1). In fact, a constant high secretion of IFN-γ by ESAT-6-specific T cells is associated with uncontrolled mycobacterial replication, and it is considered a predictor of active TB in humans (1) and cattle (32).
By contrast, we observed a decrease of both single ESAT-6- and E/C cocktail-specific IFN-γ responses at 14 weeks postinfection in all treatment groups. This IFN-γ kinetics is not in accordance with those observed in calf experiments with similar infective doses of M. bovis (32, 35). Notwithstanding, in our study the IFN-γ decrease does not seem to be related to the control of infection, but it might be an immune response failure or even an early anergic process. Therefore, the use of antigen-specific IFN-γ as a prognostic biomarker of control/severity of the infection must be employed with caution. It may be useful to determine vaccine success in the early stages of infection (<8 to 10 weeks), but it is inconclusive with regard to infection status of an animal at subsequent weeks.
Ag85A-specific IFN-γ production was not detected during the experiment. However, we found indirect correlations of Ag85A-specific humoral responses with postmortem parameters prior to challenge and after the SICCT booster effect. The kinetics of antibody responses to Ag85A confirmed the successful recognition of this antigen by the goat immune system and its usefulness as a biomarker of Ag85A expression.
We have confirmed the usefulness of DIVA candidate peptides as novel diagnostic reagents for use in goats. None of the vaccinated goats produced detectable IFN-γ to the DIVA antigens prior to challenge, whereas half of the vaccinated goats were positive in the bovine tuberculin-based IFN-γ assay, an assay that is being applied in some bovine TB eradication programs (12). The usefulness of whole-blood IFN-γ release assays (IGRAs) using ESAT-6 and CFP-10 to distinguish infected from vaccinated individuals has been reported previously for humans and cattle (7, 21). In goats infected with M. caprae, we found that the tuberculin-based IFN-γ assay and DIVA-based IFN-γ assays, where results for E/C and Rv3615c IFN-γ assays were taken together, had similar sensitivities. In this sense, the capacity of these antigen-specific assays to increase the detection of infected animals when they are used together was as previously reported for cattle (31) and goats (22).
Vaccination is the best strategy to control TB in humans and may be a valid approach in domestic and wild animal species when eradication is not feasible. Employing for the first time a goat model of TB using M. caprae infection, the goat being a natural host of this organism, we have demonstrated enhanced protection after boosting BCG-primed goats with an adenoviral vector expressing the antigen Ag85A. Furthermore, we have identified specific IgG response to be an immunomarker of protection and disease severity useful for monitoring vaccine efficacy. We believe that our current study serves as an important step for investigating immune mechanisms of protection in the goat model of TB.
ACKNOWLEDGMENTS
This study was founded by the European Union project TB-STEP (FP7-KBBE-2007-1-3-04, no. 212414). The production of AdAg85A vaccine and related murine work by Zhou Xing is supported by funds from the Canadian Institutes for Health Research.
We thank Maite Martín, Raúl Núñez, and the staff of Level 3 Biocontainment Unit of CReSA for their technical assistance. We express our appreciation to Félix García, Ana Andaluz, and Xavier Moll from the Department of Animal Medicine and Surgery of the Universitat Autònoma de Barcelona for their help with the experimental M. caprae inoculation. MTBC peptides were kindly supplied by Veterinary Laboratories Agency. The MDCT scan analysis was performed at Radiology Service of the Centro Médico Teknon.
B.P.D.V. and M.D. conceived and designed the experiments, analyzed the data, and drafted the manuscript. M.D., M.N., and S.L.-S. were responsible for the necropsy and pathological records; B.P.D.V. and F.X.A. performed the immunological and bacteriological assays; N.R. performed the MDCT and analyzed resulting data; B.V.-R., M.S., Z.X., and H.M.V. contributed substantively in scientific discussion of the study design and results.
We declare that we have no competing interests.
Footnotes
Published ahead of print 8 April 2011.
REFERENCES
- 1. Andersen P, Doherty TM, Pai M, Weldingh K. 2007. The prognosis of latent tuberculosis: can disease be predicted? Trends Mol. Med. 13:175–182 [DOI] [PubMed] [Google Scholar]
- 2. Aranaz A, Cousins D, Mateos A, Dominguez L. 2003. Elevation of Mycobacterium tuberculosis subsp. caprae Aranaz et al. 1999 to species rank as Mycobacterium caprae comb. nov., sp. nov. Int. J. Syst. Evol. Microbiol. 53:1785–1789 [DOI] [PubMed] [Google Scholar]
- 3. Brewer TF. 2000. Preventing tuberculosis with bacillus Calmette-Guérin vaccine: a meta-analysis of the literature. Clin. Infect. Dis. 31:S64–S67 [DOI] [PubMed] [Google Scholar]
- 4. Buddle BM, de Lisle GW, Pfeffer A, Aldwell FE. 1995. Immunological responses and protection against Mycobacterium bovis in calves vaccinated with a low dose of BCG. Vaccine 13:1123–1130 [DOI] [PubMed] [Google Scholar]
- 5. Buddle BM, Wedlock DN, Denis M, Skinner MA. 2005. Identification of immune response correlates for protection against bovine tuberculosis. Vet. Immunol. Immunopathol. 108:45–51 [DOI] [PubMed] [Google Scholar]
- 6. Buddle BM, et al. 2003. Revaccination of neonatal calves with Mycobacterium bovis BCG reduces the level of protection against bovine tuberculosis induced by a single vaccination. Infect. Immun. 71:6411–6419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cockle PJ, Gordon SV, Hewinson RG, Vordermeier HA. 2006. Field evaluation of a novel differential diagnostic reagent for detection of Mycobacterium bovis in cattle. Clin. Vaccine Immunol. 13:1119–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Colditz GA, et al. 1995. The efficacy of bacillus Calmette-Guerin vaccination of newborns and infants in the prevention of tuberculosis: meta-analyses of the published literature. Pediatrics 96:29–35 [PubMed] [Google Scholar]
- 9. Corner LAL, Trajstman AC. 1988. An evaluation of 1-hexadecylpyridinium chloride as a decontaminant in the primary isolation of Mycobacterium bovis from bovine lesions. Vet. Microbiol. 18:127–134 [DOI] [PubMed] [Google Scholar]
- 10. Corner LAL, Costello E, Lesellier S, O'Meara D, Gormley E. 2008. Vaccination of European badgers (Meles meles) with BCG by the subcutaneous and mucosal routes induces protective immunity against endobronchial challenge with Mycobacterium bovis. Tuberculosis (Edinb.) 88:601–609 [DOI] [PubMed] [Google Scholar]
- 11. Cvetnic Z, et al. 2007. Mycobacterium caprae in cattle and humans in Croatia. Int. J. Tuberc. Lung Dis. 11:658. [PubMed] [Google Scholar]
- 12. de la Rua-Domenech R, et al. 2006. Ante mortem diagnosis of tuberculosis in cattle: a review of the tuberculin tests, γ-interferon assay and other ancillary diagnostic techniques. Res. Vet. Sci. 81:190–210 [DOI] [PubMed] [Google Scholar]
- 13. Domingo M, et al. 2009. Effectiveness and safety of a treatment regimen based on isoniazid plus vaccination with Mycobacterium tuberculosis cells? Fragments: field-study with naturally Mycobacterium caprae-infected goats. Scand. J. Immunol. 69:500–507 [DOI] [PubMed] [Google Scholar]
- 14. Duarte EL, Domingos M, Amado A, Botelho A. 2008. Spoligotype diversity of Mycobacterium bovis and Mycobacterium caprae animal isolates. Vet. Microbiol. 130:415–421 [DOI] [PubMed] [Google Scholar]
- 15. Flynn J, et al. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249–2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Flynn JL. 2004. Immunology of tuberculosis and implications in vaccine development. Tuberculosis (Edinb.) 84:93–101 [DOI] [PubMed] [Google Scholar]
- 17. Hope JC, et al. 2005. Vaccination of neonatal calves with Mycobacterium bovis BCG induces protection against intranasal challenge with virulent M. bovis. Clin. Exp. Immunol. 139:48–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kubica T, Rusch-Gerdes S, Niemann S. 2003. Mycobacterium bovis subsp. caprae caused one-third of human M. bovis-associated tuberculosis cases reported in Germany between 1999 and 2001. J. Clin. Microbiol. 41:3070–3077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lesellier S, et al. 2009. Immunological responses and protective immunity in BCG vaccinated badgers following endobronchial infection with Mycobacterium bovis. Vaccine 27:402–409 [DOI] [PubMed] [Google Scholar]
- 20. McShane H, Hill A. 2005. Prime-boost immunisation strategies for tuberculosis. Microbes Infect. 7:962–967 [DOI] [PubMed] [Google Scholar]
- 21. Pai M, Riley LW, Colford John JM. 2004. Interferon-γ assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect. Dis. 4:761–776 [DOI] [PubMed] [Google Scholar]
- 22. Pérez de Val B, et al. 2011. Experimental model of tuberculosis in the domestic goat after endobronchial infection with Mycobacterium caprae. Clin. Vaccine Immunol. 18:1872–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Prodinger WM, et al. 2005. Characterization of Mycobacterium caprae isolates from Europe by mycobacterial interspersed repetitive unit genotyping. J. Clin. Microbiol. 43:4984–4992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rodrigues LC, Diwan VK, Wheeler JG. 1993. Protective effect of BCG against tuberculous meningitis and miliary tuberculosis: a meta-analysis. Int. J. Epidemiol. 22:1154–1158 [DOI] [PubMed] [Google Scholar]
- 25. Rodríguez E, et al. 2009. Human tuberculosis due to Mycobacterium bovis and M. caprae in Spain, 2004–2007. Int. J. Tuberc. Lung Dis. 13:1536–1541 [PubMed] [Google Scholar]
- 26. Rodriguez S, et al. 2011. Mycobacterium caprae infection in livestock and wildlife, Spain. Emerg. Infect. Dis. 17:532–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sander CR, et al. 2009. Safety and immunogenicity of a new tuberculosis vaccine, MVA85A, in Mycobacterium tuberculosis-infected individuals. Am. J. Respir. Crit. Care Med. 179:724–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Santosuosso M, McCormick S, Zhang X, Zganiacz A, Xing Z. 2006. Intranasal boosting with an adenovirus-vectored vaccine markedly enhances protection by parenteral Mycobacterium bovis BCG immunization against pulmonary tuberculosis. Infect. Immun. 74:4634–4643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sharpe SA, et al. 2009. Determination of lesion volume by MRI and stereology in a macaque model of tuberculosis. Tuberculosis (Edinb.) 89:405–416 [DOI] [PubMed] [Google Scholar]
- 30. Sharpe SA, et al. 2010. Establishment of an aerosol challenge model of tuberculosis in rhesus macaques and an evaluation of endpoints for vaccine testing. Clin. Vaccine Immunol. 17:1170–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sidders B, et al. 2008. Screening of highly expressed mycobacterial genes identifies Rv3615c as a useful differential diagnostic antigen for the Mycobacterium tuberculosis complex. Infect. Immun. 76:3932–3939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vordermeier HM, et al. 2002. Correlation of ESAT-6-specific gamma interferon production with pathology in cattle following Mycobacterium bovis BCG vaccination against experimental bovine tuberculosis. Infect. Immun. 70:3026–3032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vordermeier HM, et al. 2001. Use of synthetic peptides derived from the antigens ESAT-6 and CFP-10 for differential diagnosis of bovine tuberculosis in cattle. Clin. Diagn. Lab. Immunol. 8:571–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vordermeier HM, Huygen K, Singh M, Hewinson RG, Xing Z. 2006. Immune responses induced in cattle by vaccination with a recombinant adenovirus expressing mycobacterial antigen 85A and Mycobacterium bovis BCG. Infect. Immun. 74:1416–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vordermeier HM, et al. 2009. Viral booster vaccines improve Mycobacterium bovis BCG-induced protection against bovine tuberculosis. Infect. Immun. 77:3364–3373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang J, et al. 2004. Single mucosal, but not parenteral, immunization with recombinant adenoviral-based vaccine provides potent protection from pulmonary tuberculosis. J. Immunol. 173:6357–6365 [DOI] [PubMed] [Google Scholar]
- 37. Wedlock DN, Denis M, Vordermeier HM, Hewinson RG, Buddle BM. 2007. Vaccination of cattle with Danish and Pasteur strains of Mycobacterium bovis BCG induces different levels of IFN gamma postvaccination, but induces similar levels of protection against bovine tuberculosis. Vet. Immunol. Immunopathol. 118:50–58 [DOI] [PubMed] [Google Scholar]
- 38. Xing Z, Charters TJ. 2007. Heterologous boost vaccines for bacillus Calmette-Guerin prime immunization against tuberculosis. Expert Rev. Vaccines 6:539–546 [DOI] [PubMed] [Google Scholar]
- 39. Young D. 2009. Animal models of tuberculosis. Eur. J. Immunol. 39:2011–2014 [DOI] [PubMed] [Google Scholar]