Abstract
Humoral immunity to human papillomavirus (HPV) has not been fully characterized, and there is currently no standard serologic test for the measurement of HPV antibodies. Most HPV serologic assays developed to date are based on virus-like particles (VLPs) of the major HPV capsid protein, L1. We sought to compare the performance of a multiplex HPV L1 VLP-based serologic assay to that of an assay based on VLPs comprised of both L1 and the minor capsid, L2. We developed HPV L1 VLP and L1-L2 VLP-based multiplex seroassays for the detection of HPV type 16 (HPV16) and HPV18 virion binding antibodies using Luminex fluorescent bead technology. We compared the performance of these assays to that of established pseudovirion-based neutralization and L1 VLP-based enzyme-linked immunosorbent assays (ELISAs). A total of 391 serum specimens from unvaccinated adult males and females were tested. The L1 and L1-L2 VLP multiplex seroassays each demonstrated substantial agreement with both the neutralization assays and the ELISAs for the detection of HPV16 antibodies (κ = 0.60 to 0.64). However, the L1-L2 VLP seroassay demonstrated better agreement with neutralization assays for the detection of HPV18 antibodies than the L1 VLP seroassay (κ = 0.74 and 0.43, respectively). L1 and L1-L2 VLP seroassays showed excellent agreement with one another for the detection of HPV16 antibodies (κ = 0.86) but only moderate agreement for HPV18 antibodies (κ = 0.44). The HPV L1-L2 VLP seroassay performs well for the concurrent measurement of HPV16 and -18 antibodies in large numbers of samples and may be extended to include other HPV types.
INTRODUCTION
Humoral immunity to human papillomavirus (HPV) has not been fully characterized, and there is currently no standard serologic test for the measurement of HPV antibodies. HPV serum antibodies provide a cumulative measure of viral exposure that is useful in studies of vaccinated and unvaccinated populations. Measurement of HPV capsid antibodies for epidemiologic and clinical investigations requires assays that are capable of measuring antibodies to multiple HPV types simultaneously with high sensitivity and type specificity in a high-throughput format. In the absence of efficient methods of harvesting native antigen from culture, serologic detection of HPV has largely relied on the use of “virus-like particles” (VLPs). When expressed recombinantly, the major HPV capsid protein, L1, self-assembles into VLPs lacking viral genome and displaying conformational, type-specific epitopes that are structurally similar to authentic virions (12, 18, 20). HPV L1 VLPs induce high titers of type-specific neutralizing antibodies (13) and are the basis of prophylactic vaccines available today (23). VLP-based enzyme-linked immunosorbent assays (ELISAs) are among the most commonly used assays for HPV research (4, 8, 17, 19, 25). These assays, however, are dependent on the conformational integrity of the VLPs such that distortions can impede type-specific serologic detection (29). Moreover, HPV VLP-based ELISAs require relatively large quantities of serum, and separate assays must be run for individual HPV types. Another L1 VLP-based assay utilizes bead-based technology for multiplex detection of different HPV types in competition for L1 VLP binding by type-specific monoclonal antibodies (MAbs) directed to specific neutralizing epitopes (7, 28). The sensitivity of competitive assays is largely dependent upon the selection of MAbs capable of recognizing the immunodominant viral neutralizing epitopes. VLP-based assays in general are limited by the variable yields in their production, which can restrict their utility in high-throughput formats.
HPV pseudovirions (PsVs), like VLPs, take advantage of the self-assembly properties of the viral capsid proteins. PsVs are produced by transfection of expression plasmids containing codon-modified genes for both major and minor HPV capsids, L1 and L2, along with a reporter plasmid (1, 2). In these plasmids, the open reading frames of L1 and L2 have been modified to eradicate inhibitory elements that suppress their expression (22). HPV pseudoviruses consequently can be prepared with greater efficiency and higher yields than L1 VLPs (6). PsVs are similar to native virions and retain most of its conformation-dependent neutralizing epitopes (6).
We sought to compare the performance of a multiplex HPV L1 VLP-based serologic assay to that of an assay based on VLPs comprised of both L1 and L2. We refer to the latter as L1-L2 VLPs to distinguish them from pseudovirions, which contain a reporter construct. We hypothesize that L1-L2-based VLPs are superior to L1 VLPs for the detection of HPV virion antibodies due to their more efficient assembly and greater resemblance to native virions.
MATERIALS AND METHODS
We developed L1 VLP and L1-L2 VLP serologic assays for the concurrent detection of neutralizing antibodies to HPV types 16 (HPV16) and 18 (HPV18) using fluorescent bead technology in a multiplex format. We compared the performance of these two assays to that of an established L1-L2 PsV-based neutralization assay and an L1 VLP-based ELISA. Testing was done on stored serum specimens previously collected as part of two studies. The first was a cohort evaluating HPV transmission in 108 adult male-female sexual partners at the University of Hawaii's University Health Services and the Cancer Research Center in Honolulu, HI, from 2005 to 2006 (15). Participants had been followed at 2-month intervals, with blood collected at each visit. The second was a pilot cohort study of 83 adult females at an outpatient obstetrics-gynecology clinic in Honolulu from 1997 to 1998 (14). Study visits, including blood collection, occurred at 3- to 4-month intervals. Both studies had been approved by the Committee on Human Studies of the University of Hawaii, and all study participants provided written informed consent. Institutional Review Board (IRB) approval for the use of these stored specimens for the present study was also received.
Blood specimens were collected and processed similarly for both studies. Briefly, 30 ml of nonfasting blood was obtained from study participants at each study visit. Blood specimens were immediately refrigerated and processed within 12 h after collection. Blood specimens were spun for 15 min at 2,800 rpm in a refrigerated centrifuge, and serum and other blood components were immediately stored at −80°C. The performance of the two seroassays was compared to that of established PsV-based neutralization assays. In addition, assay results for the female pilot study samples were compared to those from an HPV16 ELISA performed shortly after blood collection in 1997 to 1998. A total of 381 serum specimens were tested. With the exception of the synthesis of VLPs for the ELISA, all work was conducted at the HPV Laboratory of the University of Hawaii Cancer Center, Honolulu, HI.
Synthesis of HPV16 and -18 PsVs and VLPs.
HPV16 VLPs for the ELISA were synthesized in the laboratory of John Schiller at the National Cancer Institute (NCI), Bethesda, MD, according to published methods (19). Briefly, sequences for HPV16 L1 were cloned into a baculovirus expression vector and used to infect Sf9 insect cells. Microwell plates were coated with purified VLPs (0.3 μg of VLP per well) and stored with phosphate-buffered saline (PBS) at −80°C until testing.
Pseudovirions (PsVs) were produced according to published methods (2, 3) using plasmids provided by J. Schiller of the NCI and M. Muller of the German Cancer Center, Heidelberg, Germany. For the neutralization assay, HPV16 and HPV18 PsVs were each prepared by transfection of codon-modified plasmid DNA of viral capsids L1 and L2 (p16sheLL and p18sheLL) and with a secreted alkaline phosphatase (SEAP) reporter plasmid (pYSEAP) into NIH 293TT cells. Bovine papillomavirus (BPV) pseudovirions used in parallel control assays were similarly prepared with pSheLL.
HPV16 and HPV18 VLPs for the L1-L2 VLP seroassay were synthesized by identical methods used for preparation of PsVs for the neutralization assay, with the exception of the exclusion of the reporter gene plasmid. For the L1 VLP seroassay, HPV16 and HPV18 VLPs were each prepared using codon-modified L1 alone (p16L1 and p18L1). Upon synthesis, all PsVs and VLPs were quantified and stored at −80°C until testing.
HPV16 and HPV18 neutralization assays.
Neutralizing antibodies to HPV16 and -18 were separately measured using PsV-based neutralization assays developed in the laboratory of John Schiller at the NCI (29) and used in a number of epidemiologic investigations (26, 27, 30, 31). Purified pseudovirion stocks were titrated to determine the minimum concentration needed to detect SEAP activity following infection of 293TT cells. Diluted serum samples (1:40 and 1:160) and diluted PsV stocks were added to 96-well plates containing 293TT cells. Following incubation, supernatant was clarified and then transferred to 96-well plates, and SEAP activity was measured via chemiluminescence (Great ESCAPE SEAP chemiluminescence kit; BD Clontech Laboratories, Inc., Mountain View, CA). Samples were read on an MLX microplate luminometer (Dynex Technologies). Samples were considered to be positive if the 1:160 titer reduced the SEAP level by greater than 50% compared to no-serum quadruplicate PsV samples and if they neutralized at a dilution at least 4-fold higher than that observed in the BPV-1 assay. All serum samples were tested in duplicate. We did not include titrations of less than 1:160 as our intention was to distinguish between seropositive and seronegative samples rather than to measure the titer of positive samples.
HPV16/18 L1 VLP multiplex seroassay.
VLPs for HPV16 and HPV18 were each coupled to fluorescent polystyrene beads or microspheres (xMap multianalyte microspheres; Luminex Corporation, Austin, TX). Each microsphere contains a specific quantity of two fluorescent dyes, which together produce a unique spectral signature. Microspheres coated with different VLPs can be used together for the detection of multiple HPV types. Conjugation of VLPs occurs via carboxyl groups on the microsphere surface, which are activated in reactions using N-hydroxysulfosuccinimide and 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride. A total of 25 μg each of the HPV16 and -18 VLPs was covalently conjugated to 6 × 106 of each bead set (sets 38 and 118, respectively), according to the manufacturer's instructions. VLP-coupled microspheres were pooled to a final concentration of 1 × 105 microspheres per ml per VLP and added to each well of a 96-well plate. Serum samples (1:50 dilution) were run in duplicate. Following incubations and washings, for secondary antibody detection, anti-human IgG conjugated to R-phycoerythrin (Sigma-Aldrich, St. Louis, MO) was added. Samples were analyzed on a Luminex 200 analyzer, and results were expressed as the median fluorescence intensity (MFI) of at least 100 microspheres per set per serum sample. Negative controls, included on each plate, consisted of pooled serum specimens from individuals who previously testing negative on the HPV16 and HPV18 neutralization assay who were also HPV DNA negative for genital infection, <23 years old, and with a history of no more than 1 sexual partner. Positive-cutoff levels were set based on the mean MFI of pooled serum specimens from samples testing borderline positive (titers of ≥1:40 and ≤1:160) on the neutralization assays. These low-level positive controls for HPV16 and HPV18 were included on each plate.
HPV16/18 L1-L2 VLP multiplex seroassays.
L1-L2 VLPs for HPV16 and HPV18 were each coupled to the fluorescent polystyrene beads using quantities and methods identical to those described above for L1 VLPs. VLP-coupled beads were pooled to a concentration of 1 × 105 microspheres/ml per VLP. The assay was conducted identically to the L1 VLP seroassay.
HPV16 VLP ELISA.
Unlike the HPV16 VLPs for the seroassay, HPV16 L1 sequences for the ELISA VLPs were not codon modified and were synthesized via a baculovirus expression vector in Sf9 insect cells. Serum samples (50 μl diluted sera) were tested by direct ELISA (19). Detection was done utilizing a secondary antibody conjugated to horseradish peroxidase and ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid] enzyme substrate. Absorbance was read on a microplate reader at 405 nm. Specimens were assayed in duplicate. For each 96-well plate, two negative controls, two positive controls, and four standards were run along with the specimen sera. Controls and standards were provided by J. Schiller. Controls consisted of previously validated negative and positive sera; the latter was used to establish the cutoff for positivity. Standards consisted of pooled sera of known seroreactivity. The optical density of each specimen and control was divided by the mean optical density of the standards in order to normalize the values. Specimens with values at or above the mean value of the positive-control sera were considered positive. Specimens with values below the mean value of the positive-control sera were considered negative.
Validation of assay cutoff levels.
The L1-L2 VLP seroassay was validated using the first international standard for HPV16 antibodies, which recently became available (10). Antibody levels of this positive serum standard are quantified at 10 IU/ml. A standard curve was generated using serial dilutions of the standard (1:50 to 1:51,200). All samples were run in quadruplicate. Negative controls, positive controls for HPV16 and HPV18, and HPV16 cutoff-level controls were included.
Statistical analyses.
All analyses were conducted with SAS version 9.2. All assay test results were classified as seropositive or seronegative. Comparisons between assay results were made utilizing the percentage of observed agreement and the kappa statistic (κ) with 95% confidence intervals (95% CIs) (11). Comparisons of serology with HPV DNA results were not included in the present evaluation.
RESULTS
A total of 391 serum specimens were tested, including 259 from the male-female HPV transmission study and 132 from the pilot female study. In the transmission study, HPV16 seropositivities were 13% (34/259) for the neutralization assay, 20% (53/259) for the L1 VLP seroassay, and 21% (55/259) for the L1-L2 VLP seroassay. When a lower cutoff (1:40) was used for the HPV16 neutralization assay, seropositivity was similar to that in the two seroassays (22%, 57/259). HPV18 seropositivities for the transmission study were 7% (18/259) for the neutralization assay, 14% (36/259) for the L1 VLP seroassay, and 5% (14/259) for the L1-L2 VLP seroassay. When a lower cutoff (1:40) was used for the HPV18 neutralization assay, seropositivity was 11% (29/259). A total of 13 of 259 (5%) samples were positive for both HPV16 and -18 antibodies based on the neutralization assays. Eleven of these 13 samples were positive for HPV16 and -18 antibodies on the L1-L2 VLP assay, and all 13 tested dually positive on the L1 VLP seroassay. However, an additional 15 samples were positive for both HPV16 and -18 antibodies on the L1 VLP seroassay.
For the female pilot study, HPV16 seropositivities were 14% (18/132) for the L1 VLP ELISA, 1.5% (2/132) for the neutralization assay, 15% (20/132) for the L1 VLP seroassay, and 13% (17/132) for the L1-L2 VLP seroassay. When a lower (1:40) cutoff was used for the HPV16 neutralization assay, the seropositivity was 9% (12/132). Female pilot study samples were retested using the neutralization assay, and similar low positivity was observed (results not shown).
The L1-L2 and L1 VLP seroassay results for the partner study were compared to those of the neutralization assays (Table 1). Both assays demonstrated comparable agreement with neutralization assays for the detection of HPV16 antibodies (κ = 0.61 and 0.60, respectively). However, the L1-L2 VLP seroassay demonstrated better agreement with neutralization assays for the detection of HPV18 antibodies than the VLP seroassay (κ = 0.74 and 0.43, respectively). When a lower cutoff (1:40) was used for the neutralization assays, agreement with the seroassays slightly increased for HPV16 (κ = 0.64 and 0.63 for the L1-L2 and L1 seroassays, respectively). For HPV18, however, a lower cutoff resulted in decreased agreement for the L1-L2 seroassay (κ = 0.62) and was unchanged for the L1 seroassay (κ = 0.42).
Table 1.
Comparison of L1 VLP and L1-L2 VLP seroassays with PsV neutralization assays for measurement of HPV16 and -18 antibodies (n = 259)
| HPV seroassay resulta | No. of HPV PsV neutralization assay samplesb: |
Overall agreement (%) | κ (95% CI) | |
|---|---|---|---|---|
| Positive | Negative | |||
| HPV16 antibody | ||||
| HPV L1 VLP seroassay | ||||
| Positive | 29 | 24 | 89 | 0.60 (0.48–0.73) |
| Negative | 5 | 201 | ||
| HPV L1-L2 VLP seroassay | ||||
| Positive | 30 | 25 | 89 | 0.61 (0.49–0.74) |
| Negative | 4 | 200 | ||
| HPV18 antibody | ||||
| HPV L1 VLP seroassay | ||||
| Positive | 13 | 23 | 89 | 0.43 (0.26–0.60) |
| Negative | 5 | 218 | ||
| HPV L1-L2 VLP seroassay | ||||
| Positive | 12 | 2 | 97 | 0.74 (0.56–0.91) |
| Negative | 6 | 239 | ||
Multiplex assay for HPV16 and -18.
Separate assays were performed for HPV16 and -18.
For female pilot study samples, results from the two seroassays and the neutralization assay were compared to those from the HPV16 ELISA (Table 2). L1 and L1-L2 VLP seroassays were equally comparable to VLP ELISAs for HPV16 antibodies (κ = 0.64 and 0.63, respectively). The HPV16 neutralization assay showed very poor agreement with the HPV16 ELISA (κ = 0.19), and agreement did not improve when a lower neutralization cutoff was employed (κ = 0.14).
Table 2.
Comparison of L1 VLP and L1-L2 VLP seroassays with L1 VLP ELISA for measurement of HPV16 antibodies (n = 132)
| HPV16 antibody assay result | No. of HPV VLP ELISA samples: |
Overall agreement (%) | κ (95% CI) | |
|---|---|---|---|---|
| Positive | Negative | |||
| HPV L1 VLP seroassaya | ||||
| Positive | 13 | 7 | 91 | 0.63 (0.44–0.82) |
| Negative | 5 | 107 | ||
| HPV L1-L2 VLP seroassaya | ||||
| Positive | 12 | 5 | 92 | 0.64 (0.44–0.84) |
| Negative | 6 | 109 | ||
| HPV neutralization assay | ||||
| Positive | 2 | 0 | 88 | 0.19 (0–0.56) |
| Negative | 16 | 114 | ||
Multiplex assay for HPV16 and -18.
Comparison of the two seroassays (Table 3) showed excellent agreement for HPV16 (κ = 0.86) and only moderate agreement for HPV18 (κ = 0.44).
Table 3.
Comparison of L1 VLP and L1-L2 VLP seroassays for measurement of HPV16 and -18 antibodies (n = 259)
| HPV L1-L2 VLP seroassay resulta | No. of HPV L1 VLP seroassay samplesa: |
Overall agreement (%) | κ (95% CI) | |
|---|---|---|---|---|
| Positive | Negative | |||
| HPV16 antibody | ||||
| Positive | 48 | 7 | 95 | 0.86 (0.78–0.94) |
| Negative | 5 | 199 | ||
| HPV18 antibody | ||||
| Positive | 12 | 2 | 92 | 0.44 (0.26–0.61) |
| Negative | 24 | 221 | ||
Multiplex assay for HPV16 and -18.
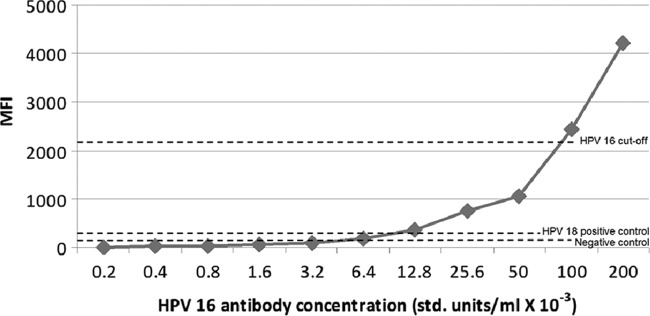
A standard curve was generated for the HPV16 L1-L2 seroassay using serial dilutions of the HPV16 international standard (Fig. 1). MFI increased with increasing antibody levels of 0.2 × 10−3 to 200 × 10−3 U/ml. The HPV16 cutoff was measured at an MFI of 2,099, corresponding to 0.1 U/ml of HPV16 antibody.
Fig 1.
HPV L1-L2 VLP seroassay showing mean fluorescence intensity (MFI) by HPV16 antibody level. std., standard.
DISCUSSION
We have developed HPV L1 VLP and L1-L2 VLP serologic assays capable of measuring virion antibodies to HPV16 and -18 simultaneously in a multiplex format. Compared to the neutralization assay, both seroassays performed well for the detection of HPV16 antibodies, but the L1-L2 seroassay performed better than the L1 seroassay for the detection of HPV18 neutralizing antibodies. The good concordance of the two immunoassays with the L1 VLP ELISA and the poor concordance between the neutralization assay and ELISA are likely due to the additional detection of nonneutralizing and potential cross-type-reactive antibodies by the ELISA and two seroassays.
The poor concordance between the neutralization assay and ELISAs may also be due to the different sources of VLPs. VLPs for the ELISA were synthesized in Sf9 insect cells, and VLPs for the neutralization assay were generated in mammalian cells. Folding of the conformation-dependent L1 surface epitopes may be slightly different in the insect cells from that in mammalian cells. The poor concordance between assays may also be due to the different lengths of specimen storage. The ELISA was performed on female pilot study samples shortly after blood collection in 1997 to 1998, while the neutralization assay was performed over 10 years later on stored samples. The very low positivity of the neutralization assay with these long-term-stored samples was not observed for the two seroassays. This indicates that the seroassays may be more robust than the neutralization assay for the detection of waning antibodies levels in samples stored over prolonged periods.
While the two seroassays showed excellent agreement for HPV16, concordance for HPV18 was much lower. Furthermore, the limited agreement of the HPV18 L1 VLP seroassay with the neutralization assay combined with the moderate agreement between the two seroassays for the detection of HPV18 antibodies may indicate that the HPV18 L1 VLP seroassay detects a greater proportion of both nonneutralizing antibodies and cross-reactive antibodies, while the L1-L2 seroassay more specifically detects type-specific neutralizing antibodies. L1 capsids may be a less-specific antigenic target than L1-L2 capsids, perhaps because they more often assume a nonnative conformation. It is well established that linear epitopes of internal L1 sequences can be broadly cross-type reactive. Alternatively, the L1-L2 capsids may have greater stability than the L1 capsid. Indeed, we observed better yields for the L1-L2 capsids than for the L1 VLPs.
The seroassays we developed are technologically simple to perform compared to neutralization assays, rendering them amenable for testing large numbers of samples. The multiplex format of the two seroassays introduced greater efficiency for the detection of multiple serologic types. The bead technology allows for the addition of other HPV types—presumably in the form of L1-L2 capsids—in order to extend serologic detection to other clinically relevant HPV types. Both assays were able to detect both HPV16 and -18 in samples that were positive for both antibodies. Nonetheless, some degree of cross-reactivity of the two HPV types cannot be entirely ruled out. Although Luminex-based assays are not directly quantitative, the availability of the first international standard for HPV16 antibodies allows for the estimation of relative antibody levels for HPV16.
The clinical significance of HPV serostatus is not fully understood. HPV neutralizing antibodies likely provide the best measure of potential protection against infection, although their role, as well as that of nonneutralizing antibodies, is unclear. HPV seropositivity is higher in cervical cancer patients than in controls, and seropositivity has been associated with other HPV-related cancers (5). In the general population, studies are conflicting as to whether naturally acquired antibodies protect against infection (16, 21, 24, 31). A major challenge of using serostatus as a measure of past or current infection is that not all infected individuals seroconvert (4, 17, 30), and seropositivity is higher in females than males (21). Among individuals who do seroconvert, antibodies may not be detectable until months after infection (4, 17). Compared to the high-titer antibody levels observed in HPV vaccines, antibody levels resulting from natural infection are very low (27, 29) and may not always persist (17).
We conclude that our newly developed HPV L1-L2 VLP seroassay performs well for the concurrent measurement of HPV16 and -18 neutralizing antibodies in large numbers of samples and can be extended to include other HPV types. Faust et al. reported the development of a similar Luminex-based assay for multiple HPV types (9). We will use the L1-L2 VLP seroassay to characterize the role of HPV antibodies in the natural history of HPV infection among males and females. We are particularly interested in examining differences in the seroconversion by anatomic site of infection and the relationship of serostatus to viral transmission.
ACKNOWLEDGMENTS
This work was supported by the Merck Investigator Studies Program, Merck and Co., Inc.
We extend our gratitude to John T. Schiller and Yuk-Ying S. Pang of the Laboratory of Cellular Oncology, Center for Cancer Research, National Cancer Institute, NIH, Bethesda, MD, for technical assistance, scientific input, and provision of reagents.
B. Y. Hernandez has received consultation and speaker fees from Merck and Co., Inc.
Footnotes
Published ahead of print 3 July 2012
REFERENCES
- 1. Buck CB, Pastrana DV, Lowy DR, Schiller JT. 2004. Efficient intracellular assembly of papillomaviral vectors. J. Virol. 78:751–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Buck CB, Pastrana DV, Lowy DR, Schiller JT. 2005. Generation of HPV pseudovirions using transfection and their use in neutralization assays. Methods Mol. Med. 119:445–462. [DOI] [PubMed] [Google Scholar]
- 3. Buck CB, Thompson CD. 1 December 2007. Unit 26.1 Production of papillomavirus-based gene transfer vectors. Curr. Protoc. Cell Biol. doi:10.1002/0471143030.cb2601s37 [DOI] [PubMed] [Google Scholar]
- 4. Carter JJ, et al. 2000. Comparison of human papillomavirus types 16, 18, and 6 capsid antibody responses following incident infection. J. Infect. Dis. 181:1911–1919 [DOI] [PubMed] [Google Scholar]
- 5. Carter JJ, et al. 2001. Human papillomavirus 16 and 18 L1 serology compared across anogenital cancer sites. Cancer Res. 61:1934–1940 [PubMed] [Google Scholar]
- 6. Conway MJ, Meyers C. 2009. Replication and assembly of human papillomaviruses. J. Dent. Res. 88:307–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dias D, et al. 2005. Optimization and validation of a multiplexed Luminex assay to quantify antibodies to neutralizing epitopes on human papillomaviruses 6, 11, 16, and 18. Clin. Diagn. Lab. Immunol. 12:959–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dillner J. 1999. The serological response to papillomaviruses. Semin. Cancer Biol. 9:423–430 [DOI] [PubMed] [Google Scholar]
- 9. Faust H, Knekt P, Forslund O, Dillner J. 2010. Validation of multiplexed human papillomavirus serology using pseudovirions bound to heparin-coated beads. J. Gen. Virol. 91:1840–1848 [DOI] [PubMed] [Google Scholar]
- 10. Ferguson M, Wilkinson DE, Heath A, Matejtschuk P. 2011. The first international standard for antibodies to HPV 16. Vaccine 29:6520–6526 [DOI] [PubMed] [Google Scholar]
- 11. Fleiss JL, Levin B, Paik MC. 2003. Statistical methods for rates and proportions. John Wiley and Sons, New York, NY [Google Scholar]
- 12. Hagensee ME, Olson NH, Baker TS, Galloway DA. 1994. Three-dimensional structure of vaccinia virus-produced human papillomavirus type 1 capsids. J. Virol. 68:4503–4505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harper DM, et al. 2004. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet 364:1757–1765 [DOI] [PubMed] [Google Scholar]
- 14. Hernandez B. 1999. Determinants of the persistence of human papillomavirus infection among a multiethnic cohort of women in Hawaii. Ph.D. dissertation. University of Hawaii, Honolulu, HI [Google Scholar]
- 15. Hernandez BY, et al. 2008. Transmission of human papillomavirus in heterosexual couples. Emerg. Infect. Dis. 14:888–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ho GY, et al. 2002. Risk factors for subsequent cervicovaginal human papillomavirus (HPV) infection and the protective role of antibodies to HPV-16 virus-like particles. J. Infect. Dis. 186:737–742 [DOI] [PubMed] [Google Scholar]
- 17. Ho GY, Studentsov YY, Bierman R, Burk RD. 2004. Natural history of human papillomavirus type 16 virus-like particle antibodies in young women. Cancer Epidemiol. Biomarkers Prev. 13:110–116 [DOI] [PubMed] [Google Scholar]
- 18. Kirnbauer R, Booy F, Cheng N, Lowy DR, Schiller JT. 1992. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc. Natl. Acad. Sci. U. S. A. 89:12180–12184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kirnbauer R, et al. 1994. A virus-like particle enzyme-linked immunosorbent assay detects serum antibodies in a majority of women infected with human papillomavirus type 16. J. Natl. Cancer Inst. 86:494–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kirnbauer R, et al. 1993. Efficient self-assembly of human papillomavirus type 16 L1 and L1-L2 into virus-like particles. J. Virol. 67:6929–6936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kreimer AR, Alberg AJ, Viscidi R, Gillison ML. 2004. Gender differences in sexual biomarkers and behaviors associated with human papillomavirus-16, -18, and -33 seroprevalence. Sex. Transm. Dis. 31:247–256 [DOI] [PubMed] [Google Scholar]
- 22. Leder C, Kleinschmidt JA, Wiethe C, Muller M. 2001. Enhancement of capsid gene expression: preparing the human papillomavirus type 16 major structural gene L1 for DNA vaccination purposes. J. Virol. 75:9201–9209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lowy DR, Schiller JT. 2006. Prophylactic human papillomavirus vaccines. J. Clin. Invest. 116:1167–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lu B, et al. 2010. Epidemiologic factors associated with seropositivity to human papillomavirus type 16 and 18 virus-like particles and risk of subsequent infection in men. Cancer Epidemiol. Biomarkers Prev. 19:511–516 [DOI] [PubMed] [Google Scholar]
- 25. Marais D, et al. 2000. Seroresponses to virus-like particles of human papillomavirus types 16, 18, 31, 33, and 45 in San people of Southern Africa. J. Med. Virol. 60:331–336 [DOI] [PubMed] [Google Scholar]
- 26. Mbulawa ZZ, et al. 2008. Association of serum and mucosal neutralizing antibodies to human papillomavirus type 16 (HPV-16) with HPV-16 infection and cervical disease. J. Gen. Virol. 89:910–914 [DOI] [PubMed] [Google Scholar]
- 27. Ochi H, et al. 2008. Neutralizing antibodies against human papillomavirus types 16, 18, 31, 52, and 58 in serum samples from women in Japan with low-grade cervical intraepithelial neoplasia. Clin. Vaccine Immunol. 15:1536–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Opalka D, et al. 2003. Simultaneous quantitation of antibodies to neutralizing epitopes on virus-like particles for human papillomavirus types 6, 11, 16, and 18 by a multiplexed Luminex assay. Clin. Diagn. Lab. Immunol. 10:108–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pastrana DV, et al. 2004. Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18. Virology 321:205–216 [DOI] [PubMed] [Google Scholar]
- 30. Steele J, et al. 2008. Measurement of the humoral immune response following an incident human papillomavirus type 16 or 18 infection in young women by a pseudovirion-based neutralizing antibody assay. Clin. Vaccine Immunol. 15:1387–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wu X, et al. 2009. Detection of HPV types and neutralizing antibodies in Gansu Province, China. J. Med. Virol. 81:693–702 [DOI] [PubMed] [Google Scholar]