Abstract
To reliably transport blood samples for cryoglobulin analysis, we have created a sample transport device containing a mixture of two waxes that solidifies at 38°C and maintains sample temperature at 38°C. Samples arriving at the laboratory at 37 to 38°C increased to 95% from 34% with the use of the device.
TEXT
Cryoglobulin is a common and simple clinical immunology laboratory test that requires the blood samples to be transported to the laboratory, without cooling, at 37°C (1, 5, 6). To achieve this, samples were generally delivered by hand to the laboratory in an insulated container containing warm water. However, the samples often arrive at the laboratory at a temperature of 36°C or below. Also, it was difficult to regulate the exact temperature of the “warm water,” resulting in occasional use of water at a temperature that was too low or even too high (4). These difficulties have prevented standardization of the cryoglobulin assay and limited use of the assay (5, 6).
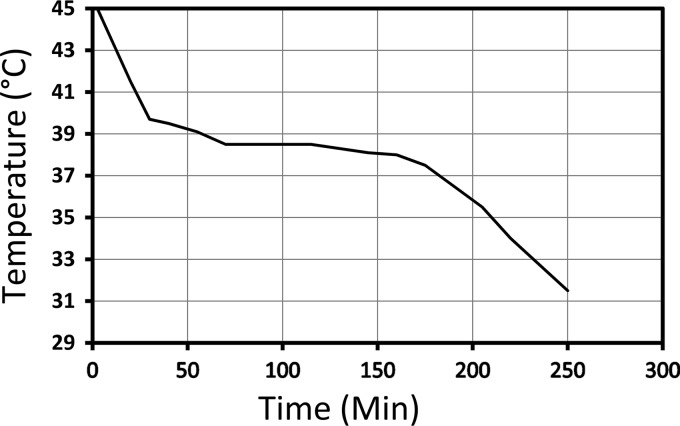
To transport blood samples at 37°C easily and reliably, we designed a carrier containing a material that solidifies at 37°C or 38°C. We created such a material with n-docosane and n-eicosane (from Sigma-Aldrich, St. Louis, MO), which are long-chain alkanes (paraffin waxes) with no specific toxic properties and which have high heat of fusion and melting at 43.5°C and 36°C (http://www.wolframalpha.com/; accessed April 2012), respectively. When they are mixed at approximately 1:1, the mixture melts at 38°C and can maintain 38°C for several hours as the material undergoes phase transition (Figure 1). The mixture was placed in a cylindrical (9-cm-diameter, 19-cm-tall) plastic container with space for two Vacutainer tubes. The container, which was named the “Cryocab,” was designed to be sent through the pneumatic tube system used in our hospital for transporting blood samples (Swisslog). The Cryocab contains about 300 g of the mixture. Since the heat of fusion values for docosane and eicosane are 37.6 and 59.1 cal/g, respectively (http://www.wolframalpha.com/; accessed April 2012), about 300 g of the mixture should be more effective than 10 liters of water in maintaining the temperature at 38°C. Indeed, the Cryocab was found to keep the sample temperature stable at 38°C for several hours (Fig. 1).
Fig 1.
Temperature of a sample (y axis) kept in a Cryocab over time (x axis).
For clinical uses, we store the Cryocab in an incubator set at 41°C. When a clinical department notifies the laboratory of a cryoglobulin order, the laboratory sends the Cryocab with two empty Vacutainer tubes through the pneumatic tube system. The phlebotomist then collects the blood in the (prewarmed) Vacutainer tubes, labels them, and sends them to the laboratory in the Cryocab through the pneumatic tube system. All users in the laboratory as well as in the patient care area have found the device to be easy to use.
Temperatures of the cryoglobulin blood samples arriving at our laboratory were measured for 15 months beginning in October 2010. The Cryocab was put into general use in August 2011 (Table 1). Prior to its use, about 34% of specimens arrived at the laboratory at 37°C or above. However, following implementation of the Cryocab technology, most of the samples have arrived at 37°C or above. Since September 2011, we have had three samples with an unacceptable temperature and were able to find explanations for two cases. In one case, the Cryocab was not used. In the second case, the Cryocab was used heavily that day and did not have enough time to reheat to 41°C. Since cryoglobulin-positive samples are uncommon, the improved delivery was not associated with an increased occurrence of cryoglobulin-positive samples (Table 1).
Table 1.
Cryocab data
| Mo and yr of sample collection | Total no. of samples | No. of samples at 36°C or below | Acceptable samples (%) | No. of cryoglobulin-positive samples |
|---|---|---|---|---|
| October 2010 | 19 | 14 | 26 | 2 |
| November 2010 | 18 | 13 | 28 | 0 |
| December 2010 | 16 | 13 | 19 | 2 (from one patient) |
| January 2011 | 27 | 20 | 26 | 0 |
| February 2011 | 16 | 10 | 38 | 0 |
| March 2011 | 26 | 16 | 38 | 0 |
| April 2011 | 23 | 16 | 30 | 0 |
| May 2011 | 24 | 11 | 54 | 1 |
| June 2011 | 39 | 23 | 41 | 0 |
| July 2011 | 18 | 10 | 44 | 0 |
| August 2011a | 29 | 3 | 90 | 0 |
| September 2011 | 23 | 1 | 96 | 0 |
| October 2011 | 17 | 1 | 94 | 0 |
| November 2011 | 30 | 0 | 100 | 0 |
| December 2011 | 20 | 1 | 95 | 0 |
The Cryocab was put into use in the middle of August 2011.
Thus, we have created a device that is easy and inexpensive to produce and effective in preserving the warm sample temperature required for reliable detection of cryoglobulins. Also, it allows us to use the pneumatic tube system and dramatically reduces the amount of labor needed for sample collection by eliminating the need to carry the sample by hand. It was previously impractical to send samples to an outside laboratory even within a city for cryoglobulin analysis. A Cryocab can be designed to maintain 37°C for a very long time and would allow one to send samples to an outside laboratory. In addition, the Cryocab is useful for transporting samples with cold agglutinins, which are present in many patients with mycoplasma pneumonia (7). Furthermore, improper deliv-ery of samples containing cryoglobulin or cold agglutinin can interfere with other assays (2, 3). We, therefore, anticipate that the Cryocab technology and method would be broadly useful in clinical laboratories.
ACKNOWLEDGMENTS
This work was supported by Public Health Service contract AI-30021 from the National Institute of Allergy and Infectious Diseases.
The University of Alabama at Birmingham has applied for a patent for the Cryocab. While we are employees of the University, we have donated all intellectual property rights associated with the Cryocab to the University of Alabama at Birmingham.
We are grateful to technical assistance provided by Lisa Carpenter, Donna Howard, Mary A. Crum, Robert Burton, and Jigui Yu.
Footnotes
Published ahead of print 18 July 2012
REFERENCES
- 1. Bakker AJ, et al. 2003. Adequate sampling in cryoglobulinaemia: recommended warmly. Clin. Chem. Lab. Med. 41:85–89 [DOI] [PubMed] [Google Scholar]
- 2. Cunningham VL, Brandt JT. 1992. Spurious thrombocytopenia due to EDTA-independent cold-reactive agglutinins. Am. J. Clin. Pathol. 97:359–362 [DOI] [PubMed] [Google Scholar]
- 3. Pham BN, et al. 2006. Quantitative measurement of hepatitis C virus core antigen is affected by the presence of cryoglobulins. Clin. Exp. Immunol. 146:211–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Presley AE, Mintz PD. 2007. Things heat up for a cold agglutinin screen sample. Transfusion 47:361 doi:10.1111/j.1537-2995.2007.01125.x [DOI] [PubMed] [Google Scholar]
- 5. Sargur R, White P, Egner W. 2010. Cryoglobulin evaluation: best practice? Ann. Clin. Biochem. 47:8–16 [DOI] [PubMed] [Google Scholar]
- 6. Vermeersch P, et al. 2008. A critical appraisal of current practice in the detection, analysis, and reporting of cryoglobulins. Clin. Chem. 54:39–43 [DOI] [PubMed] [Google Scholar]
- 7. Waites KB, Brown MB, Simecka JW. 2006. Mycoplasma: immunologic and molecular diagnostic methods, p 510–517 In Detrick B, Hamilton RG, Folds JD. (ed), Manual of molecular and clinical laboratory immunology. ASM Press, Washington, DC [Google Scholar]