SUMMARY
The multistep sequence leading to leukocyte migration is thought to be locally regulated at the inflammatory site. Here, we show that broad systemic programs involving long-range signals from the sympathetic nervous system (SNS) delivered by adrenergic nerves, regulate rhythmic recruitment of leukocytes in tissues. Constitutive leukocyte adhesion and migration in murine bone marrow (BM) and skeletal muscle microvasculature fluctuated with circadian peak values at night. Migratory oscillations, altered by experimental jetlag, were implemented by perivascular SNS fibers, acting on β-adrenoreceptors expressed on non-hematopoietic cells, and leading to tissue-specific, differential circadian oscillations in the expression of endothelial cell adhesion molecules and chemokines. We showed that these rhythms have physiological consequences by alteration of hematopoietic cell recruitment and overall survival in models of septic shock, sickle cell vaso-occlusion and BM transplantation. These data provide unique insight in the leukocyte adhesion cascade and the potential for time-based therapeutics for transplantation and inflammatory diseases.
Leukocyte recruitment is critical for combating pathogens in the periphery as well as for bone marrow (BM) repopulation after transplantation. Much progress has been made in the past two decades in our understanding of the major molecular mechanisms involved in leukocyte recruitment in response to an inflammatory challenge. Leukocytes initially tether and roll on endothelial cell P- and E-selectins, allowing signals from chemokines and endothelial receptors to activate leukocyte integrins to bind to intercellular cell adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1). These high affinity interactions lead to leukocyte arrest on endothelial cells and subsequently diapedesis toward an inflammatory site or for engraftment in the BM (Butcher, 1991; Ley et al., 2007; Muller, 2011; Springer, 1994; Vestweber and Blanks, 1999; Wagner and Frenette, 2008). This sequential multistep process is regulated by signals in situ from adhesion receptors and by soluble factors (e.g. cytokines and chemoattractants), thereby enabling endothelial cells to serve as gatekeepers at the interface of blood and tissues.
While leukocyte migration in inflammatory scenarios has been intensely studied, the regulation of leukocyte trafficking under homeostasis is less understood. Steady-state migration of hematopoietic stem cells (HSCs) and lymphocytes in lymphoid and non-lymphoid tissues has been described as part of normal immunosurveillance to maximize encounters with potential pathogens (Massberg et al., 2007; Sigmundsdottir and Butcher, 2008; von Andrian and Mackay, 2000). It has been assumed that similar surveillance mechanisms exist for myeloid cells whose migration to tissues exposed to the external environment (e.g. skin, gut) keeps pathogens at bay. Constitutive, low level expression of endothelial adhesion molecules likely regulates myeloid cell trafficking because mice lacking major adhesion pathways are susceptible to spontaneous bacterial infections (Bullard et al., 1996; Forlow et al., 2002; Frenette et al., 1996). Since leukocytes play key roles in regenerative processes, one would predict that the organism also possesses broad “housekeeping” programs to maintain the integrity of all tissues, irrespective of infectious probabilities.
Circadian rhythms regulate several vital biological processes through internal molecular clocks (Dibner et al., 2010; Green et al., 2008). Blood leukocyte numbers have long been known to exhibit circadian oscillations (Haus and Smolensky, 1999) and more recent studies have revealed that the release of hematopoietic stem and progenitor cells from the BM follows similar rhythms (Lucas et al., 2008; Mendez-Ferrer et al., 2008). Interestingly, specific circadian times have been linked with the onset of acute diseases, notably in the cardio-vascular system (Muller et al., 1985; Willich et al., 1987). Emerging data, in turn, indicate that chronic perturbations of circadian rhythms promote vascular diseases (Anea et al., 2009; Brown et al., 2009). Although the mechanisms are still undefined, numerous studies have demonstrated strong associations between high leukocyte counts and various ischemic vascular diseases (Coller, 2005; Margolis et al., 2005). Here, we tested the hypothesis that circadian-controlled neural signals influence leukocyte behavior and the inflammatory response. We show that leukocyte recruitment to tissues under homeostasis was not a continuous process, but rather exhibited circadian oscillations, and that these rhythms, orchestrated by the molecular clock via adrenergic nerves, can impact disease outcome.
EXPERIMENTAL PROCEDURES
Animals
Bmal1−/− (gift from C. A. Bradfield), Adrb2tm1Bkk (gift from G. Karsenty), Berkeley SCD mice [Tg(Hu-miniLCRα1GγAγδβS) Hba−/− Hbb−/−] (gift from N. Mohandas), Sele−/−Selp−/−, FVB/N-Adrb3tm1Lowl/J, Icam1tm1Jcgr/J, Vav1-creA2Kio/J, STOCK Tg(TIE2GFP)287Sato/J mice (all from Jackson Laboratories), and ROSA26Sortm2(ACTB−Luc)Tyj and the inbred FVB/NJ and C57BL/6-CD45.1/2 congenic strains (all from the National Cancer Institute) were used in this study. See Extended Experimental Procedures for references and details. All mice used were males, housed on a 12h-light/dark cycle (lights on/off at 7am/7pm) with food ad libitum. All experimental procedures were approved by the Animal Care and Use Committees of Albert Einstein College of Medicine and Mount Sinai School of Medicine.
Reagents
Details can be found in Extended Experimental Procedures.
Whole-mount immunofluorescence
Whole-mount immunostaining of cremaster muscle and BM tissues was performed as previously detailed (Scheiermann et al., 2007). See Extended Experimental Procedures for details.
Intravital microscopy
BIM determination of leukocyte/vessel wall interactions in murine cremasteric venules, hemodynamic characteristics, and image analyses were studied as previously detailed (Chang et al., 2008; Scheiermann et al., 2009). For MFIM, animals were prepared as previously detailed for the cremaster (Chiang et al., 2007) and the BM (Mazo et al., 1998). See Extended Experimental Procedures for details.
Surgical denervation techniques
The genitofemoral nerve (Lucio et al., 2001) and the superior cervical ganglion (Alito et al., 1987; Walton and Buono, 2003) were dissected as described. See Extended Experimental Procedures for details.
BM transplantation and generation of chimeric mice
Transplantation procedures were carried out using congenic CD45.1/2 mice. Chimeric mice were generated by i.v. injection of 1×106 donor mice bone marrow nucleated cells into lethally irradiated (12 Gy, split dose, 3h apart) mice as previously detailed (Katayama et al., 2006). For survival studies, the recovery of donor mature WBC in peripheral blood and survival were monitored as indicated. In some experiments, recipient mice were irradiated with 4 Gy of sublethal irradiation and donor/recipient chimerism in mature peripheral leukocytes were analyzed by flow cytometry.
Flow cytometry, sorting and Q-PCR
Tissues were harvested from Tie2-GFP animals, minced and digested in type IV collagenase (Clostridium histolyticum, Sigma). Single cell suspensions were stained with DAPI and fluorescence-conjugated antibodies and analyzed by flow cytometry using an LSRII (BD). Data were analyzed with FlowJo (Tree Star) or FACS Diva 6.1 software. Endothelial cells were sorted into Q-PCR Dynabead lysis buffer (Invitrogen) and processed with SYBR green as previously described (Mendez-Ferrer et al., 2008). Details can be found in Extended Experimental Procedures.
Adoptive transfer of labeled BM cells
BM cells were labeled with carboxyfluorescein succinimidyl ester (CFSE) (Invitrogen) as per manufacturer’s instructions and injected into non-irradiated recipient mice (at ZT4 and ZT12). Cells were allowed to circulate for 1h. The trafficking of labeled BM cells was assessed by MFIM of the BM immediately after 10µg of R6G was injected to visualize BM sinusoids. In some experiments, BM cells were labeled with VT680 (Visen MEDICAL). The subsets of adoptively transferred cells in the recipient BM were analyzed by flow cytometry.
Homing assay of HSPCs
Experiments were performed as previously described (Katayama et al., 2003). See Extended Experimental Procedures for details.
β-adrenergic agonist treatment
Isoproterenol, the β3 adrenergic agonist, BRL37344, or the β2 adrenergic agonist, clenbuterol (5 mg/kg) were injected i.p. for 5 days and an in vitro homing assay or BM transplantation was performed 24 hours after the last injection to minimize the direct effect of the drugs on the injected donor cells.
CXCL12 ELISA
Details can be found in Extended Experimental Procedures.
Induction of light regime
To induce changes in light regime mice were placed in a light cycler (Park Bioservices) with a 12h-inverted light cycle for 12h (to induce jetlag), a minimum of 2 weeks to completely establish an inverse light cycle or kept for 3 weeks in complete darkness.
Histopathology
Details can be found in Extended Experimental Procedures.
Statistics
All data are represented as mean ± SEM. Comparisons between two samples were done using the paired and unpaired Student’s t tests. One-way ANOVA analyses followed by Tukey’s multiple comparison tests were used for multiple group comparisons. Statistical analyses were performed with Graph Pad Prism 5. Log-rank analyses were used for Kaplan-Meier survival curves. *P<0.05, **P<0.01, ***P<0.001.
RESULTS
Hematopoietic cell recruitment in the BM operates under circadian control
Circulating total leukocyte counts oscillate in murine blood, peaking ~5 h after the onset of light (zeitgeber time, ZT5) and exhibiting a trough at ZT13 (Figure 1A), confirming previous reports (Haus and Smolensky, 1999). These rhythms are observed for HSCs (Mendez-Ferrer et al., 2008) as well as all major leukocyte subsets (Figure S1A) but not erythrocytes and platelets (not shown).
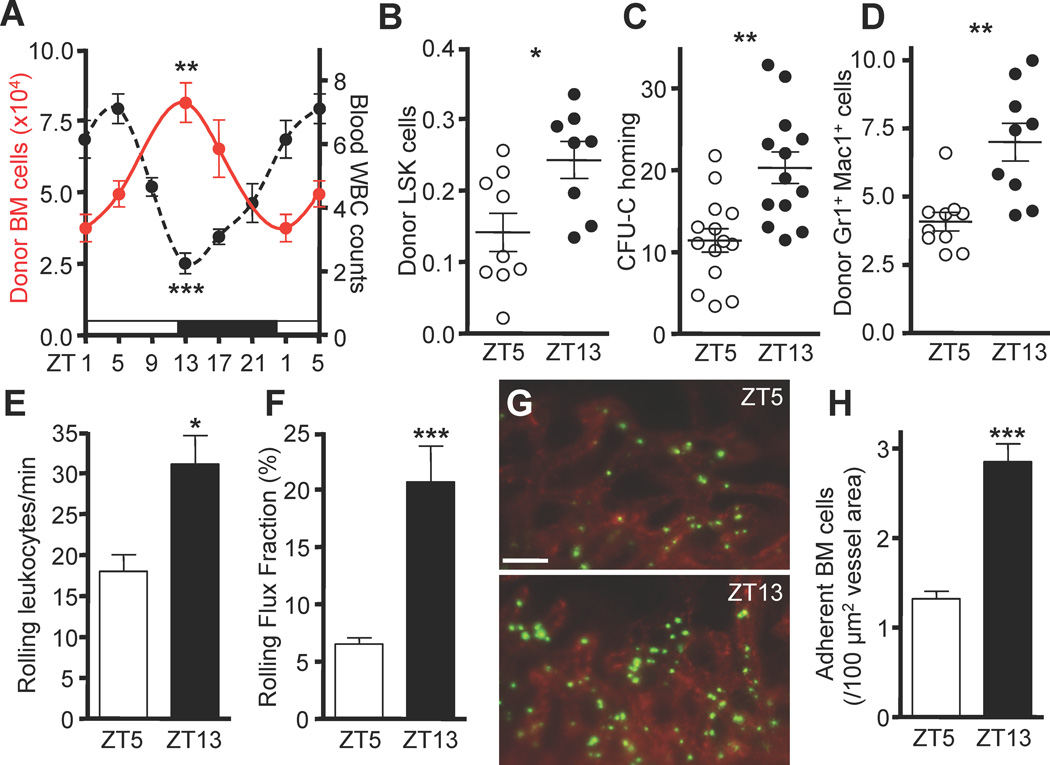
Figure 1. Circadian oscillations of hematopoietic cell recruitment to the bone marrow.
(A) Time course of homed adoptively transferred BM cells (red) and corresponding WBC numbers in blood (×103/µl, black). Light and dark cycles are indicated by white and black bars, respectively. n = 4–15. (B) Quantification of homing of donor LSK cells (×103) 3h after 5×106 BM cells were transferred to recipients. n = 8–9. (C–D) Circadian oscillations in homing of colony-forming units in culture (CFU-C) (in %) (C) and neutrophils (×104) (D). n = 9–14. (E–F) Quantifications of absolute numbers of rolling leukocytes in BM sinusoids (E) and the rolling flux fraction (F). n = 33–43 vessels from 8–9 mice per group. (G–H) In vivo images (G) and quantification (H) of fluorescently labeled adherent BM cells (green) after adoptive transfer. BM sinusoids were identified with rhodamine 6G (red). n = 60 areas from 7 mice per group. *P<0.05, **P<0.01, ***P<0.001. Scale bar: 50 µm. See also Figure S1 and Table S1.
To test whether the recruitment of leukocytes from the blood to tissues also exhibited circadian preferences, we initially investigated hematopoietic cell recruitment to the BM. When we assessed the recruitment potential of adoptively transferred BM cells throughout the day, we observed significant circadian oscillations in homing of total hematopoietic cells to the BM whose pattern ran in antiphase with that of blood exhibiting a peak at ZT13 and a trough in the daytime (ZT5) (Figure 1A). Circadian recruitment was observed for Lineage− Sca-1+ c-kit+ (LSK) cells (Figure 1B), colony-forming units in culture (CFU-C, Figure 1C), as well as neutrophils (Figure 1D).
To investigate the sequence of events in vivo, we visualized the interactions between circulating leukocytes and BM sinusoidal endothelium using multichannel fluorescence intravital microscopy (MFIM) of the calvarial BM as previously described (Chiang et al., 2007; Mazo et al., 1998). The number of endogenous rolling leukocytes in BM sinusoids was increased at ZT13 compared to ZT5 (Figure 1E and F) as was the number of adherent cells after adoptive transfer (Figure 1G–H and Figure S1B–C). These findings suggested that the circadian time has a significant impact on the rhythmic recruitment of different hematopoietic cell populations to the BM.
Rhythmic leukocyte recruitment to skeletal muscle
We next aimed to investigate whether circadian oscillations also dictated leukocyte recruitment to peripheral tissues using the cremasteric muscle as a model to investigate leukocyte-endothelial cell interactions in real time. Initial investigations by whole-mount immunofluorescence staining of unstimulated muscle tissues for the pan-leukocyte marker CD45 and myeloid antigens F4/80 or Gr-1, revealed numerous extravascular CD45+ cells alongside postcapillary venules identified by expression of platelet-endothelial cell adhesion molecule-1 (PECAM-1), vascular-endothelial cadherin (VE-Cadherin) and ephrin-receptor B4 (EphB4) (Figure 2A and S2A). Interestingly, extravascular leukocyte numbers exhibited circadian fluctuations, with CD45+F480+ macrophages representing the predominant leukocyte population in muscle (81% of CD45+ cells vs 2% for neutrophils, data not shown), peaking at ZT13 along postcapillary venules, where leukocyte infiltration mainly occurs (Figure 2B–C and S2B–C), but not arterioles or capillaries (Figure S2D). Staining for Ki67 revealed very few positive leukocytes with no detectable circadian rhythm (not shown) suggesting that the observed leukocyte increase at night was not due to proliferation.
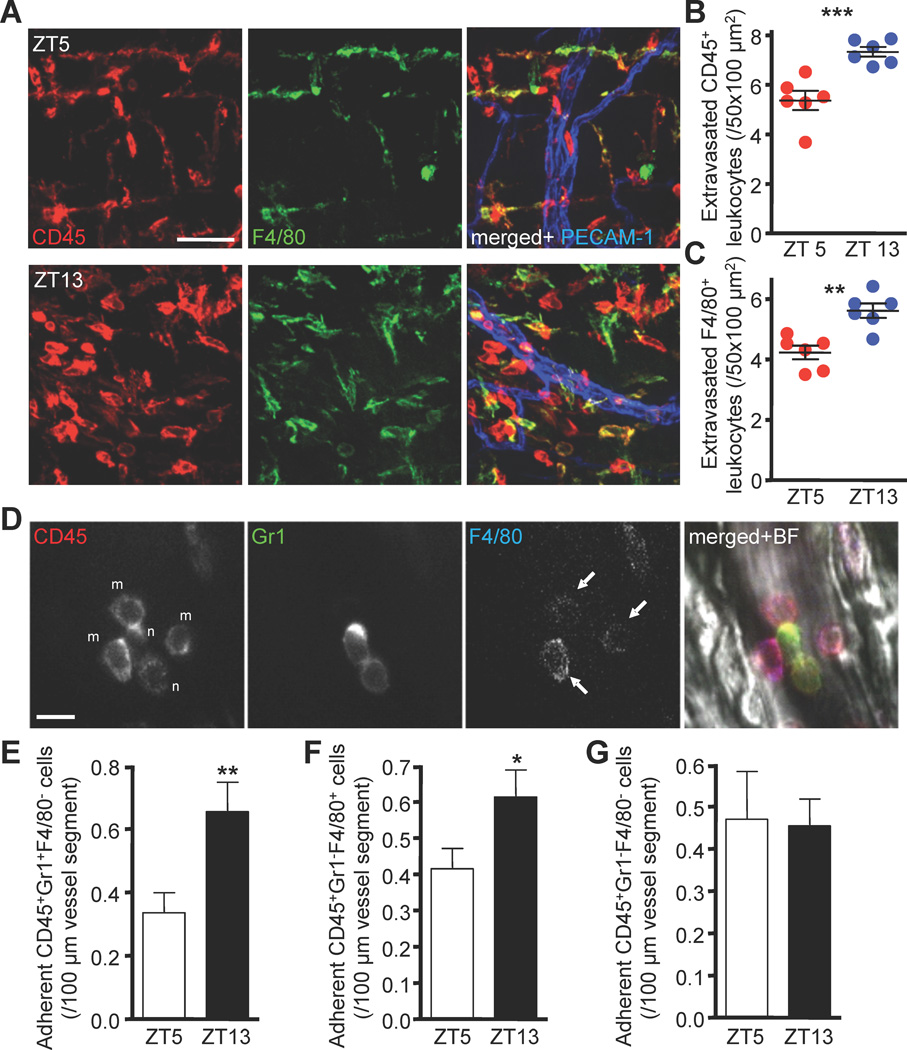
Figure 2. Circadian oscillations of leukocyte recruitment to skeletal muscle.
(A–C) Ex vivo images (A) and quantifications (B–C) of extravasated CD45+ and F4/80+ leukocytes situated around postcapillary venules as analyzed by whole-mount immunofluorescence staining of cremaster muscle tissues. n = 6. (D–G) Adherent leukocyte subsets as determined by multichannel fluorescent intravital microscopy. (D) In vivo images showing antibody-stained adherent leukocytes. Adherent neutrophils, n (E), monocytes, arrows, m (F) and lymphocytes (G). n = 142–148 vessels quantified from 7 mice per group. *P<0.05, **P<0.01, ***P<0.001. Scale bars, A: 50 µm, D: 10 µm. See also Figure S2 and Table S1.
We next quantified leukocyte recruitment in real-time in the exteriorized cremaster muscle by MFIM after injection of very low doses of fluorescently-conjugated antibodies to identify leukocyte populations in vivo (Figure 2D) (Chiang et al., 2007). Under surgically induced trauma conditions, the numbers of adherent neutrophils and monocytes significantly increased at night, while lymphocyte numbers remained unchanged (Figure 2E–G) and overall leukocyte rolling was not affected (not shown). No circadian differences in hemodynamic parameters such as blood flow or wall shear rates were observed at both time points in BM or skeletal muscle (Table S1). Thus, even when an inflammatory response is triggered, the experimental time can influence the recruitment of myeloid cells in trauma-stimulated tissues. Together, these data argue for analogous mechanisms orchestrating circadian recruitment to BM and skeletal muscle across a wide range of hematopoietic lineages and differentiation stages.
Oscillations of adhesion molecules and chemokines mediate circadian leukocyte recruitment
The circadian differences in leukocyte rolling and/or adhesion in the BM and skeletal muscle suggest substantial diurnal changes in the ability of endothelial cells to recruit leukocytes. We used transgenic mice expressing GFP under the Tie-2 promoter (Tie2-Gfp) to isolate endothelial cells (Motoike et al., 2000). Whole-mount staining of cremaster muscle and BM tissues from Tie2-Gfp mice as well as flow cytometry of collagenase IV-digested tissues using antibodies directed against PECAM-1 and VE-Cadherin confirmed endothelial GFP expression (Figure S3A–D). We therefore sorted the CD45− VE-Cadherin+ PECAM-1+ GFP+ subset for quantitative PCR (Q-PCR) analyses (Figure S3C–D). Interestingly, we observed significant fluctuations in Icam1 expression in sorted cremasteric endothelial cells with peak and trough values overlapping with those of leukocyte recruitment (Figure 3A). By contrast, and consistent with the circadian differences observed in adhesion but not rolling in the muscle (Figure 2E–F and data not shown), there was no difference in the expression of endothelial selectins (Sele and Selp), Vcam1, Icam2 or Cd44 (Figure 3A and S3E). Immunofluorescence staining of frozen tissue sections corroborated these data at the protein level (Figure 3B and Figure S3F). We confirmed circadian fluctuations of ICAM-1 on the endothelial cell surface in vivo by quantifying the numbers of i.v. injected adherent green or red fluorescent microspheres coated with anti-ICAM-1 or isotype control antibodies using MFIM (Figure 3C, left). Oscillations were also seen in hematopoietic Icam1−/− radiation chimeras, excluding the potential contribution from ICAM-1-bearing blood leukocytes (Figure 3C, right). We also investigated circadian expression levels of chemokines known to be involved in myeloid cell trafficking in cremasteric endothelial cells by Q-PCR. We detected a clear circadian rhythm for Ccl2 (Figure 3D) but not for other chemokines tested, including Ccl3, Ccl5, Cxcl1, Cxcl2 and Cx3cl1 (Figure S3G). In contrast to the muscle, BM endothelial cells exhibited circadian differences in P-selectin, E-selectin and VCAM-1, but not ICAM-1 at both the RNA and protein levels (Figure 3E and S3H).
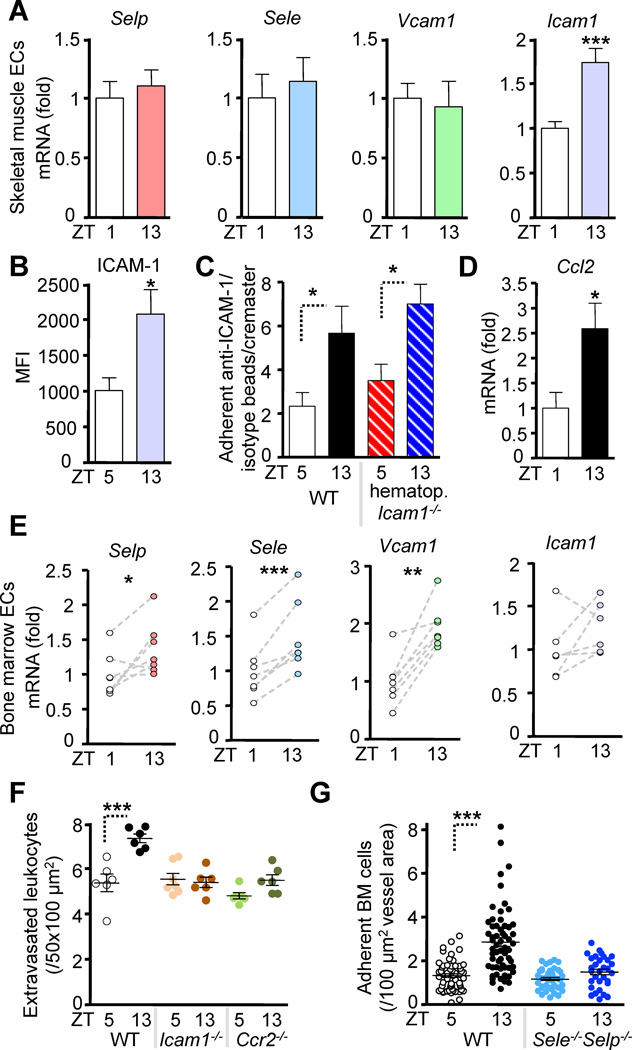
Figure 3. Oscillations of promigratory factors mediate circadian leukocyte recruitment.
(A) Q-PCR analysis of sorted cremasteric endothelial cells (ECs) for P-selectin (Selp), E-selectin (Sele), Vcam1 and Icam1. n = 12–16. (B) Quantification of ICAM-1 protein expression in muscle by confocal immunofluorescence imaging of frozen sections. n = 9. (C) MFIM quantification of specific (anti-ICAM-1-coated) vs. non-specific (IgG-coated) fluorescent microsphere adhesion in the cremasteric microvasculature in WT and hematopoietic Icam1−/−/WT BM chimeras. n = 5–6. (D) Q-PCR analysis of sorted cremasteric endothelial cells for Ccl2. n = 6. (E) Q-PCR of sorted BM endothelial cells. n = 6–7. (F) Numbers of extravasated CD45+ leukocytes as analyzed by whole-mount immunofluorescence staining of cremaster muscle tissues in WT control, Icam1−/− and Ccr2−/− animals. n = 6–7. (G) Quantification of adherent fluorescently labeled cells to BM sinusoids in WT and Selp−/−Sele−/− mice after adoptive transfer. n = 31–75 areas from 4–8 mice per group. *P<0.05, **P<0.01, ***P<0.001. See also Figure S3.
To investigate the functional relevance of ICAM-1 and CCL2 fluctuations in skeletal muscle we examined the numbers of extravasated leukocytes in Icam1−/− mice and mice deficient in the CCL2 receptor (Ccr2−/−) by whole-mount ex vivo immunofluorescence. Consistent with our expression results, no circadian rhythm was apparent, demonstrating the critical requirement of these molecules in this activity (Figure 3F).
To assess the functional relevance of fluctuations in adhesion molecule expression in BM, we examined the role of P- and E-selectins. MFIM of BM microvessels of Sele−/−Selp−/− mice revealed that in these mice circadian oscillations in leukocyte recruitment were ablated (Figure 3G). In stark contrast and in agreement with the expression data, circadian leukocyte recruitment to the BM was independent of ICAM-1 since Icam1−/− mice exhibited no alterations in circadian oscillations in the BM relative to WT animals (Figure S3I).
Taken together, these data demonstrate that tissue-specific expression of key promigratory factors fluctuates within endothelial cells of these tissues. Of importance, the differences in their molecular signature correlate with the oscillations observed in their respective functions, i.e. adhesion in the cremaster muscle (ICAM-1, CCL2) and both rolling and adhesion in the BM (endothelial selectins, VCAM-1).
Requirements of local adrenergic nerves
Most prior studies have focused on the relevance of humoral factors in synchronizing circadian rhythms (Dimitrov et al., 2009; Haus, 2007). We have recently described a key role for local innervation in the circadian release of HSCs from the BM (Mendez-Ferrer et al., 2008). To assess the function of local innervation in hematopoietic cell recruitment to tissues, we adopted two surgical approaches to denervate the cremaster muscle and BM. For the cremaster muscle, we denervated mice unilaterally by microsection of the genitofemoral nerve (GFNx) (Lucio et al., 2001) that innervates this tissue (Zempoalteca et al., 2002). For the BM, we sympathectomized mice by unilateral surgical ablation of the superior cervical ganglion (SCGx) (Alito et al., 1987), which resulted in ptosis on the denervated side (Figure S4A) as seen in the Horner’s syndrome (Walton and Buono, 2003). Denervation was confirmed by whole-mount immunofluorescence staining for the sympathetic nerve marker tyrosine hydroxylase (TH) (Zhou et al., 1995), which revealed marked reductions in the number of TH+ fibers in the neurectomized sides of the tissues compared to the sham-operated contralateral sides (Figure 4A–B). Thus, these approaches allowed us to investigate the role of local innervation in nerve-intact and denervated tissues of the same mouse.
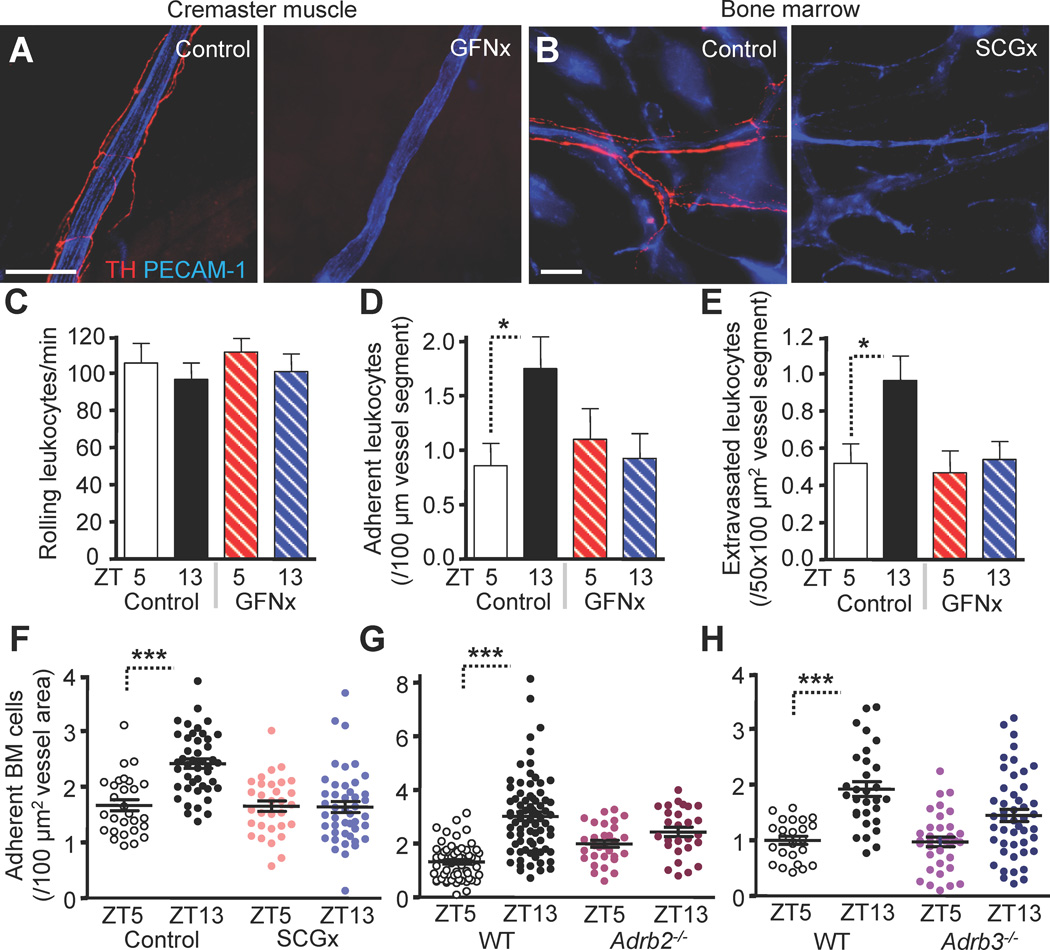
Figure 4. Requirements of local adrenergic nerves.
(A–F) Unilateral surgical denervation of the genitofemoral nerve (GFNx) or the superior cervical ganglion (SCGx). (A–B) Images of TH+ fibers (red) and associated vessels (PECAM-1, blue) in cremaster muscle (A) or calvarium (B) 4 weeks after GFNx or SCGx of the contralateral control and operated sides. (C–E) BIM microscopy of exteriorized cremaster muscle tissues quantifying rolling (C), adhesion (D) and transmigration (E) after GFNx. n = 28–40 vessels from 3–4 mice per group. (F–H) Quantification of adherent fluorescently labeled cells to BM sinusoids after SCGx (F) or in Adrb2−/− (G) or Adrb3−/− (H) mice after adoptive transfer. n = 28–45 vessels from 5–6 mice per group. *P<0.05, ***P<0.001. Scale bars: 100 µm. See also Figure S4 and Table S2.
While brightfield intravital microscopy (BIM) studies of the cremasteric microvasculature revealed no circadian oscillations in leukocyte rolling (Figure 4C), leukocyte adhesion (Figure 4D) and extravasation (Figure 4E) were significantly elevated at night in contralateral muscle tissues with no oscillations observed in the denervated side. In addition, GFNx completely abolished the nightly increase in ICAM-1 expression (Figure S4B), suggesting that the night surge in ICAM-1 is controlled locally by adrenergic sympathetic nerve fibers. Adoptively transferred BM cells in SCGx animals exhibited circadian fluctuations in the numbers of adherent cells in the nerve-intact side, whereas the denervated side did not show any oscillations (Figure 4F). No circadian differences were observed in hemodynamic parameters, tissue weight or vascular density in neurectomized animals (Figure S4C–E and Table S2). Together, these studies demonstrate the importance of local innervation for circadian oscillations in hematopoietic cell recruitment to both skeletal muscle and BM.
Signals from the SNS are transmitted from the brain to peripheral tissues by norepinephrine through adrenoreceptors (Elenkov et al., 2000). To examine which adrenergic receptors were important for the circadian regulation of hematopoietic cell trafficking, we adoptively transferred fluorescently labeled WT BM cells into β2-(Adrb2) or β3-(Adrb3) adrenergic receptor-deficient recipients. We found that circadian oscillations in cell recruitment to the BM were significantly reduced in both recipient strains (Figure 4G–H). In addition, circadian differences in the expression of BM endothelial cell adhesion molecules were ablated in these mice (Figure S4F–G). Similarly, no circadian oscillations were observed in numbers of extravascular leukocytes in the cremaster muscle of Adrb2−/− or Adrb3−/− animals at steady state (Figure S4H–I) and BM chimeras for both strains revealed the critical role of non-hematopoietic β2- and β3-adrenoreceptors in circadian leukocyte recruitment to this tissue (Figure S4J–L). Furthermore, the night surge in ICAM-1 expression was averted when mice were treated with the β3-adrenoreceptor specific antagonist SR59230A (Figure S4M). These data clearly indicate that the endothelial oscillations in adhesion molecule expression require local delivery of adrenaline and signaling through β-adrenoreceptors
Biological relevance of circadian BM recruitment
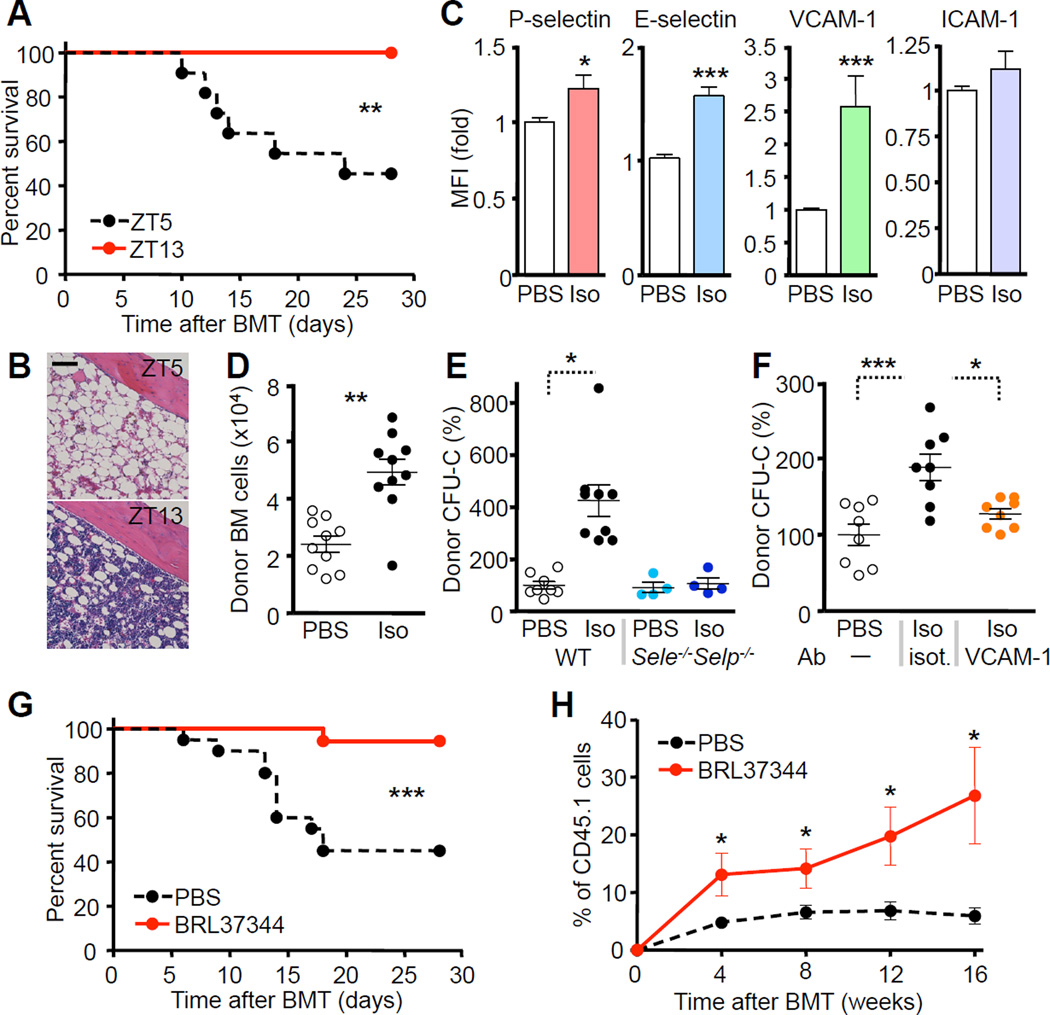
We next examined whether the circadian regulation of hematopoietic cell recruitment to the BM could be exploited in the setting of BM transplantation. Injection of lethally irradiated mice with limiting numbers of BM cells (25,000 cells), led to marked differences in survival. While most animals transplanted in the morning succumbed to death due to hematopoietic failure (Figure S5A), all animals survived the procedure when transplanted at ZT13 (Figure 5A–B). To test whether mimicking enhanced adrenergic tone at night could emulate this phenotype, we treated mice with the pan-β-adrenergic receptor agonist isoproterenol, which significantly upregulated expression of P- and E-selectins and VCAM-1 but not ICAM-1 in irradiated recipients (Figure 5C). After isoproterenol treatment, the number of recruited donor BM cells and progenitors was significantly increased compared to control (Figure 5D–E), while this effect was abrogated in Selp−/−Sele−/− or anti-VCAM-1-treated animals (Figure 5E–F). Furthermore, survival was markedly improved in the isoproterenol-treated animals when limiting numbers of BM cells were injected for transplantation (Figure S5B). To determine whether this effect was dependent on Adrβ2 or Adrβ3, we treated mice with specific agonists (clenbuterol or BRL37344, respectively). We found that only BRL37344 exhibited a similar effect as isoproterenol (Figure 5G, S5A and data not shown), indicating that β3-adrenergic receptor signaling is sufficient to promote hematopoietic recruitment to the BM. BRL37344 treatment also enhanced the homing of long-term repopulating hematopoietic stem cells (LT-HSC) (Figure 5H). Since we performed bone marrow transplantation 24hr after treatment with isoproterenol or BRL37344, the enhanced recruitment was likely due an effect on the stroma, rather than on the infused BM cells. We found no differences in CXCL12 levels between groups at the time of BMT (Figure S5C–D). These data suggested that transplantation performed at night or after pharmacological treatment with β3-agonists could potentially reduce the number of HSPCs needed for successful long-term engraftment.
Figure 5. β-adrenergic stimulation enhances hematopoietic progenitor reconstitution after transplantation.
(A) Survival curves after circadian BM transplantation with limiting numbers of BM cells (2.5×104) into lethally irradiated recipients. n = 10. (B) Representative H&E micrographs 30 days after circadian BMT. (C) Flow cytometric analysis of endothelial cell adhesion molecule expression after PBS or isoproterenol (iso) treatment in recipients. n = 5. (D) Quantification of donor BM cells that homed to the BM of recipients treated with PBS or isoproterenol. n = 10. (E–F) Quantification of donor CFU-Cs that homed to the BM of recipients treated with PBS or isoproterenol in WT or Selp−/−Sele−/− mice (E) or after treatment with an isotype (isot.) or anti-VCAM-1 blocking antibody (F). n = 4–9. (G) Survival curves after transplantation with limiting numbers of BM cells (2.5×104) into lethally irradiated recipients pre-treated with PBS or BRL37344. n = 18–20. (H) Effect of BRL37344 on homing of long-term repopulating HSCs. n = 6. *P<0.05, **P<0.01, ***P<0.001. Scale bar: 100 µm. See also Figure S5.
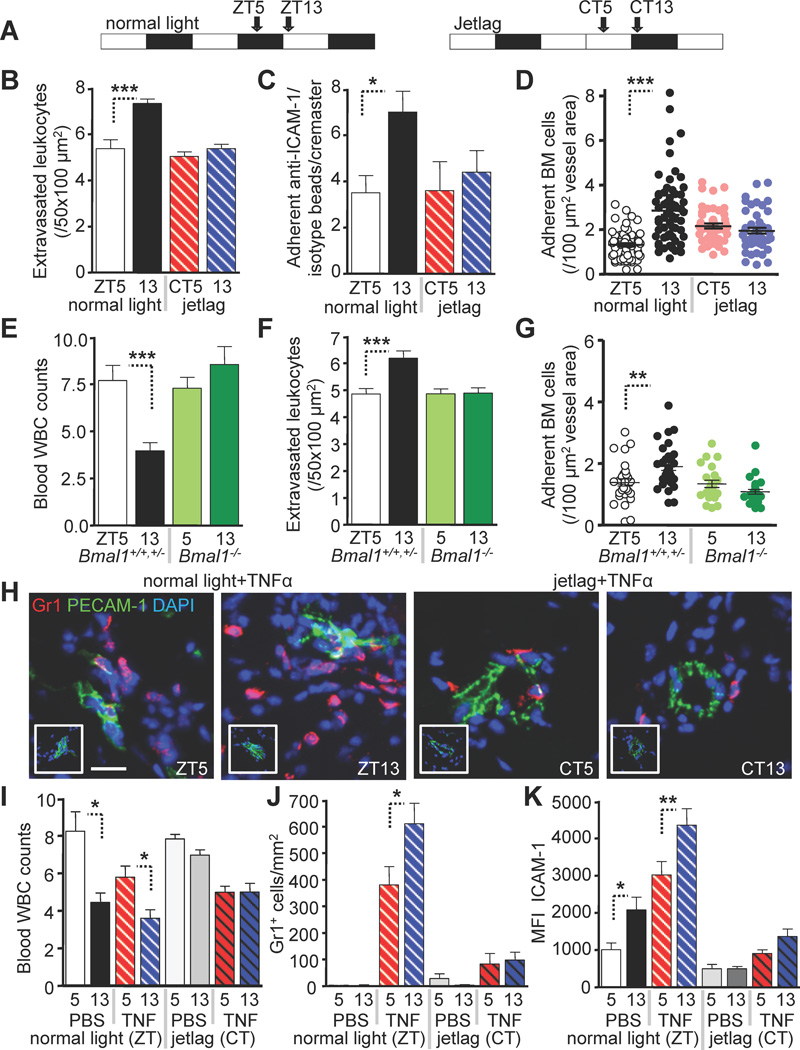
Circadian rhythms in leukocyte recruitment are entrained by photic cues
Circadian rhythms are entrained by light where photic input is interpreted by suprachiasmatic nuclei (SCN), the fundamental timekeeper of the mammalian clock (Klein et al., 1991; Ralph et al., 1990). We thus tested whether alterations in photic input by induction of an experimental jetlag would be sufficient to modify rhythms in leukocyte recruitment. Advancing the light regime by 12h after the light phase (Figure 6A) completely abolished circadian rhythms in leukocyte recruitment to skeletal muscle (Figure 6B), and the oscillations in endothelial ICAM-1 expression (Figure 6C). Experimental jetlag also eliminated the increased homing to the BM at night (Figure 6D). To assess further the role of the molecular clock in the circadian fluctuations of leukocytes, we evaluated the role of BMAL-1, a key transcription factor required to entrain circadian oscillations (Levi and Schibler, 2007). We found that circadian oscillations of leukocyte numbers in both blood and tissues were completely abolished in Bmal1−/− animals kept in constant darkness for three weeks, in contrast to normal oscillations observed in heterozygous and WT littermates (Figure 6E–G). These results underscore the importance of light as an environmental cue required for synchronizing the clock machinery that entrains rhythms in leukocyte recruitment to tissues.
Figure 6. Circadian rhythms in leukocyte recruitment are entrained by photic cues.
(A) Light regime under normal and jetlag conditions as entrained using a light cycler. (B) Quantification of extravasated CD45+ leukocytes in the cremaster muscle as analyzed by whole-mount immunofluorescence staining in mice under normal light and jetlag conditions. n = 3–6. (C) Quantification of specific vs. non-specific sphere adhesion in hematopoietic Icam1−/−/WT BM chimeras under normal light and jetlag conditions. n = 5–6. (D) Quantification of adherent fluorescently labeled cells to BM sinusoids in mice under normal light and jetlag conditions. n = 46–61 areas quantified from 4–5 mice per group. (E–G) Circadian oscillations in Bmal1−/− mice and control littermates after 3 weeks of constant darkness. (E) Blood leukocyte counts (×103/µl). n = 4–8. (F) Numbers of extravasated CD45+ leukocytes as analyzed by whole-mount immunofluorescence staining of cremaster muscle tissues. n = 24–30 vessels from 4 mice per group. (G) Numbers of adherent fluorescently labeled cells to BM sinusoids after adoptive transfer. n = 22–31 areas quantified from 3 mice per group. (H–J) Representative images (PBS in insets) (H), blood leukocyte counts (×103/µl) (I) and quantification of TNFα-induced neutrophil infiltration in sections of the cremaster muscle (J) under normal light and jetlag conditions. n = 3–9. (K) Quantification of ICAM-1 protein expression in frozen sections harvested from the same mice. *P<0.05, **P<0.01, ***P<0.001. Scale bar: 50 µm. See also Figure S6.
Circadian time influences leukocyte recruitment in inflammation
To assess whether circadian leukocyte recruitment could alter the inflammatory response, we injected mice with TNF-α and analyzed cremaster muscle tissues 8h later, either at ZT5 or ZT13. While leukocyte rhythms in blood were significantly blunted after stimulation (Figure 6I, left), neutrophil infiltration showed a dramatic increase compared to PBS treated animals (Figure 6H, left and 6J, left). Circadian rhythms in leukocyte recruitment remained intact in inflammation, with ZT13 showing significantly higher neutrophil infiltration than ZT5 (Figure 6H, left and 6J, left). In addition, ICAM-1 expression on endothelial cells was significantly increased after TNF-α-stimulation and exhibited circadian oscillations (Figure 6K, left). We also observed induction of endothelial cell adhesion molecule expression (P- and E-selectin, VCAM-1 and Cd44) as well as chemokines (Ccl2, Ccl5, Cxcl1, Cxcl2) after TNF-α-stimulation (Figure S6A–H), but no detectable rhythm. Icam2, Ccl3 or Cx3cl1 levels were not altered after inflammation and did not exhibit oscillations (Figure S6I–K).
When we induced jetlag and treated mice with TNF-α using the same protocol, circadian rhythms in WBC counts were inhibited (Figure 6I, right) and neutrophil infiltration was almost completely abolished (Figure 6H, right and 6J, right) while the expression of endothelial cell adhesion molecules was dramatically reduced compared to controls kept under a normal light regime (Figure 6K, right and S6A–C). These experiments further illustrate the significance of circadian time and light in leukocyte recruitment under both steady-state and inflammatory conditions.
Inflammatory leukocyte recruitment oscillates in sickle cell disease
We next aimed to evaluate the relevance of circadian leukocyte recruitment in inflammation in models of sickle cell disease (SCD) and septic shock, pathologies in which neutrophil recruitment has been shown to play a critical role (Frenette and Atweh, 2007; Hewett et al., 1992; Thomas et al., 1992). SCD mice challenged with a TNF-α-induced model of vaso-occlusion (Figure S7A) exhibited significantly increased leukocyte recruitment to the cremaster muscle when the experiment was performed at night compared to the daytime (Figure S7B–C). In addition, adherent leukocytes showed marked elevations in Mac-1 integrin activation at night (Figure S7D), a key molecule mediating heterotypic interactions between activated adherent leukocytes and circulating RBCs (Hidalgo et al., 2009). This translated to a ~2-fold increase in WBC-RBC interactions (Figure S7E) and a significant decrease of the mean venular blood flow rates (Figure S7F). Ultimately, the overall survival of SCD mice was significantly reduced at night. Lethality was due to disseminated intravascular coagulation as evidenced by microthrombi in the lungs as well as scattered foci of liver necrosis (Figure S7G–I and data not shown). Importantly, we did not observe circadian differences in TNF-receptor expression at the investigated time points (Figure S7J). These data indicate that circadian leukocyte recruitment can exacerbate sickle cell vaso-occlusion and compromise survival.
Ciradian time governs leukocyte recruitment and survival in septic shock
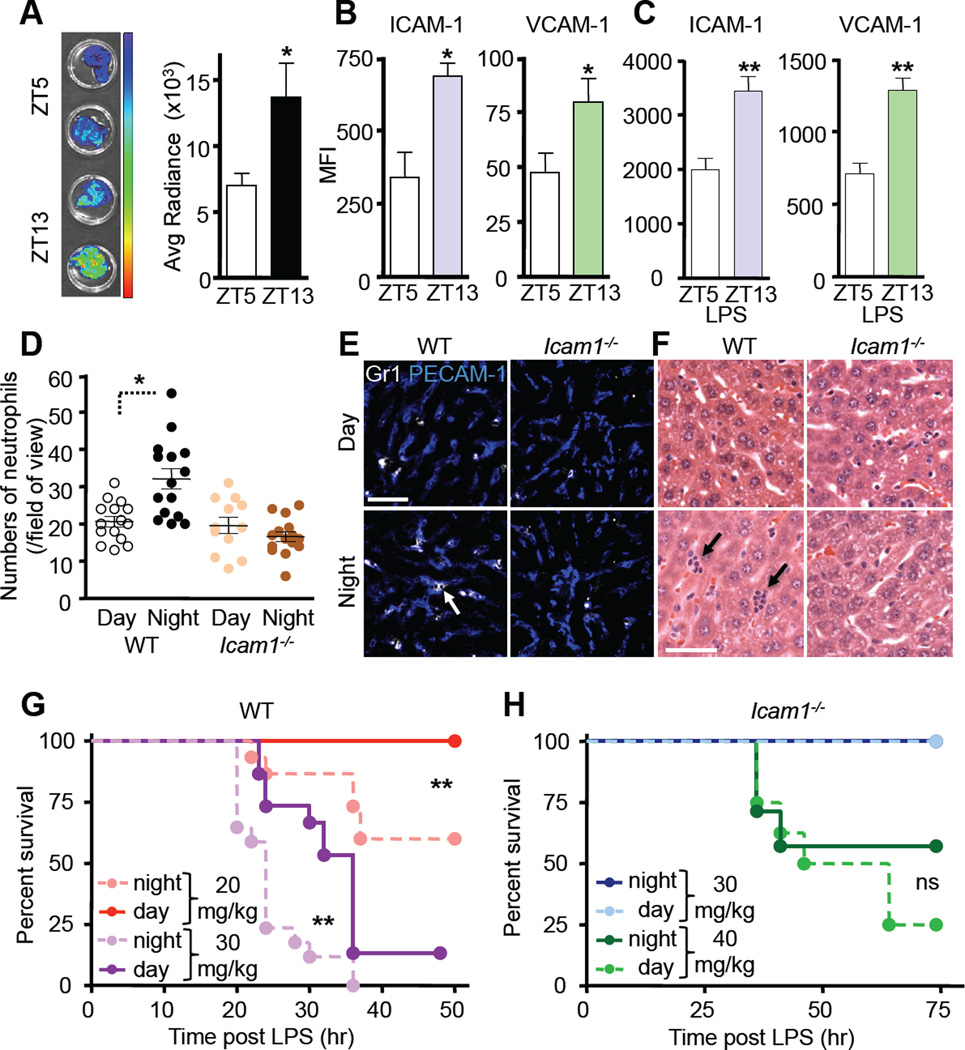
To gain further insight into the relevance of circadian leukocyte recruitment to vital organs, we performed adoptive transfer studies using BM harvested from Vav1-Cre; Rosa26-lsl-Luc mice, expressing luciferase selectively in hematopoietic cells. Consistent with our studies in the muscle and bone marrow, we observed strong recruitment of adoptively transferred cells to the liver with a circadian rhythm peaking at night (Figure 7A). These rhythms coincided with oscillations in endothelial ICAM-1 and VCAM-1 expression (Figure 7B) whereas expression of endothelial selectins was unchanged (data not shown). To test further the relevance of these fluctuations, we challenged mice with a lethal dose of endotoxin (LPS) in the morning or at night. Consistent with our results with TNFα, both adhesion molecule expression (Figure 7C) and leukocyte recruitment exhibited strong oscillations, with neutrophil infiltration into liver peaking when LPS was administered at night (Figure 7D–F). In contrast, diurnal variations in neutrophil recruitment to this tissue were ablated in Icam1−/− animals (Figure 7D–F). Similarly to TNF-receptor expression, we also did not observe circadian rhythms of TLR4 (the principal receptor for LPS) at these timepoints (not shown). In line with prior studies (Feigin et al., 1969; Halberg et al., 1960), survival was greatly reduced when mice were challenged with LPS in the night compared to the morning (Figure 7G). Mortality was highly dependent on leukocyte recruitment since Icam1−/− animals were significantly more resistant to LPS-induced lethality (Xu et al., 1994) (Figure 7H). Additionally, the impaired survival of night-challenged animals was abrogated in Icam1−/− mice, indicating that oscillating ICAM-1 levels are critical for the observed diurnal rhythm (Figure 7H). Icam+/− animals exhibited an intermediate phenotype in survival compared to WT and Icam1−/− mice indicating the functional importance of differences in ICAM-1 expression observed in this model (Figure S7K). Taken together, these results demonstrate that circadian rhythms in leukocyte recruitment can directly impact the outcome of inflammatory diseases.
Figure 7. Circadian time influences leukocyte recruitment in septic shock.
(A) Bioluminescence imaging and quantifications of harvested liver after adoptive transfer of Vav-Cre:luciferase-expressing BM cells to recipients at ZT5 or ZT13. n = 7. (B–C) Quantification of endothelial cell adhesion molecule expression levels in liver at steady state (B) and after 12h stimulation with LPS (C) by immunofluorescence confocal imaging of frozen tissue sections. n = 4–7. (D) Quantification of LPS-induced neutrophil infiltration in frozen liver sections of WT or Icam1−/− animals. n = 6. (E–F) Representative images of neutrophil infiltration in liver sections as assessed by immunofluorescence confocal imaging (E) and H&E staining (F) showing neutrophils clusters (arrows). (G–H) Survival curves of WT (G) and Icam1−/− (H) animals after LPS-induced septic shock. n = 7–17. *P<0.05, **P<0.01. Scale bars: 50 µm. See also Figure S7.
DISCUSSION
Here, we show that the emigration of leukocytes to tissues is regulated by signals from the autonomic nervous system where the peak recruitment occurs at night in rodents, during a period of activity. Circadian hematopoietic cell recruitment is synchronized by the molecular clock via sympathetic nerves, which induce through β-adrenoreceptors oscillations in endothelial cell expression of ICAM-1 and CCL2 in skeletal muscle and endothelial selectins, VCAM-1 as well as CXCL12 (Mendez-Ferrer et al., 2008) in the BM. In addition, our data suggest that rhythmic leukocyte recruitment is not restricted to these tissues but occurs in many vital organs. The difference in the molecular signature of circadian expression of endothelial cell adhesion molecules and chemokines in tissues is likely responsible for the diurnal preference in hematopoietic cell populations recruited to various organs.
Changes in the light cycle, a major zeitgeber for circadian rhythms (Golombek and Rosenstein, 2010), were sufficient to ablate oscillations in hematopoietic cell recruitment. This finding, along with the involvement of BMAL-1, strongly indicates bona fide circadian rhythms and suggests their central orchestration by the molecular clock (Dibner et al., 2010; Green et al., 2008). Additionally, the surprising anti-inflammatory effect seen after acute jetlag suggests a potent and broad interference of light in the normally rhythmically generated inflammatory response. It is possible that chronic jetlag may perturb circadian immune-surveillance mechanisms and contribute to the higher incidence of cancer in shift workers (Filipski et al., 2004). How jetlag exerts these broad changes in the inflammatory response should be the subject of future investigations.
Most prior studies on the regulation of circadian rhythms have focused on the involvement of humoral factors, notably of glucocorticoid (GC) levels, which peak at night in mice and reach trough values during the day (Dickmeis, 2009). Since GCs exhibit anti-inflammatory properties, we would expect that GC hormones might antagonize the rhythms described herein which depend on local innervation. It is possible however, that neural and hormonal pathways complement each other to fine tune the physiological and inflammatory responses (Elenkov et al., 2000). Thus, higher levels of GCs at night in mice may in fact keep the night surge in leukocyte infiltration in check.
Although catecholamine treatment of donor progenitors in vitro enhanced their engraftment ability through the β2-adrenoreceptor (Spiegel et al., 2007), we have excluded a role for adrenergic receptors on transplantable hematopoietic cells in our studies using radiation chimeras and adoptive transfer experiments. Therefore, endothelial cells in BM and skeletal muscle, which can express both the β2- and β3-receptors (Steinle et al., 2003), likely represent a logical cellular target since they also express the necessary adhesion molecules to mediate hematopoietic cell/endothelial cell interactions. Our results indicate that BM transplantation performed after stimulation with β3-adrenergic agonists in the recipient may improve clinical outcomes through enhanced engraftment efficiency. Since adrenergic stimulation can also induce the mobilization of HSPCs (Mendez-Ferrer et al., 2008), it remains unclear as to how the cells targeted by adrenergic signals interpret these signals at different times. The fact that the same adrenergic receptors can lead to distinct adhesion molecule expression in different vascular beds, suggests tissue-specific signaling responses that remain undefined. The present findings are consistent with previous studies in which circadian oscillations of BM CXCL12 with trough expression levels of the chemokine occurring in the morning were associated with the peak of HSC egress (Mendez-Ferrer et al., 2008). It is likely that the nightly increase of CXCL12 in the BM cooperates with endothelial upregulation of adhesion molecules for maximal hematopoietic cell homing in the evening. In the bone marrow, β3-adrenoreceptor expression is enriched in rare Nestin+ perivascular mesenchymal stem cells, which express high amounts of CXCL12 and are targeted by the SNS in the stem cell niche (Mendez-Ferrer et al., 2010). How microvascular pericytes collaborate with endothelial cells to entrain rhythms in extramedullary tissues will be the subject of future studies in the laboratory.
The rhythmic pattern of leukocyte recruitment may have evolved for the benefit of having readily available tissue phagocytes to enhance the response to pathogens during the period of activity, when injuries and encounters with microorganisms are more likely to occur. Although macrophages represent the major leukocyte subset oscillating under homeostasis, we show that neutrophil recruitment after trauma or in the setting of inflammatory diseases is significantly influenced by endogenous rhythms of endothelial cell adhesion. It is conceivable that the diurnal clearance of senescent neutrophils by the BM and liver is mediated by these circadian mechanisms. Whether the HSPC pool is rejuvenated by diurnal migratory incentives is possible. We have presented clear evidence, however, that circadian timing can influence the engraftment efficiency when HSPC counts are sub-optimal, demonstrating the significance of circadian hematopoietic cell recruitment to the BM. As such, given the inverted rhythms between rodents and humans (Lucas et al., 2008), we would predict that human hematopoietic stem cell transplantation is optimal early in the morning, rather than later in the day.
We postulate that the diurnal mechanisms of keeping neutrophil numbers in check may affect cardiovascular diseases – which are influenced by circadian time – as their levels are known to correlate positively with the degree of severity (Coller, 2005; Muller et al., 1985). When exaggerated, these mechanisms may exert detrimental effects. Indeed, in models of TNF-α-induced vaso-occlusion in SCD mice and LPS-induced lethality, we have found that circadian rhythms modulate the robustness of the inflammatory response with implications on survival. While we recognize potential mechanistic differences between atherosclerosis and inflammatory models studied herein, these data are in line with prior studies that have shown persistent oscillations in the susceptibility of mice to bacterial infection and inflammatory leukocyte trafficking (Castanon-Cervantes et al., 2010; Feigin et al., 1969; Gibbs et al., 2011; Halberg et al., 1960; House et al., 1997; Keller et al., 2009; Liu et al., 2006; Shackelford and Feigin, 1973; Silver et al., 2012). Sensitivity to inflammatory stimuli at times of higher SNS tone likely interacts at multiple levels encompassing local neural input as we describe herein, endogenous rhythms within tissues as well as interactions with humoral factors and the parasympathetic nervous system.
Given the reported critical role of blood leukocytes in ischemic vascular diseases, the present results argue that circadian leukocyte adhesion or an exaggeration thereof might contribute to triggering acute vascular diseases in the morning. Our own as well as published studies have clearly shown that leukocyte recruitment promotes LPS-induced lethality (Xu et al., 1994) and SCD vaso-occlusion (Hidalgo et al., 2009; Turhan et al., 2002), which led us to evaluate experimentally the effect of circadian time in inflammatory diseases. Our results provide the proof-of-principle that time-dependent differences in leukocyte recruitment translate into significant changes in survival. Thus, a better understanding of rhythms in leukocyte migration will allow the design of targeted chronotherapy that may lead to meaningful clinical impact on disease outcome.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. Rani Sellers for histopathological examinations, Dr. Lydia Tesfa for cell sorting experiments and Colette Prophete, Matt Huggins and Neepa Dholakia for technical assistance. This work was supported by the National Institutes of Health (NIH) (R01 grants HL097700; HL069438; DK056638) to P.S.F. We are grateful for fellowship support from the German Academic Exchange Service (DAAD) to C.S, the Japan Society for the Promotion of Science (Y.K.), the Fundación Ramón Areces (D.L.), a Ruth L. Kirschstein National Research Service Award from NHLBI (F30HL099028) to A.C. and a Founders Affiliate Predoctoral Fellowship from the American Heart Association (J-E.J.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alito AE, Romeo HE, Baler R, Chuluyan HE, Braun M, Cardinali DP. Autonomic nervous system regulation of murine immune responses as assessed by local surgical sympathetic and parasympathetic denervation. Acta Physiol Pharmacol Latinoam. 1987;37:305–319. [PubMed] [Google Scholar]
- Anea CB, Zhang M, Stepp DW, Simkins GB, Reed G, Fulton DJ, Rudic RD. Vascular disease in mice with a dysfunctional circadian clock. Circulation. 2009;119:1510–1517. doi: 10.1161/CIRCULATIONAHA.108.827477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DL, Feskanich D, Sanchez BN, Rexrode KM, Schernhammer ES, Lisabeth LD. Rotating night shift work and the risk of ischemic stroke. Am J Epidemiol. 2009;169:1370–1377. doi: 10.1093/aje/kwp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullard DC, Kunkel EJ, Kubo H, Hicks MJ, Lorenzo I, Doyle NA, Doerschuk CM, Ley K, Beaudet AL. Infectious susceptibility and severe deficiency of leukocyte rolling and recruitment in E-selectin and P-selectin double mutant mice. The Journal of experimental medicine. 1996;183:2329–2336. doi: 10.1084/jem.183.5.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- Castanon-Cervantes O, Wu M, Ehlen JC, Paul K, Gamble KL, Johnson RL, Besing RC, Menaker M, Gewirtz AT, Davidson AJ. Dysregulation of inflammatory responses by chronic circadian disruption. J Immunol. 2010;185:5796–5805. doi: 10.4049/jimmunol.1001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Shi PA, Chiang EY, Frenette PS. Intravenous immunoglobulins reverse acute vaso-occlusive crises in sickle cell mice through rapid inhibition of neutrophil adhesion. Blood. 2008;111:915–923. doi: 10.1182/blood-2007-04-084061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang EY, Hidalgo A, Chang J, Frenette PS. Imaging receptor microdomains on leukocyte subsets in live mice. Nat Methods. 2007;4:219–222. doi: 10.1038/nmeth1018. [DOI] [PubMed] [Google Scholar]
- Coller BS. Leukocytosis and ischemic vascular disease morbidity and mortality: is it time to intervene? Arterioscler Thromb Vasc Biol. 2005;25:658–670. doi: 10.1161/01.ATV.0000156877.94472.a5. [DOI] [PubMed] [Google Scholar]
- Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517–549. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- Dickmeis T. Glucocorticoids and the circadian clock. J Endocrinol. 2009;200:3–22. doi: 10.1677/JOE-08-0415. [DOI] [PubMed] [Google Scholar]
- Dimitrov S, Benedict C, Heutling D, Westermann J, Born J, Lange T. Cortisol and epinephrine control opposing circadian rhythms in T cell subsets. Blood. 2009;113:5134–5143. doi: 10.1182/blood-2008-11-190769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52:595–638. [PubMed] [Google Scholar]
- Feigin RD, San Joaquin VH, Haymond MW, Wyatt RG. Daily periodicity of susceptibility of mice to pneumococcal infection. Nature. 1969;224:379–380. doi: 10.1038/224379a0. [DOI] [PubMed] [Google Scholar]
- Filipski E, Delaunay F, King VM, Wu MW, Claustrat B, Grechez-Cassiau A, Guettier C, Hastings MH, Francis L. Effects of chronic jet lag on tumor progression in mice. Cancer Res. 2004;64:7879–7885. doi: 10.1158/0008-5472.CAN-04-0674. [DOI] [PubMed] [Google Scholar]
- Forlow SB, Foley PL, Ley K. Severely reduced neutrophil adhesion and impaired host defense against fecal and commensal bacteria in CD18−/−P-selectin−/− double null mice. Faseb J. 2002;16:1488–1496. doi: 10.1096/fj.02-0230com. [DOI] [PubMed] [Google Scholar]
- Frenette PS, Atweh GF. Sickle cell disease: old discoveries, new concepts, and future promise. J Clin Invest. 2007;117:850–858. doi: 10.1172/JCI30920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenette PS, Mayadas TN, Rayburn H, Hynes RO, Wagner DD. Susceptibility to infection and altered hematopoiesis in mice deficient in both P- and Eselectins. Cell. 1996;84:563–574. doi: 10.1016/s0092-8674(00)81032-6. [DOI] [PubMed] [Google Scholar]
- Gibbs JE, Blaikley J, Beesley S, Matthews L, Simpson KD, Boyce SH, Farrow SN, Else KJ, Singh D, Ray DW, et al. The nuclear receptor REVERBalpha mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc Natl Acad Sci U S A. 2011;109:582–587. doi: 10.1073/pnas.1106750109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golombek DA, Rosenstein RE. Physiology of circadian entrainment. Physiol Rev. 2010;90:1063–1102. doi: 10.1152/physrev.00009.2009. [DOI] [PubMed] [Google Scholar]
- Green CB, Takahashi JS, Bass J. The meter of metabolism. Cell. 2008;134:728–742. doi: 10.1016/j.cell.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberg F, Johnson EA, Brown BW, Bittner JJ. Susceptibility rhythm to Ecoli endotoxin and bioassay. Proc Soc Exp Biol Med. 1960;103:142–144. doi: 10.3181/00379727-103-25439. [DOI] [PubMed] [Google Scholar]
- Haus E. Chronobiology in the endocrine system. Adv Drug Deliv Rev. 2007;59:985–1014. doi: 10.1016/j.addr.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Haus E, Smolensky MH. Biologic rhythms in the immune system. Chronobiol Int. 1999;16:581–622. doi: 10.3109/07420529908998730. [DOI] [PubMed] [Google Scholar]
- Hewett JA, Schultze AE, VanCise S, Roth RA. Neutrophil depletion protects against liver injury from bacterial endotoxin. Laboratory investigation; a journal of technical methods and pathology. 1992;66:347–361. [PubMed] [Google Scholar]
- Hidalgo A, Chang J, Jang JE, Peired AJ, Chiang EY, Frenette PS. Heterotypic interactions enabled by polarized neutrophil microdomains mediate thromboinflammatory injury. Nat Med. 2009;15:384–391. doi: 10.1038/nm.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House SD, Ruch S, Koscienski WF, 3rd, Rocholl CW, Moldow RL. Effects of the circadian rhythm of corticosteroids on leukocyte-endothelium interactions in the AM and PM. Life Sci. 1997;60:2023–2034. doi: 10.1016/s0024-3205(97)00167-7. [DOI] [PubMed] [Google Scholar]
- Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, Frenette PS. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- Katayama Y, Hidalgo A, Furie BC, Vestweber D, Furie B, Frenette PS. PSGL-1 participates in E-selectin-mediated progenitor homing to bone marrow: evidence for cooperation between E-selectin ligands and alpha4 integrin. Blood. 2003;102:2060–2067. doi: 10.1182/blood-2003-04-1212. [DOI] [PubMed] [Google Scholar]
- Keller M, Mazuch J, Abraham U, Eom GD, Herzog ED, Volk HD, Kramer A, Maier B. A circadian clock in macrophages controls inflammatory immune responses. Proc Natl Acad Sci U S A. 2009;106:21407–21412. doi: 10.1073/pnas.0906361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DC, Moore RY, Reppert SM. Suprachiasmatic nucleus: the mind's clock. New York, NY: 1991. [Google Scholar]
- Levi F, Schibler U. Circadian rhythms: mechanisms and therapeutic implications. Annu Rev Pharmacol Toxicol. 2007;47:593–628. doi: 10.1146/annurev.pharmtox.47.120505.105208. [DOI] [PubMed] [Google Scholar]
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- Liu J, Malkani G, Shi X, Meyer M, Cunningham-Runddles S, Ma X, Sun ZS. The circadian clock Period 2 gene regulates gamma interferon production of NK cells in host response to lipopolysaccharide-induced endotoxic shock. Infect Immun. 2006;74:4750–4756. doi: 10.1128/IAI.00287-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas D, Battista M, Shi PA, Isola L, Frenette PS. Mobilized hematopoietic stem cell yield depends on species-specific circadian timing. Cell Stem Cell. 2008;3:364–366. doi: 10.1016/j.stem.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucio RA, Flores-Rojas G, Aguilar F, Zempoalteca R, Pacheco P, Velazquez-Moctezuma J. Effects of genitofemoral nerve transection on copulatory behavior and fertility in male rats. Physiol Behav. 2001;73:487–492. doi: 10.1016/s0031-9384(01)00437-1. [DOI] [PubMed] [Google Scholar]
- Margolis KL, Manson JE, Greenland P, Rodabough RJ, Bray PF, Safford M, Grimm RH, Jr, Howard BV, Assaf AR, Prentice R. Leukocyte count as a predictor of cardiovascular events and mortality in postmenopausal women: the Women's Health Initiative Observational Study. Arch Intern Med. 2005;165:500–508. doi: 10.1001/archinte.165.5.500. [DOI] [PubMed] [Google Scholar]
- Massberg S, Schaerli P, Knezevic-Maramica I, Kollnberger M, Tubo N, Moseman EA, Huff IV, Junt T, Wagers AJ, Mazo IB, et al. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131:994–1008. doi: 10.1016/j.cell.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazo IB, Gutierrez-Ramos JC, Frenette PS, Hynes RO, Wagner DD, von Andrian UH. Hematopoietic progenitor cell rolling in bone marrow microvessels: parallel contributions by endothelial selectins and vascular cell adhesion molecule 1. The Journal of experimental medicine. 1998;188:465–474. doi: 10.1084/jem.188.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442–447. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma'ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motoike T, Loughna S, Perens E, Roman BL, Liao W, Chau TC, Richardson CD, Kawate T, Kuno J, Weinstein BM, et al. Universal GFP reporter for the study of vascular development. Genesis. 2000;28:75–81. doi: 10.1002/1526-968x(200010)28:2<75::aid-gene50>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Muller JE, Stone PH, Turi ZG, Rutherford JD, Czeisler CA, Parker C, Poole WK, Passamani E, Roberts R, Robertson T, et al. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med. 1985;313:1315–1322. doi: 10.1056/NEJM198511213132103. [DOI] [PubMed] [Google Scholar]
- Muller WA. Mechanisms of leukocyte transendothelial migration. Annual review of pathology. 2011;6:323–344. doi: 10.1146/annurev-pathol-011110-130224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247:975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- Scheiermann C, Colom B, Meda P, Patel NS, Voisin MB, Marrelli A, Woodfin A, Pitzalis C, Thiemermann C, Aurrand-Lions M, et al. Junctional adhesion molecule-C mediates leukocyte infiltration in response to ischemia reperfusion injury. Arterioscler Thromb Vasc Biol. 2009;29:1509–1515. doi: 10.1161/ATVBAHA.109.187559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiermann C, Meda P, Aurrand-Lions M, Madani R, Yiangou Y, Coffey P, Salt TE, Ducrest-Gay D, Caille D, Howell O, et al. Expression and function of junctional adhesion molecule-C in myelinated peripheral nerves. Science. 2007;318:1472–1475. doi: 10.1126/science.1149276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackelford PG, Feigin RD. Periodicity of susceptibility to pneumococcal infection: influence of light and adrenocortical secretions. Science. 1973;182:285–287. doi: 10.1126/science.182.4109.285. [DOI] [PubMed] [Google Scholar]
- Sigmundsdottir H, Butcher EC. Environmental cues, dendritic cells and the programming of tissue-selective lymphocyte trafficking. Nature immunology. 2008;9:981–987. doi: 10.1038/ni.f.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver AC, Arjona A, Walker WE, Fikrig E. The circadian clock controls toll-like receptor 9-mediated innate and adaptive immunity. Immunity. 2012;36:251–261. doi: 10.1016/j.immuni.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel A, Shivtiel S, Kalinkovich A, Ludin A, Netzer N, Goichberg P, Azaria Y, Resnick I, Hardan I, Ben-Hur H, et al. Catecholaminergic neurotransmitters regulate migration and repopulation of immature human CD34+ cells through Wnt signaling. Nature immunology. 2007;8:1123–1131. doi: 10.1038/ni1509. [DOI] [PubMed] [Google Scholar]
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Steinle JJ, Booz GW, Meininger CJ, Day JN, Granger HJ. Beta 3- adrenergic receptors regulate retinal endothelial cell migration and proliferation. J Biol Chem. 2003;278:20681–20686. doi: 10.1074/jbc.M300368200. [DOI] [PubMed] [Google Scholar]
- Thomas JR, Harlan JM, Rice CL, Winn RK. Role of leukocyte CD11/CD18 complex in endotoxic and septic shock in rabbits. J Appl Physiol. 1992;73:1510–1516. doi: 10.1152/jappl.1992.73.4.1510. [DOI] [PubMed] [Google Scholar]
- Turhan A, Weiss LA, Mohandas N, Coller BS, Frenette PS. Primary role for adherent leukocytes in sickle cell vascular occlusion: a new paradigm. Proc Natl Acad Sci U S A. 2002;99:3047–3051. doi: 10.1073/pnas.052522799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestweber D, Blanks JE. Mechanisms that regulate the function of the selectins and their ligands. Physiol Rev. 1999;79:181–213. doi: 10.1152/physrev.1999.79.1.181. [DOI] [PubMed] [Google Scholar]
- von Andrian UH, Mackay CR. T-cell function and migration. Two sides of the same coin. N Engl J Med. 2000;343:1020–1034. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- Wagner DD, Frenette PS. The vessel wall and its interactions. Blood. 2008;111:5271–5281. doi: 10.1182/blood-2008-01-078204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton KA, Buono LM. Horner syndrome. Curr Opin Ophthalmol. 2003;14:357–363. doi: 10.1097/00055735-200312000-00007. [DOI] [PubMed] [Google Scholar]
- Willich SN, Levy D, Rocco MB, Tofler GH, Stone PH, Muller JE. Circadian variation in the incidence of sudden cardiac death in the Framingham Heart Study population. Am J Cardiol. 1987;60:801–806. doi: 10.1016/0002-9149(87)91027-7. [DOI] [PubMed] [Google Scholar]
- Xu H, Gonzalo JA, St Pierre Y, Williams IR, Kupper TS, Cotran RS, Springer TA, Gutierrez-Ramos JC. Leukocytosis and resistance to septic shock in intercellular adhesion molecule 1-deficient mice. J Exp Med. 1994;180:95–109. doi: 10.1084/jem.180.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zempoalteca R, Martinez-Gomez M, Hudson R, Cruz Y, Lucio RA. An anatomical and electrophysiological study of the genitofemoral nerve and some of its targets in the male rat. J Anat. 2002;201:493–505. doi: 10.1046/j.1469-7580.2002.00112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou QY, Quaife CJ, Palmiter RD. Targeted disruption of the tyrosine hydroxylase gene reveals that catecholamines are required for mouse fetal development. Nature. 1995;374:640–643. doi: 10.1038/374640a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.