Abstract
Infants are susceptible to infections in early life and must rely on their innate immune system for protection. β-Glucans potentiate immune responses. Therefore, we evaluated the influence of purified yeast (1,3/1,6)-β-d-glucan (Wellmune WGP, here referred to as WGP) on the development of the gastrointestinal tract and the intestinal and systemic immune systems in neonatal piglets. Piglets were fed formula containing 0 (control), 1.8, 18, or 90 mg WGP/kg body weight (BW) and were vaccinated against human influenza. Piglets were euthanized at 7 or 21 days of age. Piglet weight and small intestinal length and weight were unaffected by dietary WGP. In addition, WGP did not affect ileal crypt depth, villus height, or ascending colon cuff depth. Immune parameters not affected by WGP supplementation included T cell phenotypes, cytokine gene expression, and cell proliferation. However, vaccination and developmental effects were seen. Overall, the doses of 1.8, 18, and 90 mg/kg BW of dietary WGP had no effect on intestinal or immune development and did not improve the antibody response to vaccination in neonatal piglets.
INTRODUCTION
Infants must rely on their innate immune systems for protection against infections to a significant extent during early life. Active adaptive immunity must develop rapidly and appropriately in the neonate because immune protection acquired by the fetus from the mother via placental transfer, colostrum, and breast milk does not confer protection against antigens to which the mother has not been exposed. The process of immune maturation is even more important for infants who are not breast-fed, since they receive passive immunity only from placental transfer and no further immune protection through their diet. The U.S. Centers for Disease Control and Prevention currently recommend that infants receive six vaccinations prior to 3 months of age. For these vaccinations to be effective, it is important for the neonate to produce adequate amounts of antigen-specific antibodies and circulating memory cells specific for the antigens in the vaccinations being received. Therefore, studies which examine the effects of dietary supplementation with immune-stimulating compounds on the overall T helper status of the immune system and on the immune response to vaccination are valuable. Furthermore, the identification and characterization of compounds that enhance the growth, development, and health of those infants remains a priority.
The rate of maturation of the immune system is influenced by exposure to commensal bacteria and to dietary antigens (4, 18, 21–23, 48). Modifications to infant formula are hypothesized to enhance the process of immune maturation in formula-fed infants (38). Although an emphasis has been placed on identifying and replicating the components found in breast milk, other compounds can stimulate immune development. One such class of compounds is β-glucans (βG). β-Glucans are a family of homopolysaccharides of glucose commonly found in fungi, yeasts, plants, and seaweeds. They boost the natural defense mechanisms of the adult host by stimulating both innate (16) and adaptive (1) immune responses. βG are recognized by the pattern recognition receptors of the innate immune system (13). At least four receptors have been identified for the recognition of βG: complement receptor 3, lactosylceramide, scavenger receptors, and dectin-1, the latter of which is considered the most important βG receptor (16, 40). Thus, dietary βG, through binding to innate immune receptors, has the potential to enhance the infant's ability to fight infections and respond to challenges.
Others have examined the effects of yeast βG on immunity in young animals (42, 43). Dietary yeast βG supplementation improved the humoral immunity of pigs and modulated cellular immunity of weanling pigs by mitigating the elevation of proinflammatory cytokines and increasing the production of anti-inflammatory cytokines after an immunological challenge with lipopolysaccharide (LPS) (28). Furthermore, yeast βG exerted antiviral effects against both swine influenza virus in 5-day-old piglets (20) and porcine reproductive and respiratory virus in weanling pigs (47). In both studies, yeast βG administration was associated with an increase in circulating gamma interferon (IFN-γ) concentrations.
To our knowledge, no studies have examined chronic, enteral βG supplementation in a neonatal population. Herein we test a clinically relevant βG, (1,3/1,6)-β-d-glucan (Wellmune WGP, herein referred to as WGP), which is generally recognized as safe (GRAS) by the U.S. FDA, in a developmentally appropriate model. Based on data obtained in experiments in older populations and shorter-term studies in βG-supplemented neonates, we hypothesized that chronic dietary WGP would alter the development of the intestinal mucosal and systemic immunity in the neonatal piglet. Our data demonstrate that WGP did not affect intestinal or immune development in neonatal piglets.
MATERIALS AND METHODS
Chemicals.
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise indicated.
Dietary yeast β-glucan.
(1,3/1,6)-β-d-Glucan (Wellmune WGP, herein referred to as WGP) was obtained from Biothera, Inc. (Eagan, MN). This compound was extracted from Saccharomyces cerevisiae using a process that produces a whole glucan particle in which the outer surface of mannoprotein and inner cellular contents are removed (2). WGP existed as a particulate suspension in the bovine milk-based formulas used herein. WGP was added to formula to provide doses at 1.8 mg/kg of body weight (BW)/day, 18 mg/kg BW/day, or 90 mg/kg BW/day. The lowest dose provided an average WGP intake of 5 mg/day, slightly exceeding Biothera's recommendation of 2 mg/kg BW/day. The middle dose provided an average WGP intake of 50 mg/day. The highest dose provided an average WGP intake of 250 mg/day. The two lowest doses do not surpass the level generally recognized as safe by the U.S. FDA (200 mg/serving; GRAS GRN 239; www.FDA.gov, accessed 27 October 2010). Furthermore, these levels are within the range that has been shown to result in no observed adverse effects in toxicological testing (2 to 100 mg/kg BW/day) (2).
Dietary treatment and animal protocol.
Piglets (n = 68) were obtained at 48 h postpartum to allow for consumption of colostrum. The piglets were randomized to one of four dietary treatment groups: (i) a medicated sow milk replacer formula (WGP0) (formula) (Milk Specialties Global Animal Nutrition, Carpentersville, IL), (ii) formula plus 5 WGP mg/liter (WGP1.8), (iii) formula plus 50 WGP mg/liter (WGP18), or (iv) formula plus 250 WGP mg/liter (WGP90). Piglets were individually housed in environmentally controlled rooms (25°C) in cages capable of maintaining six piglets separated by Plexiglas partitions. Radiant heaters were attached to the tops of the cages to maintain an ambient temperature of 30°C. Formula was offered 14 times daily at a rate of 360 ml/kg BW/day. The piglets were monitored daily for normal growth and food intake, as well as the presence of fever, diarrhea, or lethargy. All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Illinois.
Vaccination.
On day 7 postpartum, a subset of piglets from each treatment group (WGP0, n = 5; WGP1.8, n = 6; WGP18, n = 5; WGP90, n = 6) were vaccinated with a 0.25-ml intramuscular (i.m.) injection of human influenza vaccine (Fluzone; Sanofi Pasteur, Swiftwater, PA). A blood sample was drawn on day 7 from the jugular vein prior to administration of the vaccine. Vaccinated animals were boosted on day 14 with the same dose of influenza vaccine. Blood samples were collected longitudinally from all piglets on day 14 and day 21 from the jugular vein or following euthanasia, respectively.
Sample collection.
On day 7 (WGP0, n = 5; WGP1.8, n = 5; WGP18, n = 5; WGP90, n = 5) or day 21 (WGP0, n = 12; WGP1.8, n = 13; WGP18, n = 11; WGP90, n = 12) postpartum, piglets were sedated with an intramuscular injection of tiletamine HCl and zolazepam HCl, 3.5 mg/kg BW each [Telazol]; Pfizer Animal Health, Fort Dodge, IA). After sedation, blood was collected by cardiac puncture into plain vials for serum isolation and into heparin-laced vials (BD Biosciences, Franklin Lakes, NJ) for isolation of mononuclear cells. Piglets were then euthanized by an intravenous injection of sodium pentobarbital (Fatal Plus, 72 mg/kg BW; Vortech Pharmaceuticals, Dearborn, MI). After death, a laparotomy was performed. The small intestine was quickly excised from the pyloric sphincter to the ileocecal valve, and its length was measured. The small intestine was cut into three segments: duodenum (first 10%), jejunum (middle 75%), and ileum (final 15%). All sections were flushed with ice-cold saline and weighed. The portion of the ascending colon (ASC) most proximal to the ileocecal junction was also excised and rinsed with ice-cold saline. Sections (1 to 2 cm) of each intestinal section were fixed in Bouin's solution or snap-frozen in liquid nitrogen. Remaining ileal segments were opened longitudinally, and ileal Peyer's patches (IPP) were isolated for the preparation of cells and snap-frozen in liquid nitrogen for RNA analysis. Spleen and mesenteric lymph nodes (MLN) were also collected for isolation of cells and snap-frozen for RNA analysis.
Intestinal histomorphology and immunohistochemistry.
Bouin's solution-fixed ileum and ASC samples were embedded in paraffin, sliced to approximately 5 μm with a microtome, and mounted on glass microscope slides. Slides were hematoxylin and eosin (H&E) stained (University of Illinois Veterinary Diagnostic Laboratory). Slides were visualized in the University of Illinois Institute of Genomic Biology Imaging Facility using the NanoZoomer Digital Pathology System (Hamamatsu Corporation, Bridgewater, NJ) and analyzed using AxioVision 4.8 digital image processing software (Carl Zeiss MicroImaging, Inc., Thornwood, NY). To assess ileal villus length and crypt depth, 10 measurements for each parameter were taken per piglet. Additionally, 10 ASC cuff depths were measured per piglet. Ileum samples were also stained for T cells (University of Illinois Veterinary Diagnostic Laboratory). Because the tissues were paraffin embedded and bouin fixed, antigen retrieval was done. A citrate buffer (pH 6.0) was used to break protein cross-links formed by fixation, allowing the antibody to recognize the CD3 protein. After antigen retrieval, slides were incubated with a rabbit anti-human CD3 polyclonal antibody (Biocare Medical, Concord, CA). This antibody cross-reacts with pig CD3 and is extensively used by the Veterinary Diagnostic Histology Laboratory at the University of Illinois College of Veterinary Medicine (whose staff has verified this cross-reactivity by achieving the expected staining of pig lymph node sections). Staining was visualized using a horseradish peroxidase-diaminobenzidine (HRP/DAB) system (Super Sensitive polymer-HRP detection system; Biogenex, San Ramon, CA), following the manufacturer's instructions. Briefly, slides were blocked (casein-phosphate-buffered saline [PBS]) for 20 min, and secondary anti-rabbit-polymer-HRP antibodies were incubated with slides for 30 min. HRP was visualized by DAB exposure for 5 min. Slides were counterstained with hematoxylin for 1 min. The NanoZoomer digital pathology system was used to image slides. Images were analyzed using AxioVision 4.8 digital image processing software. CD3+ cells were counted in 10 villus areas (area = 100 μm2) per piglet. Care was taken to include only the lamina propria area and exclude the epithelial layer and IPP in the areas analyzed. CD3+ cell numbers are expressed relative to 100-μm2 villus area.
Isolation of mononuclear cells from peripheral blood and total cells from immune tissues.
Peripheral blood mononuclear cells (PBMC) were isolated by density gradient centrifugation. Briefly, 10 ml of heparinized blood was diluted in 25 ml of RPMI 1640 (Life Technologies Invitrogen, Grand Island, NY) and layered over Ficoll-Paque Plus lymphocyte separation medium (GE Healthcare, Uppsala, Sweden). The PBMC were recovered after centrifugation (400 × g, 30 min) across the density gradient. Isolated PBMC were placed in complete medium (RPMI 1640 containing 10% fetal calf serum [Life Technologies Invitrogen, Carlsbad, CA], 2 mM l-glutamine [Life Technologies Invitrogen, Carlsbad, CA], 100 μg/ml penicillin, 100 μg/ml streptomycin, and 50 μg/ml gentamicin [Life Technologies Invitrogen, Carlsbad, CA]). Red blood cells were lysed using ammonium chloride lysing buffer. Cells from spleen, MLN, and IPP were obtained by cutting tissues into small pieces and dissociating using a Gentle Macs dissociator (Miltenyi Biotec, Auburn, CA). Cells were then sequentially passed through 100-μm and 40-μm cell strainers (BD Biosciences, Bedford, MA) to form single-cell suspensions. Cells were counted using a Countess automated cell counter (Life Technologies Invitrogen, Carlsbad, CA). The number of viable cells was assessed by trypan blue (Life Technologies Invitrogen, Eugene, OR) exclusion. Isolated cells were kept in complete medium until use.
Phenotypic identification of cells.
The phenotypes of T lymphocyte subpopulations from PBMC, MLN, IPP, and spleen were monitored using fluorescently labeled monoclonal antibodies (MAbs). Lymphocytes were identified by anti-swine CD45 (clone K252-1E4; AbD Serotec, Raleigh, NC). Anti-CD45 was conjugated to Alexa 647 with a Zenon mouse antibody labeling kit (Invitrogen Molecular Probes, Eugene, OR). T lymphocytes were identified by mouse anti-pig CD3-biotin (clone BB23-8E6; Southern Biotech, Birmingham, AL), which was visualized with streptavidin-phycoerythrin (PE)-Cy7 (Southern Biotech). To further differentiate T cell populations, cells were stained with mouse anti-pig CD4-fluorescein isothiocyanate (FITC) (clone 74-12-4; Southern Biotech) and mouse anti-pig CD8-PE (clone 76-2-11; Southern Biotech) antibodies. All staining procedures took place on ice, and care was taken to prevent unnecessary exposure to light. Briefly, one million cells per tube were blocked with anti-pig CD16 (clone G-7; AbD Serotec) for 5 min. Next, cells were incubated for 20 min in a total of 10 μl CD3-biotin. Cells were then centrifuged at 2,000 rpm for 5 min at 4°C. Supernatants were removed. Then, cells were incubated for 20 min in a total volume of 40 μl (10 μl of each MAb: CD45, CD4, and CD8, as well as 10 μl of streptavidin-PE-Cy7). Cells were washed twice with PBS–1% bovine serum albumin (BSA)–0.1% sodium azide and then fixed with 2% paraformaldehyde. Staining was assessed using an LSRII flow cytometer (BD Biosciences, San Jose, CA). The relative number of T cell subpopulations was determined using the FlowJo 7.0 software program (FlowJo, Ashland, OR). CD45+ CD3+ events were considered T cells. CD45+ CD3+ CD4+ CD8− events were considered T helper cells. CD45+ CD3+ CD8+ CD4− events were considered cytotoxic T cells. CD45+ CD3+ CD4+ CD8+ events were considered double-positive T cells.
Mitogenic cell stimulation.
PBMC, spleen, and MLN cells were plated in 96-well plates (2 × 105 cells/well) in a final volume of 200 μl complete medium at 37°C under 5% CO2. Twenty microliters of concanavalin A (ConA) (25 μg/ml) or 20 μl of LPS (20 μg/ml) was added on day 0 (n = 3 wells per sample per stimulant). [3H]thymidine (Perkin Elmer, Boston, MA) was added 72 h after the initiation of the mitogenic stimulation at a concentration of 1 μCi per well, and plates were incubated for an additional 24 h. Plates were stored at −80°C until analysis. Cells were harvested (Harvester 96 Mach III M; TomTec, Hamden, CT) onto 1.5-μm glass fiber filter paper (Skatron Instruments, Sterling, VA) and put into vials containing 7 ml Ultima Gold F scintillation fluid (Perkin Elmer, Waltham, MA). Samples were counted on a Beckman Coulter, LS 6500 scintillation system (Brea, CA). Data are expressed as a change in counts per minute (Δcpm), which was obtained by subtracting counts from unstimulated control wells from counts for wells with mitogens. Samples were analyzed in triplicate. Data analysis was performed on log-transformed Δcpm values.
Influenza vaccine preparation for ex vivo analyses.
Prior to use in ex vivo assays, influenza vaccine was dialyzed to remove additives that inhibit cell proliferation. The vaccine solution was placed in Spectra/Por 4 dialysis membranes (Spectrum Laboratories, Rancho Dominguez, CA) and submerged in PBS for 24 h at 4°C. The protein content of predialyzed and dialyzed influenza vaccine was assessed using a Bradford assay (Quick Start Bradford; Bio-Rad, Hercules, CA). Both samples contained 130 μg protein/ml.
Assessment of cell-mediated response to influenza vaccine antigen.
The cell-mediated immune (CMI) response of the piglets was monitored by stimulating PBMC and spleen cells with the dialyzed influenza vaccine solution ex vivo. This strategy had previously been used to assess CMI in response to the influenza vaccine in human subjects (24). Cells (2 × 105) were added to each well of round-bottom microtiter plates in 150 μl of complete cell culture medium. Fifty microliters of dialyzed influenza vaccine (0.875, 1.75, 3.5, or 7 μg/ml) was added to each well (n = 3 wells per sample per stimulant). On day 4, [3H]thymidine (Perkin Elmer, Boston, MA) was added at a concentration of 1 μCi per well, and plates were incubated overnight. For this antigen-specific assay, cells were treated and analyzed as described above for mitogen stimulation.
Assessment of influenza vaccine-specific antibody response.
An enzyme-linked immunosorbent assay (ELISA) was used to detect swine IgG specific for influenza virus antigens. Flat-bottom plates (Nunc, Rochester, NY) were coated with dialyzed influenza vaccine at a 1:80 dilution in coating buffer (0.5 M carbonate-bicarbonate buffer, pH 9.6) and incubated overnight at 4°C. Following incubation, 200 μl of PBS–10% fetal bovine serum (FBS) was added to each well to block nonspecific binding. Following incubation (1 h at 4°C), the plate was washed three times with PBS–0.05% Tween 20. Duplicate serum samples (50 μl) diluted in PBS–10% FBS were added to each well, and plates were incubated for 1 h at 37°C. Positive stock serum was run on each plate in dilutions ranging from 1:100 to 1:1,600. A standard curve was made using these dilutions. Samples were diluted to fall within the linear range of the standard curve. Plates were washed three times with PBS–Tween. Fifty microliters of goat anti-pig IgG (GeneTex, Irvine, CA), not gamma chain specific, conjugated to peroxidase, was added to each well at a dilution of 1:400 in PBS–10% FBS. Following 1 h of incubation at 37°C, the plate was washed three times with PBS–0.05% Tween. Fifty microliters of tetramethylbenzidine (TMB) substrate reagent (BD Biosciences) was added, and plates were incubated at room temperature (RT) for 20 min. Then, 50 μl 2 N sulfuric acid was added. The plate was analyzed on a spectrophotometer (SpectraMax M2e; Molecular Devices, Sunnyvale, CA) at 450 nm. Influenza vaccine-specific IgG is expressed in arbitrary units calculated from the linear portion of the standard curve.
Assessment of serum total immunoglobulin levels.
Total serum immunoglobulin levels were detected by ELISA using porcine IgG, IgM, and IgA quantification sets (Bethyl Laboratories, Montgomery, TX). A 96-well, flat-bottom plate (Nunc, Rochester, NY) was coated with 100 μl coating antibody (no. of μg of coating antibody as suggested by the manufacturer, diluted in 0.05 M carbonate-bicarbonate buffer, pH 9.6) and incubated overnight at 4°C. The antibody solution was poured off, and the plate was washed three times with PBS–0.05% Tween 20. The plates were blocked with 300 μl of 3% BSA-PBS for 1 h at RT. The plates were washed as before. Serum samples were serially diluted in 0.05% gelatin-PBS and added to the wells in duplicate (100 μl per well), and plates were incubated for 1 h at RT. Samples for standard curves were used as directed. Plates were washed as before, and 100 μl HRP-conjugated detection antibody (concentration as recommended by the manufacturer) in 0.05% gelatin-PBS was added to each well. Plates were protected from light and incubated for 1 h at RT. Plates were washed four times with PBS-Tween 20. One hundred microliters TMB reagent solution (OptEIA; BD Biosciences, San Diego, CA) was added to each well and allowed to develop protected from light at RT for the time recommended by the manufacturer. The reaction was stopped with 100 μl 2 N sulfuric acid per well. The absorbance was read at 450 nm with 570-nm correction using a spectrophotometer (SpectraMax M2e; Molecular Devices, Sunnyvale, CA). Total immunoglobulin values were determined based on a standard curve that was run on each plate.
Tissue mRNA expression.
Total RNA was extracted from snap-frozen IPP, ASC, MLN, and spleen samples with TRIzol reagent (Invitrogen, Carlsbad, CA). RNA was quantified by spectrophotometry (absorbance at 260 nm) using a Nanodrop 1000 instrument (Thermo Scientific, Rockford, IL). The RNA concentration was adjusted to 0.25 μg/ml using RNase-free water. RNA quality was assessed by using a 2100 bioanalyzer (Agilent Technologies, Inc., Santa Clara, CA). All samples had an RNA integrity number (RIN) greater than 6. Reverse transcription was performed on 3 μg total RNA in a reaction volume of 20 μl (high-capacity cDNA reverse transcription kit; Applied Biosystems, Foster City, CA). Quantitative real-time PCR was conducted using SYBR green (Applied Biosystems Inc.), and data were collected using the TaqMan ABI 7900 PCR system (Applied Biosystems Inc.). A total of 40 PCR cycles were run. Primers used are listed in Table 1, and final primer concentrations were 300 nM. The relative standard curve method was used for quantitation. Standard curves consisted of dilutions of pooled spleen cDNA. β-Actin was used as the internal standard reporter gene. Normalized values for each target were calculated by dividing the target quantity mean by the β-actin quantity mean. A fold difference was calculated for each measurement by dividing the normalized target values by the normalized calibrator sample. Tissue from the day 21, formula-fed, nonvaccinated (NV) animals was used as the calibrator in each instance. All samples that were statistically compared to each other were run on the same plate.
Table 1.
Primers used for quantitative RT-PCR
| Gene product | Forward primer (5′ to 3′) | Reverse primer (5′ to 3′) | GenBank accession no. |
|---|---|---|---|
| β-Actina | CACGCCATCCTGCGTCTGGA | AGCACCGTGTTGGCGTAGAG | DQ845171.1 |
| Dectinb | CTCTCACAACCTCACCAGGAGAT | CAGTAATGGGTCGCCAATAAGG | FJ386384.1 |
| IL-2c | TCAACTCCTGCCACAATGT | CTTGAAGTAGGTGCACCGT | EU139160.1 |
| IL-12 p35c | CGTGCCTCGGGCAATTATAA | CAGGTGAGGTCGCTAGTTTGG | NM_213993.1 |
| IL-4d | TTGCTGCCCCAGAGAAC | TGTCAAGTCCGTCAGG | AY294020 |
| IL-10d | CAGATGGGCGACTTGTTG | ACAGGGCAGAATTGATGAC | L20001 |
| IL-6c | CTGGCAGAAAACAACCTGAACC | TGATTCTCATCAAGCAGGTCTCC | AB194100.1 |
| IL-1βc | AACGTGCAGTCTATGGAGT | GAACACCACTTCTCTCTTCA | NM 214055.1 |
| TNF-αc | AACCTCAGATAAGCCCGTCG | ACCACCAGCTGGTTGTCTTT | EU682384.1 |
| TGF-β2c | TGTGTGCTGAGCGCTTTTCT | GAGCGTGCTGCAGGTAGACA | L08375.1 |
Statistical analysis.
Statistical analyses were performed using the software program SAS (SAS, Cary, NC). Body weight, formula intake, and assessment of antibody response were tested between groups by repeated-measures analysis of variance (ANOVA). All other data were analyzed by 2-way ANOVA using the general linear model (GLM) procedure to determine the effects of diet and vaccination or time and their interactions. In the event of a significant main effect being significant, a post hoc least-significant-difference test was used. Statistical significance was defined as a P value of <0.05, and trends were reported as a P value of <0.10. Data are presented as means ± standard deviations (SD).
RESULTS
Because dietary WGP had no effects on the outcomes measured, the results, tables, and figures for all measurements except WGP intake, body weight, intestinal weight, and intestinal length include only control (WGP0) and high-dose WGP (WGP90) treatments.
Formula intake, body weight, intestinal weight, and intestinal length.
Daily formula intake averaged 808 ± 167 ml/day/pig for the first week and 1,488 ± 129 ml/day/pig during the last 2 weeks of the study. Mean WGP intakes over the 21-day period were 0 ± 0, 4.6 ± 0.6, 46.1 ± 7.7, and 225.1 ± 26.6 (mg/day) for WGP0, WGP1.8, WGP18, and WGP90, respectively. At day 21, as planned, mean WGP intakes were 0 ± 0, 1.7 ± 0.2, 16.5 ± 1.6, and 86.1 ± 10.0 (mg/kg BW) for WGP0, WGP1.8, WGP18, and WGP90, respectively (Table 2). Body weight, small intestinal weight, and small intestinal length were not affected by WGP supplementation (Table 2). Additionally, vaccination did not influence formula intake or body weights. Thus, addition of WGP to formula did not affect BW or intestinal growth.
Table 2.
Body weight, WGP intake averaged over time and absolute WGP intake, and total small intestinal weight and length
| Measurementa | Value for diet groupb |
|||||||
|---|---|---|---|---|---|---|---|---|
| Day 7 |
Day 21 |
|||||||
| WGP0 | WGP1.8 | WGP18 | WGP90 | WGP0 | WGP1.8 | WGP18 | WGP90 | |
| Avg body wt (kg) | 2.3 ± 0.4 | 2.3 ± 0.7 | 2.2 ± 0.3 | 2.3 ± 0.5 | 4.1 ± 0.7 | 4.5 ± 0.7 | 4.5 ± 0.8 | 4.3 ± 0.5 |
| Avg WGP intake over 7- or 21-day period (mg/day) | 0 ± 0 | 2.7 ± 0.7 | 25.5 ± 3.7 | 45.0 ± 9.9 | 0 ± 0 | 4.6 ± 0.6 | 46.1 ± 7.7 | 225.1 ± 26.6 |
| WGP intake at d7 or d21 (mg/kg body wt) | 0 ± 0 | 1.8 ± 0.0 | 17.6 ± 0.3 | 88.2 ± 1.1 | 0 ± 0 | 1.7 ± 0.2 | 16.5 ± 1.6 | 86.1 ± 10.0 |
| Small intestinal wt (g kg body wt) | 40 ± 9 | 46 ± 4 | 39 ± 7 | 42 ± 4 | 43 ± 6 | 42 ± 4 | 40 ± 4 | 41 ± 9 |
| Small intestinal length (cm/kg body wt) | 229 ± 28 | 212 ± 67 | 223 ± 23 | 255 ± 39 | 160 ± 39 | 160 ± 21 | 156 ± 29 | 164 ± 21 |
d, day.
Data are expressed as means ± SD.
Immunohistochemistry and histomorphology.
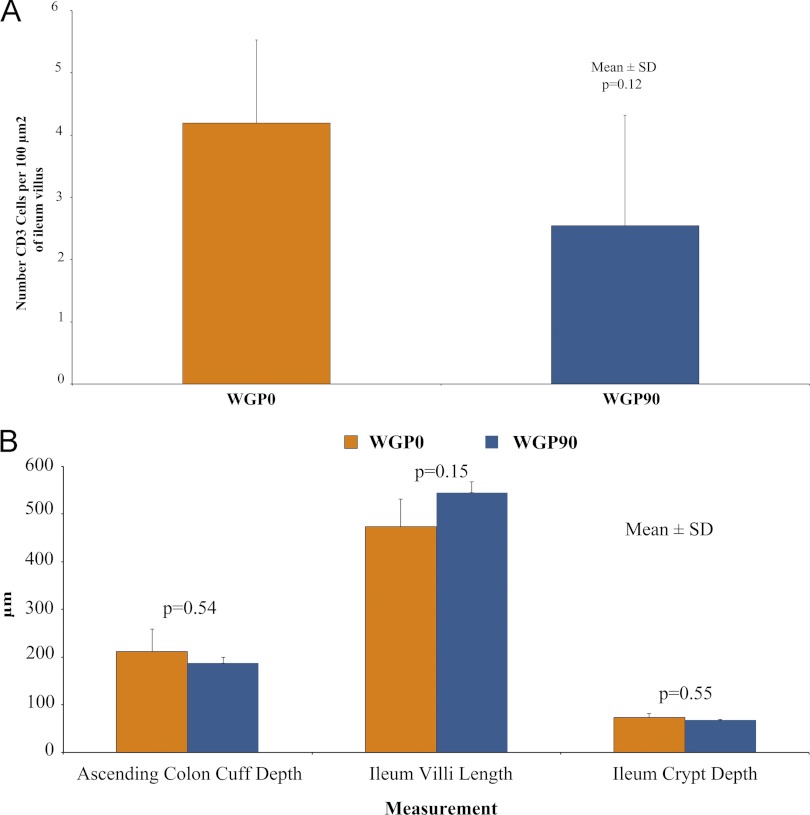
To determine if WGP had an effect on intestinal development, ileal CD3+ T lymphocytes, as well as ileal villus length and ASC cuff depth, were measured. There was a tendency (P = 0.1218) for increasing dietary WGP to decrease numbers of CD3+ cells in the ileal villi of 21-day-old animals (Fig. 1A). Additionally, ASC cuff depth, ileal villus length, and ileal crypt depth at day 21 were not affected by dietary WGP supplementation (Fig. 1B). Therefore, intestinal growth and intestinal T cell numbers were unaffected by chronic enteral WGP administration.
Fig 1.
Immunohistochemistry and histomorphology for 21-day animals. (A) Number of CD3 cells per 100 μm2 of ileum villus did not differ by dietary treatment (P = 0.12). (B) Dietary WGP did not affect ASC colon cuff depth (P = 0.54), ileal villus length (P = 0.15), or ileal crypt depth (P = 0.55). Values are expressed as means ± SD.
T cell populations.
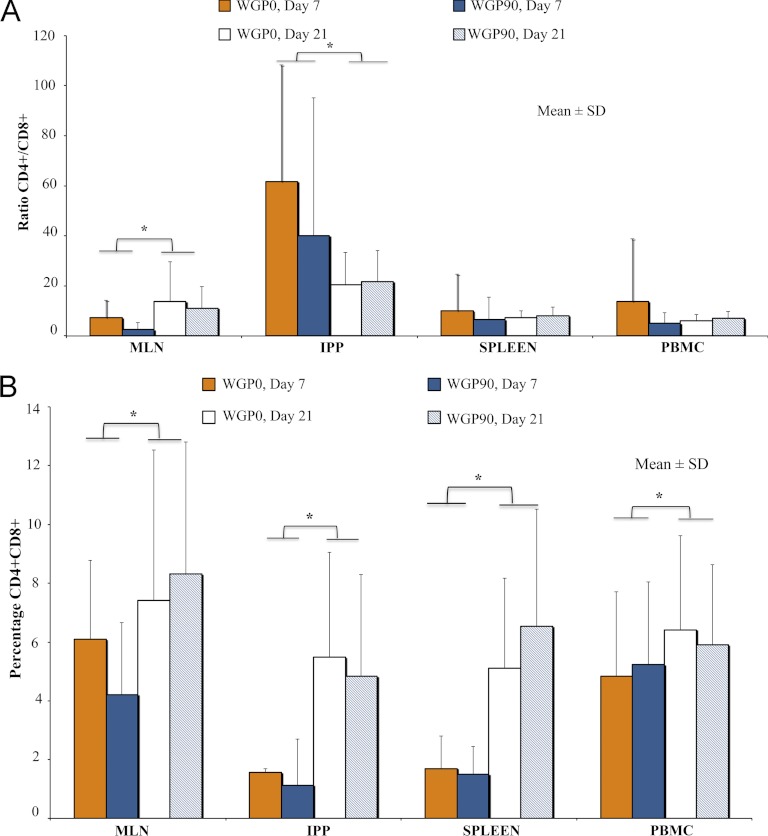
T cell phenotypes in PBMC, spleen, MLN, and IPP were evaluated by flow cytometry. No diet or vaccination effects were seen in T cell populations for T helper cells, but T helper cells were significantly increased on day 21 compared to levels on day 7 in all tissues (Table 3). Conversely, cytotoxic T cells were significantly decreased on day 21 compared to levels on day 7 in all tissues (Table 4). The T helper/cytotoxic T cell ratio was higher at day 21 in MLN, but in IPP it was higher at day 7 (Fig. 2A). As previously reported (49), the doubly positive T cell populations were greater on day 21 than on day 7 in MLN, IPP, spleen, and PBMC (Fig. 2B).
Table 3.
T helper cell populationsa
| Tissue | Day | % T helper cells |
P value | ||||
|---|---|---|---|---|---|---|---|
| WGP0 |
WGP90 |
||||||
| NV | V | NV | V | Modelb (day) | Modelc | ||
| PBMC | 7 | 35.8 ± 22.9 | 34.2 ± 26.1 | ||||
| PBMC | 21 | 48.6 ± 5.8 | 41.6 ± 20.1 | 53.0 ± 16.1 | 45.4 ± 14.5 | 0.5476 (0.0294) | 0.8875 |
| Spleen | 7 | 32.6 ± 24.3 | 24.2 ± 22.8 | ||||
| Spleen | 21 | 40.7 ± 11.9 | 33.4 ± 16.8 | 43.3 ± 14.3 | 45.7 ± 11.5 | 0.3909 (0.0358) | 0.5992 |
| MLN | 7 | 46.2 ± 24.8 | 35.4 ± 30.1 | ||||
| MLN | 21 | 64.3 ± 10.2 | 60.8 ± 7.9 | 65.0 ± 11.3 | 62.0 ± 9.9 | 0.0255 (0.0003) | 0.5500 |
| IPP | 7 | 45.5 ± 33.6 | 24.2 ± 22.4 | ||||
| IPP | 21 | 50.7 ± 13.7 | 44.4 ± 21.7 | 60.1 ± 21.1 | 44.4 ± 23.6 | 0.3216 (0.0481) | 0.9481 |
Data are expressed as means ± SD. Values are CD45+ CD3+ CD4+ CD8− events as a percentage of CD45+ CD3+ events. NV, nonvaccinated; V, vaccinated.
“Model” included diet, day, and interaction: model P value (day P value). All diet and interaction P values > 0.05.
“Model” included diet, vaccination, and interaction. All diet, vaccination, and interaction P values > 0.05.
Table 4.
Cytotoxic T cell populationsa
| Tissue | Day | % cytotoxic T cells |
P value | ||||
|---|---|---|---|---|---|---|---|
| WGP0 |
WGP90 |
||||||
| NV | V | NV | V | Modelb (day) | Modelc | ||
| PBMC | 7 | 23.8 ± 28.6 | 24.6 ± 24.9 | ||||
| PBMC | 21 | 10.4 ± 5.8 | 7.3 ± 3.7 | 7.1 ± 2.3 | 8.8 ± 6.4 | 0.0443 (0.0006) | 0.7515 |
| Spleen | 7 | 21.6 ± 26.8 | 24.1 ± 23.0 | ||||
| Spleen | 21 | 6.2 ± 3.0 | 4.9 ± 2.9 | 5.6 ± 2.7 | 6.8 ± 3.1 | 0.0015 (<0.0001) | 0.7926 |
| MLN | 7 | 19.5 ± 24.1 | 30.4 ± 32.3 | ||||
| MLN | 21 | 8.4 ± 4.3 | 8.7 ± 6.7 | 6.2 ± 2.7 | 9.6 ± 5.5 | 0.0058 (<0.0001) | 0.7894 |
| IPP | 7 | 12.8 ± 24.5 | 19.4 ± 25.7 | ||||
| IPP | 21 | 3.6 ± 2.3 | 5.5 ± 4.3 | 5.2 ± 6.4 | 3.8 ± 2.1 | 0.5446 (0.0323) | 0.5301 |
Data are expressed as means ± SD. Values are CD45+ CD3+ CD8+ CD4− events as a percentage of CD45+ CD3+ events. NV, nonvaccinated; V, vaccinated.
Model included diet, day, and interaction: model P value (day P value). All diet and interaction P values > 0.05.
Model included diet, vaccination, and interaction: model P value (vaccination P value). All diet, vaccination, and interaction P values > 0.05.
Fig 2.
T helper cells, cytotoxic T cells, and double-positive T cells in 7-day and 21-day piglets. (A) The ratio of CD45+ CD3+ CD4+ CD8−/CD45+ CD3+ CD8+ CD4− T cells was significantly higher on day 21 than on day 7 (P = 0.009) in MLN and significantly higher on day 7 than on day 21 (P = 0.046) in IPP. (B) The percentage of double-positive (CD45+ CD3+ CD4+ CD8+) T cells was significantly higher on day 21 in MLN, IPP, spleen, and PBMC (P = 0.0113, 0.03, 0.045, and 0.004, respectively). Values are expressed as means ± SD. ∗, P < 0.05.
Mitogenic cell stimulation and assessment of cell-mediated response.
Proliferation was evaluated to determine if cells isolated from animals fed WGP or vaccinated with influenza vaccine showed increased proliferation to mitogens. LPS- or ConA-stimulated PBMC, spleen, and MLN cells from all animals proliferated more than unstimulated cells (P < 0.0001). Data are expressed as changes in counts per minute (Δcpm), which were obtained by subtracting counts from unstimulated control wells from counts for wells with mitogens, which is why unstimulated data are not shown. All animals responded to LPS or ConA similarly, independent of in vivo treatment of WGP or vaccination (Table 5). Influenza vaccine treatment of 0.875, 1.75, 3.5, and 7 μg/ml did not stimulate proliferation compared to results for unstimulated cells in the blood and spleen for vaccinated or nonvaccinated piglets (P > 0.05) (data not shown).
Table 5.
Proliferation of PBMC, spleen, and MLN cells at day 21a
| Cell type | Proliferation (log Δcpm) with stimulation by: |
|||||
|---|---|---|---|---|---|---|
| LPS |
ConA |
|||||
| WGP0 | WGP90 | Modelb P value | WGP0 | WGP90 | Modelb P value | |
| PBMC | 3.0 ± 3.3 | 2.7 ± 2.7 | 0.9465 | 4.8 ± 4.9 | 4.5 ± 4.3 | 0.8181 |
| Spleen | 3.6 ± 3.8 | 4.1 ± 4.1 | 0.1541 | 4.6 ± 4.6 | 4.7 ± 4.7 | 0.8690 |
| MLN | 2.9 ± 3.4 | 3.3 ± 3.5 | 0.2212 | 5.1 ± 4.4 | 5.1 ± 4.4 | 0.7964 |
Data are expressed as means ± SD.
Model included diet as a main effect.
Assessment of influenza vaccine-specific antibody response.
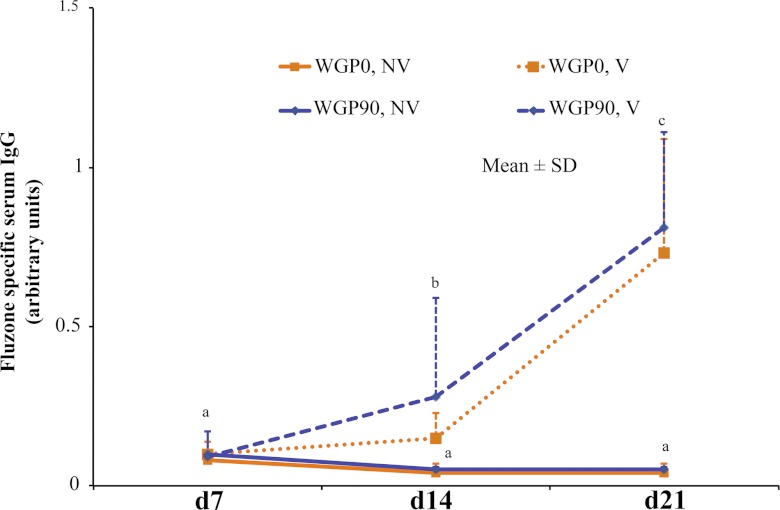
Vaccine-specific serum IgG levels were measured to determine if dietary WGP would improve the humoral response to vaccination. Serum influenza vaccine-specific IgG was similar in all vaccinated animals at all time points regardless of dietary treatment (Fig. 3). Vaccinated animals had a significantly larger amount of influenza vaccine-specific IgG than nonvaccinated animals at day 14 and day 21 (P < 0.0001). Day 21 piglets had a greater vaccine response than day 14 piglets (P < 0.0001). Influenza vaccine-specific IgG is low on day 7, which implies that sows and piglets were negative for influenza virus antibodies at the start of the study.
Fig 3.
Influenza vaccine-specific serum IgG levels. Piglets were vaccinated on day 7 and day 14 with a 0.25-ml i.m. injection of human influenza vaccine. A prevaccination blood sample was drawn on day 7, and blood samples were collected longitudinally from all piglets on day 14 and day 21. On day 14 and day 21, vaccinated piglets had greater serum influenza vaccine-specific IgG than nonvaccinated piglets (P < 0.0001). Vaccinated piglets had greater serum influenza vaccine-specific IgG levels at day 21 than at day 14 (P < 0.0001). Values are expressed as means ± SD. Repeated-measures ANOVA: overall model, P = 0.02; diet, P = 0.17; day, P < 0.001; and diet-day interaction, P = 0.68.
Assessment of total serum immunoglobulin levels.
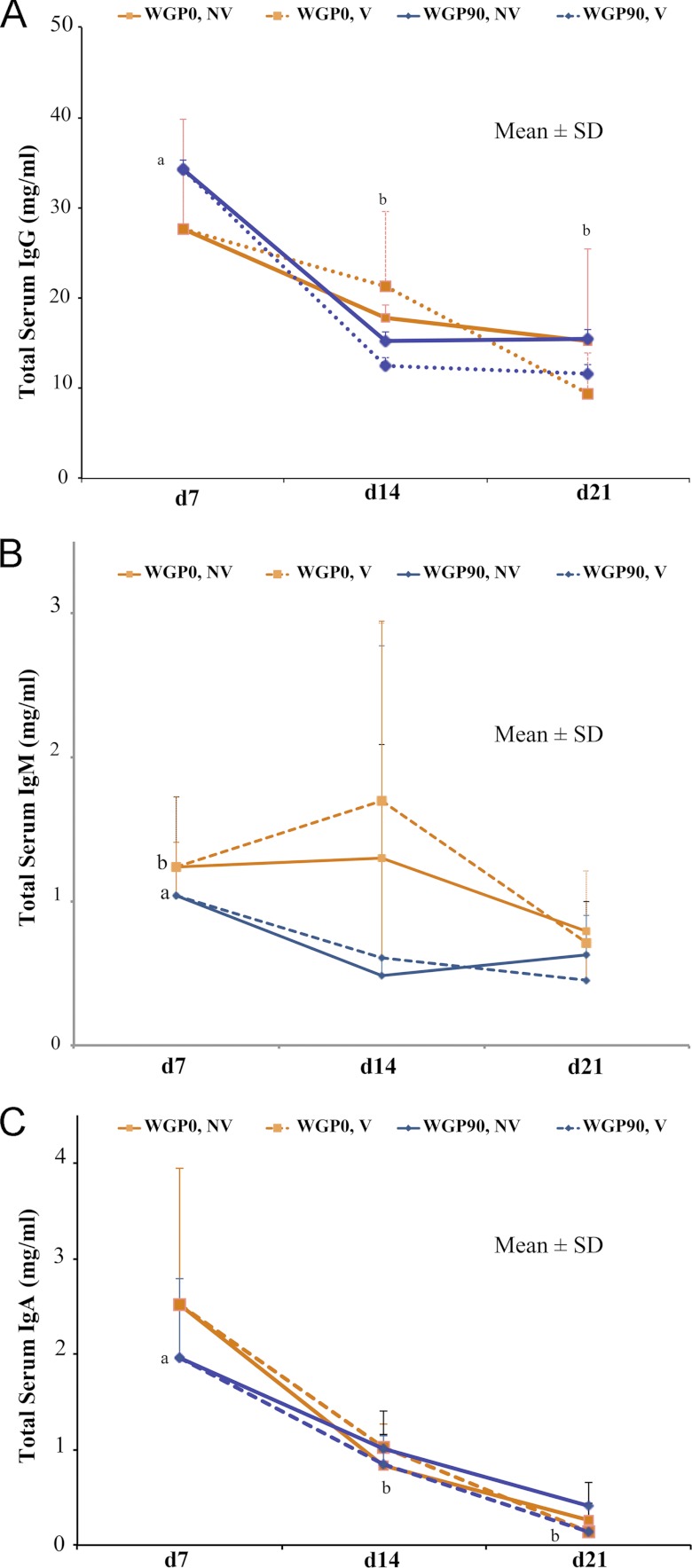
To determine if dietary WGP could increase total immunoglobulin concentrations, sera from the WGP0 and WGP90 groups were analyzed for serum IgG, IgM, and IgA concentrations at day 7, day 14, and day 21. Serum IgG concentrations were higher (P < 0.05) at day 7 than at day 14 and day 21 (Fig. 4A). However, serum IgG concentrations were unaffected by diet and vaccination. WGP0 piglets had higher (P = 0.04) serum IgM concentrations than WGP90 piglets (Fig. 4B). However, serum IgM concentrations were unaffected by vaccination and day. Serum IgA concentrations were higher (P < 0.05) at day 7 than at day 14 and day 21 (Fig. 4C). However, serum IgA concentrations were unaffected by vaccination and diet.
Fig 4.
Total serum IgG, IgM, and IgA concentrations. Data are expressed as means ± SD. Data with different letters are significantly different. (A) Total serum IgG concentrations were higher on day 7 than on day 14 and day 21 (P = 0.002). No diet (P = 0.6) or vaccination (P = 0.88) differences were detected. Model, P = 0.002; day, P < 0.0001; diet, P = 0.6; and day-diet interaction, P = 0.49. (B) Total serum IgM concentrations were higher in WGP0 piglets than in WGP90 piglets (P = 0.04). No day (P = 0.09) or vaccination (P = 0.23) differences were detected. Model, P = 0.04; day, P = 0.09; diet, P = 0.01; and day-diet interaction, P = 0.80. (C) Total serum IgA concentrations were higher on day 7 than on day 14 or day 21 (P < 0.0001). No diet (P = 0.49) or vaccination (P = 0.17) differences were detected. Model, P < 0.0001; day, P < 0.0001; diet, P = 0.49; and day-diet interaction, P = 0.32.
Tissue mRNA abundance.
Based on previous studies where βG influenced cytokines (20, 47), we examined cytokine mRNA expression. In addition, mRNA levels of dectin-1, a primary receptor for βG, was measured to test if dietary WGP would regulate the receptor's expression. Measurement of mRNA levels also enabled the determination of developmental or vaccination effects. Expression of dectin-1 did not change in IPP, MLN, ASC, or spleen in response to chronic dietary WGP administration (Tables 6 and 7). No dietary or vaccination differences in the relative abundances of cytokine mRNAs were detected in whole-tissue samples of MLN, IPP, or ASC from 21-day animals (Table 6). In addition, neither WGP supplementation nor day affected mRNA abundance in the spleen. However, vaccination significantly increased (P < 0.05) interleukin 2 (IL-2), IL-4, IL-6, and dectin mRNA abundance in whole spleen tissue from 21-day animals (Table 7). IL-1β, IL-10, IL-12, tumor necrosis factor alpha (TNF-α), or TGF-β2 mRNA abundance in whole spleen tissue was not altered.
Table 6.
Cytokine and dectin mRNA expression in MLN, IPP, and ASC of nonvaccinated and influenza vaccine-treated 21-day-old pigletsa
| Sample and cytokine or dectin | mRNA expression |
Model P valueb | |||
|---|---|---|---|---|---|
| NV |
V |
||||
| WGP0 | WGP90 | WGP0 | WGP90 | ||
| MLN | |||||
| IL-2 | 1.0 ± 0.7 | 0.5 ± 0.5 | 1.3 ± 0.3 | 0.5 ± 0.4 | 0.1601 |
| IL-12 | 1.0 ± 0.6 | 0.5 ± 0.4 | 0.4 ± 0.4 | 0.7 ± 0.3 | 0.4369 |
| IL-4 | 1.0 ± 0.4 | 0.6 ± 0.3 | 1.1 ± 0.3 | 1.1 ± 0.3 | 0.4978 |
| Dectin | 1.0 ± 0.3 | 0.6 ± 0.5 | 0.8 ± 0.5 | 0.9 ± 0.3 | 0.6668 |
| IPP | |||||
| IL-2 | 1.0 ± 0.6 | 0.8 ± 0.3 | 1.2 ± 0.4 | 3.6 ± 0.2 | 0.3157 |
| IL-12 | 1.0 ± 0.3 | 0.8 ± 0.2 | 1.0 ± 0.6 | 1.5 ± 0.2 | 0.7665 |
| IL-4 | 1.0 ± 0.3 | 1.4 ± 0.1 | 1.6 ± 0.2 | 1.0 ± 0.2 | 0.1392 |
| Dectin | 1.0 ± 0.2 | 2.5 ± 0.2 | 2.0 ± 0.1 | 1.2 ± 0.1 | 0.4426 |
| ASC | |||||
| IL-2 | 1.0 ± 0.2 | 0.8 ± 0.7 | 0.6 ± 2.0 | 1.0 ± 0.2 | 0.5407 |
| IL-12 | 1.0 ± 0.4 | 0.9 ± 0.4 | 0.4 ± 0.8 | 1.1 ± 1.3 | 0.7454 |
| IL-4 | 1.0 ± 0.3 | 0.2 ± 1.0 | 0.3 ± 0.8 | 1.1 ± 0.9 | 0.9978 |
| Dectin | 1.0 ± 1.1 | 0.4 ± 0.5 | 0.2 ± 0.3 | 0.3 ± 0.2 | 0.4514 |
Data are expressed as means ± SD relative to fold difference of gene expression in nonvaccinated, formula diet samples at day 21. NV, nonvaccinated; V, vaccinated.
Model included diet and vaccination. Interaction between diet and vaccination was previously run and was found to be nonsignificant. All diet and vaccination P values > 0.05.
Table 7.
Cytokine and dectin mRNA expression in spleen of nonvaccinated and human influenza vaccine-treated 21-day-old pigletsa
| Cytokine or dectin | mRNA expression |
Model P valueb (vaccination) | |||
|---|---|---|---|---|---|
| NV |
V |
||||
| WGP0 | WGP90 | WGP0 | WGP90 | ||
| IL-2 | 1.0 ± 1.1 | 1.3 ± 1.1 | 12.4 ± 5.9 | 2.5 ± 2.4 | 0.0321 (0.0056) |
| IL-12 | 1.0 ± 1.1 | 13.8 ± 4.7 | 6.3 ± 3.9 | 2.8 ± 1.4 | 0.4981 (0.6394) |
| IL-4 | 1.0 ± 0.5 | 0.6 ± 0.4 | 3.8 ± 1.5 | 1.3 ± 0.7 | 0.0004 (<0.0001) |
| IL-6 | 1.0 ± 0.5 | 1.0 ± 0.4 | 3.1 ± 1.1 | 2.2 ± 1.6 | 0.0299 (0.001) |
| TNF-α | 1.0 ± 0.3 | 3.3 ± 2.7 | 5.3 ± 1.5 | 2.1 ± 1.7 | 0.1388 (0.0451) |
| Dectin | 1.0 ± 0.6 | 0.6 ± 0.4 | 4.3 ± 2.8 | 1.4 ± 0.6 | 0.0226 (0.0046) |
| TGF-β2 | 1.0 ± 0.3 | 1.9 ± 2.7 | 6.4 ± 4.5 | 1.6 ± 1.0 | 0.0500 (0.084) |
Data are expressed as mean ± SD, relative to fold difference of gene expression in day 21 nonvaccinated formula diet group. NV, nonvaccinated; V, vaccinated.
Model included diet, vaccination, and interaction: model P value (vaccination P value). All diets and interaction were nonsignificant (P > 0.05).
DISCUSSION
At birth, the neonatal immune system is Th2 polarized (14). Formula-fed neonates also produce less-robust antibody responses to vaccination than breast-fed infants (31, 32, 34, 36, 38). It was hypothesized that supplementation of formula with a yeast product, WGP, would potentiate immune system responses to vaccination and boost the overall immune system in formula-fed piglets. However, dietary WGP had no effect on intestinal or systemic immune development in full-term, colostrum-fed, neonatal piglets. Importantly, no adverse effects of orally administered WGP were observed, and WGP did not negatively impact the expected immune system development. Studies examining the effects of βG on the local intestinal immune response in neonatal pigs are scarce. The experiment presented herein allowed for the local intestinal immune response to be examined in tissues in addition to being monitored in the blood.
Other studies have shown βG to impact immune development. In our study, lymphocyte populations (PBMC, MLN, spleen, or IPP) were not altered by WGP supplementation, nor was proliferation affected (PBMC, MLN, spleen). Previous literature has shown βG effects on lymphocyte populations. Levels of CD4+ and CD8+ T cells were higher in weaned pigs fed Saccharomyces cerevisiae-derived βG-supplemented diets than in pigs fed control diets (17). However, it took 8 weeks for this effect to be detected. In contrast, animals in our study were fed WGP for a total of 3 weeks. We vaccinated and boosted such young pigs in a close interval because we are interested in early immune development. In another study looking at older animals, spleen cells from mice supplemented with one to five intragastric βG treatments (obtained from culture filtrate of the fungus Sclerotinia sclerotiorum IFO 9395) proliferated more strongly when stimulated with T or B cell mitogens than did those from control mice (44). Not only were the animals older in that study, but also βG was administered in a more concentrated form over a shorter time period than in our study. Others have shown that cells directly treated with some forms of βG proliferate more than untreated cells (41). In other studies, βG from S. cerevisiae has been shown to enhance the activities of natural killer cells and peritoneal macrophages, as well as stimulating cytotoxic T lymphocytes, B cells, and macrophages in mice (11). However, these effects were seen only when βG was applied to cells in vitro or administered intraperitoneally (i.p.). We did not evaluate the effects of WGP added to cells in culture because we are primarily interested in assessing the effects from dietary exposure in vivo and not direct effects on immune cells in vitro. In addition, WGP may be a less biologically active form of βG than those used in studies mentioned above. However, the WGP used in our study is a clinically relevant βG, since it is generally recognized as safe for infants by the U.S. FDA. Thus, differences in our results from those previously reported could be due to the ages of the experimental subjects, the mode/length of administration of WGP, or the specific βG used.
It is possible that an immunocompromised immune system is needed to see the effects of WGP. As such, our ability to detect significant changes in body weight, intestinal growth, and immune status with dietary WGP treatment may have been limited by the fact that full-term, colostrum-fed piglets rather than diseased or immunocompromised animals were used in our studies. In fact, dietary supplementation of weaned pigs with βG Original XPC yeast culture (Diamond V, Cedar Rapids, IA) improved growth in pigs challenged with Salmonella (35), and WGP given orally increased the survival rate of mice infected with anthrax (26). Finally, WGP has demonstrated effectiveness by switching a Th2 response to a protective Th1 cell-mediated response in tumor-bearing mice when it was given orally and combined with antitumor monoclonal antibodies (3). Thus, the health of our animals may have limited our ability to detect effects of WGP supplementation, but the study was designed to assess effects in healthy formula-fed infants using an FDA-approved βG.
However, some differences in mRNA levels based on the vaccination status of an animal were observed. Tissue mRNA abundances of IL-2, IL-4, IL-6, and dectin were significantly higher in whole spleen tissue from 21-day vaccinated animals compared to those from nonvaccinated animals. Previously these cytokines have not been found to be significantly increased in serum concentrations of adults vaccinated against influenza (6). However, blood cultures from infants who had been vaccinated against tuberculosis (TB) had a greater cytokine response when exposed to TB for 15 out of 21 cytokines tested, including IL-2, IL-4, and IL-6 (27). Most cytokines tested tended to be increased, although not all were increased significantly, for vaccinated animals than for nonvaccinated animals. Because we collected samples on day 7, prior to vaccination, and on day 21, 1 week after the last vaccination, our ability to detect some cytokine differences was compromised.
Although cytokine expression was increased due to vaccination in our study, a WGP effect on the influenza vaccine vaccination response was not seen. We used an ELISA to measure antibody concentrations, since it is more sensitive than the hemagglutination assay (45). Our lab has shown that an influenza virus hemagglutination inhibition (HI) assay was not able to detect influenza virus antibodies in our nonvaccinated or vaccinated piglets, confirming the lack of sensitivity of the HI assay (data not shown). Our current WGP study suggests that dietary WGP does not improve immune response to influenza vaccination in a full-term, colostrum-fed, and otherwise healthy neonatal piglet population. This is surprising, since a number of studies have shown that βG supplementation improves immune responses. A number of other studies, however, have shown little effect of βG supplementation on the antibody response to systemic immunization. For instance, yeast βG did not enhance the efficacy of vaccinations for porcine reproductive and respiratory syndrome (PRRS) virus (19) or different enterotoxinogenic Escherichia coli (ETEC) antigens (12). Additionally, dietary WGP did not affect antibody or cell-mediated immune responses to influenza virus vaccination in mice (33). βG from S. cerevisiae supplemented to piglets vaccinated with an atrophic rhinitis vaccine actually caused pigs to produce fewer antigen-specific antibodies (17), but pigs supplemented with βG from S. cerevisiae injected with ovalbumin produced more antibodies (28). An oral dietary supplement may have a greater chance of affecting an oral vaccination than an i.m. vaccination. However, most vaccinations are administered i.m., with the exception of the rotavirus vaccine. Additionally, we have tried to use an oral rotavirus vaccine with our piglet model, but we could not detect an effect of vaccination on antibody titer (unpublished observation). This is likely because rotavirus commonly infects pig herds, and so maternally transferred antirotavirus antibody levels were high. Ultimately, we are interested in the effects of oral βG on immune development, and our functional outcome was antibody production in response to an i.m. vaccination, since the majority of neonatal vaccinations are given i.m.
To gain a perspective of systemic immune development of piglets, total serum IgG, IgM, and IgA concentrations were measured. Our developmental study showed results similar to the findings of Bourne et al. (9). We found that the serum IgG concentrations were higher (P < 0.05) at day 7 than at day 14 and day 21. Serum IgM concentrations were higher (P < 0.05) in WGP0 piglets than in WGP90 piglets. IgA serum concentrations were higher at day 7 than at day 14 and day 21 (P < 0.05). Our previous developmental study, as well as Bourne's research, suggests that this is a typical developmental pattern in pigs receiving colostrum (9), where maternal antibody is transferred to the piglets' circulation early and disappears over time due to normal protein turnover. WGP supplementation only moderately affected total IgM and had no effect on total IgG or IgA levels.
One potential source of variation seen in these studies may be the form of βG utilized. βG can be extracted from a number of sources, and these forms have been shown to have various activity levels. Although the molecular structures of the various βG chains with branch points at 1,3/1,6 are highly conserved, the three-dimensional structure of the molecule can be different among organisms and depends on the chain length, branch type, and branch frequency. The method by which βG are processed or manufactured can also have a significant impact on their immune stimulatory activity (7, 29) (37) (39) (46). There are also data that indicate that more water-soluble polymers are more active, and related to that, the immunopotentiating activity of βG can depend on the helical conformation and on the presence of hydrophilic groups located on the outside surface of the helix. Evidence also suggests that the activity is dependent on the size, with high-molecular-weight (100,000 to 200,000) fractions being most active, while fractions from the same source with molecular weights of 5,000 to 10,000 show no activity (5, 15, 25). Thus, the physical structure of WGP may have limited its ability to affect change when administered orally. Again, we utilized WGP since it is a clinically relevant βG, which is generally recognized as safe by the FDA for the population we wanted to target.
In conclusion, the effects of orally administered βG on immunity vary by βG source and animal model. Further studies are needed to examine the effectiveness of modulating the neonate's immune system with different forms of βG.
ACKNOWLEDGMENTS
This work was funded by Abbott Nutrition, Columbus, OH.
We thank Barbara Pilas and Ben Montez from the University of Illinois Roy J. Carver Biotechnology Center, Flow Cytometry Core Facility, for their expertise and guidance on flow staining and analysis. Special thanks also go to the members of the Donovan laboratory for assistance with piglet care and tissue processing.
Sharon Donovan and Jeff Woods have received grant funding from Abbott Nutrition. Sharon Donovan has served as a paid consultant for Abbott Nutrition. Shelly Hester was a part-time intern at Abbott Nutrition. No other authors have conflicts to disclose.
Footnotes
Published ahead of print 18 July 2012
REFERENCES
- 1. Akramiene D, Kondrotas A, Didziapetriene J, Kevelaitis E. 2007. Effects of beta-glucans on the immune system. Medicina (Kaunas) 43:597–606 [PubMed] [Google Scholar]
- 2. Babicek K, Cechova I, Simon RR, Harwood M, Cox DJ. 2007. Toxicological assessment of a particulate yeast (1,3/1,6)-beta-D-glucan in rats. Food Chem. Toxicol. 45:1719–1730 [DOI] [PubMed] [Google Scholar]
- 3. Baran J, Allendorf DJ, Hong F, Ross GD. 2007. Oral beta-glucan adjuvant therapy converts nonprotective Th2 response to protective Th1 cell-mediated immune response in mammary tumor-bearing mice. Folia Histochem. Cytobiol. 45:107–114 [PubMed] [Google Scholar]
- 4. Bauer E, Williams BA, Smidt H, Verstegen MW, Mosenthin R. 2006. Influence of the gastrointestinal microbiota on development of the immune system in young animals. Curr. Issues Intest. Microbiol. 7:35–51 [PubMed] [Google Scholar]
- 5. Blaschek W, Kasbauer J, Kraus J, Franz G. 1992. Pythium aphanidermatum: culture, cell-wall composition, and isolation and structure of antitumour storage and solubilised cell-wall (1–3),(1–6)-beta-D-glucans. Carbohydr. Res. 231:293–307 [DOI] [PubMed] [Google Scholar]
- 6. Bogomolov SV, Zhirova SN, Kostinov MP. 2008. Cytokine profile and level of antibodies after administration of split-vaccine against influenza to adults. Zh. Mikrobiol. Epidemiol. Immunobiol. 5:57–61 (In Russian.) [PubMed] [Google Scholar]
- 7. Bohn JA BJ. 1995. (1,3)-B-D-glucans as biological response modifiers: a review of structure-functional activity relationships. Carbohydr. Polym. 28:3 [Google Scholar]
- 8. Borca MV, Gudmundsdottir I, Fernandez-Sainz IJ, Holinka LG, Risatti GR. 2008. Patterns of cellular gene expression in swine macrophages infected with highly virulent classical swine fever virus strain Brescia. Virus Res. 138:89–96 [DOI] [PubMed] [Google Scholar]
- 9. Bourne FJ. 1973. The immunoglobulin system of the suckling pig. Proc. Nutr. Soc. 32:205–215 [DOI] [PubMed] [Google Scholar]
- 10. Collado-Romero M, Arce C, Ramirez-Boo M, Carvajal A, Garrido JJ. 2010. Quantitative analysis of the immune response upon Salmonella typhimurium infection along the porcine intestinal gut. Vet. Res. 41:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cross GG, et al. 2001. Immunostimulant oxidized beta-glucan conjugates. Int. Immunopharmacol. 1:539–550 [DOI] [PubMed] [Google Scholar]
- 12. Decuypere J, Dierick N, Boddez S. 1998. The potentials for immunostimulatory substances (B-1,3/1,6-glucans) in pig nutrition. J. Anim. Feed Sci. 7(Suppl. 1):259 [Google Scholar]
- 13. Descroix K, Ferrieres V, Jamois F, Yvin JC, Plusquellec D. 2006. Recent progress in the field of beta-(1,3)-glucans and new applications. Mini Rev. Med. Chem. 6:1341–1349 [DOI] [PubMed] [Google Scholar]
- 14. Desselberger U, et al. 2009. Rotaviruses and rotavirus vaccines. Br. Med. Bull. 90:37–51 [DOI] [PubMed] [Google Scholar]
- 15. Fabre I, Bruneteau M, Ricci P, Michel G. 1984. Isolation and structural studies of glucans from Phytophthora parasitica. Eur. J. Biochem. 142:99–103 [DOI] [PubMed] [Google Scholar]
- 16. Goodridge HS, Wolf AJ, Underhill DM. 2009. Beta-glucan recognition by the innate immune system. Immunol. Rev. 230:38–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hahn TW, Lohakare JD, Lee SL, Moon WK, Chae BJ. 2006. Effects of supplementation of beta-glucans on growth performance, nutrient digestibility, and immunity in weanling pigs. J. Anim. Sci. 84:1422–1428 [DOI] [PubMed] [Google Scholar]
- 18. Helgeland L, Vaage JT, Rolstad B, Midtvedt T, Brandtzaeg P. 1996. Microbial colonization influences composition and T-cell receptor V beta repertoire of intraepithelial lymphocytes in rat intestine. Immunology. 89:494–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hiss S, Sauerwein H. 2003. Influence of dietary ss-glucan on growth performance, lymphocyte proliferation, specific immune response and haptoglobin plasma concentrations in pigs. J. Anim. Physiol. Anim. Nutr. (Berl.) 87:2–11 [DOI] [PubMed] [Google Scholar]
- 20. Jung K, et al. 2004. Antiviral effect of Saccharomyces cerevisiae beta-glucan to swine influenza virus by increased production of interferon-gamma and nitric oxide. J. Vet. Med. B Infect. Dis. Vet. Public Health 51:72–76 [DOI] [PubMed] [Google Scholar]
- 21. Kaplan JL, Shi HN, Walker WA. 2011. The role of microbes in developmental immunologic programming. Pediatr. Res. 69:465–472 [DOI] [PubMed] [Google Scholar]
- 22. Kelly D, Coutts AG. 2000. Early nutrition and the development of immune function in the neonate. Proc. Nutr. Soc. 59:177–185 [DOI] [PubMed] [Google Scholar]
- 23. Kelly D, King T, Aminov R. 2007. Importance of microbial colonization of the gut in early life to the development of immunity. Mutat. Res. 622:58–69 [DOI] [PubMed] [Google Scholar]
- 24. Keylock KT, et al. 2007. Higher antibody, but not cell-mediated, responses to vaccination in high physically fit elderly. J. Appl. Physiol. 102:1090–1098 [DOI] [PubMed] [Google Scholar]
- 25. Kojima T, Tabata K, Itoh W, Yanaki T. 1986. Molecular weight dependence of the antitumor activity of schizophyllan. Agric. Biol. Chem. 50:231 [Google Scholar]
- 26. Kournikakis B, Mandeville R, Brousseau P, Ostroff G. 2003. Anthrax-protective effects of yeast beta 1,3 glucans. MedGenMed 5:1. [PubMed] [Google Scholar]
- 27. Lalor MK, et al. 2010. Complex cytokine profiles induced by BCG vaccination in UK infants. Vaccine 28:1635–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li J, et al. 2005. Effects of beta-glucan extracted from Saccharomyces cerevisiae on humoral and cellular immunity in weaned piglets. Arch. Anim. Nutr. 59:303–312 [DOI] [PubMed] [Google Scholar]
- 29. Mueller A, et al. 2000. The influence of glucan polymer structure and solution conformation on binding to (1→3)-beta-D-glucan receptors in a human monocyte-like cell line. Glycobiology 10:339–346 [DOI] [PubMed] [Google Scholar]
- 30. Nygard AB, Jorgensen CB, Cirera S, Fredholm M. 2007. Selection of reference genes for gene expression studies in pig tissues using SYBR green qPCR. BMC Mol. Biol. 8:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ogra SS, Weintraub D, Ogra PL. 1977. Immunologic aspects of human colostrum and milk. III. Fate and absorption of cellular and soluble components in the gastrointestinal tract of the newborn. J. Immunol. 119:245–248 [PubMed] [Google Scholar]
- 32. Pabst HF, Spady DW. 1990. Effect of breast-feeding on antibody response to conjugate vaccine. Lancet 336:269–270 [DOI] [PubMed] [Google Scholar]
- 33. Pence BD, Hester SN, Donovan SM, Woods JA. 2012. Dietary whole glucan particles do not affect antibody or cell-mediated immune responses to influenza virus vaccination in mice. Immunol. Invest. 41:275–289 [DOI] [PubMed] [Google Scholar]
- 34. Pickering LK, et al. 1998. Modulation of the immune system by human milk and infant formula containing nucleotides. Pediatrics. 101:242–249 [DOI] [PubMed] [Google Scholar]
- 35. Price KL, et al. 2010. Use of Saccharomyces cerevisiae fermentation product on growth performance and microbiota of weaned pigs during Salmonella infection. J. Anim. Sci. 88:3896–3908 [DOI] [PubMed] [Google Scholar]
- 36. Sabirov A, Casey JR, Murphy TF, Pichichero ME. 2009. Breast-feeding is associated with a reduced frequency of acute otitis media and high serum antibody levels against NTHi and outer membrane protein vaccine antigen candidate P6. Pediatr. Res. 66:565–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Saito H, et al. 1991. Relationship between conformation and biological response for (1–3)-beta-D-glucans in the activation of coagulation factor G from limulus amebocyte lysate and host-mediated antitumor activity. Demonstration of single-helix conformation as a stimulant. Carbohydr. Res. 217:181–190 [DOI] [PubMed] [Google Scholar]
- 38. Schaller JP, et al. 2004. Effect of dietary ribonucleotides on infant immune status. Part 1. Humoral responses. Pediatr. Res. 56:883–890 [DOI] [PubMed] [Google Scholar]
- 39. Seijelid R, Bogwald J, Lundwall A. 1981. Glycan stimulation of macrophages in vitro. Exp. Cell Res. 131:121. [DOI] [PubMed] [Google Scholar]
- 40. Sonck E, Stuyven E, Goddeeris B, Cox E. 2009. Identification of the porcine C-type lectin dectin-1. Vet. Immunol. Immunopathol. 130:131–134 [DOI] [PubMed] [Google Scholar]
- 41. Sonck E, Stuyven E, Goddeeris B, Cox E. 2010. The effect of beta-glucans on porcine leukocytes. Vet. Immunol. Immunopathol. 135:199–207 [DOI] [PubMed] [Google Scholar]
- 42. Stuyven E, et al. 2009. Effect of beta-glucans on an ETEC infection in piglets. Vet. Immunol. Immunopathol. 128:60–66 [DOI] [PubMed] [Google Scholar]
- 43. Stuyven E, Van den Broeck W, Nauwynck H, Goddeeris BM, Cox E. 2010. Oral administration of beta-1,3/1,6-glucan Macrogard fails to enhance the mucosal immune response following oral F4 fimbrial immunisation in gnotobiotic pigs. Vet. Immunol. Immunopathol. 137:291–297 [DOI] [PubMed] [Google Scholar]
- 44. Suzuki I, Hashimoto K, Ohno N, Tanaka H, Yadomae T. 1989. Immunomodulation by orally administered beta-glucan in mice. Int. J. Immunopharmacol. 11:761–769 [DOI] [PubMed] [Google Scholar]
- 45. Takemae N, et al. 2011. Swine influenza virus infection in different age groups of pigs in farrow-to-finish farms in Thailand. Virol. J. 8:537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Willment JA, Gordon S, Brown GD. 2001. Characterization of the human beta-glucan receptor and its alternatively spliced isoforms. J. Biol. Chem. 276:43818–43823 [DOI] [PubMed] [Google Scholar]
- 47. Xiao Z, Trincado CA, Murtaugh MP. 2004. Beta-glucan enhancement of T cell IFNgamma response in swine. Vet. Immunol. Immunopathol. 102:315–320 [DOI] [PubMed] [Google Scholar]
- 48. Zanetti M. 1992. Ontogeny of the immune system and the invisible frontier to immune regulation. Int. Rev. Immunol. 8:209–218 [DOI] [PubMed] [Google Scholar]
- 49. Zuckermann FA, Husmann RJ. 1996. Functional and phenotypic analysis of porcine peripheral blood CD4/CD8 double-positive T cells. Immunology 87:500–512 [PMC free article] [PubMed] [Google Scholar]