Abstract
Objective
To evaluate whether a dietary intervention designed to reduce fat intake and increase intake of fruit, vegetables, and whole grains, and weight loss, reduce vasomotor symptoms (VMS, i.e., hot flashes or night sweats) in postmenopausal women.
Methods
We included 17,473 postmenopausal U.S. women, ages 50–79 at baseline who participated in the Women’s Health Initiative Dietary Modification (DM) trial and were not taking menopausal hormone therapy. Logistic regression was used to evaluate associations.
Results
In multivariate-adjusted analyses, with simultaneous adjustment for the intervention and weight change, assignment to dietary intervention vs. control arm was significantly (OR=1.14, 95%CI: 1.01–1.28) related to a higher likelihood of symptom elimination among women with VMS at baseline. Additionally, women with symptoms at baseline who lost ≥10 lbs (OR=1.23, 95%CI: 1.05–1.46) or lost ≥10% of their baseline body weight (OR=1.56, 95%CI: 1.21–2.02) between baseline and year 1 were significantly more likely to eliminate VMS compared with those who maintained weight. Upon examining the joint effect of the dietary modification and weight loss, compared to women in the control arm who maintained weight, women who lost substantial weight (≥10%) as a part of the intervention (OR=1.89, 95%CI: 1.39–2.57), but not control arm (OR=1.40, 95%CI: 0.92–2.13), were significantly more likely to end VMS, though these two groups did not differ significantly from each other. Large weight loss (>22 lbs) but not dietary changes were related to elimination of moderate/severe VMS.
Conclusions
Weight loss as part of a healthy dietary modification may help to eliminate vasomotor symptoms among postmenopausal women.
Keywords: Hot flashes, night sweats, vasomotor symptoms, weight, body mass, weight change, women
Introduction
Vasomotor symptoms (VMS) include hot flashes and night sweats and are thought to result from vasodilation of the blood vessels close to the skin. Up to 80% of peri- and postmenopausal women report having VMS1–13 with up to half reporting moderate or severe symptoms. 14 Vasomotor symptoms peak during the menopausal transition, but they can persist for years—longer than a decade in some women 15, 16. Up to one quarter of women in their 60s and 70s report having hot flashes 17, 18.
Vasomotor symptoms can negativelyaffect a women’s quality of life 19 by disrupting sleep 14, 20, interfering with work and leisure activities, and exacerbating anxiety and depression21. In multiple observational studies, women with a larger body size, either higher body mass index (BMI) or greater percent body fat, have reported more frequent or severe vasomotor symptoms compared with women with a lower BMI or percent body fat4, 8, 22–25.
Although high body weight is linked to greater vasomotor symptomatology, little is known about weight change and VMS—whether weight gain might exacerbate and weight loss might ameliorate symptoms. Two studies have reported that weight gain26, or fat gain27 is associated with reporting more VMS, and one small randomized weight loss intervention trial (N=338) reported that women in the intervention group had fewer and less bothersome hot flashes than women in the control group at the end of the trial28.
Since most women tend to gain weight with age, weight loss or prevention of weight gain, in addition to other potential health benefits, may offer a viable strategy to help alleviate VMS. In addition, dietary patterns might also influence VMS. Most studies have focused on the effect of soy and other isoflavonoid intake on symptoms 4, 29, 30 and there is evidence that high fiber 31 and low fat intake 32 may be related to a reduced likelihood of these symptoms; nonetheless, there is limited research on dietary patterns and VMS.
The Women’s Health Initiative (WHI) Dietary Modification (DM) trial consisted of a dietary intervention aimed at reducing fat intake and increasing fruit, vegetable, and whole grain intake. Although weight loss was not a goal, participants assigned to the intervention lost on average ~2 kg between baseline and Year 1 compared to the control group, providing the opportunity to examine whether this dietary intervention, weight change, and weight change resulting from a low fat dietary pattern intervention, was associated with the reduction or elimination of VMS.
METHODS
Study population
The design and recruitment methods of the WHI Dietary Modification (DM) trial have been previously described. 33–35 Briefly, the WHI DM trial enrolled a diverse group of 48,835 post-menopausal women between 1993–1998 at 40 US clinical centers who agreed to be randomly assigned to a dietary intervention (40%) or control arm (60%). Eligibility criteria included: 1)ages 50–79; 2)postmenopausal status; 3)willingness to provide informed consent; and 4)at least a three-year life expectancy. The purpose of the dietary trial was to evaluate the effects of a low-fat dietary pattern on heart disease, breast and colorectal cancer and fracture in postmenopausal women. Exclusions from the DM trial included any prior breast or colorectal cancer, other cancer except non-melanoma skin cancer within the past 10 years, adherence or retentionconcerns, or a baseline diet estimated to have less than 32% of energy from fat, as assessed by the WHI food-frequency questionnaire (FFQ). Human Subjects Review Committees at each participating institution approved the protocol. All participants provided written informed consent.
The 40% of DM trial women who were assigned to the intervention (DM-I) were asked to change their diet to a low-fat dietary pattern (20% of energy from fat) with increased intake of fruits and vegetables (5 servings per day) and whole grains (6 servings per day). DM-I participants also received an intensive behavioral modification program to assist themin achieving the dietary intervention goals. This included 18 group sessions in the first year and quarterly maintenance sessions thereafter. In these sessions, groups of 8–15 women were led by specially trained and certified nutritionists/registered dietitians. Each session included both nutritional and behavioral topics. In contrast, the controls received a copy of the Dietary Guidelines for Americans36, as well as other health-related materials, but had no contact with the nutrition interventionists. Detailed descriptions of the intervention and methodology of the WHI DM trial have been published37. DM-I participants were encouraged to adjust their diet to maintain their baseline weight; however, 21% of women in the intervention portion of the trial lost weight, as did 7% of women in the control group.
Of the 48,835 DM trial participants, we excluded 8,050 who also participated in the WHI Hormone Trial and 21,335 reporting baseline intake of hormone therapy because of possible confounding by hormone therapy which is known to relieve VMS. We also excluded 2,271 women missing data on VMS or weight at baseline or year 1. The final sample size included 17,473 women.
Data collection
Weight change
Weight change was computed in two ways—absolute change in weight (lbs) between baseline and year 1 of follow-up and percent change from baseline weight. Weight change was categorized to enable evaluation of small, medium, and large weight changes, compared to a reference group of women within the analytic cohort who maintained their weight 38. Categories of weight change included: weight loss ≥10 lbs, weight loss from >5-<10 lbs, weight maintenance within 5 lbs of baseline (reference), weight gain of >5-<10 lbs, and weight gain ≥10 lbs. Categories of percent weight change were: Weight loss ≥ 10%, weight loss between 7-<10% of baseline, weight loss of >3-<7% of baseline, weight maintenance within 3% of baseline (reference), weight gain of >3-<7% of baseline, weight gain between 7-<10% of baseline, and weight gain ≥10% of baseline. Continuous measures of weight change were also evaluated.
Vasomotor symptoms
Reports of hot flashes and night sweats at baseline and Year 1 were obtained from a 34-item self-reported symptom inventory, which asked individually about hot flash and night sweat occurrence/severity in the prior 4 weeks (scored 0 = none to 3 = severe). Mild symptoms were considered those that did not interfere with usual activities while moderate symptoms interfered somewhat and severe symptoms were so bothersome that usual activities could not be performed.
We created a variable to characterize the presence and severity of VMS from the two hot flash and night sweats variables, based on the common definition of VMS and women were assigned a value for VMS (0–3) based on the highest reported value for either hot flashes or night sweats.
Covariates
Information on covariates was self-reported at the baseline assessment through questionnaires. Covariates included demographic factors (ethnicity, education), years since menopause, hysterectomy, oophorectomy, depressive symptomatology based on the Center for Epidemiologic Studies Depression scale (CES-D) and several behavioral factors (physical activity, alcohol intake, smoking status) (see Tables 1–3). We chose covariates considered to be important risk factors for VMS.
Table 1.
Baseline characteristics by vasomotor symptom status and severity (N=17,473)
| Level of vasomotor symptoms | |||||
|---|---|---|---|---|---|
| None | Mild | Moderate | Severe | p-value* | |
| N (%) | 11,369 (65%) | 4,371 (25%) | 1,393 (8%) | 340 (2%) | |
| Age at randomization, mean (SD) | 64.8 (6.7) | 61.3 (6.7) | 60.4 (6.8) | 59.6 (6.5) | |
| Randomization arm (%) | |||||
| Dietary modification (N=7,039) | 65.5 | 24.5 | 8.0 | 2.0 | 0.64 |
| Control (N=10,434) | 64.8 | 25.3 | 8.0 | 1.9 | |
| Body mass index (%) | |||||
| <18.5 kg/m2 | 73.6 | 17.0 | 9.4 | 0.0 | <0.001 |
| 18.5-<25 kg/m2 | 67.6 | 24.7 | 6.3 | 1.4 | |
| 25-<30 kg/m2 | 64.9 | 25.3 | 8.0 | 1.9 | |
| 30-<35 kg/m2 | 64.3 | 24.5 | 9.0 | 2.3 | |
| 35-<40 kg/m2 | 62.0 | 26.5 | 9.3 | 2.3 | |
| ≥40 kg/m2 | 64.8 | 24.3 | 8.4 | 2.4 | |
| Ethnicity (%) | |||||
| Caucasian (N=13,794) | 68.6 | 23.5 | 6.6 | 1.3 | <0.001 |
| African-American (N=2,415) | 45.9 | 34.0 | 14.7 | 5.4 | |
| Hispanic/Latino (N=550) | 57.1 | 25.8 | 13.1 | 4.0 | |
| Asian (N=386) | 73.8 | 20.5 | 4.9 | 0.8 | |
| American Indian (N=74) | 58.1 | 31.1 | 8.1 | 2.7 | |
| Other (N=206) | 62.1 | 24.3 | 11.2 | 2.4 | |
| College graduate (%) | |||||
| No (N=10,880) | 63.3 | 25.3 | 9.0 | 2.4 | <0.001 |
| Yes (N=6,496) | 68.2 | 24.5 | 6.2 | 1.2 | |
| Oophorectomy (%) | |||||
| No (N=13,696) | 65.3 | 25.2 | 7.7 | 1.8 | <0.001 |
| Yes (N=3,561) | 63.9 | 24.8 | 8.9 | 2.4 | |
| Hysterectomy (%) | |||||
| No (N=12,275) | 65.7 | 25.0 | 7.6 | 1.7 | <0.001 |
| Yes (N=5,196) | 63.6 | 25.0 | 8.8 | 2.6 | |
| Smoking status (%) | |||||
| Current (N=1,164) | 57.2 | 28.8 | 10.4 | 3.6 | <0.001 |
| Former (N=7,015) | 65.0 | 24.6 | 8.4 | 2.0 | |
| Never (N=9,111) | 66.2 | 24.8 | 7.3 | 1.7 | |
| Depression (%) | |||||
| CESD<0.06 (on a scale of 0–1) (N=15,880) | 66.5 | 24.5 | 7.4 | 1.6 | <0.001 |
| CESD≥0.06 (N=1,593) | 50.4 | 30.6 | 13.8 | 5.2 | |
| Years since menopause, median (IQR**) | 15 (9–22) | 11 (5–18) | 10 (4–19) | 9 (4–17) | 0.004† |
| Alcohol intake (servings/wk), median (IQR) | 0.4 (0.0–2.3) | 0.4 (0.0–2.0) | 0.2 (0.0–1.4) | 0.0 (0.0–1.0) | 0.003† |
| Physical activity (METS‡/wk), median (IQR) | 6.8 (1.5–15.0) | 6.0 (1.3–14.0) | 5.3 (0.8–13.5) | 6.1 (0.5–13.2) | 0.38† |
p-value, Mantel-Haenszel χ2 test, not for trend, age-adjusted
Interquartile range
p-value, continuous variable
Metabolic equivalents
Table 3.
Multivariate-adjusted odds ratios of weight change over one year and at least 1 Likert category reduction of vasomotor symptoms (VMS) among 6,104 postmenopausal women with symptoms at baseline
| N | At least 1 Likert category reduction** | Age- adjusted OR† | 95% CI | MV- adjusted OR‡ | 95% CI | |
|---|---|---|---|---|---|---|
| Any vasomotor symptoms at baseline | ||||||
| DM arm | ||||||
| Control | 3,672 | 1,508 | 1.00 | |||
| Intervention | 2,432 | 1,103 | 1.19 | (1.08–1.32) | 1.15 | (1.03–1.28) |
| p-value | <0.001 | 0.01 | ||||
| Weight change (lbs) | ||||||
| Gain ≥ 10 lbs | 399 | 162 | 0.99 | (0.80–1.22) | 1.00 | (0.44–1.26) |
| Gain >5-<10 lbs | 663 | 266 | 0.96 | (0.81–1.14) | 0.97 | (0.82–1.16) |
| Maintain weight | 3,173 | 1,316 | 1.00 | 1.00 | ||
| Loss >5-<10 lbs | 963 | 438 | 1.18 | (1.02–1.36) | 1.12 | (0.97–1.30) |
| Loss ≥ 10 lbs | 906 | 429 | 1.28 | (1.10–1.48) | 1.20 | (1.03–1.40) |
| p-value, gain* | 0.84 | 0.98 | ||||
| p-value, loss* | 0.04 | 0.07 | ||||
| Weight change (%) | ||||||
| Gain ≥ 10% | 124 | 46 | 0.85 | (0.59–1.23) | 0.85 | (0.58–1.23) |
| Gain >7-<10% | 143 | 64 | 1.17 | (0.83–1.64) | 1.16 | (0.83–1.64) |
| Gain >3-7% | 785 | 313 | 0.95 | (0.81–1.11) | 0.95 | (0.81–1.12) |
| Maintain within 3% | 3230 | 1341 | 1.00 | 1.00 | ||
| Loss >3-7% | 1140 | 510 | 1.14 | (0.99–1.30) | 1.07 | (0.93–1.23) |
| Loss >7-<10% | 389 | 181 | 1.23 | (0.99–1.52) | 1.14 | (0.91–1.41) |
| Loss ≥ 10% | 293 | 156 | 1.62 | (1.28–2.07) | 1.50 | (1.17–1.92) |
| p-value, gain* | 0.81 | 0.70 | ||||
| p-value, loss* | 0.002 | 0.01 | ||||
| Moderate/severe symptoms at baseline | ||||||
| DM arm | ||||||
| Control | 1,028 | 1,508 | 1.00 | 1.00 | ||
| Intervention | 705 | 1,103 | 1.10 | (0.90–1.34) | 1.09 | (0.89–1.34) |
| p-value | 0.36 | 0.41 | ||||
| Weight change (lbs) | ||||||
| Gain ≥ 10 lbs | 140 | 77 | 0.74 | (0.51–1.06) | 0.75 | (0.52–1.08) |
| Gain >5-<10 lbs | 206 | 123 | 0.89 | (0.65–1.22) | 0.87 | (0.63–1.19) |
| Maintain weight | 842 | 528 | 1.00 | 1.00 | ||
| Loss >5-<10 lbs | 283 | 180 | 1.04 | (0.79–1.38) | 1.01 | (0.75–1.34) |
| Loss ≥ 10 lbs | 262 | 163 | 0.98 | (0.74–1.31) | 0.94 | (0.70–1.27) |
| p-value, gain* | 0.97 | 0.56 | ||||
| p-value, loss* | 0.75 | 0.67 | ||||
continuous variable
Signifies reduction of vasomotor symptoms by one or more Likert categories (i.e., severe to moderate, severe to mild, or severe to no symptoms, or moderate to mild or moderate to no symptoms)
Age-adjusted model adjusted for age (continuous).
Multivariate-adjusted models were adjusted for age (continuous), ethnicity (White (ref), Black, Hispanic, Asian, Indian, other), education (≤HS, ≤ college, graduate school (ref)), years since menopause (0-<10, 10-<20, 20+ (ref)), depressive symptoms (no (ref), yes), physical activity (0, >0-5, >5-10, >20-15, >15 METS/wk (ref)), alcohol intake (0 (ref), >0-0.5, >0.5-2, >2 svg/wk), smoking (never (ref), past, current). Models were also simultaneously adjusted for DM arm (intervention, control (ref)) and weight change (percent change categories used in intervention analyses).
Statistical analysis
We examined baseline characteristics by categories of VMS severity, using age-adjusted Mantel-Haenzsel (MH) χ2 tests to evaluate associations between covariates and VMS (Table 1).
Analyses of dietary intervention, weight change and changes in VMS
For these analyses (except for the proportional odds models), we examined the 6,104 women with VMS at baseline, separately and simultaneously evaluating whether the dietary modification (intervention vs. control) and weight change were associated with elimination of symptoms by Year 1. Analyses then evaluated whether each of these variables was independently associated with the relative odds of a reduction of symptoms (diminishing bother) by at least one Likert category (i.e., severe to moderate, severe to mild, or severe to none; moderate to mild, or moderate to none; or mild to none), compared to those who maintained weight. Subsequent analyses focused on those with moderate or severe symptoms at baseline (N=1,733).
Initial analyses were adjusted for age. Additional analyses were adjusted for selected covariates of interest (Table 1).
To determine if the effect of the dietary modification and weight change variables on VMS could be characterized as cumulative across the range of severity, we employed proportional odds logistic models in the total group of 17,473 women to evaluate associations of the dietary modification, categories of weight change, and the four category ordinal level VMS outcome at year 1. A model adjusted for age was compared to a multivariate-adjusted model, testing for non-proportional odds using the score test. For the multivariate-adjusted model, adjustments for covariates that did not cause the model to violate the proportional odds assumption included age, smoking, and years since menopause. We then compared this model to one adjusted for all covariates included in general analyses.
Finally, the joint effect of the dietary modification and weight change and changes in VMS was evaluated with outcomes (i.e., elimination of symptoms, diminishing bother) regressed against the cross-classification of the intervention and categories of weight change.
RESULTS
Seventy-four percent of women in the study reported no hot flashes at baseline and 19% reported mild, 6% reported moderate, and 1% reported severe hot flashes. Similarly, 73% reported no night sweats and 20% reported mild, 6% reported moderate, and 1% reported severe night sweats. Table 1 shows the prevalence of women with VMS. Those reporting severe VMS were younger and more recently menopausal. Presence and severity of VMS were positively associated with African-American ethnicity, less education, current smoking, depressive symptomatology, and lower alcohol intake. There were no significant differences by randomization arm or level of physical activity (Table 1).
Of the 17,473 women with vasomotor data at both baseline and year 1, over half (56.0%) had no VMS at either baseline or year 1. Of the remaining women, 14.9% reduced and 12.5% saw a worsening of their symptoms, while 16.5% reported similar VMS at both visits.
DM-I participants were three times more likely to lose weight (≥5 lbs) compared to the control arm (OR=3.3, 95% CI: 3.1–3.5) whereas women in the control group were over two times more likely to gain weight (≥5 lbs) (OR=2.3, 95% CI: 2.1–2.5) as DM-I participants (Figure 1).
Figure1.
Weight change over one year by dietary modification arm. Women in the intervention were three times more likely to lose weight (>5 lbs) than women in the control arm (OR=3.3 (95% CI: 3.1–3.5). Women in the control arm were two times more likely to gain weight (>5 lbs) than women in the intervention (OR=2.3, 95% CI: 2.1–2.5).
Dietary modification and changes in vasomotor symptoms
Overall, women assigned to the DM intervention were more likely to eliminate VMS by Year 1, compared with controls, in age-adjusted (OR=1.19, 95%CI: 1.06–1.33) and multivariate-adjusted (OR=1.14, 95%CI: 1.01–1.28) analyses (Table 2). The intervention was similarly associated with an increased likelihood of at least a one Likert category reduction of symptoms (Table 3). However, the intervention was not significantly related to the elimination or reduction of symptoms among women with moderate or severe symptoms at baseline (Tables 2 and 3).
Table 2.
Multivariate-adjusted odds ratios of the intervention and changes in weight over one year and elimination of vasomotor symptoms (VMS) among 6,104 postmenopausal women with symptoms at baseline
| N | Elimination of VMS | Age-adjusted OR** | 95% CI | MV-adjusted OR† | 95% CI | |
|---|---|---|---|---|---|---|
| Any vasomotor symptoms at baseline | ||||||
| DM arm | ||||||
| Controls | 3,672 | 1,014 | 1.00 | 1.00 | ||
| Intervention | 2,432 | 757 | 1.19 | (1.06–1.33) | 1.14 | (1.01–1.28) |
| p-value | 0.003 | 0.04 | ||||
| Weight change (lbs) | ||||||
| Gain ≥ 10 lbs | 399 | 106 | 0.96 | (0.76–1.21) | 1.02 | (0.80–1.30) |
| Gain >5-<10 lbs | 663 | 164 | 0.85 | (0.70–1.03) | 0.88 | (0.73–1.08) |
| Maintain weight | 3,173 | 902 | 1.00 | 1.00 | ||
| Loss >5-<10 lbs | 963 | 290 | 1.09 | (0.93–1.27) | 1.02 | (0.87–1.20) |
| Loss ≥ 10 lbs | 906 | 309 | 1.32 | (1.13–1.55) | 1.23 | (1.05–1.46) |
| p-value, gain* | 0.27 | 0.36 | ||||
| p-value, loss | 0.005 | 0.008 | ||||
| Weight change (%) | ||||||
| Gain ≥ 10% | 124 | 30 | 0.86 | (0.57–1.31) | 0.87 | (0.57–1.32) |
| Gain >7-<10% | 143 | 43 | 1.16 | (0.80–1.68) | 1.20 | (0.83–1.74) |
| Gain >3-7% | 785 | 194 | 0.87 | (0.73–1.04) | 0.88 | (0.73–1.06) |
| Maintain within 3% | 3230 | 904 | 1.00 | 1.00 | ||
| Loss >3-7% | 1140 | 359 | 1.17 | (1.01–1.36) | 1.08 | (0.94–1.27) |
| Loss >7-<10% | 389 | 125 | 1.22 | (0.97–1.53) | 1.10 | (0.87–1.38) |
| Loss ≥ 10% | 293 | 116 | 1.73 | (1.35–2.22) | 1.56 | (1.21–2.02) |
| p-value, gain* | 0.54 | 0.47 | ||||
| p-value, loss* | <0.001 | <0.001 | ||||
| Moderate/severe symptoms at baseline | ||||||
| DM arm | ||||||
| Controls | 1,028 | 132 | 1.00 | 1.00 | ||
| Intervention | 705 | 99 | 1.10 | (0.83–1.46) | 1.10 | (0.81–1.48) |
| p-value | 0.50 | 0.54 | ||||
| Weight change (lbs) | ||||||
| Gain ≥ 10 lbs | 140 | 21 | 1.19 | (0.72–1.97) | 1.31 | (0.78–2.21) |
| Gain >5-<10 lbs | 206 | 21 | 0.75 | (0.46–1.23) | 0.73 | (0.44–1.21) |
| Maintain weight | 842 | 114 | 1.00 | 1.00 | ||
| Loss >5-<10 lbs | 283 | 32 | 0.81 | (0.54–1.24) | 0.77 | (0.50–1.18) |
| Loss ≥ 10 lbs | 262 | 43 | 1.28 | (0.87–1.87) | 1.23 | (0.82–1.84) |
| p-value, gain* | 0.79 | 0.26 | ||||
| p-value, loss | 0.10 | 0.12 | ||||
p-value, continuous variable
Age-adjusted model adjusted for age (continuous).
Multivariate-adjusted models were adjusted for age (continuous), ethnicity (White (ref), Black, Hispanic, Asian, Indian, other), education (≤HS, ≤ college, graduate school (ref)), years since menopause (0-<10, 10-<20, 20+ (ref)), depressive symptoms (no (ref), yes), physical activity (0, >0-5, >5-10, >20-15, >15 METS/wk (ref)), alcohol intake (0 (ref), >0-0.5, >0.5-2, >2 svg/wk), smoking (never (ref), past, current). Models were also simultaneously adjusted for DM arm (intervention, control (ref)) and weight change (percent change categories used in intervention analyses).
In the proportional odds model, women in the intervention were significantly less likely than controls to report VMS at the Year 1 follow-up, in the model that did not violate the proportional odds assumption (see below) (OR=0.70, 95% CI: 0.60–0.82) or in the fully adjusted model (OR=0.75, 95% CI: 0.63–0.90). Among women with moderate or severe symptoms, associations were nonsignificant (data not shown).
Weight change and changes in vasomotor symptoms
Women who lost weight were more likely to eliminate VMS (Table 2). Similar associations were seen when the outcome was defined as at least one category reduction in symptoms (Table 3). Associations were apparent whether weight change was defined in terms of absolute change (lbs) or percent change in weight from baseline. However, associations were not apparent among women with moderate or severe VMS at baseline. Also, reductions in VMS were not apparent among women who gained vs. those who maintained weight.
In a longitudinal proportional odds model of vasomotor outcome at year 1, women who gained a large, OR=1.16 (95% CI: 0.93–1.44), or a small, OR=1.17 (95% CI: 1.06–1.29), amount of weight had a higher odds of VMS at year 1, compared to those who maintained weight though women who gained a medium amount of weight, OR=1.04 (95% CI: 0.84–1.28), had no higher odds. The continuous weight change variable among these women was nonsignificant (p-continuous weight gain=0.72). Those who lost a small OR=0.96 (95% CI: 0.88–1.05) or medium amount of weight, OR=0.97 (95% CI: 0.85–1.11) were not less likely to have VMS than those who maintained weight. However, those who lost a large amount of weight (≥10% of baseline) had a lower odds (OR=0.70, 95% CI: 0.60–0.82) of VMS at year 1, compared to those who maintained weight (p-continuous weight loss=0.001). A test of the proportional odds assumption indicated no significant departure from the model, χ2=37.2, df=28, p=0.11, when adjusted for a partial set of covariates. Results were similar when adjusted for all covariates considered.
Joint effect of dietary modification and weight change and changes in VMS
Analysis of study arm and absolute weight change with elimination of VMS
In the analysis of the cross-classification of the dietary intervention with weight change categories and changes in VMS, DM-I participants who lost ≥10 lbs were more likely to eliminate (OR=1.43, 95% CI: 1.17–1.74) or reduce (OR=1.38, 95% CI: 1.15–1.67) symptoms than women in the control group who maintained weight. However, women in the intervention group who gained ≥10 lbs were also more likely to eliminate (OR=1.52, 95% CI: 1.02–2.27) or reduce (OR=1.49, 95% CI: 1.03–2.15) symptoms. Women in the control group who either lost or gained weight were not more likely than those who maintained weight to eliminate or reduce symptoms. Other weight categories for women in the intervention group were unrelated to outcomes. Median changes in weight for each weight change group were highly similar by study arm (data not shown).
Analysis of study arm and percent weight change with elimination of VMS
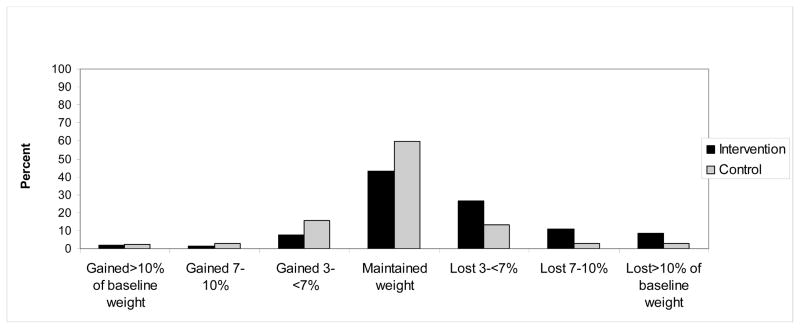
Among women in the dietary intervention, women who lost large amounts of weight (≥ 10% of baseline) were significantly more likely to eliminate their symptoms compared with controls who maintained weight (OR=1.89, 95% CI: 1.39–2.57). Among the controls, women who lost ≥10% of baseline weight appeared somewhat more likely than those who maintained their weight, to eliminate VMS, but the finding was not significant (OR=1.40, 95% CI: 0.92–2.13). However, these two groups did not differ significantly from each other (p-contrast=0.56). There were no other significant associations (Figure 2).
DISCUSSION
As anticipated, women who lost weight over one year were more likely to reduce or eliminate their hot flash or night sweat symptoms compared with those who maintained their weight. Additionally, women who participated in a dietary modification trial that encouraged them to decrease fat and increase fruit, vegetable, and whole grain intake, were significantly more likely to eliminate VMS. The effects were statistically significant and independent though the association of weight loss and elimination of VMS appeared somewhat stronger in the DM-I arm. The surprising finding that women in the intervention who gained >10 lbs also reduced VMS suggests that the beneficial impact of healthy diet was not restricted to those who lost weight. Adjustment for factors known to be related to VMS did not explain associations. This is the largest report to date of the effect of weight loss on change in vasomotor symptom status, and the first trial to examine the influence of a healthy dietary change on VMS changes. These findings suggest that weight loss and healthy dietary changes could each help to reduce or eliminate VMS.
We report similar findings for weight loss and VMS to those in another trial of weight loss and hot flashes 28. However, that study was limited because it included only women with urinary incontinence. This study is considerably larger and should generalize more broadly to the population of postmenopausal women. Given the possible adverse effects of hormone therapy on breast cancer and cardiovascular disease risk, intentional weight loss following a healthy diet may be an important, alternative means of reducing VMS without exacerbating risk of disease.
Diet also appeared to reduce VMS beyond the effects of weight change. Although analyses suggested the potential benefit of weight loss with regard to VMS in both the DM-I and control groups, the association between weight loss and elimination of symptoms appeared stronger in the intervention group. Interestingly, the beneficial effect of diet on VMS also appeared among DM-I participants who gained weight. Though weight gain occurred less often in the intervention than in controls, we considered that weight gain in the intervention could have resulted from an increased intake of fruit, vegetables, and whole grains without a commensurate reduction in fat intake. However, in post-hoc analysis, women in the DM-I who gained weight reported significant reductions in fat and increases in carbohydrate (but not protein) intake, although those who lost weight had greater changes than those who maintained or gained weight. At the end of year 1, compared with the controls, women in the intervention had higher intakes of fiber (17.9 vs. 14.9 g/d), fruit (2.5 vs. 1.8 s/d), vegetables (2.6 vs. 2.1 s/d), genistein (0.73 vs. 0.65 mg/d) and vegetable protein (21.4 vs. 19.0 g/d) and lower intake of total fat (24.7 vs. 34.9% of kcal) and Ω-3 fatty acids (1.1 vs. 1.5 g/d) (all differences, p<0.05). Stratifying by weight change and comparing women in the DM-I with the controls, there were differences in fat intake in all weight categories and differences in vegetable, vegetable protein, and fiber intake among those who lost weight, maintained weight, or gained a small amount of weight (all differences, p<0.05), but not among those who gained a medium or large amount of weight (data not shown). However, no differences in dietary components or changes could explain the apparently stronger effect in DM-I women who gained vs. maintained weight and this result may be due to chance. Additional research is needed to evaluate the aspects of diet that reduce VMS.
An alternative explanation, the placebo effect, i.e., the increased well-being due to the positive healing attention provided by nutritionists to women in this intervention39, may explain some of the intervention’s effect on this subjective outcome 40. Related to this but aside from the direct social benefit of the intervention, it is possible that engagement in the intervention augmented feelings of empowerment associated with one’s own attempts to improve one’s health 41 which could diminish VMS 42. Feelings of taking control of one’s diet and thus one’s health could help to explain the suggested benefit among controls who lost substantial weight as well as the suggested benefits in the intervention group, regardless of weight change.
Although models employing the full sample suggested benefit across the range of VMS, in analyses restricting to those with VMS at baseline, the greatest benefits accrued to those with mild, rather than moderate or severe VMS. In a sensitivity analysis, we evaluated whether more extreme weight changes might influence associations. In an analysis of weight change in kilograms, comparable to the analysis of weight change in pounds, we noted an increased odds of eliminating symptoms (OR=2.36, 95% CI: 1.14–4.92) among those with moderate or severe symptoms at baseline who lost ≥10 kg (~22 lbs), compared to those who maintained weight within 5 kg (~11 lbs). In fact, women who were most likely to see relief of their symptoms lost a substantial amount of weight—10% or more of their baseline weight, suggesting the possible need to lose considerable weight for this strategy to succeed. Given that the average weight among overweight women was 79.2 kg (~175 lbs), a woman of this weight who has moderate or severe symptoms may need to lose at least 7.92 kg (17.5 lbs) to clearly experience an effect. However, this weight loss should ideally be a loss of fat and not lean mass.
With regard to treatment, reduction in severity of symptoms and not simply the elimination of symptoms would be considered desirable. Nevertheless, although small weight or dietary changes may make little measurable difference in severity of symptoms, larger changes may bring substantial relief. Both weight loss and diet reduced symptoms over the course of a year. Given that 2/3 of the population is overweight, weight loss and healthy dietary changes could be broadly useful strategies for reducing VMS in addition to improving other health outcomes.
Proposed mechanisms for weight and VMS include greater insulation against heat loss due to increased peripheral fat43, abnormal sympathetic neural activity associated with increased visceral fat44, and alterations in leptin and other cytokines expressed by adipocytes that affect thermoregulatory function 45. Women who are overweight or obese may also differ with regard to social or psychological46 factors that affect subjective experience of somatic symptoms. Our results for weight loss and VMS are consistent with the thermoregulatory model and suggest that weight loss may reduce adipose tissue, a potent insulator 47, and may thus reduce VMS because of the reduced need to dissipate excess body heat that occurs in the context of the menopausal transition 48, 49. However, further research is needed to evaluate mechanistic relationships between diet and VMS.
Study strengths include a large sample size in a well-characterized cohort of postmenopausal women and the ability to control for numerous covariates. Because of longitudinal assessment, we were also able to assess changes in VMS over time. While we report on subjective and not objective measures of hot flashes and night sweats, a woman’s perception of the bother of symptoms is a valid indicator of symptoms 50. We did not measure data on frequency of hot flashes or night sweats, a common measure in assessment of VMS. We had limited statistical power to fully examine the influence of diet and weight change, particularly on those with moderate to severe symptoms. Women were not selected for the trial based on bothersome vasomotor symptoms, so future clinical trials should focus on this population.
Conclusion
In summary, women who lost weight during participation in a dietary modification trial designed to reduce fat and increase fruit, vegetable, and fiber intake reported a reduction or elimination of VMS over one year. The dietary intervention appeared to ameliorate symptoms over and above the effect of weight change. These results support the use of weight loss and healthy dietary change as alternative approaches to hormone therapy for the relief of vasomotor symptoms.
Table 4.
Multivariate-adjusted odds ratios of the cross-classification of the intervention and changes in weight over one year and elimination of vasomotor symptoms (VMS) among 6,104 postmenopausal women with VMS at baseline
| N | Elimination of VMS | MV-adjusted OR† | 95% CI | |
|---|---|---|---|---|
| Any VMS at baseline | ||||
| Weight change (%) and study arm | ||||
| Gain ≥ 10%, Controls | 87 | 19 | 0.78 | (0.46–1.31) |
| Gain ≥ 10%, Intervention | 37 | 11 | 1.27 | (0.61–2.63) |
| Gain >7-<10%, Controls | 583 | 145 | 0.89 | (0.72–1.10) |
| Gain >7-<10%, Intervention | 42 | 16 | 1.75 | (0.92–3.33) |
| Gain >3-7%, Controls | 101 | 27 | 1.08 | (0.68–1.70) |
| Gain >3-7%, Intervention | 202 | 49 | 0.99 | (0.70–1.39) |
| Maintain within 3%, Controls | 2,160 | 593 | 1.00 | |
| Maintain within 3%, Intervention | 1,070 | 311 | 1.15 | (0.97–1.35) |
| Loss >3-7%, Controls | 514 | 160 | 1.17 | (0.95–1.45) |
| Loss >3-7%, Intervention | 626 | 199 | 1.16 | (0.96–1.41) |
| Loss >7-<10%, Controls | 121 | 34 | 1.07 | (0.71–1.63) |
| Loss >7-<10%, Intervention | 268 | 91 | 1.26 | (0.96–1.65) |
| Loss ≥ 10%, Controls | 106 | 36 | 1.40 | (0.92–2.13) |
| Loss ≥ 10%, Intervention | 187 | 80 | 1.89 | (1.39–2.57) |
p-value, continuous variable
Age-adjusted model adjusted for age (continuous).
Multivariate-adjusted models were adjusted for age (continuous), ethnicity (White (ref), Black, Hispanic, Asian, Indian, other), education (≤HS, ≤ college, graduate school (ref)), years since menopause (0-<10, 10-<20, 20+ (ref)), depressive symptoms (no (ref), yes), physical activity (0, >0-5, >5-10, >20-15, >15 METS/wk (ref)), alcohol intake (0 (ref), >0-0.5, >0.5-2, >2 svg/wk), smoking (never (ref), past, current). Models were also simultaneously adjusted for DM arm (intervention, control (ref)) and weight change (percent change categories used in intervention analyses).
Acknowledgments
The work in this paper was supported by an NHLBI grant (Contract #HHSN26820110003C, Subcontract #26534020-50957-B)
Footnotes
The authors report no conflicts of interest.
References
- 1.Williams RE, Kalilani L, DiBenedetti DB, et al. Frequency and severity of vasomotor symptoms among peri- and postmenopausal women in the United States. Climacteric. 2008;11:32–43. doi: 10.1080/13697130701744696. [DOI] [PubMed] [Google Scholar]
- 2.Stearns V, Ullmer L, Lopez JF, Smith Y, Isaacs C, Hayes D. Hot flushes. Lancet. 2002;360:1851–61. doi: 10.1016/s0140-6736(02)11774-0. [DOI] [PubMed] [Google Scholar]
- 3.Bastian LA, Smith CM, Nanda K. Is this woman perimenopausal? JAMA. 2003;289:895–902. doi: 10.1001/jama.289.7.895. [DOI] [PubMed] [Google Scholar]
- 4.Gold EB, Block G, Crawford S, et al. Lifestyle and demographic factors in relation to vasomotor symptoms: baseline results from the Study of Women’s Health Across the Nation. Am J Epidemiol. 2004;159:1189–99. doi: 10.1093/aje/kwh168. [DOI] [PubMed] [Google Scholar]
- 5.Gold EB, Sternfeld B, Kelsey JL, et al. Relation of demographic and lifestyle factors to symptoms in a multi-racial/ethnic population of women 40–55 years of age. Am J Epidemiol. 2000;152:463–73. doi: 10.1093/aje/152.5.463. [DOI] [PubMed] [Google Scholar]
- 6.McKinlay SM, Jefferys M. The menopausal syndrome. Br J Prev Soc Med. 1974;28:108–15. doi: 10.1136/jech.28.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whiteman MK, Staropoli CA, Benedict JC, Borgeest C, Flaws JA. Risk factors for hot flashes in midlife women. J Womens Health (Larchmt) 2003;12:459–72. doi: 10.1089/154099903766651586. [DOI] [PubMed] [Google Scholar]
- 8.Whiteman MK, Staropoli CA, Langenberg PW, McCarter RJ, Kjerulff KH, Flaws JA. Smoking, body mass, and hot flashes in midlife women. Obstet Gynecol. 2003;101:264–72. doi: 10.1016/s0029-7844(02)02593-0. [DOI] [PubMed] [Google Scholar]
- 9.Staropoli CA, Flaws JA, Bush TL, Moulton AW. Predictors of menopausal hot flashes. J Womens Health. 1998;7:1149–55. doi: 10.1089/jwh.1998.7.1149. [DOI] [PubMed] [Google Scholar]
- 10.Li C, Wilawan K, Samsioe G, Lidfeldt J, Agardh CD, Nerbrand C. Health profile of middle-aged women: The Women’s Health in the Lund Area (WHILA) study. Hum Reprod. 2002;17:1379–85. doi: 10.1093/humrep/17.5.1379. [DOI] [PubMed] [Google Scholar]
- 11.Brown WJ, Mishra GD, Dobson A. Changes in physical symptoms during the menopause transition. Int J Behav Med. 2002;9:53–67. doi: 10.1207/s15327558ijbm0901_04. [DOI] [PubMed] [Google Scholar]
- 12.Sukwatana P, Meekhangvan J, Tamrongterakul T, Tanapat Y, Asavarait S, Boonjitrpimon P. Menopausal symptoms among Thai women in Bangkok. Maturitas. 1991;13:217–28. doi: 10.1016/0378-5122(91)90196-w. [DOI] [PubMed] [Google Scholar]
- 13.Chim H, Tan BH, Ang CC, Chew EM, Chong YS, Saw SM. The prevalence of menopausal symptoms in a community in Singapore. Maturitas. 2002;41:275–82. doi: 10.1016/s0378-5122(01)00299-7. [DOI] [PubMed] [Google Scholar]
- 14.Ohayon MM. Severe hot flashes are associated with chronic insomnia. Arch Intern Med. 2006;166:1262–8. doi: 10.1001/archinte.166.12.1262. [DOI] [PubMed] [Google Scholar]
- 15.Col NF, Guthrie JR, Politi M, Dennerstein L. Duration of vasomotor symptoms in middle-aged women: a longitudinal study. Menopause. 2009;16:453–7. doi: 10.1097/gme.0b013e31818d414e. [DOI] [PubMed] [Google Scholar]
- 16.Politi MC, Schleinitz MD, Col NF. Revisiting the duration of vasomotor symptoms of menopause: a meta-analysis. J Gen Intern Med. 2008;23:1507–13. doi: 10.1007/s11606-008-0655-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barnabei VM, Cochrane BB, Aragaki AK, et al. Menopausal symptoms and treatment-related effects of estrogen and progestin in the Women’s Health Initiative. Obstet Gynecol. 2005;105:1063–73. doi: 10.1097/01.AOG.0000158120.47542.18. [DOI] [PubMed] [Google Scholar]
- 18.Barnabei VM, Grady D, Stovall DW, et al. Menopausal symptoms in older women and the effects of treatment with hormone therapy. Obstet Gynecol. 2002;100:1209–18. doi: 10.1016/s0029-7844(02)02369-4. [DOI] [PubMed] [Google Scholar]
- 19.Avis NE, Ory M, Matthews KA, Schocken M, Bromberger J, Colvin A. Health-related quality of life in a multiethnic sample of middle-aged women: Study of Women’s Health Across the Nation (SWAN) Med Care. 2003;41:1262–76. doi: 10.1097/01.MLR.0000093479.39115.AF. [DOI] [PubMed] [Google Scholar]
- 20.Kravitz HM, Ganz PA, Bromberger J, Powell LH, Sutton-Tyrrell K, Meyer PM. Sleep difficulty in women at midlife: a community survey of sleep and the menopausal transition. Menopause. 2003;10:19–28. doi: 10.1097/00042192-200310010-00005. [DOI] [PubMed] [Google Scholar]
- 21.Freeman EW, Sammel MD, Liu L, Gracia CR, Nelson DB, Hollander L. Hormones and menopausal status as predictors of depression in women in transition to menopause. Arch Gen Psychiatry. 2004;61:62–70. doi: 10.1001/archpsyc.61.1.62. [DOI] [PubMed] [Google Scholar]
- 22.Freeman EW, Sammel MD, Grisso JA, Battistini M, Garcia-Espagna B, Hollander L. Hot flashes in the late reproductive years: risk factors for Africa American and Caucasian women. J Womens Health Gend Based Med. 2001;10:67–76. doi: 10.1089/152460901750067133. [DOI] [PubMed] [Google Scholar]
- 23.Gold EB, Colvin A, Avis N, et al. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopausal transition: study of women’s health across the nation. Am J Public Health. 2006;96:1226–35. doi: 10.2105/AJPH.2005.066936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thurston RC, Sowers MR, Chang Y, et al. Adiposity and reporting of vasomotor symptoms among midlife women: the study of women’s health across the nation. Am J Epidemiol. 2008;167:78–85. doi: 10.1093/aje/kwm244. [DOI] [PubMed] [Google Scholar]
- 25.Thurston RC, Sowers MR, Sutton-Tyrrell K, et al. Abdominal adiposity and hot flashes among midlife women. Menopause. 2008;15:429–34. doi: 10.1097/gme.0b013e31815879cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su HI, Sammel MD, Springer E, Freeman EW, DeMichele A, Mao JJ. Weight gain is associated with increased risk of hot flashes in breast cancer survivors on aromatase inhibitors. Breast Cancer Res Treat. 2010;124:205–11. doi: 10.1007/s10549-010-0802-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thurston RC, Sowers MR, Sternfeld B, et al. Gains in body fat and vasomotor symptom reporting over the menopausal transition: the study of women’s health across the nation. Am J Epidemiol. 2009;170:766–74. doi: 10.1093/aje/kwp203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang AJ, Subak LL, Wing R, et al. An intensive behavioral weight loss intervention and hot flushes in women. Arch Intern Med. 2011;170:1161–7. doi: 10.1001/archinternmed.2010.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levis S, Strickman-Stein N, Ganjei-Azar P, Xu P, Doerge DR, Krischer J. Soy isoflavones in the prevention of menopausal bone loss and menopausal symptoms: a randomized, double-blind trial. Arch Intern Med. 2011;171:1363–9. doi: 10.1001/archinternmed.2011.330. [DOI] [PubMed] [Google Scholar]
- 30.Evans M, Elliott JG, Sharma P, Berman R, Guthrie N. The effect of synthetic genistein on menopause symptom management in healthy postmenopausal women: a multi-center, randomized, placebo-controlled study. Maturitas. 2011;68:189–96. doi: 10.1016/j.maturitas.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 31.Gold EB, Flatt SW, Pierce JP, et al. Dietary factors and vasomotor symptoms in breast cancer survivors: the WHEL Study. Menopause. 2006;13:423–33. doi: 10.1097/01.gme.0000185754.85328.44. [DOI] [PubMed] [Google Scholar]
- 32.Appling S, Paez K, Allen J. Ethnicity and vasomotor symptoms in postmenopausal women. J Womens Health (Larchmt) 2007;16:1130–8. doi: 10.1089/jwh.2006.0033. [DOI] [PubMed] [Google Scholar]
- 33.Matthews KA, Shumaker SA, Bowen DJ, et al. Women’s health initiative. Why now? What is it? What’s new? Am Psychol. 1997;52:101–16. doi: 10.1037//0003-066x.52.2.101. [DOI] [PubMed] [Google Scholar]
- 34.Women’s Health Initiative Study Group. Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 35.Hays J, Hunt JR, Hubbell FA, et al. The Women’s Health Initiative recruitment methods and results. Ann Epidemiol. 2003;13:S18–77. doi: 10.1016/s1047-2797(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 36.US Department of Agriculture. Dietary Guidelines for Americans. Washington, DC: US Dept of Health and Human Services; 1990. [Google Scholar]
- 37.Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 38.Caan BJ, Kwan ML, Hartzell G, et al. Pre-diagnosis body mass index, post-diagnosis weight change, and prognosis among women with early stage breast cancer. Cancer Causes Control. 2008;19:1319–28. doi: 10.1007/s10552-008-9203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller FG, Colloca L, Kaptchuk TJ. The placebo effect: illness and interpersonal healing. Perspect Biol Med. 2009;52:518–39. doi: 10.1353/pbm.0.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wechsler ME, Kelley JM, Boyd IO, et al. Active albuterol or placebo, sham acupuncture, or no intervention in asthma. N Engl J Med. 2011;365:119–26. doi: 10.1056/NEJMoa1103319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Syme SL. Social and economic disparities in health: thoughts about intervention. Milbank Q. 1998;76:493–505. 306–7. doi: 10.1111/1468-0009.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elavsky S, McAuley E. Personality, Menopausal Symptoms, and Physical Activity Outcomes in Middle-Aged Women. Pers Individ Dif. 2009;46:123–8. doi: 10.1016/j.paid.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Greendale GA, Gold EB. Lifestyle factors: are they related to vasomotor symptoms and do they modify the effectiveness or side effects of hormone therapy? Am J Med. 2005;118(Suppl 12B):148–54. doi: 10.1016/j.amjmed.2005.09.049. [DOI] [PubMed] [Google Scholar]
- 44.Swartzman LC, Edelberg R, Kemmann E. Impact of stress on objectively recorded menopausal hot flushes and on flush report bias. Health Psychol. 1990;9:529–45. doi: 10.1037//0278-6133.9.5.529. [DOI] [PubMed] [Google Scholar]
- 45.Alexander C, Cochran CJ, Gallicchio L, Miller SR, Flaws JA, Zacur H. Serum leptin levels, hormone levels, and hot flashes in midlife women. Fertil Steril. 2010;94:1037–43. doi: 10.1016/j.fertnstert.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li C, Borgfeldt C, Samsioe G, Lidfeldt J, Nerbrand C. Background factors influencing somatic and psychological symptoms in middle-age women with different hormonal status. A population-based study of Swedish women. Maturitas. 2005;52:306–18. doi: 10.1016/j.maturitas.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 47.Anderson GS. Human morphology and temperature regulation. Int J Biometeorol. 1999;43:99–109. doi: 10.1007/s004840050123. [DOI] [PubMed] [Google Scholar]
- 48.Freedman RR, Krell W. Reduced thermoregulatory null zone in postmenopausal women with hot flashes. Am J Obstet Gynecol. 1999;181:66–70. doi: 10.1016/s0002-9378(99)70437-0. [DOI] [PubMed] [Google Scholar]
- 49.Freedman RR. Pathophysiology and treatment of menopausal hot flashes. Semin Reprod Med. 2005;23:117–25. doi: 10.1055/s-2005-869479. [DOI] [PubMed] [Google Scholar]
- 50.Miller HG, Li RM. Measuring hot flashes: summary of a National Institutes of Health workshop. Mayo Clin Proc. 2004;79:777–81. doi: 10.4065/79.6.777. [DOI] [PubMed] [Google Scholar]