Abstract
Human visceral leishmaniasis (VL) is routinely diagnosed by detecting IgG that specifically binds to Leishmania antigens. The enzyme-linked immunosorbent assay (ELISA) remains a widely used method. However, the biggest challenge remains the choice of antigen with the highest specificity and sensitivity. This study is aimed at assessing the diagnostic performances of crude Leishmania histone (CLH) protein-based ELISAs in Mediterranean VL patients. The CLH proteins were biochemically purified from promastigote nuclear extracts. Their reactivities were analyzed by Western blotting (WB) using rabbit polyclonal antibodies against Leishmania recombinant histones and sera from VL patients, respectively. Then, the diagnostic potential of CLH proteins was validated by the CLH-based ELISA using 42 infantile VL patients' sera and 70 control subjects. The CLH-based ELISA performance was compared to that of the soluble Leishmania antigen (SLA)- and the recombinant K39 (rK39)-based ELISAs. Analysis of the WB profile with the use of polyclonal antibodies confirmed the histone origin of low molecular mass proteins (12 to 16 kDa). All VL samples tested presented antibodies reacting against different antigen fractions; however, recognition patterns were different depending on the reactivity of each serum. CLH-based ELISA showed an excellent ability to discriminate between VL cases and healthy controls (97.6% sensitivity and 100% specificity). It had a diagnostic performance similar to that of rK39-based ELISA (97.6% sensitivity and 97.1% specificity, P = 0.5) and a better serodiagnosis accuracy than the SLA-based ELISA (85.7% sensitivity and 90% specificity, P < 0.05). Therefore, crude Leishmania histone extract could be a valuable antigen for clinical use.
INTRODUCTION
Zoonotic visceral leishmaniasis (VL), caused by Leishmania infantum (syn. Leishmania chagasi), is an important emerging parasitic disease in countries around the Mediterranean basin, in the Middle East, and in Latin America. The parasite is transmitted by the bite of female sandflies, and dogs are the principal reservoir hosts (1, 13). Human VL occurs as a sporadic life-threatening disease mainly in children (3) and immunocompromised patients (9). Clinical manifestations include fever, anemia, and spleen enlargement (27). Symptomatic infection is usually fatal if left untreated (27). Some of the infected individuals, however, develop a few mild symptoms which are often self-resolving. Asymptomatic forms usually outnumber VL cases and are evidenced by a positive Leishmania skin test (LST) and/or PCR, and to a lesser extent by seroconversion (31).
Parasitological diagnosis remains the gold standard in infantile VL owing to its high specificity. It is generally based on the detection of Leishmania parasites in bone marrow aspirates (37, 38). Recently quantitative real-time PCR technology (qPCR), using primers designed on kinetoplast DNA (kDNA), was successfully performed on blood samples with high sensitivity (2). Human VL is also diagnosed by detecting IgG that specifically binds to Leishmania antigens. Several serological tests are used, such as indirect fluorescent antibody assay, direct agglutination assay, Western blotting (WB), and enzyme-linked immunosorbent assay (ELISA). However, the main limitation of conventional crude antigen-based tests is the cross-reactivity with other endemic diseases (37, 38). Out of the myriad of potential recombinant proteins, a fragment of a kinesin protein known as K39 (4) has enabled the development of accurate VL serodiagnosis assays (23). On the other hand, purified parasite fractions, including different parasite antigens, were revealed as an alternative for the development of sensitive and specific VL diagnostic tests (38).
Several studies demonstrated that Leishmania histones are immunogenic. In spite of being among the most highly conserved proteins along the evolutionary scale, Leishmania histones have accumulated substantial sequence differences to trigger a specific immune response (11, 30). The starting point for most of the studies concerning the antigenicity of Leishmania histones was the screening of expression libraries with infected dogs' sera. The identification of the L. infantum histone H2A, after immunoscreening with a canine VL (CVL) serum, was the first report of a specific immune response against histones during parasitic infection (33). The subsequent characterization of histone H3, strongly recognized by CVL sera, showed that this parasite owns the most divergent histone H3 described to date (34). It was also demonstrated that the other two histones, H2B and H4, belonging to the nucleosomal core of L. infantum are recognized by CVL sera (36). In addition, antibodies against rH2A and rH2B were detected in the sera of a large group of VL patients (22, 24, 29). However, these previous reports did not evaluate the accuracy of serodiagnosis of these histone fractions in Mediterranean VL. Likewise, there are no data concerning the serodiagnosis value of H1, H3, and H4 histones in human VL and no study employing crude histone extract in VL immunodiagnosis. The purpose of this work was to assess and compare the diagnostic performance of the crude Leishmania histone (CLH)-based ELISA with those of soluble Leishmania antigen (SLA)- and recombinant K39 (rK39)-based ELISAs for Mediterranean VL patients.
MATERIALS AND METHODS
VL patients and controls.
A total of 112 sera were collected from 42 VL Tunisian patients and 70 matched controls hospitalized in a Kairouan hospital. They ranged in age from 4 months to 6 1/2 years. They did not present with immunosuppressive diseases or risk factors of human immunodeficiency infection. VL diagnosis was suspected upon clinical signs and confirmed by both the microscopic observation of Leishmania amastigotes in Giemsa-stained bone marrow smears and real-time PCR performed on blood samples (2). Matched controls were selected among patients hospitalized in the same period for other diseases. Matching was conducted according to age and geographical origin. Children with a previous history of leishmaniasis who were hospitalized for malnutrition or inflammatory diseases were excluded from the control population. A second control group was composed of 65 serum samples collected from healthy women in the setting of toxoplasmosis screening. The study was reviewed and approved by the Pasteur Institute of Tunis (PIT) ethics committee.
Antigens.
The recombinant protein of L. infantum, namely, rK39 (a 39 amino acid repetitive immunodominant B-cell epitope of kinesin-related antigen) (6) was kindly provided by the Infectious Disease Research Institute, Seattle, WA. The SLA extracted from L. infantum promastigotes after ultrasonic treatment was prepared as described elsewhere (25). The CLH proteins, however, were extracted by following a standard approach of histone protein isolation with some modifications (32). Briefly, mid-log- phase L. infantum promastigotes (2 × 107/ml) were pelleted, washed in cold PBS buffer and freeze-thawed three times before lysis in hypotonic buffer [10 mM Tris-HCl (pH 8), 1 mM KCl, 1.5 mM MgCl2, 1 mM dithiothreitol] supplemented with 1× protease inhibitor (cocktail tablets from Roche Applied Science, Mannheim, Germany). Released nuclei were pelleted by centrifugation at 10,000 × g for 10 min at 4°C, resuspended in ice-cold 0.4 N H2SO4 and incubated on a rotator overnight at 4°C. Centrifugation at 16,000 × g for 10 min was performed to remove nuclear debris. Histone precipitation was obtained after transferring the supernatant into clean tubes, addition of ice-cold acetone, and overnight incubation at −20°C. After centrifugation (16,000 × g for 10 min) the pellet was air-dried and resuspended in distilled water. The CLH protein concentration was estimated by the Bradford method (5). The histone extract (1, 5, and 10 μg) was separated by 15% SDS-PAGE and stained with Coomassie brilliant blue solution to characterize the histone concentration before SDS-PAGE Western blotting. Polyclonal antibodies against Leishmania recombinant histones (rH2A, rH2B, rH3, and rH4) were raised in immunized rabbits as previously described (7). They were used as WB controls for Leishmania histone extraction.
Western blotting.
The CLH proteins (50 μg per single well) were resuspended in Laemmli's buffer (20), heated for 5 min and separated by 15% SDS-PAGE by using a Bio-Rad protein electrophoresis minigel system (Hercules, CA). The gels were transferred onto nitrocellulose membranes (GE Healthcare, United Kingdom). The blots were probed individually with the human sera (1:100), anti-rH2A, anti-rH2B, anti-rH3 (1:1,000), and anti-rH4 (1:500) polyclonal rabbit antibodies. The secondary antibody, horseradish peroxidase-conjugated anti-human IgG (1:5,000) or anti-rabbit IgG (1:7,500) (Sigma-Aldrich, St. Louis, MO) were used. The protein bands were revealed with H2O2 and diaminobenzidine (40).
Enzyme-linked immunosorbent assays.
Nunc MaxiSorp ELISA microplates (Thermo Fisher Scientific, Roskilde, Denmark) were individually coated with three different antigens, rK39, SLA, and CLH, as follows: 100 μl per well of either rK39, SLA, or CLH protein was diluted in ELISA coating buffer [0.1 M NaHCO3-Na2CO3 (pH 9.6)] at a concentration of 0.25 μg/ml, 5 μg/ml, or 5 μg/ml, respectively. Incubation lasted 1 h and overnight at 37°C and 4°C, respectively. ELISA microplates were then rinsed three times with a washing buffer (PBS, 0.1% Tween 20) and blocked with a PBS buffer (pH 7.2) containing 0.1% Tween 20 and 5% skim milk. Sera were diluted to 1:100 into the blocking buffer and incubated for 1 h at 37°C. All samples were tested in duplicate. Plates were then rinsed five times and incubated with anti-human IgG antibody (Fc specific) (Sigma-Aldrich, St. Louis, MO) conjugated with horseradish peroxidase for 1 h. The substrate solution [0.4 mg/ml ortho-phenylenediamine dihydrochloride (Sigma-Aldrich, Steinheim, Germany) in 0.1 M citrate buffer (pH 5) and 0.03% H2O2] was added at a volume of 100 μl/well. The reaction, developed at room temperature, was stopped with 50 μl of 4 N H2SO4. Optical densities were read at dual wavelengths (492 and 620 nm) by the ELISA reader (Anthos 2020, Biochrom Ltd., United Kingdom). For each serum, the mean optical density (OD) value, computed from the duplicate, was recorded.
Statistical analysis.
Statistical analysis was performed by the MedCalc statistical software. The receiver operating characteristic (ROC) curves were used to analyze the diagnostic performances of each test in discriminating patients with VL from those without (14) and to assess the sensitivities and specificities of diagnostic assays. Analysis of the areas under the ROC curves (AUCs) allowed comparison of the performance characteristics of one test with those of another. The AUCs were compared as described by Hanley and McNeil (15). A test was considered significant if the P value was <0.05.
RESULTS
CLH antigen.
Coomassie blue-stained SDS gel with acid-extracted histones showed 3 major bands of low molecular mass with approximate molecular sizes of 12, 14 to 16, and 17 kDa. These migrating bands probably constitute the four Leishmania core histone proteins and the Leishmania histone linker H1, which were previously described (28). However, higher molecular weight proteins were also apparent (data not shown). Rabbit antibodies anti-rH2A, anti-rH2B, and anti-rH3 reacted with specific bands located between 14 and 16 kDa, whereas anti-rH4 reacted with a specific band of 12 kDa, confirming the histone origin of these low molecular mass proteins (Fig. 1). High molecular weight proteins do not seem to be histone aggregates, since they are not recognized by the antihistone polyclonal antibodies. Preimmune sera, used as negative controls, showed no specific signal.
Fig 1.
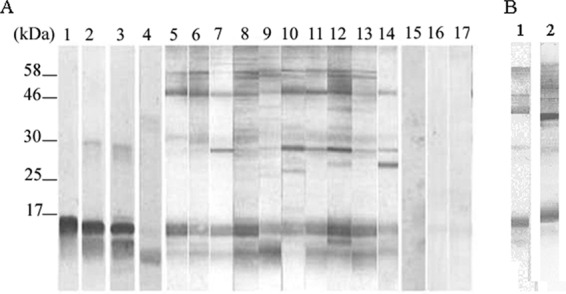
Western blotting of CLH proteins. (A) CLH protein extract incubated with rabbit antibodies anti-rH2A, anti-rH2B, anti-rH3, and anti-rH4 protein (lanes 1, 2, 3, and 4, respectively), with sera of VL patients (lanes 5 to 14) and with sera from control subjects (lanes 15 to 17). Molecular mass markers are shown on the left side of the Western blot in kilodaltons. (B) Recognition pattern of a positive serum against 2 batches of Leishmania histone extracts.
CLH antigenicity during human VL.
In order to analyze the antigenicity of the CLH proteins, sera of 10 VL patients and 3 controls were incubated with a nitrocellulose membrane containing the L. infantum CLH proteins (Fig. 1A). The VL serum samples recognized several protein bands. Recognition patterns were different depending on the reactivity of each serum. However, despite the obtained variability, 3 immunodominant regions (12- to 16-kDa, 26- to 29-kDa, and 50- to 70-kDa proteins) reacted with positive samples. The sera from control subjects were negative, and only a few protein bands were faintly detected. On the other hand, comparison of recognition patterns of different sera against 2 different batches of Leishmania histone extracts indicated that purification seems to be reproducible (Fig. 1B).
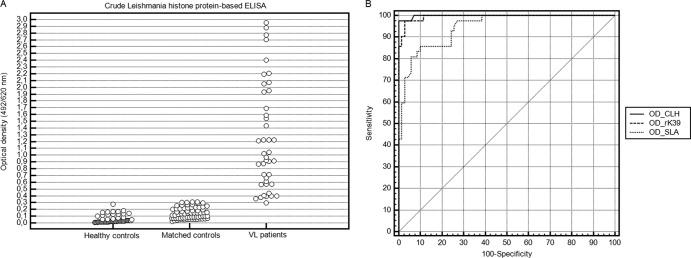
The immunoreactivity of sera against the CLH was subsequently measured by ELISA. Figure 2A shows the absorbance values of the VL patients' sera (n = 42) and sera from controls (n = 135). Absorbance values were above the cutoff in 97.6% of VL sera, whereas 100% of the control sera ODs were under the same threshold.
Fig 2.
Sensitivities and specificities of CLH-, rK39-, and SLA-based ELISAs. (A) ELISA reactivity against CLH proteins. Each dot corresponds to the optical density obtained with an individual serum. Optical densities found in the 2 control groups are shown separately. (B) ROC curves for rK39-, SLA-, and CLH-based ELISAs applied on VL cases versus matched controls.
Comparison of rK39-, SLA-, and CLH-based ELISAs for serodiagnosis of VL.
ROC curves were plotted to compare clinical values of rK39-, SLA-, and CLH-based ELISAs applied for human VL diagnosis (Fig. 2B). Optimal sensitivities of rK39 and SLA were 97.6% and 85.7%, respectively, whereas optimal specificities were 97.1% and 90%, respectively. There was no statistical difference between the AUCs for VL diagnostic assays based on CLH proteins and rK39 (P = 0.6). However, there was a significant difference between AUCs for VL diagnostic assays based on CLH proteins and SLA (P < 0.05). These results suggest that CLH-based ELISA has the same performance in discriminating VL cases from healthy controls as an rK39-based ELISA but gave better discrimination between VL cases and controls than the SLA-based ELISA (Fig. 2B).
DISCUSSION
Recombinant histones, rH2A, rH2B, rH3, and rH4, obtained from L. infantum have been successfully applied in the field of veterinary medicine and particularly in leishmaniasis serodiagnosis (36). Recombinant histone-based ELISA revealed specificity but little sensitivity, especially when recombinant antigens were individually tested (sensitivities of 72%, 60%, 68%, and 44% for H2A, H2B, H3, and H4, respectively) (35, 36). On the other hand, only 88% of CVL sera reacted with at least one of these 4 recombinant histone proteins (35, 36). Lower sensitivities of recombinant fractions were related to the heterogeneous humoral response elicited against parasite proteins observed in each infected dog (36). However, less sensitivity could also be associated with a loss of antigenicity. In fact, heterologous proteins produced by bacterial expression systems, such as Escherichia coli, might not correctly handle posttranslational modifications (8). Previous studies reported that Leishmania histone proteins share many posttranslational modifications, including acetylation, methylation, and phosphorylation (18, 39). These modifications take place on the N-terminal tail domains (18, 39) and probably modulate immune recognition of Leishmania histone proteins. Therefore, the use of purified native parasite antigen, including the different Leishmania histone fractions, seems to be an alternative for improving ELISA performance.
Most of the histone extraction work was based on histone solubility in acid conditions (HCl or H2SO4) under which most of the other nuclear proteins and nucleic acids could precipitate (26). This method is easy to perform, is reproducible, with a good yield, and allows a routine and reliable extraction of Leishmania histone proteins (32). WB using anti-rH2A, anti-rH2B, anti-rH3, and anti-rH4 showed that the CLH protein preparation contains at least the four core histones H2A, H2B, H3, and H4, whose approximate molecular sizes range from 12 to 16 kDa. These proteins were recognized by most VL patients' sera. The additional bands revealed in WB could be explained by the recognition of either histone isoforms, variants, or other histone-coextracted nucleoproteins (41). In that way, histone-like proteins and high-mobility group proteins are the major components of the general family of acid-extractable proteins (12, 16).
Parasite histone proteins were employed as the source of antigen for ELISA. This technique is precise, sensitive and more adapted for the screening of large sample numbers. CLH-based ELISA showed an excellent ability to discriminate between VL patients and controls. In the present work, the CLH-based ELISA was revealed to be more accurate than the SLA-based ELISA in VL diagnosis, and it had the same sensitivity but was more specific. In fact, the SLA-based ELISA specificity largely depends on the antigen preparation, which may give some false-positive results when tested with sera of patients affected with VL-coendemic diseases (17). CLH-based ELISA was revealed to be as accurate as the rK39-based ELISA in VL diagnosis, with no statistical differences between sensitivity and specificity in both tests. It is important to note that rK39 antigen is known to be specific, highly sensitive, and predictive of acute disease onset (10, 19).
In conclusion, CLH-based ELISA showed high accuracy in VL diagnosis, making CLH a promising antigen for clinical use. The identification and characterization of Leishmania histone antigenic protein fractions, specifically those involved in immune response, and the production of the corresponding recombinant proteins in yeast expression systems such as Pichia pastoris could provide interesting perspectives (21). In fact, this expression system allows eukaryotic protein folding and posttranslational modifications of target proteins, which may offer more specific and sensitive tools for VL diagnosis.
ACKNOWLEDGMENTS
We thank M. Soto, Universidad Autónoma de Madrid, Spain, for providing recombinant proteins H2A, H3, and H4 of L. infantum; M. Chenik, Institut Pasteur de Tunis, for providing recombinant protein H2B; S. G. Reed, Infectious Disease Research Institute (IDRI), Seattle, WA, for providing recombinant K39 of L. infantum; and all the collaborators from the pediatrics department who allowed and facilitated VL sample collection. We thank Inès Ben Sghaier for her technical support.
This study was supported by the Ministry of Higher Education and Research in Tunisia and carried out within the framework of the Medical Parasitology, Biotechnology and Biomolecules research lab (LR 11 IPT 06).
The authors report that they have no conflicts of interest.
Footnotes
Published ahead of print 18 July 2012
REFERENCES
- 1. Alvar J, Canavate C, Molina R, Moreno J, Nieto J. 2004. Canine leishmaniasis. Adv. Parasitol. 57:1–88 [DOI] [PubMed] [Google Scholar]
- 2. Aoun K, et al. 2009. Contribution of quantitative real-time PCR to follow-up of visceral leishmaniasis patients treated with meglumine antimoniate. Am. J. Trop. Med. Hyg. 81:1004–1006 [DOI] [PubMed] [Google Scholar]
- 3. Aoun K, Jeddi F, Amri F, Ghrab J, Bouratbine A. 2009. Current epidemiological data on visceral leishmaniasis in Tunisia. Med. Mal. Infect. 39:775–779 (In French) [DOI] [PubMed] [Google Scholar]
- 4. Badaró R, et al. 1996. rK39: a cloned antigen of Leishmania chagasi that predicts active visceral leishmaniasis. J. Infect. Dis. 173:758–761 [DOI] [PubMed] [Google Scholar]
- 5. Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 7:248–254 [DOI] [PubMed] [Google Scholar]
- 6. Burns JM, Jr, et al. 1993. Molecular characterization of a kinesin-related antigen of Leishmania chagasi that detects specific antibody in African and American visceral leishmaniasis. Proc. Natl. Acad. Sci. U. S. A. 90:775–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chenik M, et al. 2006. Vaccination with the divergent portion of the protein histone H2B of Leishmania protects susceptible BALB/c mice against a virulent challenge with Leishmania major. Vaccine 24:2521–2529 [DOI] [PubMed] [Google Scholar]
- 8. Demain AL, Vaishnav P. 2009. Production of recombinant proteins by microbes and higher organisms. Biotechnol. Adv. 27:297–306 [DOI] [PubMed] [Google Scholar]
- 9. Desjeux P, Alvar J. 2003. Leishmania/HIV co-infections: epidemiology in Europe. Ann. Trop. Med. Parasitol. 97(Suppl 1):S3–S15 [DOI] [PubMed] [Google Scholar]
- 10. Galaï Y, et al. 2011. Diagnosis of Mediterranean visceral leishmaniasis by detection of Leishmania antibodies and Leishmania DNA in oral fluid samples collected using an Oracol device. J. Clin. Microbiol. 49:3150–3153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Galanti N, Galindo M, Sabaj V, Espinoza I, Toro GC. 1998. Histone genes in Trypanosomatids. Parasitol. Today 14:64–70 [DOI] [PubMed] [Google Scholar]
- 12. Galasinski SC, Resing KA, Ahn NG. 2003. Protein mass analysis of histones. Methods 31:3–11 [DOI] [PubMed] [Google Scholar]
- 13. Gramiccia M, Gradoni L. 2005. The current status of zoonotic leishmaniases and approaches to disease control. Int. J. Parasitol. 35:1169–1180 [DOI] [PubMed] [Google Scholar]
- 14. Hanley JA, McNeil BJ. 1982. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 143:29–36 [DOI] [PubMed] [Google Scholar]
- 15. Hanley JA, McNeil BJ. 1983. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 148:839–843 [DOI] [PubMed] [Google Scholar]
- 16. Jenson JC, Chin-Lin P, Gerber-Jenson B, Litman GW. 1980. Structurally unique basic protein coextracted with histones from calf thymus chromatin. Proc. Natl. Acad. Sci. U. S. A. 77:1389–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kohanteb J, Ardehali S. 2005. Cross-reaction of sera from patients with various infectious diseases with Leishmania infantum. Med. Princ. Pract. 14:79–82 [DOI] [PubMed] [Google Scholar]
- 18. Kouzarides T. 2007. Chromatin modifications and their function. Cell 128:693–705 [DOI] [PubMed] [Google Scholar]
- 19. Kumar R, Pai K, Pathak K, Sundar S. 2001. Enzyme-linked immunosorbent assay for recombinant K39 antigen in diagnosis and prognosis of Indian visceral leishmaniasis. Clin. Diagn. Lab. Immunol. 8:1220–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- 21. Lau YL, Thiruvengadam G, Lee WW, Fong MY. 2011. Immunogenic characterization of the chimeric surface antigen 1 and 2 (SAG1/2) of Toxoplasma gondii expressed in the yeast Pichia pastoris. Parasitol. Res. 109:871–878 [DOI] [PubMed] [Google Scholar]
- 22. Maalej IA, et al. 2003. Comparative evaluation of ELISAs based on ten recombinant or purified Leishmania antigens for the serodiagnosis of Mediterranean visceral leishmaniasis. Am. J. Trop. Med. Hyg. 68:312–320 [PubMed] [Google Scholar]
- 23. Maia Z, et al. 13 January 2012. Comparative study of rK39 Leishmania antigen for serodiagnosis of visceral leishmaniasis: systematic review with meta-analysis. PLoS Negl. Trop. Dis. 6:e1484 doi:10.137/pntd.0001484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Meddeb-Garnaoui A, et al. 2010. Cellular and humoral responses induced by Leishmania histone H2B and its divergent and conserved parts in cutaneous and visceral leishmaniasis patients, respectively. Vaccine 28:1881–1886 [DOI] [PubMed] [Google Scholar]
- 25. Melby PC, Neva FA, Sacks DL. 1989. Profile of human T cell response to Leishmanial antigens. Analysis by immunoblotting. J. Clin. Invest. 83:1868–1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Murray K. 1966. The acid extraction of histones from calf thymus deoxyribonucleoprotein. J. Mol. Biol. 15:409–419 [DOI] [PubMed] [Google Scholar]
- 27. Murray HW, Berman JD, Davies CR, Saravia NG. 2005. Advances in leishmaniasis. Lancet 366:1561–1577 [DOI] [PubMed] [Google Scholar]
- 28. Noll TM, Desponds C, Belli SI, Glaser TA, Fasel NJ. 1997. Histone H1 expression varies during the Leishmania major life cycle. Mol. Biochem. Parasitol. 84:215–227 [DOI] [PubMed] [Google Scholar]
- 29. Passos S, et al. 2005. Recombinant Leishmania antigens for serodiagnosis of visceral leishmaniasis. Clin. Diagn. Lab. Immunol. 12:1164–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Requena JM, Alonso C, Soto M. 2000. Evolutionarily conserved proteins as prominent immunogens during Leishmania infections. Parasitol. Today 16:246–250 [DOI] [PubMed] [Google Scholar]
- 31. Riera C, et al. 2008. Asymptomatic infection by Leishmania infantum in blood donors from the Balearic Islands (Spain). Transfusion 48:1383–1389 [DOI] [PubMed] [Google Scholar]
- 32. Shechter D, Dormann HL, Allis CD, Hake SB. 2007. Extraction, purification and analysis of histones. Nat. Protoc. 2:1445–1457 [DOI] [PubMed] [Google Scholar]
- 33. Soto M, Requena JM, Gomez LC, Navarrete I, Alonso C. 1992. Molecular characterization of a Leishmania donovani infantum antigen identified as histone H2A. Eur. J. Biochem. 205:211–216 [DOI] [PubMed] [Google Scholar]
- 34. Soto M, Requena JM, Morales G, Alonso C. 1994. The Leishmania infantum histone H3 possesses an extremely divergent N-terminal domain. Biochim. Biophys. Acta 1219:533–535 [DOI] [PubMed] [Google Scholar]
- 35. Soto M, et al. 1995. Mapping of the linear antigenic determinants from the Leishmania infantum histone H2A recognized by sera from dogs with leishmaniasis. Immunol. Lett. 48:209–214 [DOI] [PubMed] [Google Scholar]
- 36. Soto M, et al. 1999. Antigenicity of the Leishmania infantum histones H2B and H4 during canine viscerocutaneous leishmaniasis. Clin. Exp. Immunol. 115:342–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Srivastava P, Dayama A, Mehrotra S, Sundar S. 2011. Diagnosis of visceral leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 105:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Srividya G, Kulshrestha A, Singh R, Salotra P. 2012. Diagnosis of visceral leishmaniasis: developments over the last decade. Parasitol. Res. 110:1065–1078 [DOI] [PubMed] [Google Scholar]
- 39. Sullivan WJ, Jr, Naguleswaran A, Angel SO. 2006. Histones and histone modifications in protozoan parasites. Cell. Microbiol. 8:1850–1861 [DOI] [PubMed] [Google Scholar]
- 40. Towbin H, Staehein T, Gordon J. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. U. S. A. 76:4350–4354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zougman A, Wiœniewski JR. 2006. Beyond linker histones and high mobility group proteins: global profiling of perchloric acid soluble proteins. J. Proteome Res. 5:925–934 [DOI] [PubMed] [Google Scholar]