Abstract
Rapid shallow breathing (RSB) is mainly mediated by bronchopulmonary C-fibers (PCFs). We asked whether this RSB could be modulated by opioid. In anesthetized rats right atrial bolus injection of phenylbiguanide (PBG) to evoke RSB was repeated after: 1) intravenously giving fentanyl (μ-receptor agonist), DPDPE (δ-receptor agonist), or U-50488H (κ-receptor agonist); 2) fentanyl (iv) following naloxone methiodide, a peripheral opioid receptor antagonist; 3) bilateral microinjection of fentanyl into the nodose ganglia; 4) fentanyl (iv) with pre-blocking histamine H1 and H2 receptors by diphenhydramine and ranitidine. Systemic fentanyl challenge, but not DPDPE or U-50488H, switched the PBG-induced RSB to a long lasting apnea. This switch was blocked by naloxone methiodide rather than diphenhydramine and ranitidine. After microinjecting fentanyl into the nodose ganglia, PBG also produced an apnea. Our results suggest that activating μ-receptors is capable of turning the PCF-mediated RSB into an apnea, at least partly, via facilitating PCFs’ activity and this switching effect appears independent of the released histamine.
1. Introduction
Tachypnea, especially RSB, can occur in patients suffering from pulmonary inflammation, congestion, infection, and edema (Churchill & Cope, 1929; Hatridge et al., 1989; Roussos & Koutsoukou, 2003). RSB is thought to result mainly from activating bronchopulmonary C-fibers (PCFs) under the pulmonary disorders (see reviews (Coleridge & Coleridge, 1994; Kubin et al., 2006)) although rapidly adapting receptors (Coleridge & Coleridge, 1986) or high threshold delta fibers in the airways (Yu et al., 2007; Lin et al., 2011) may be also involved. PCFs innervating the lungs and airways constitute approximately 75% of the sensory fibers in the pulmonary branches of the vagus nerve and play an important role in modulating the respiratory rhythm (see the review (Lee et al., 2003)).
Interestingly, opioid receptors are expressed in both vagal nerves (Chang, 2005) and the nucleus tractus solitarius (NTS) (Haber & Elde, 1982; Carter & Lightman, 1985; Ding et al., 1996) where PCFs terminate. Opioids are widely used in clinic as analgesics. There are at least three distinct opioid receptors including mu (μ), kappa (κ), and delta (δ) (Santiago & Edelman, 1985). It is unknown whether systemic activation of these opioid receptors is able to modulate the PCF-mediated RSB. Opioids are capable of producing a naloxone-preventable excitation of unmyelinated C-type neural activity in the nodose ganglion of rabbits (Higashi et al., 1982; Crain & Shen, 1990; Huang, 1992) although they generally inhibit central neural activities (Werz & Macdonald, 1983; Pan et al., 1990). In agreement, bolus injection of opioids into the right atrium can excite PCFs in vivo (Willette & Sapru, 1982). Thus, we tested to what extent the modulatory effect of systemic opioid challenge on the PCF-mediated RSB was dependent on activating peripheral opioid receptors. We further determined whether local activation of opioid receptors on nodose ganglia neurons, cell bodies of vagus nerves afferent fibers, could modulate the PCF-mediated RSB, similar to the result from systemic opioid challenge.
Vagal nerves contain histamine receptors (Sampson & Vidruk, 1975). Opioids can act on lung mast cells to promote the pulmonary release of histamine (Barke & Hough, 1993) that is capable of sensitizing PCFs (Lee & Morton, 1993; Undem & Weinreich, 1993). Thus, it is possible that opioids may be able to indirectly change the PCFs-mediated RSB via promoting pulmonary mast cells’ release of histamine. In fact, exposure to aerosol histamine in baboons has been shown to cause a rapid breathing by acting on histamine H1 or H2 receptors of pulmonary sensory fibers (Yeates & Hameister, 1992). Therefore, our overall hypothesis was that systemic administration of opioids could affect the PCF-mediated RSB, and this effect was triggered directly by acting on PCF opioid receptors and/or indirectly by the opioid-induced release of histamine.
2. Materials and Methods
Fifty pathogen-free Sprague-Dawley male rats (400–500 g) were purchased from Charles River Laboratories, Inc. (Wilmington, MA), housed in the animal facility at Lovelace Respiratory Research Institute (LRRI) in filter top cages, and provided with water and food ad libitum. The room was constantly ventilated and the temperature was kept at 23°C. The experimental protocols were conducted in accordance with the Guide for the Care and Use of Laboratory Animals and approved by LRRI’s Institutional Animal Care and Use Committee, which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International, USA.
2.1 General Animal Preparation
The rats were anesthetized with urethane [1200 mg/kg, intraperitoneal (ip)]. As needed, supplemental urethane (300 mg/kg, ip) was administered to completely eliminate eye-blink and limb-withdrawal reflex throughout the experiment. The general animal preparation was the same as we previously reported in rats (Zhang et al., 2007). Briefly, the right femoral vein was cannulated for solution infusion and the right femoral artery cannulated for monitoring of arterial blood pressure (BP) and heart rate (HR). The right jugular vein was also cannulated for the bolus injection of phenylbiguanide (PBG) into the pulmonary circulation with the catheter tip placed just above right atrium. The trachea below the larynx was exposed through a midline incision, tracheotomized, and cannulated. The tracheal cannula was connected to a pneumotachograph to record airflow. End-tidal pressure of carbon dioxide (PETCO2) was measured via a carbon dioxide analyzer (MicroCapStar end-tidal carbon dioxide analyzer, Model 15–10000; CWE, Inc. Ardmore, PA) connected to a side-port of the tracheal cannula. In those paralyzed preparations, the animal received an intravenous infusion of pancuronium (0.1–0.3 mg kg−1 for induction and 0.1 mg kg−1 h−1 for maintenance). For phrenic nerve recording, the left cervical phrenic nerve was isolated, cut, and its central end recorded as detailed in our previous reports (Xu et al., 1995; Xu & Frazier, 1997) while the tracheal pressure (Pt) was measured via a side port of the tracheal cannula. The animal was exposed to 50% O2 in nitrogen throughout the experiment and the core temperature was monitored with a rectal probe and maintained at 36.5–37.5°C by a water heating pad and radiant heat lamp.
2.2. Drugs preparation
All drugs were purchased from Sigma Corporation and prepared in saline. Stock solutions of PBG (1 mg/ml) and fentanyl (1 mg/ml) were made and the desired concentrations prepared daily based on the animal’s body weight.
2.3. PCFs’ Stimulation
PCFs were stimulated to induce RSB by a bolus injection of PBG (3–6 μg/kg), a PCF selective stimulant (Wilson & Bonham, 1997), into the right atrium. PBG (0.05 ml) was first slowly loaded into the catheter with a volume of 0.08 ml and then quickly flushed into the right atrium with 0.12 ml saline, which was the same for the following studies. A previous study has shown that 3 μg/kg of PBG leads to a maximal tachypnea (Dutta & Deshpande, 2010). Therefore, this dose was chosen as the initial dose to evoke the RSB in this study. If 3 μg/kg of PBG failed to cause RSB, 4.5 μg/kg or 6.0 μg/kg (if 4.5 μg/kg still failed) was utilized.
2.4. Microinjection of fentanyl or vehicle into nodose ganglia
In some rats, bilateral nodose ganglia were exposed through side approach. As reported previously (Hermann et al., 2005), a glass micropipette (broken tip with diameter of ~50 μm) was filled with fentanyl (1 μg/ml, 1 μl) or the same volume of vehicle. The micropipette was inserted into the nodose ganglion under a stereomicroscope and agents were injected slowly (within 5 min) using a computerized microinfusion pump (Model 55–1111, Harvard Apparatus, USA).
2.5. Experiment Protocol
Study Series I was designed to test whether systemic administration of μ-, δ- or κ-receptor agonist could modulate the PBG-induced RSB in 26 rats. To this end, PBG was injected into the right atrium to evoke RSB in anesthetized and spontaneously breathing rats. This protocol was repeated in three groups of rats (n = 7/group) 5 min after systemic administration of fentanyl (μ-receptor agonist, 8 μg/kg (Brookes et al., 2006)), DPDPE (δ-receptor agonist, 2 mg/kg (Yilmaz et al., 1998)), or U-50488H (κ-receptor agonist, 4 mg/kg (Pei et al., 2006)). Owing to that fentanyl rather than DPDPE or U-50488H was capable of switching the RSB into an apnea in our pilot studies, three-fold higher concentrations of DPDPE and U-50488H were applied in two other rats to ensure no effect of them. Activating μ-receptor could contribute to airway obstruction (McCrimmon & Alheid, 2003). Because the upper airway was cannulated in our preparation, the fentanyl-induced apnea we observed is likely central but not obstructive. To further confirm this view, the same protocols (PBG alone and coupled with fentanyl) were carried out while the phrenic nerve activity was recorded in three paralyzed and artificially ventilated rats as reported before (Xu et al., 2001).
Study Series II was carried to test whether fentanyl exerted this switch depending on activating peripheral μ-receptors (n = 6). The procedure was similar to Study Series I with the exception that naloxone methiodide (0.1 mg/kg, iv), a selective peripheral opioid receptor antagonist (Hayashida et al., 2004), was administered 5 min prior to fentanyl.
Study series III was performed to determine whether locally activating μ-receptors on nodose ganglia neurons could also make a similar switch. Intra-atrium injection of PBG was performed before and 5 min after bilateral microinjection of fentanyl into the nodose ganglia in 6 rats. In addition, microinjection of vehicle was conducted in five other rats to serve as a sham-operation control.
Study series IV was conducted to test whether fentanyl switched the PBG-induced RSB into an apnea via promoting the release of histamine, i.e., an indirect effect of fentanyl. The same intra-atrium injection of PBG was administered (n = 7) before and after intravenously giving combined histamine H1 and H2 receptor antagonists diphenhydramine (DPH, 1 mg/kg) and ranitidine (RTD, 5 mg/kg), followed by fentanyl (iv). The same dose of DPH and RTD has been used previously to block histamine H1 or H2 receptors in rats (Zochodne & Ho, 1993). We chose to block H1 or H2 receptors because they are mainly responsible for the lung and airway responses to histamine (Braude et al., 1984; Bryce et al., 2006).
2.6. Data Acquisition and Analysis
Raw data of the airflow, mean arterial blood pressure (MBP), HR, phrenic neurogram, Pt, and rectal temperature were digitized, monitored, and recorded using a computer-based data acquisition and analysis system (PowerLab/8sp; AD Instruments Inc., Colorado Springs, CO) with Chart 5 software. The airflow signals were integrated to generate expiratory duration (TE), tidal volume (VT), respiratory frequency (f), and minute ventilatory volume (VE). The baseline cardiorespiratory variables were collected for 1 min immediately before and 5 min after agents administration. These variables were expressed as absolute values. With respect to the responses to PBG, baseline TE and associated cardiovascular values were averaged 1 min before PBG administration as controls, and the values from the PBG-evoked four fast breaths (before fentanyl) and the apneic response (after fentanyl) were measured as the responses. These responses were presented as percentage changes from the control (Δ%). In the present study, the VT decrease > 15% and f increase > 15% compared with control were defined as RSB, while a TE value that was three-fold longer than the control defined as an apnea (Peng et al., 2007). Paired-t test was used to test the effects of a given treatment on the baseline cardiorespiratory variables, while repeated one-way ANOVA was employed to detect significant changes in the evoked cardiorespiratory responses to PBG and significant effect of a given treatment on the responses. If an overall test was significant, Tukey test was used for specific comparisons between individual groups. The software Statistica 6.0 (StatSoft, Inc., Tulsa, OK) was used for statistical analysis. All data are presented as means ± standard error (SE). The difference was considered significant at a P value < 0.05.
3. Results
3.1. Fentanyl, rather than DPDPE and U-50488H, switches the RSB into an apnea
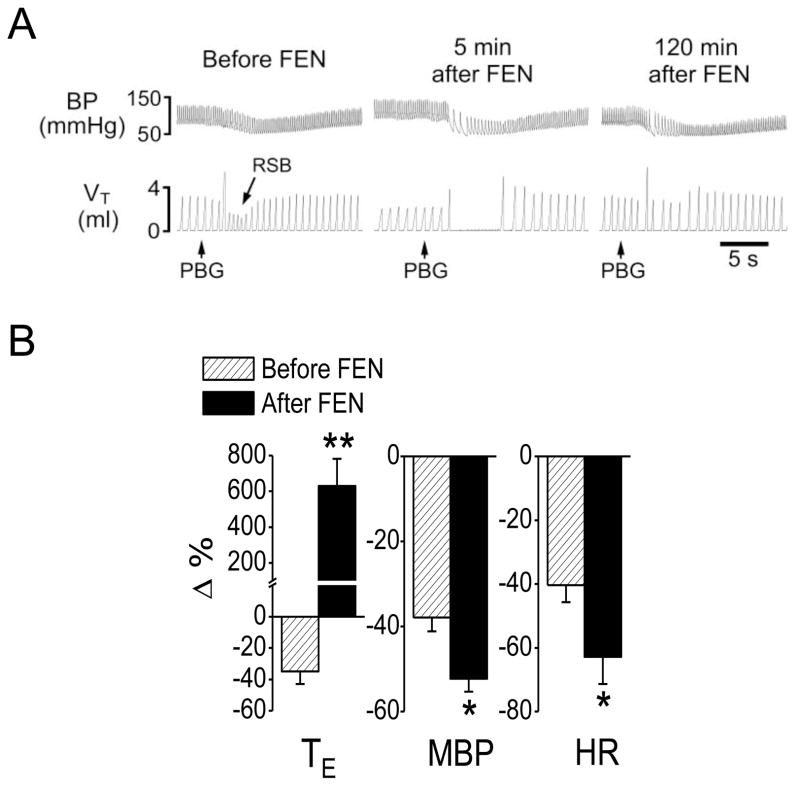
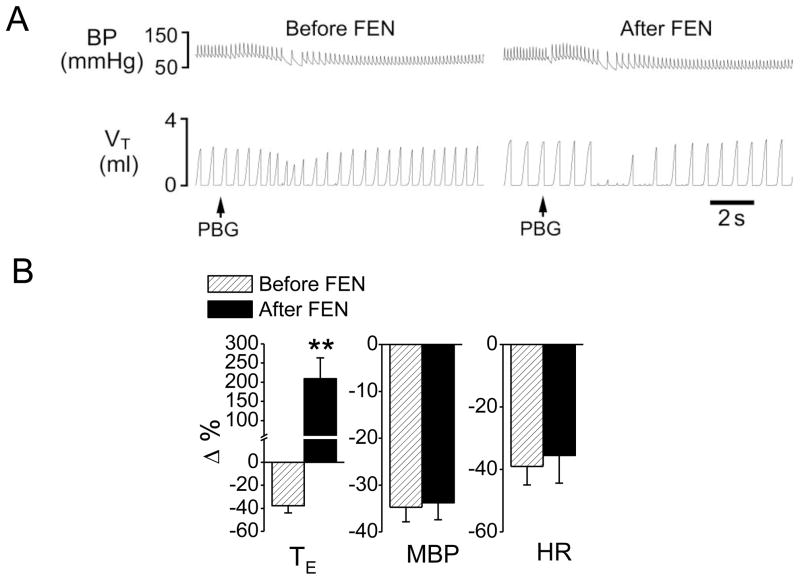
We first tested whether systemically activating μ, δ, or κ receptors could affect the PCF-mediated RSB. As shown in Fig. 1, right atrial bolus injection of PBG induced RSB associated with bradycardia and hypotension, consistent with a previous report in rats (Dutta & Deshpande, 2010). The cardiorespiratory responses to PBG in this study were characterized by: 1) a shortened TE (−35%, from 0.40 ± 0.05 s to 0.26 ± 0.03 s, P < 0.01) with a latency of 1.88 ± 0.03 s; 2) a decreased MBP (−38%, from 88 ± 5 to 54 ± 6 mmHg, P < 0.01) and 3) a lowed HR (−40%, from 336 ± 23 to 201 ± 21 beats/min, P < 0.05). Fentanyl (8 μg/kg, iv) significantly inhibited VE, f and VT; moderately prolonged TE; increased PETCO2 from 35 ± 2 to 39 ± 3 mmHg (P < 0.05); and elevated MBP without effect on HR (Table 1). After systemic fentanyl administration, the same dose of PBG produced a long-lasting apnea with TE 6.5-fold longer than the control (increasing from 0.52 ± 0.06 s to 3.9 ± 0.63 s, P < 0.01). Furthermore, the PBG-induced hypotension (−38%) and bradycardia (−40%) were markedly aggravated after giving fentanyl (−52% and −63%, respectively, P < 0.01). The modulatory impact of fentanyl on the PBG-induced cardiorespiratory responses usually disappeared 2.5 h later. In contrast, intravenous injection of vehicle did not significantly alter the PBG-induced RSB.
Fig 1.
Fentanyl-induced changes in cardiorespiratory responses to PBG. A: A representative recording showing that fentanyl (FEN, 8 μg/kg, iv) converted the PBG (4.5 μg/kg)-induced RSB (left) into a long-lasting apnea (middle) and this facilitating effect disappeared 2 h later (right). The traces from the top to bottom are arterial blood pressure (BP) and tidal volume (VT). B: Group data of the effects of FEN on the cardiorespiratory responses to PBG. N = 7; mean ± SE. Note: all the cardiorespiratory responses to PBG were significant (P < 0.01). * P < 0.05 and ** P < 0.01 compared with before fentanyl. TE, expiratory duration; MBP, mean arterial blood pressure; HR, heart rate.
Table 1.
Effect of fentanyl alone or coupled with a given pretreatment on baseline cardiorespiratory variables
| VE (ml/min) | f (breaths/min) | VT (ml) | TE (s) | MBP (mmHg) | HR (beats/min) | ||
|---|---|---|---|---|---|---|---|
| FEN (iv) (n = 7) | Before | 249 ± 30 | 101 ± 5 | 2.5 ± 0.2 | 0.40 ± 0.05 | 87 ± 6 | 336 ± 23 |
| After | 167 ± 25 ** | 77 ± 5 ** | 2.1 ± 0.3 ** | 0.52 ± 0.06** | 104 ± 5 ** | 315 ± 26 | |
| NXM+FEN (iv) (n = 6) | Before | 256 ± 21 | 102 ± 7 | 2.5 ± 0.3 | 0.36 ± 0.05 | 90 ± 7 | 332 ± 9 |
| After | 176 ± 27 ** | 86 ± 9 ** | 2.1 ± 0.3 ** | 0.45 ± 0.07* | 109 ± 5 ** | 325 ± 11 | |
| DPH/RTD+FEN (iv) (n = 7) | Before | 235 ± 30 | 97 ± 7 | 2.4 ± 0.2 | 0.38 ± 0.03 | 92 ± 7 | 356 ± 29 |
| After | 154 ± 26 ** | 73 ± 6 ** | 2.1 ± 0.2 ** | 0.51 ± 0.04** | 111 ± 7 ** | 350 ± 26 | |
| FEN (local) (n = 6) | Before | 215 ± 19 | 105 ± 5 | 2.1 ± 0.2 | 0.36 ± 0.03 | 92 ± 7 | 375 ± 10 |
| After | 192 ± 21* | 83 ± 6 * | 2.3 ± 0.3 | 0.48 ± 0.05* | 90 ± 9 | 402 ± 25 * |
Data are shown as mean ± SE;
P < 0.05,
P < 0.01 compared with “before”. DPH/RTD, diphenhydramine/ranitidine; FEN, fentanyl; iv, intravenous; local, injection into the nodose ganglia; f, respiratory frequency; HR, heart rate; MBP, mean arterial blood pressure; NXM, naloxone methiodide; TE, expiratory duration; VE, minute ventilation; VT, tidal volume.
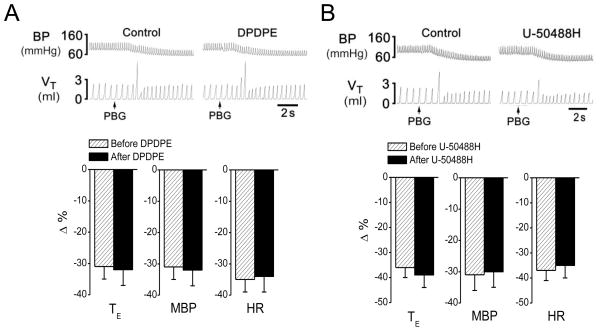
We also examined the influences of δ- or κ-receptor agonists (DPDPE or U-50488H) on the PBG-induced RSB, respectively. Different from μ-receptor agonist fentanyl, DPDPE and U-50488H neither switched the PBG-induced RSB into an apnea nor altered the PBG-induced BP and HR responses (Fig. 2). In fact, the same results were also observed in the two rats when triple-dosed DPDPE or U-50488H was injected, in which the PBG-induced TE was shorten by −37% and−38% before and after DPDPE; and by−40% and−41% before and after U-50488H (P > 0.05). Additionally, DPDPE had no significant effect on cardiorespiratory baseline variables, while U-50488H decreased VE via lowering VT without change in cardiovascular activity (Table 2).
Fig 2.
Opioid δ- (A) and κ-receptor agonist (B) failed to convert the PBG-induced RSB into an apnea. The top panes present representative recordings showing that fentanyl (FEN, 8 μg/kg, iv) failed to converted the PBG (4.5 μg/kg)-induced RSB into a apnea and the bottom panes are the corresponding group data. In the top panel, the traces are arterial blood pressure (BP) and tidal volume (VT). N = 7 for each agonist; mean ± SE; Note:all the cardiorespiratory responses to PBG were significant (P < 0.01). T E, expiratory duration; MBP, mean arterial blood pressure; and HR, heart rate.
Table 2.
Comparison of baseline cardiorespiratory variables before and after intravenous DPDPE, U-50488H, histamine blockers, peripheral opioid receptor blocker and local injecting vehicle into the nodose ganglia.
| VE (ml/min) | f (breaths/min) | VT (ml) | TE (s) | MBP (mmHg) | HR (beats/min) | ||
|---|---|---|---|---|---|---|---|
| DPDPE (iv) (n = 7) | Before | 267 ± 34 | 97 ± 7 | 2.8 ± 0.2 | 0.39 ± 0.04 | 96 ± 7 | 315 ± 22 |
| After | 270 ± 39 | 103 ± 8 | 2.6 ± 0.3 | 0.38 ± 0.05 | 91 ± 8 | 311 ± 18 | |
| U-50488H (iv) (n = 7) | Before | 238 ± 11 | 92 ± 8 | 2.6 ± 0.2 | 0.42 ± 0.04 | 94 ± 9 | 331 ± 17 |
| After | 210 ± 22 ** | 97 ± 8 | 2.1 ± 0.2 ** | 0.40 ± 0.05 | 96 ± 10 | 330 ± 9 | |
| NXM (iv) (n = 6) | Before | 251 ± 19 | 98 ± 6 | 2.5 ± 0.3 | 0.39 ± 0.04 | 93 ± 6 | 329 ± 7 |
| After | 256 ± 21 | 102 ± 7 | 2.5 ± 0.3 | 0.36 ± 0.05 | 90 ± 7 | 332 ± 9 | |
| DPH/RTD (iv) (n = 6) | Before | 216 ± 11 | 87 ± 6 | 2.5 ± 0.3 | 0.45 ± 0.05 | 82 ± 7 | 363 ± 19 |
| After | 235 ± 30* | 97 ± 7* | 2.4 ± 0.2 | 0.38 ± 0.03** | 92 ± 7* | 356 ± 29 | |
| Vehicle (local) (n = 5) | Before | 231 ± 14 | 98 ± 7 | 2.4 ± 0.3 | 0.40 ± 0.05 | 98 ± 7 | 343 ± 17 |
| After | 226 ± 21 | 93 ± 9 | 2.4 ± 0.3 | 0.42 ± 0.05 | 97 ± 8 | 341 ± 25 |
Data are shown as mean ± SE;
P < 0.05,
P < 0.01 compared with “before”.
DPH/RTD, diphenhydramine/ranitidine; iv, intravenous; local, injection into the nodose ganglia; f, respiratory frequency; HR, heart rate; MBP, mean arterial blood pressure; NXM, naloxone methiodide; TE, expiratory duration; VE, minute ventilation; VT, tidal volume.
3.2. Fentanyl switches the PBG-induced RSB into a central apnea
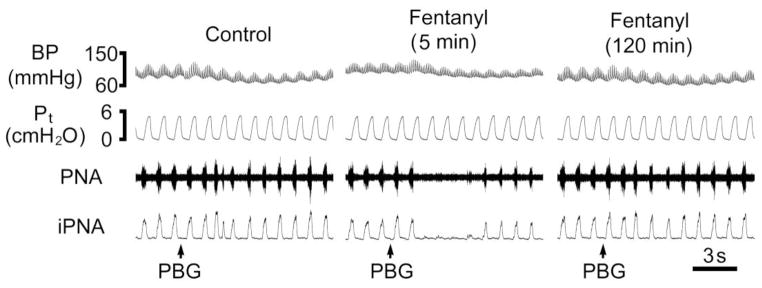
We recorded the phrenic nerve firing in response to the same PBG before and after fentanyl (iv) to verify that the apnea induced by the switching effect of fentanyl is central but not obstructive. As shown in Fig. 3, fentanyl switched the PBG-induced RSB into an apnea denoted on the phrenic nerve recording. In three rats tested, TE was shortened by PBG from 0.76 ± 0.01 s to 0.52 ± 0.12 s, indicating the PBG-induced RSB. The PBG-induced RSB became an apnea after fentanyl, i.e., the TE was profoundly prolonged by 6.1-fold (from 0.77 ± 0.01 s to 4.68 ± 0.61 s). PBG injection did not change Pt either before fentanyl (4.97 ± 0.23 vs. 5.01 ± 0.21 cmH2O) or after fentanyl (5.03 ± 0.24 vs. 5.02 ± 0.24 cmH2O).
Fig 3.
A representative phrenic nerve recording showing that fentanyl (8 μg/kg, iv) turns the PBG (3 μg/kg)-induced RSB into an apnea without changing the tracheal pressure and the partial recovery from the apnea in a paralyzed and ventilated rat. The traces from the top to bottom are arterial blood pressure (ABP), tracheal pressure (Pt), phrenic nerve activity (PNA) and integrated PNA (iPNA).
3.3. Fentanyl-mediated switch is dependent on peripheral μ-receptors
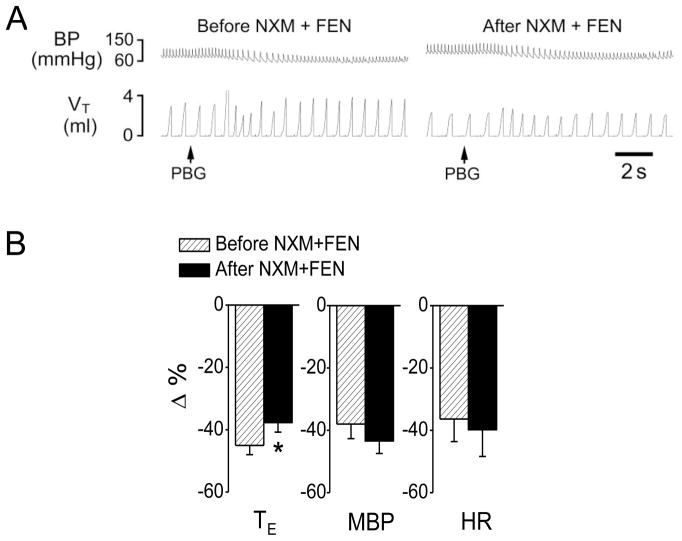
To test whether fentanyl (iv) triggered the switch via acting peripheral μ-receptors, naloxone methiodide, a peripheral μ-receptor antagonist, was administered 5 min prior to fentanyl. This pretreatment with naloxone methiodide failed to alter: 1) the baseline respiratory activities (Table 2), and 2) the fentanyl-induced cardiorespiratory changes including inhibition of VE, f and VT, moderate prolongation of TE, and increase in BP (Table 1). However, this pretreatment prevented: 1) the switching effect of fentanyl although the TE of the PBG-induced RSB after fentanyl was slightly prolonged (Fig. 4), and 2) fentanyl’s aggravating effect on the PBG-induced hypotension and bradycardia response (Fig. 4).
Fig 4.
The effect of peripheral opioid receptor antagonist naloxone methiodide (NXM) and fentanyl (FEN) on the cardiorespiratory responses to PBG. A, a representative recording showing that fentanyl (8 μg/kg, iv) failed to convert the PBG (6 μg/kg)-induced RSB into an apnea after NXM was administered. The traces from the top to bottom are arterial blood pressure (BP) and tidal volume (VT). B, the corresponding group data. N = 6; mean ± SE. Note: all the cardiorespiratory responses to PBG were significant (P < 0.01). * P < 0.05 compared with before fentanyl. TE, expiratory duration; MBP, mean arterial blood pressure; and HR, heart rate.
3.4. Microinjecting fentanyl into the nodose ganglia turns on the switch
After confirming that peripherally activating μ-receptors is necessary for fentanyl to switch the PBG-induced RSB into an apnea, we further examined whether selectively activating μ-receptors in the nodose ganglia where the cell bodies of vagal sensory fibers reside could produce a similar switch. Local application of fentanyl decreased baseline VE significantly (PETCO2 changed from 35 ± 2 to 38 ± 3 mmHg, P < 0.05) although this inhibition of VE was less than that induced by systemic fentanyl challenge (−11 ± 3% vs. −33 ± 5%, P < 0.05) (Table 1). As illustrated in Fig 5, this local fentanyl treatment also switched the PBG-induced RSB into an apnea (3.1-fold prolongation of TE from 0.48 ± 0.03 s to 1.49 ± 0.26 s) though the latter was significantly shorter than that induced by systemically injected (6.5-fold prolongation). Different from systemic administration, local fentanyl failed to change PBG-induced decrease in MBP and HR (Fig. 5). As a sham-operation control, local microinjection of vehicle into the nodose ganglia neither changed the baseline cardiorespiratory values (Table 2) nor produced such switching effect (−39 ± 4% vs. −34 ± 6% for TE response to PBG, P > 0.05).
Fig 5.
The effect of intra-nodose ganglia microinjection of fentanyl (FEN) on cardiorespiratory responses to PBG. A, a representative recording showing that fentanyl microinjected into the nodose ganglia converted the PBG (4.5 μg/kg)-induced RSB into an apnea. The traces from the top to bottom are arterial blood pressure (BP) and tidal volume (VT). B, the corresponding group data. N = 6; mean ± SE. Note: all the cardiorespiratory responses to PBG were significant (P < 0.01). ** P < 0.01 compared with before fentanyl. TE, expiratory duration; MBP, mean arterial blood pressure; and HR, heart rate.
3.5. Fentanyl-mediated switch is independent of histamine H1 and H2receptors
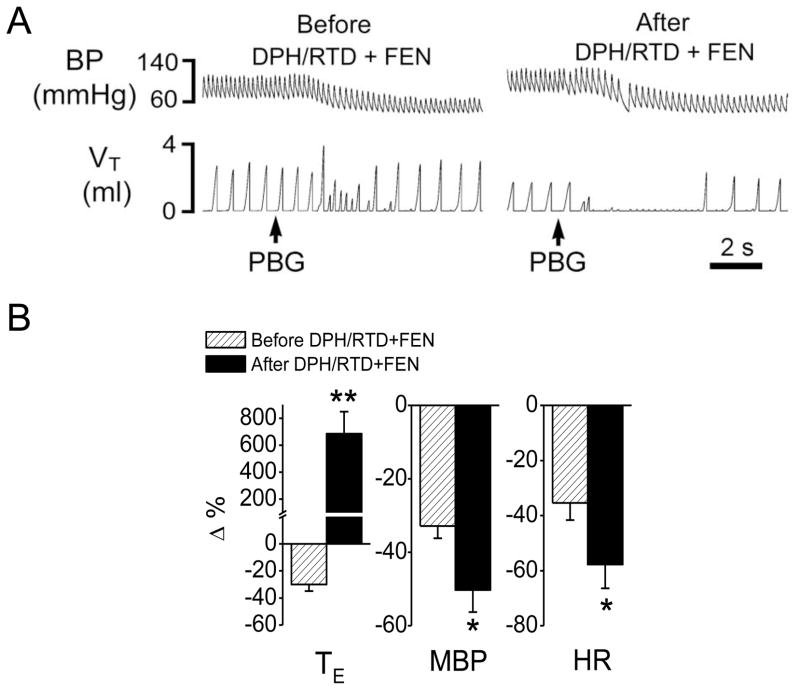
The data mentioned above showed a relatively smaller switching effects induced by local fentanyl injection compared with the result from systemic administration of fentanyl, suggesting an involvement of other mechanisms in full expression of the systemic fentanyl-induced switch. Here we sought to define the possible role of the indirect effect of fentanyl via promoting histamine release in the switch. We tested whether fentanyl failed to switch the PBG-induced RSB into an apnea after blocking both histamine H1 and H2 receptors. As a result, similar to fentanyl alone, fentanyl following blockade of both types of histamine receptors by combination of DPH and RTD still evoked a similar RSB-apnea switch and aggravated hypotension and bradycardia (Fig. 6). DPH/RTD per se slightly increased the baseline VE by 8% due to the elevation of f, and increased MBP with no change of HR (Table 2). After DPH/RTD, fentanyl still depressed VE by 34% (PETCO2 changed from 34 ± 2 to 39 ± 3 mmHg, P < 0.05) due to the inhibition of both VT and f, and increased MBP (Table 1).
Fig 6.
The effect of fentanyl (FEN) on cardiorespiratory responses to PBG after blocking histamine H1 and H2 receptors. A, a representative recording showing that fentanyl still converted the PBG (4.5 μg/kg)-induced RSB into an apnea after the blockage of histamine H1 and H2 receptors by diphenhydramine and ranitidine (DPH/RTD). The traces from the top to bottom are arterial blood pressure (BP) and tidal volume (VT). B, the corresponding group data. N = 7; mean ± SE. Note: all the cardiorespiratory responses to PBG were significant (P < 0.01). * P < 0.05 and ** P < 0.01 compared with before fentanyl. HR, heart rate; MBP, mean arterial blood pressure; TE, expiratory duration.
4. Discussion
The major finding of this study is that systemically activating μ-receptors, but not δ- and κ-receptors, is able to switch the PCF-mediated RSB into a long-lasting apnea. RSB commonly occurs in patients with pulmonary inflammation (infection), congestion, and edema (Churchill & Cope, 1929; Hatridge et al., 1989; Roussos & Koutsoukou, 2003). These patients are more vulnerable to suffering from respiratory depression and even respiratory failure than normal subjects when opioids are administered as analgesics, mainly due to acting on μ-receptors, in the clinical setting (Gruber & Tschernko, 2003; Horton & Barber, 2009). However, the reason for this vulnerability is not clear. PCFs can be activated and/or sensitized in patients by pulmonary inflammation (infection), congestion, and edema (Churchill & Cope, 1929; Hatridge et al., 1989; Tepper et al., 1990; Roussos & Koutsoukou, 2003), and these fibers are thought to be the main factor in generating RSB under the pulmonary disorders (Widdicombe, 1982; Coleridge & Coleridge, 1994; Kubin et al., 2006). Therefore, our result that fentanyl could switch the PCFs-mediated RSB into an apnea may benefit our understanding why opioids are much more depressant to respiration in these patients though several other mechanisms may also be involved.
An important finding of this study is that the activation of peripheral μ-receptors, most likely those on PCFs, is the prerequisite for this switch. As shown in Fig. 4, switching the PBG-induced RSB into a long lasting apnea by intravenous administration of fentanyl was fully prevented after using naloxone methiodide, a peripheral opioid receptor antagonist. One of the most likely peripheral mechanisms for this switching is that fentanyl directly facilitate PCFs’ activity to significantly augment PBG-induced PCF excitation, and consequently, turn the PBG-evoked RSB to an apnea. This assumption is supported by several lines of evidence. First, the production of RSB or an apnea is dependent on the stimulating intensity of PCFs. The RSB was induced by intra-atrial injection of a low dose of PBG while an apnea was also evoked if a high dose of PBG was used, and both types of responses disappeared after bilateral vagotomy (Coleridge & Coleridge, 1994; Moreira et al., 2007; Dutta & Deshpande, 2010). Second, μ-receptor agonists can excite unmyelinated C type neurons in the nodose ganglion of rabbits (Higashi et al., 1982; Crain & Shen, 1990) in vitro and stimulate PCFs to induce an apnea in vivo (Willette & Sapru, 1982). Third, more importantly, our data showed that fentanyl microinjected into nodose ganglia also switched the RSB to an apnea. It is worthy to note that local fentanyl treatment-induced 3.1-fold prolongation of TE is shorter than that induced by systemic administration (6.5-fold TE prolongation). This discrepancy may be due to an insufficient stimulation of all PCFs by the pretreatment of nodose ganglia with fentanyl in this study. On the other hand, it is also possible that the central μ-receptors may contribute to the switch response to systemic administration of fentanyl. μ-receptors are abundant in the central pathways underlying the PCF-mediated respiratory responses (Kubin et al., 2006), including those in the nucleus tractus solitarius and pre-Botzinger complex (Ding et al., 1996; Haji et al., 2003). In fact, the result that after naloxone methiodide, fentanyl failed to induce the switch but still attenuated the PBG-induced RSB (Fig. 4B) points to an involvement of central μ-receptors in this modulation. Nevertheless, the two lines of results from blocking peripheral μ receptors and local injection of fentanyl into the nodose ganglia indicate that peripheral μ receptors, especially those on PCFs, are necessary in triggering this switch.
Opioids including fentanyl and its derivatives can promote the pulmonary release of histamine (Kaye et al., 2006a; Kaye et al., 2006b), which was reported to sensitize PCFs (Lee & Morton, 1993; Undem & Weinreich, 1993). Furthermore, inhaling histamine causes a rapid breathing by activating histamine H1 or H2 receptors of pulmonary sensory fibers in baboons (Yeates & Hameister, 1992). Dutta and Deshpande (Dutta & Deshpande, 2011) have also shown the involvement of histaminergic system to the augmentation of PBG reflexes by scorpion venom. These data, together with a relative shorter PBG-induced apnea after fentanyl locally injected into the nodose ganglion, suggest a possible involvement of secondarily released histamine by systemic fentanyl in the switch. We compared the fentanyl effect on the PBG-induced RSB with and without blocking both H1 and H2 receptors. As the result, switching the RSB to the apnea by systemic fentanyl was not significantly affected by blocking both histamine receptors, not supporting a histamine H1/H2 receptors’ involvement in this switch. Because fentanyl may also release other mediators stimulatory to PCFs, such as adenosine (Gu et al., 2003), our data in this study can not rule out their involvement in the switch.
Another interesting finding in the present study was that systemic fentanyl augmented the PBG-induced bradycardia and hypotension that could be prevented by the blockage of peripheral opioid receptors. Intra-atrium injection of PBG induced a transient hypotension and bradycardia as reported previously (Dutta & Deshpande, 2010). Fentanyl augmented the cardiovascular response to PBG seemingly not through acting on vagal μ-receptors because the local treatment of nodose ganglia with fentanyl failed to exhibit such augmenting effect. In our study systemic administration of fentanyl increased BP, which may be due to its easy access to the central nervous system and its central impact on pressor (Bellet et al., 1980). This centrally mediated hypertension was related to activation of sympathetic tone as it was abolished after bilateral adrenalectomy and application of ganglion blocking agents (Bellet et al., 1980).
In summary, our results allow us to conclude that the systemic activation of μ-receptors rather than δ- or κ-receptors is capable of switching the PCF-mediated RSB into a central apnea in anesthetized rats. Moreover, this switching effect is triggered by acting peripheral μ-receptors, at least partially, through acting PCF μ-receptors.
Fentanyl switched pulmonary C-fibers (PCFs)-mediated rapid shallow breathing (RSB) into an apnea in anesthetized rats.
The switching effect of fentanyl could be prevented by blocking peripheral μ-receptors.
Microinjection of fentanyl into nodose ganglia also switched PCFs-mediated RSB into an apnea.
Fentanyl’s switching effect was not dependent on the histamine receptors
Acknowledgments
Funding: This study is supported by RO1 HL107462 from the National Heart, Lung, and Blood Institute, Bethesda, MD, and American Lung Association Biomedical Research Grant RG-191095-N, New York, NY
Supported by HL 107462 and ALA RG-191095-N
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barke KE, Hough LB. Opiates, mast cells and histamine release. Life Sci. 1993;53:1391–1399. doi: 10.1016/0024-3205(93)90581-m. [DOI] [PubMed] [Google Scholar]
- Bellet M, Elghozi JL, Meyer P, Pernollet MG, Schmitt H. Central cardiovascular effects of narcotic analgesics and enkephalins in rats. British journal of pharmacology. 1980;71:365–369. doi: 10.1111/j.1476-5381.1980.tb10949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braude S, Royston D, Coe C, Barnes PJ. Histamine increases lung permeability by an H2-receptor mechanism. Lancet. 1984;2:372–374. doi: 10.1016/s0140-6736(84)90542-7. [DOI] [PubMed] [Google Scholar]
- Brookes ZL, Reilly CS, Lawton BK, Brown NJ. Intravenous anesthesia inhibits leukocyte-endothelial interactions and expression of CD11b after hemorrhage. Shock (Augusta, Ga. 2006;25:492–499. doi: 10.1097/01.shk.0000209541.76305.8e. [DOI] [PubMed] [Google Scholar]
- Bryce PJ, Mathias CB, Harrison KL, Watanabe T, Geha RS, Oettgen HC. The H1 histamine receptor regulates allergic lung responses. The Journal of clinical investigation. 2006;116:1624–1632. doi: 10.1172/JCI26150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter DA, Lightman SL. Selective cardiovascular and neuroendocrine effects of a kappa-opioid agonist in the nucleus tractus solitarii of rats. J Physiol. 1985;367:363–375. doi: 10.1113/jphysiol.1985.sp015829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K-J. History of Delta Receptors. In: Chang K-J, Porreca F, Woods JH, editors. The Delta Receptor. Marcel Dekker; New York: 2005. pp. 3–14. [Google Scholar]
- Churchill ED, Cope O. The rapid shallow breathing resulting from pulmonary congestion and edema. The Journal of Experimental Medicine. 1929;49:531–537. doi: 10.1084/jem.49.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleridge HM, Coleridge JC. Pulmonary reflexes: neural mechanisms of pulmonary defense. Annual review of physiology. 1994;56:69–91. doi: 10.1146/annurev.ph.56.030194.000441. [DOI] [PubMed] [Google Scholar]
- Coleridge HM, Coleridge JCG. Reflexes evoked from the tracheobronchial tree and lungs. In: Cherniack N, Widdicombe JG, editors. Handbook of Physiology, section 3, Respiratory System. part 1. II. American Physiological Society; Bethesda: 1986. pp. 395–429. [Google Scholar]
- Crain SM, Shen KF. Opioids can evoke direct receptor-mediated excitatory effects on sensory neurons. Trends in pharmacological sciences. 1990;11:77–81. doi: 10.1016/0165-6147(90)90322-y. [DOI] [PubMed] [Google Scholar]
- Ding YQ, Kaneko T, Nomura S, Mizuno N. Immunohistochemical localization of mu-opioid receptors in the central nervous system of the rat. J Comp Neurol. 1996;367:375–402. doi: 10.1002/(SICI)1096-9861(19960408)367:3<375::AID-CNE5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Dutta A, Deshpande S. Cardio-respiratory reflexes evoked by phenylbiguanide in rats involve vagal afferents which are not sensitive to capsaicin. Acta Physiol (Oxf) 2010;200:87–95. doi: 10.1111/j.1748-1716.2010.02105.x. [DOI] [PubMed] [Google Scholar]
- Dutta A, Deshpande SB. Indian red scorpion venom-induced augmentation of cardio-respiratory reflexes and pulmonary edema involve the release of histamine. Toxicon. 2011;57:193–198. doi: 10.1016/j.toxicon.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Gruber EM, Tschernko EM. Anaesthesia and postoperative analgesia in older patients with chronic obstructive pulmonary disease: special considerations. Drugs & aging. 2003;20:347–360. doi: 10.2165/00002512-200320050-00004. [DOI] [PubMed] [Google Scholar]
- Gu Q, Ruan T, Hong JL, Burki N, Lee LY. Hypersensitivity of pulmonary C fibers induced by adenosine in anesthetized rats. J Appl Physiol. 2003;95:1315–1324. doi: 10.1152/japplphysiol.00107.2003. discussion 1314. [DOI] [PubMed] [Google Scholar]
- Haber S, Elde R. The distribution of enkephalin immunoreactive fibers and terminals in the monkey central nervous system: an immunohistochemical study. Neuroscience. 1982;7:1049–1095. doi: 10.1016/0306-4522(82)91118-6. [DOI] [PubMed] [Google Scholar]
- Haji A, Yamazaki H, Ohi Y, Takeda R. Distribution of mu receptors in the ventral respiratory group neurons; immunohistochemical and pharmacological studies in decerebrate cats. Neurosci Lett. 2003;351:37–40. doi: 10.1016/s0304-3940(03)00951-0. [DOI] [PubMed] [Google Scholar]
- Hatridge J, Haji A, Perez-Padilla JR, Remmers JE. Rapid shallow breathing caused by pulmonary vascular congestion in cats. J Appl Physiol. 1989;67:2257–2264. doi: 10.1152/jappl.1989.67.6.2257. [DOI] [PubMed] [Google Scholar]
- Hayashida K, Takeuchi T, Ozaki T, Shimizu H, Ando K, Miyamoto A, Harada E. Bovine lactoferrin has a nitric oxide-dependent hypotensive effect in rats. American journal of physiology. 2004;286:R359–365. doi: 10.1152/ajpregu.00214.2003. [DOI] [PubMed] [Google Scholar]
- Hermann GE, Nasse JS, Rogers RC. Alpha-1 adrenergic input to solitary nucleus neurones: calcium oscillations, excitation and gastric reflex control. J Physiol. 2005;562:553–568. doi: 10.1113/jphysiol.2004.076919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi H, Shinnick-Gallagher P, Gallagher JP. Morphine enhances and depresses Ca2+-dependent responses in visceral primary afferent neurons. Brain Res. 1982;251:186–191. doi: 10.1016/0006-8993(82)91291-4. [DOI] [PubMed] [Google Scholar]
- Horton R, Barber C. Opioid-induced respiratory depression resulting from transdermal fentanyl-clarithromycin drug interaction in a patient with advanced COPD. Journal of pain and symptom management. 2009;37:e2–5. doi: 10.1016/j.jpainsymman.2009.02.230. [DOI] [PubMed] [Google Scholar]
- Huang LM. The excitatory effects of opioids. Neurochemistry international. 1992;20:463–468. doi: 10.1016/0197-0186(92)90024-l. [DOI] [PubMed] [Google Scholar]
- Kaye AD, Baluch A, Phelps J, Baber SR, Ibrahim IN, Hoover JM, Zhang C, Fields A. An analysis of remifentanil in the pulmonary vascular bed of the cat. Anesth Analg. 2006a;102:118–123. doi: 10.1213/01.ane.0000184826.02943.70. [DOI] [PubMed] [Google Scholar]
- Kaye AD, Hoover JM, Ibrahim IN, Phelps J, Baluch A, Fields A, Huffman S. Analysis of the effects of fentanyl in the feline pulmonary vascular bed. American journal of therapeutics. 2006b;13:478–484. doi: 10.1097/01.mjt.0000178338.43545.3a. [DOI] [PubMed] [Google Scholar]
- Kaye AD, Phelps J, Baluch A, Ibrahim IN, Hoover JM, Baber SR, Zhang C, Armstrong C, Huffman S, Fields A. The effects of sufentanil in the feline pulmonary vascular bed. European journal of pharmacology. 2006c;534:159–164. doi: 10.1016/j.ejphar.2006.01.039. [DOI] [PubMed] [Google Scholar]
- Kubin L, Alheid GF, Zuperku EJ, McCrimmon DR. Central pathways of pulmonary and lower airway vagal afferents. J Appl Physiol. 2006;101:618–627. doi: 10.1152/japplphysiol.00252.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LY, Morton RF. Histamine enhances vagal pulmonary C-fiber responses to capsaicin and lung inflation. Respir Physiol. 1993;93:83–96. doi: 10.1016/0034-5687(93)90070-q. [DOI] [PubMed] [Google Scholar]
- Lee LY, Shuei Lin Y, Gu Q, Chung E, Ho CY. Functional morphology and physiological properties of bronchopulmonary C-fiber afferents. Anat Rec A Discov Mol Cell Evol Biol. 2003;270:17–24. doi: 10.1002/ar.a.10005. [DOI] [PubMed] [Google Scholar]
- Lin S, Li H, Xu L, Moldoveanu B, Guardiola J, Yu J. Arachidonic acid products in airway nociceptor activation during acute lung injury. Exp Physiol. 2011;96:966–976. doi: 10.1113/expphysiol.2011.058263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrimmon DR, Alheid GF. On the opiate trail of respiratory depression. American journal of physiology. 2003;285:R1274–1275. doi: 10.1152/ajpregu.00428.2003. [DOI] [PubMed] [Google Scholar]
- Moreira TS, Takakura AC, Colombari E, Guyenet PG. Activation of 5-hydroxytryptamine type 3 receptor-expressing C-fiber vagal afferents inhibits retrotrapezoid nucleus chemoreceptors in rats. J Neurophysiol. 2007;98:3627–3637. doi: 10.1152/jn.00675.2007. [DOI] [PubMed] [Google Scholar]
- Pan ZZ, Williams JT, Osborne PB. Opioid actions on single nucleus raphe magnus neurons from rat and guinea-pig in vitro. J Physiol. 1990;427:519–532. doi: 10.1113/jphysiol.1990.sp018185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei JM, Sun X, Guo HT, Ma S, Zang YM, Lu SY, Bi H, Wang YM, Ma H, Ma XL. U50,488H depresses pulmonary pressure in rats subjected to chronic hypoxia. Journal of cardiovascular pharmacology. 2006;47:594–598. doi: 10.1097/01.fjc.0000211737.55583.15. [DOI] [PubMed] [Google Scholar]
- Peng W, Zhuang J, Harrod KS, Xu F. Respiratory syncytial virus infection in anesthetized weanling rather than adult rats prolongs the apneic responses to right atrial injection of capsaicin. J Appl Physiol. 2007;102:2201–2206. doi: 10.1152/japplphysiol.01436.2006. [DOI] [PubMed] [Google Scholar]
- Roussos C, Koutsoukou A. Respiratory failure. Eur Respir J. 2003:3s–14s. doi: 10.1183/09031936.03.00038503. [DOI] [PubMed] [Google Scholar]
- Sampson SR, Vidruk EH. Properties of ‘irritant’ receptors in canine lung. Respiration physiology. 1975;25:9–22. doi: 10.1016/0034-5687(75)90047-x. [DOI] [PubMed] [Google Scholar]
- Santiago TV, Edelman NH. Opioids and breathing. J Appl Physiol. 1985;59:1675–1685. doi: 10.1152/jappl.1985.59.6.1675. [DOI] [PubMed] [Google Scholar]
- Tepper JS, Wiester MJ, Weber MF, Menache MG. Measurements of cardiopulmonary response in awake rats during acute exposure to near-ambient concentrations of ozone. J Appl Toxicol. 1990;10:7–15. doi: 10.1002/jat.2550100103. [DOI] [PubMed] [Google Scholar]
- Undem BJ, Weinreich D. Electrophysiological properties and chemosensitivity of guinea pig nodose ganglion neurons in vitro. Journal of the autonomic nervous system. 1993;44:17–33. doi: 10.1016/0165-1838(93)90375-5. [DOI] [PubMed] [Google Scholar]
- Werz MA, Macdonald RL. Opioid peptides with differential affinity for mu and delta receptors decrease sensory neuron calcium-dependent action potentials. The Journal of pharmacology and experimental therapeutics. 1983;227:394–402. [PubMed] [Google Scholar]
- Widdicombe JG. Pulmonary and respiratory tract receptors. The Journal of experimental biology. 1982;100:41–57. doi: 10.1242/jeb.100.1.41. [DOI] [PubMed] [Google Scholar]
- Willette RN, Sapru HN. Peripheral versus central cardiorespiratory effects of morphine. Neuropharmacology. 1982;21:1019–1026. doi: 10.1016/0028-3908(82)90116-2. [DOI] [PubMed] [Google Scholar]
- Wilson CG, Bonham AC. Effect of cardiopulmonary C fibre activation on the firing activity of ventral respiratory group neurones in the rat. J Physiol. 1997;504 (Pt 2):453–466. doi: 10.1111/j.1469-7793.1997.453be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Frazier DT. Respiratory-related neurons of the fastigial nucleus in response to chemical and mechanical challenges. J Appl Physiol. 1997;82:1177–1184. doi: 10.1152/jappl.1997.82.4.1177. [DOI] [PubMed] [Google Scholar]
- Xu F, Owen J, Frazier DT. Hypoxic respiratory responses attenuated by ablation of the cerebellum or fastigial nuclei. J Appl Physiol. 1995;79:1181–1189. doi: 10.1152/jappl.1995.79.4.1181. [DOI] [PubMed] [Google Scholar]
- Xu F, Zhang Z, Frazier DT. Microinjection of acetazolamide into the fastigial nucleus augments respiratory output in the rat. J Appl Physiol. 2001;91:2342–2350. doi: 10.1152/jappl.2001.91.5.2342. [DOI] [PubMed] [Google Scholar]
- Yeates DB, Hameister WM. Alveolar epithelial permeability in baboons: histamine and capsaicin. J Physiol. 1992;450:363–374. doi: 10.1113/jphysiol.1992.sp019131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz B, Gilmore DP, Wilson CA. Effects of DPDPE (a specific delta-opioid receptor agonist) and naloxone on hypothalamic monoamine concentrations during the pre-ovulatory LH surge in the rat. European journal of endocrinology/European Federation of Endocrine Societies. 1998;139:546–551. doi: 10.1530/eje.0.1390546. [DOI] [PubMed] [Google Scholar]
- Yu J, Lin S, Zhang J, Otmishi P, Guardiola JJ. Airway nociceptors activated by pro-inflammatory cytokines. Respir Physiol Neurobiol. 2007;156:116–119. doi: 10.1016/j.resp.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Xu F, Zhang C, Liang X. Activation of opioid mu receptors in caudal medullary raphe region inhibits the ventilatory response to hypercapnia in anesthetized rats. Anesthesiology. 2007;107:288–297. doi: 10.1097/01.anes.0000270760.46821.67. [DOI] [PubMed] [Google Scholar]
- Zochodne DW, Ho LT. Evidence that capsaicin hyperaemia of rat sciatic vasa nervorum is local, opiate-sensitive and involves mast cells. J Physiol. 1993;468:325–333. doi: 10.1113/jphysiol.1993.sp019774. [DOI] [PMC free article] [PubMed] [Google Scholar]