Abstract
Aims and Objectives
(1) To determine the incidence of inferior alveolar nerve (IAN) deficits following surgical extraction of mandibular third molar.
(2) To document recovery of IAN injuries.
Materials and Methods
A total of 400 lower third molars were extracted, 205 male patients and 195 females. All underwent extraction by the prescribed buccal approach. All cases were examined by one examiner preoperatively and postoperatively, at 7 days, one month, two months and six months. Two-point discrimination test (2-pd), brush stroke direction (BSD), contact detection, pin prick and thermal testing was carried out.
Results and Conclusion
One patient presented with IAN injury (0.25%). This single case of nerve injury was mesioangular, Level B, Class 2, impaction with a difficulty rating of 5. Levels A and B tests (2PD, BSD, Contact detection) were altered. In these tests, the IAN did not show any signs of recovery by six months. Level C tests (pin prick test, sharp blunt detection) showed that the nerve had recovered completely by two months.
Keywords: Neurosensory deficits, Inferior alveolar nerve, Nerve injury, Impacted third molars
Changes in feeling in the orofacial region may interfere with speaking, chewing and social interactions [1]. Even apparently minor changes can significantly affect a patient’s quality of life [2]. Trauma to a peripheral nerve may result in a deficiency ranging from total loss of sensation (anesthesia) to a mild decrease in feeling (mild hypoesthesia). These sensory deficits may be either temporary or permanent. Some patients may also experience dysesthesia, which is characterized by abnormally painful sensations. Such pain may be caused by a neuroma located at the site of the trauma, changes in the autonomic nervous system (sympathetically mediated pain) or alterations in the central nervous system (central neuropathic pain).
Maxillofacial neurosensory deficits may be caused by various surgical procedures like tooth extraction, orthognathic surgeries, preprosthetic procedures, excision of cysts and tumours, surgery of temporomandibular joint, facial fractures [3–7].
The pathophysiology of these neuropathies is complex, and treatment results are often disappointing [8]. The presence of anesthesia, dysesthesia or spontaneous pain also indicates poor prospects for recovery without surgical intervention. Overall, 25% of patients with iatrogenic paraesthesia suffer permanent effects [3].
To evaluate nerve dysfunction, it is important to use objective testing rather than to simply ask a patient subjectively to report neuropathic changes. Objective data can be obtained by clinical neurosensory tests or by more complicated electrophysiologic tests.
Materials and Methods
The patients enrolled in the study were patients reporting to the outpatient department of Department of Oral and Maxillofacial surgery. 400 cases were included in the study after written informed consent.
Excluded from the study were patients incapable of completing adequate neurosensory examination, patients with history of head injury, mentally retarded patients with a history of neurological disorders, collagen vascular diseases, craniomandibular disorders, patients on psychotropic drugs, patients who had undergone orthognathic surgery and also with a history of facial fractures.
All the preoperative neurosensory evaluations showed normal values. All extractions were performed under local anaesthesia. A buccal mucoperiosteal flap was raised; lingual flap was raised only minimally. Sterile low speed handpiece and saline solution was used for ostectomy and crown sectioning, where necessary. Wound was closed with 3–0 silk. Sutures were removed after 7 days, and patients were questioned about lower lip sensitivity.
All cases were examined by one examiner preoperatively and postoperatively at 7 days, one month, two months and six months.
To assess the function, the protocol based on Zuniga and Essick [9] was followed. All clinical tests were done in a peaceful room while the patient was sitting with the eyes closed. First the patient was asked to describe any changes in the sensation over the face. Next the areas perceived abnormal were mapped. This was accomplished by touching the centre of the dermatome supplied by the respective nerve and asking the patient whether it felt normal, if not the stimulus was moved preferably until the patient said that normal sensation was felt. In this manner, the outline was drawn of the area that represented the altered sensation as subjectively by the patient (Fig. 1). After mapping the following tests were carried out in the same order.
Fig. 1.
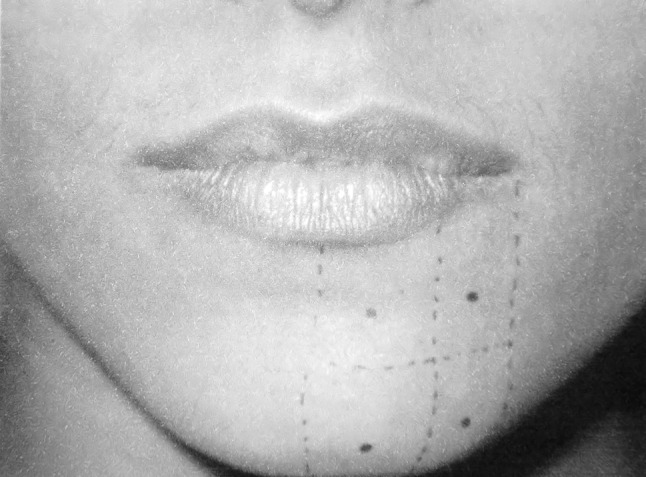
Mapping the area of altered sensation
Armamentarium used consisted of Sensory filament mounted on Perspex handles, dental probe with a rubber stopper, Vernier callipers, Water at 50°C and 15°C in glass test tubes, camel hair brush “0” number, thermometer to assess the temperature of water.
Level A Tests
This test is used to determine the response of the slowly adapting larger myelinated fibres (A-α) [9, 10].
Two-Point Discrimination Test (2-pd)
The patient’s ability to discriminate between two points was measured with a sliding calliper (Fig. 2). The two pointed, but not sharp, tips of the calliper touched the skin simultaneously with light pressure while the patient’s eyes were closed. The separation of the two points was gradually reduced from 20 mm at the chin and 10 mm at the lips to the moment where the patient could feel one point only. The minimum separation at which two points could be reported was recorded. Distances 2 mm greater than the preoperative values were considered abnormal [9, 10].
Fig. 2.
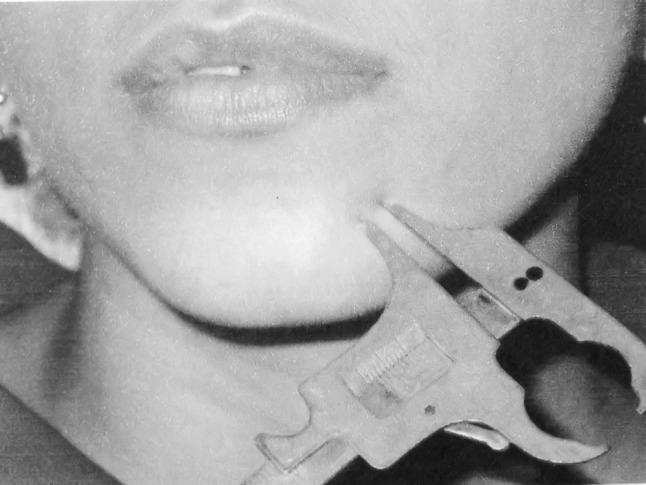
Two point discrimination test
Brush Stroke Direction (BSD)
This test is used to determine the response of the slowly adapting larger myelinated fibres (A-α) and A-β myelinated axons. The sensory modalities for these receptors are vibration, touch and flutter. Moving stimuli was delivered with the soft brush (0 number camel hair brush) at a fairly constant velocity (2–3 cm/s) (Fig. 3). Ten, two interval forced choice trials are then delivered to verify that the direction of motion is identified correctly [9, 10].
Fig. 3.
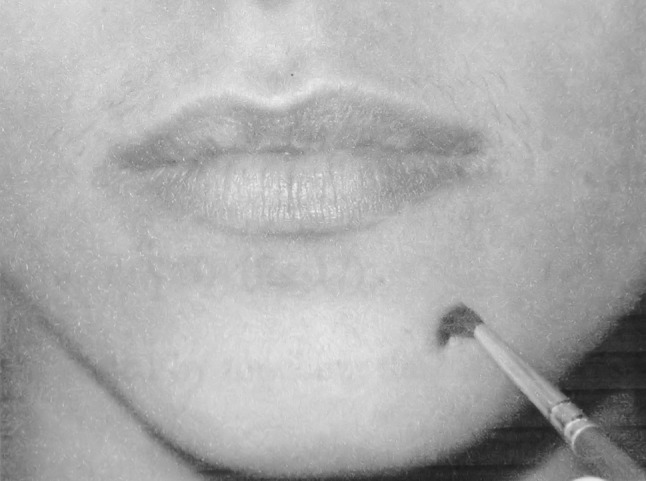
Brush stroke direction
Level B Test
Assesses the quickly adapting large myelinated (A-α) fibres.
Contact Detection
The contact detection threshold, the minimum force of contact against the skin that is felt, was measured with the use of prolene monofilament mounted onto end of a plastic handle [10]. The maximum force applied was made to be 2 g by adjusting the length of the suture material at the point at which it was bending (Fig. 4). A positive or negative response was the only option at each different point.
Fig. 4.
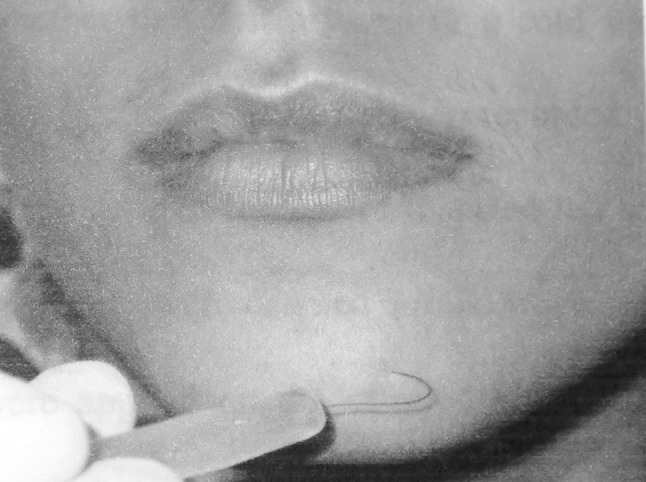
Contact detection
Level C Tests
These tests assess the small myelinated A-δ and c fibres.
Sharp Blunt Discrimination
This is done by touching the test area randomly with a sharp or a blunt head of the mechanical probe. A rubber stopper is centred at the end of the dental probe so that when the tip is pressed to the skin, a constant degree of skin indentation was caused [11] (Fig. 5). The patient has to decide whether the stimulus was sharp or blunt.
Fig. 5.
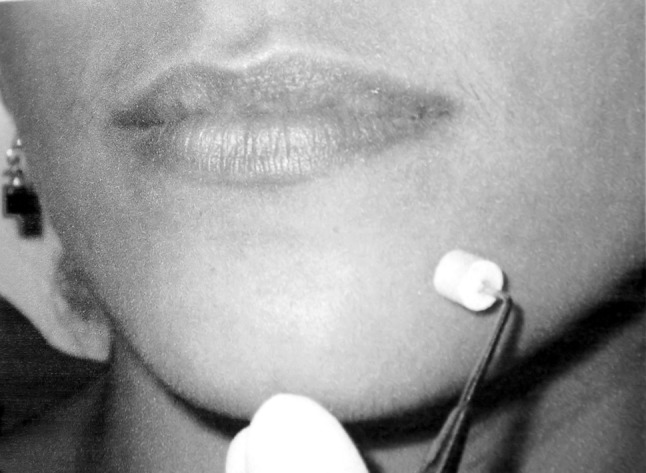
Sharp-blunt detection
Thermal Testing
Perception of warmth is attributed to the integrity of A-δ fibres and cold to C fibres. Two small glass tubes containing water at 50°C (warm) and 15°C (cold) were used. The report of each stimulus i.e. cold versus hot was recorded [9].
All tests were repeated ten times and the results of BSD, sharp blunt discrimination and thermal testing were considered normal when 80% of the answers were correct.
Ordinal scores were assigned as follows.
4-normal
3-Level A impaired
2-Level B impaired
1-Level C impaired
0-Anesthetic
Results
A total of 400 lower third molars were extracted, 205 male patients and 195 females. All underwent extraction by the prescribed buccal approach. Out of 400 sites, 182 were left sided and 218 were right sided. Only one patient presented with inferior alveolar nerve (IAN) injury (0.25%). This single case of nerve injury was mesioangular, Level B, Class 2 impaction with a difficulty rating of five.
Levels A and B tests (2PD, BSD, Contact detection) were altered. In these tests, the IAN did not show any signs of recovery by six months. Level C tests (pin prick test, sharp blunt detection) showed that the nerve had recovered completely by two months.
Discussion
The reported incidence of paraesthesia after extraction of impacted third molars varies between 0.4 to 8% for the lower alveolar nerve [12–14]. In our study the incidence was 0.25%. These variations can be explained by differences in procedures and technique, in particular with regard to clinical evaluation and diagnostic criteria, as well as differences in the surgeon’s experience. The risk of paraesthesia depends on the clinical situation. It may be almost non-existent under the best conditions (young patient, incompletely formed roots, mandibular canal not in close proximity) but could exceed 50% in other circumstances (elderly patient, unfavourable position of the tooth, proximity of the mandibular canal). But in our study age, sex, angulation, depth, space available or difficulty rate did not positively correlate with the occurrence of neurosensory disturbances. A good clinical evaluation can be used to inform the patient about the potential risks of surgery. Written informed consent, after the patient has received a complete description of these risks, must be obtained in all cases. Among patients with iatrogenic paraesthesia in the third division of the trigeminal nerve, 75% regain normal sensitivity without further treatment [3]. In most cases, complete recovery occurs 6 to 8 weeks after the trauma, although it may take up to 24 months. If paraesthesia is not completely resolved within about two months, the probability of a permanent deficit increases significantly; it is unlikely that complete resolution will occur if the deficit is still present after nine months [15]. In our study also the nerve recovered very quickly for the first three months, and has been static since then.
The rate of recovery is supposed to increase after six months and again after nine months, exhibiting a bimodal pattern, with a continuous increase in the recovery rate up until 12–15 months. This could be explained by the fact that IAN injuries differ in type. Lesions that recover within the first three months are probably neuropraxias or Sunderland 1st or 2nd degree injuries [16, 17]. Long standing injuries could represent more severe form of injuries. Recovery from IAN after one year has also been reported in the literature [18]. It is difficult to identify the injuries with poor prognosis especially if the nerve damage is not been seen at the time of surgery. Nevertheless compression of the nerve does not cause damage for more than four months, and partial sectioning should recover by eight months. Most permanent injuries are hypoesthesias and are thankfully well tolerated by patients unlike dysesthesias.
Conclusion
Most cases of iatrogenic paraesthesia can be prevented. This can be achieved by buccal approach technique of third molar removal. However, when paraesthesia occurs, follow-up must be initiated quickly, since the first few months may determine the degree of nerve healing. In our study, we observed that the IAN nerve recovers faster in gross neurosensory assessment i.e. Level C tests. Complete recovery, as tested by the fine neurosensory assessing tests like, Levels A and B tests is slower and may be impaired permanently also.
References
- 1.Ziccardi VB, Assael LA. Mechanisms of trigeminal nerve injuries. Atlas Oral Maxillofac Surg Clin North Am. 2001;9(2):1–11. [PubMed] [Google Scholar]
- 2.Sandstedt P, Sorensen S. Neurosensory disturbances of the trigeminal nerve: a long-term follow-up of traumatic injuries. J Oral Maxillofac Surg. 1995;53(5):498–505. doi: 10.1016/0278-2391(95)90055-1. [DOI] [PubMed] [Google Scholar]
- 3.Zuniga JR, LaBank JP. Advancses in microsurgical nerve repair. J Oral Maxillofac Surg. 1993;51(suppl 1):62–68. doi: 10.1016/0278-2391(93)90010-B. [DOI] [PubMed] [Google Scholar]
- 4.Blackburn CW, Bramley PA. Lingual nerve damage associated with the removal of third molars. Br Dent J. 1989;167:103–107. doi: 10.1038/sj.bdj.4806922. [DOI] [PubMed] [Google Scholar]
- 5.Jaaskelainen SK, Petola JK, Lehtinen R. The mental nerve blink reflex in the diagnosis of lesions of the inferior alveolar nerve following orthognathic surgery of the mandible. Br J Oral Maxillofac Surg. 1996;34:87–95. doi: 10.1016/S0266-4356(96)90143-6. [DOI] [PubMed] [Google Scholar]
- 6.Vriens JPM, Moos KJ. Morbidity of infraorbital nerve following orbitozygomatic complex fractures. J Craniomaxillofac Surg. 1995;23:363–368. doi: 10.1016/S1010-5182(05)80131-3. [DOI] [PubMed] [Google Scholar]
- 7.Yoshida T, Nagamine T, Kobayashi T, Michimi N, Nakajima T, Sasakura H, Hanada K. Impairment of the inferior alveolar nerve after sagittal split osteotomy. J Craniomaxillofac Surg. 1989;17:271–278. doi: 10.1016/S1010-5182(89)80095-2. [DOI] [PubMed] [Google Scholar]
- 8.Cooper BY, Sessle BJ. Anatomy, physiology, and pathophysiology of trigeminal system paresthesias and dysesthesias. Oral Maxillofac Clin North Am. 1992;4(2):297–322. [Google Scholar]
- 9.Zuniga JR, Essick GK. A contemporary approach to the clinical evaluation of trigeminal nerve injuries. Oral Maxillofac Clin North Am. 1992;4(2):353–367. [Google Scholar]
- 10.Ghali GE, Epker BN. Clinical neurosensory testing: practical applications. J Oral Maxillofac Surg. 1989;47:1074–1078. doi: 10.1016/0278-2391(89)90184-5. [DOI] [PubMed] [Google Scholar]
- 11.Levant BA. Mental anesthesia and its prognosis. Br J Oral Surg. 1967;4:206. doi: 10.1016/s0007-117x(66)80038-0. [DOI] [PubMed] [Google Scholar]
- 12.Rood JP. Lingual split technique. Damage to inferior alveolar and lingual nerves during the removal of impacted mandibular third molars. Br Dent J. 1983;154:402–403. doi: 10.1038/sj.bdj.4805103. [DOI] [PubMed] [Google Scholar]
- 13.Rood JP. Degrees of injury to inferior alveolar nerve sustained during the removal of the impacted mandibular third molar by lingual split technique. Br Dent J. 1983;21:103–116. doi: 10.1016/0007-117x(83)90054-9. [DOI] [PubMed] [Google Scholar]
- 14.Carmicheal FA, McGowan DA. Incidence of nerve damage following third molar removal: a West of Scotland oral surgery Research Group study. Br J Oral Maxillofac Surg. 1992;30:78–82. doi: 10.1016/0266-4356(92)90074-S. [DOI] [PubMed] [Google Scholar]
- 15.Robinson PP. Observations on the recovery of sensation following inferior alveolar nerve injuries. Br J Oral Maxillofac Surg. 1988;26(3):177–189. doi: 10.1016/0266-4356(88)90161-1. [DOI] [PubMed] [Google Scholar]
- 16.Seddon HJ. Three types of nerve injury. Brain. 1943;66:237–288. doi: 10.1093/brain/66.4.237. [DOI] [Google Scholar]
- 17.Sunderland S. A classification of peripheral nerve injuries producing loss of function. Brain. 1951;74:491. doi: 10.1093/brain/74.4.491. [DOI] [PubMed] [Google Scholar]
- 18.Alling CC., 3rd Dysesthesia of the lingual and inferior alveolar nerves following third molar surgery. J Oral Maxillofac Surg. 1986;44:454–457. doi: 10.1016/S0278-2391(86)80010-6. [DOI] [PubMed] [Google Scholar]


