Abstract
The disposition of actinides, most recently 239Pu from dismantled nuclear weapons, requires effective containment of waste generated by the nuclear fuel cycle. Because actinides (e.g., 239Pu and 237Np) are long-lived, they have a major impact on risk assessments of geologic repositories. Thus, demonstrable, long-term chemical and mechanical durability are essential properties of waste forms for the immobilization of actinides. Mineralogic and geologic studies provide excellent candidate phases for immobilization and a unique database that cannot be duplicated by a purely materials science approach. The “mineralogic approach” is illustrated by a discussion of zircon as a phase for the immobilization of excess weapons plutonium.
The disposition of “waste” generated by the nuclear fuel cycle is one of the most pressing, and potentially costly, environmental problems for the 21st century, a heritage from the atomic age of the 20th century. Proposed strategies are complicated, not only by the large volumes and activities of waste, but by the political and public policy issues associated with the long times considered for containment and disposal (104-106 years). Furthermore, the waste includes fissile material, e.g., 239Pu, of high energy content. Three primary sources of actinide-bearing waste in the United States are as follows.
High-level waste (HLW) resulting from reprocessing to reclaim fissile materials for weapons production.
Approximately 380,000 m3 (100 million gallons) of HLW have a total radioactivity of 960 million curies (1 Ci = 37 GBq) (1). The greatest volumes (340,000 m3) are stored in tanks at Hanford, WA, and Savannah River, SC. Over 99 percent of the present activity is from nonactinide radionuclides with half-lives <50 years (reprocessing has removed much of the actinide content); however, after 500 years, the total activity will be substantially reduced, and the primary radionuclides will be 238Pu, 131Sm, and 241Am. After 50,000 years, most of the activity will be associated with longer-lived radionuclides, such as 239Pu and 240Pu. Also related to reprocessing are much lower activity waste contaminated with transuranic elements, TRU waste. These are defined as containing 100 nanocuries of α-emitting transuranic isotopes, with half-lives >20 years, per gram of waste. Over 60,000 m3 are stored retrievably at Department of Energy sites, destined for disposal at the Waste Isolation Pilot Plant in New Mexico (2). The estimated cost of remediation and restoration actives in the Department of Energy complex during the next decades is in the order of 200 billion dollars (3).
Used or spent nuclear fuel resulting from commercial power generation.
Just over 20 percent of the electricity generated in the United States is produced by nuclear power reactors. In 1995, 32,200 metric tons of spent fuel with a total activity of 30,200 MCi were stored by the electric utilities at 70 sites (either in pools or in dry storage systems) (2, 4). By 2020, the projected inventory will be 77,100 metric tons of heavy metal (MTHM) with a total activity of 34,600 MCi. Although the volume of the spent fuel is only a few percent of the volume of HLW, >95% of the total activity (defense-related plus commercially generated waste) is associated with the commercially generated spent nuclear fuel (3). At present, none of the spent fuel will be reprocessed, and all is destined for direct disposal in a geologic repository.
The dismantlement of nuclear weapons.
Under the first and second Strategic Arms Reduction treaties, as well as unilateral pledges made by both the United States and Russia, several thousand nuclear weapons will be dismantled. Initially, this will result in an estimated 100 metric tons of weapons plutonium that will require long-term disposition. The disposition strategy should not only protect the public and the environment but must also ensure that the plutonium is not readily recoverable for use in weapons (5). Present U.S. strategy calls for “burning” the Pu as a mixed-oxide fuel in existing or modified reactors followed by direct disposal with commercially generated spent fuel in a geologic repository (6, 7). A smaller portion of the Pu (tens of metric tons) is destined for immobilization into a durable solid followed by geologic disposal. The present program has an anticipated cost of two billion dollars.
World-wide, since the first creation of milligram quantities of plutonium by Glenn Seaborg in 1941, the global inventory of plutonium has reached 1,350 tons and continues to increase by ≈70 tons/year (Table 1). This commercially generated plutonium is in two forms: (i) incorporated in spent fuel destined for direct geologic disposal (>600 metric tons of plutonium is in the spent fuel in the U.S.); and (ii) plutonium separated by reprocessing of commercial fuel, which is estimated to reach 300 tons by the year 2000. This is greater than the amount of plutonium presently in nuclear weapons (9). Considering that the bare critical mass for weapons grade plutonium is 15 kg of metal (this number is substantially reduced in the presence of a neutron reflector), safe-guarding this plutonium is essential. In fact, the need for safeguards to protect against the diversion of separated plutonium applies equally to all grades of plutonium (10). The peaceful use of nuclear energy will inevitably require a strategy for the disposition and disposal of actinides.
Table 1.
Estimated global plutonium inventory (metric tons) at the end of 1996 (8)
| In spent fuel | |
| USA | 650 |
| Japan | 90 |
| France | 70 |
| Germany | 50 |
| Total | 860 |
| In operating reactors | 80 |
| Separated by civilian reprocessing | 150 |
| Military inventories | |
| Former USSR | 140 |
| USA | 100 |
| France | 6 |
| China | 5 |
| Israel, India, Pakistan | 1 |
| Total | 252 |
| Estimated world inventory, +70 metric tons/year production | 1,350 |
Why Are Actinides Important?
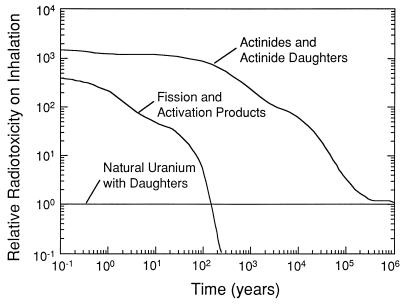
Although there are a number of fission product radionuclides of high activity (137Cs and 90Sr) and long half-life (99Tc, 200,000 years; 129I, 1.6 × 107 years) in spent nuclear fuel, actinides and their daughter products account for most of the radiotoxicity of nuclear waste after the first 500 years of disposal (Fig. 1). After several hundred years, radiotoxicity is dominated by 239Pu (half-life = 24, 100 years) and 237Np (half-life = 2,000,000 years). Thus, a major part of the long-term risk is directly related to the fate of these two actinides in the geosphere (natural, crustal concentrations of Pu are on the order of 10−11 ppm for 239Pu).
Figure 1.
Relative radiotoxicity on inhalation of spent nuclear fuel with a burnup of 38 megawatt days/kg U. The radiotoxicity values are relative to the radiotoxicity (horizontal line) of the quantity of uranium ore that was originally mined to produce the fuel (eight tons of natural uranium yields one ton of enriched uranium, 3.5% 235U) (11).
Plutonium has several important and unique properties: (i) 239Pu is fissile; (ii) 239Pu with a half-life of 24,100 years decays to 235U, another fissile radionuclide with a half-life of 700 million years (the bare critical mass of weapons grade uranium is ≈50 kg); and (iii) Pu has four oxidation states (3+, 4+, 5+, and 6+) in natural water–rock systems. Although crystalline PuO2 has a low solubility, Pu may exist as PuO2+ or PuO22+ aqueous species, with the former predominating in oxidized natural waters. Additionally, in the 3+, 5+, and 6+ oxidation states, Pu forms strong carbonate complexes (pH >5) (12). Actual plutonium concentrations in solution are further complicated by the possibility of disproportionation among oxidation states, α-radiolysis of water to produce oxidants, such as H2O2, α-decay-induced amorphization of the solid that increases leach rates, and the formation of intrinsic actinide or actinide-bearing colloids that can increase actinide concentrations in ground waters by several orders of magnitude. Thus, the geochemistry of Pu has the full array of dissolution, transport, and precipitation mechanisms that are typical in geologic systems, in addition to radiation effects; as with other multivalent elements (e.g., insoluble U4+ vs. mobile U6+), this can lead to either dispersal or concentration in the geosphere. Thus, it is essential to evaluate the long-term behavior of Pu either as it exists in spent nuclear fuel or is immobilized in solid waste forms. The purpose of this paper is to illustrate the unique contributions that mineralogy and geochemistry can make in the design and selection of durable waste forms for the long term disposal of plutonium.
Durable Waste Forms for Plutonium
Although the development of waste forms for plutonium poses special problems and requirements for long-term durability, weapons Pu presents special opportunities: (i) As compared with high-level waste, the volumes are relatively small. For example, if Pu is immobilized in a typical waste form with a waste loading of 10 wt %, the 100 metric tons of weapons Pu can be immobilized in a volume of several hundred cubic meters. (ii) Weapons plutonium is remarkably pure, consisting of a Pu-Ga alloy (0.5–2% Ga) coated with a corrosion-resistant layer, generally nickel. This high purity provides a materials engineer with a wide range of potential processing techniques and the possibility of the production of phase pure waste forms at prescribed waste loading levels. The absence of highly active fission products, such as 137Cs and 90Sr, that are the primary source of ionizing radiation makes handling the material tractable, utilizing technologies comparable to those used to fabricate mixed-oxide fuels. (iii) Although the half-life of 239Pu (24,100 years) is much longer than that of the much higher activity fission products, a substantial amount of decay occurs over relatively short geologic time scales (e.g., containment of Pu for 10 half-lives requires on the order of 200,000 years). Thus, immobilization over the time required for substantial radioactive decay is short relative to the durability of some geologic materials, measured in many millions of years. The fact that 239Pu decays to 235U poses additional challenges for durability of the waste form, but the greater critical mass of 235U combined with the possibility of dilution of 235U by 238U can provide additional barriers to the potential for criticality.
The essential question to mineralogists is, Are there naturally occurring, actinide-bearing phases of demonstrable chemical and physical durability that can be used for the long-term immobilization and disposal of weapons plutonium? This question is posed against a backdrop of 50 years of research and development of radioactive waste forms, mainly borosilicate glass and spent fuel. The first proposal for a waste form was made 45 years ago by Hatch (13), who suggested clays for the fixation of radionuclides into crystalline phases. Roy and McCarthy (14) were guided in their selection of appropriate phases by reference to minerals that are resistant to both geochemical alteration and radiation effects, and systematic studies were already in progress by the early 1980s (15). The most enduring proposal for a waste form was made by Ringwood et al. (16, 17) with the development of the Synroc concept, a Ti-based polyphase assemblage. The Synroc phase assemblage presently provides two of the candidate phases, pyrochlore and zirconolite, for Pu-immobilization (Table 2). These alternative concepts for waste forms led to a peak of research and development activity, mainly for the immobilization of the HLW in the tanks at the Savannah River site, during the late 1970s and early 1980s. This was a highly contentious period (18–20) when a wide variety of “alternative” waste forms competed with borosilicate glass as the “reference” waste form (21). Although vitrification was finally selected for the Savannah River HLW (the Defense Waste Processing Facility began vitrifying waste in 1996), work on alternative waste forms has continued to the present day and provides the present basis for the discussion of waste forms for weapons plutonium (22).
Table 2.
Actinide-bearing phases that are candidates presently under consideration for plutonium immobilization
| Mineral | Ideal formula | GeoRef citations, 1998 | Durable heavy mineral* |
|---|---|---|---|
| Pyrochlore† | (Ca,REE)Ti2O7 | 494 | |
| Zirconolite | CaZrTi2O7 | 62 | |
| Apatite | Ca4−xREE6+x(SiO4)6−y(PO4)y(O,F)2 | 5,098 | Yes |
| Zircon | ZrSiO4 | 8,055 | Yes |
| Monazite | CePO4 | 1,711 | Yes |
| Baddelyite‡ | ZrO2 | 244 |
Heavy minerals identified and discussed in monograph on heavy mineral occurrences (23). Heavy minerals that were noted, but which are not included in this table, are gadolinite, allanite, thorite, titanite, and xenotime. Thorite (ThSiO4) and xenotime (YPO4) are isostructural with zircon; gadolinite, allanite, and titanite are either relatively rare or less durable than the minerals listed in the table.
Presently receiving the greatest attention within the U.S. program for the disposition of fissile materials.
Cubic and tetragonal polymorphs of ZrO2 have been considered as waste form phases; however, naturally occurring ZrO2 is most commonly monoclinic baddelyite. Although not cited in ref. 23, it is included in this list because of its known durability and because it is considered both as a waste form and as inert matrix fuel for burning plutonium (9).
Rather than selecting a specific waste form in preference to others for Pu-immobilization, I want to illustrate what “mineralogical thinking” can bring to the table of such a discussion. The critical aspects of the question are the determination of “demonstrable, long-term physical and chemical durability.” Durability refers to a wide variety of properties: mechanical strength, thermodynamic stability, slow kinetics for corrosion processes, or retention of trace elements because of low diffusivity (in this case, actinides and neutron absorbers, such as Gd and Hf).
The qualitative, geologic answer to such a question is obvious: e.g., the heavy minerals that survive weathering, erosion, transport, and deposition (sometimes many cycles) and persist as placer deposits in stream beds. The classic paper on heavy detrital minerals by Hutton (23) provides a detailed list of heavy minerals (Table 2). Note the absence of potentially important actinide-bearing phases: pyrochlore, zirconolite, and baddeleyite, because of either their relative rarity or lower durability. The second issue is how much geologic and mineralogic data exist for these phases. Table 2 gives the number of citations for these phases as taken from GeoRef (American Geological Institute). The result is not surprising; those phases that are important to geochronology (zircon, apatite, and monazite) account for a major portion (95%) of the published literature. These studies are of two types: (i) laboratory studies to determine the ability of the minerals to retain isotopic signatures as a result of their physical and chemical durability in a variety of geologic environments; and (ii) age-dating studies that essentially confirm the results of the laboratory studies in actual, long-term geologic environments. Both types of studies provide the essential data required for waste form design and selection.
Present research on waste forms for Pu-immobilization includes a relatively short list of phases (Table 2). In the U.S., most of the effort within the Materials Disposition program of the Department of Energy focuses on immobilization in a ceramic (24), particularly cubic pyrochlore and its monoclinic derivative, zirconolite, because there are considerable data on these materials as waste form phases (22). However, the Department of Energy evaluation and selection process (24) is very different from the mineralogic approach presented in this paper.
To illustrate the mineralogic approach, I review relevant work on zircon, drawn mainly from the mineralogic and geochemical literature, to demonstrate the utility of the mineralogic approach, as well as the extensive amounts of data that are already available in the literature. Although the structures of zircon (25) and actinide orthosilicates (26, 27) have been known for 30 years, I focus on studies applicable to the analysis of zircon as an actinide host phase.
Zircon as an Actinide Waste Form
Zircon (I41/amd; Z = 4) occurs in nature with uranium and thorium concentrations typically up to 5,000 ppm, but reaching 10 wt %. Zircon is an extremely durable mineral (28), often found as a heavy mineral in stream sediments, that, after transport over great distances, shows limited chemical alteration or physical abrasion (23, 29). The widespread distribution of zircon in the continental crust, its tendency to concentrate trace elements (lanthanides and actinides), its use in age dating, and its resistance to chemical and physical degradation (30–33) have made zircon probably the most useful accessory mineral in geologic studies.
Zircon has been identified as an actinide-bearing phase in polyphase ceramic waste forms (34). Zircon also occurs in the Chernobyl lavas as an important actinide-bearing phase (6–12 atomic percent uranium) (35). The propensity to incorporate actinides and its durability have lead to the suggestion that zircon be used to immobilize actinides (36–38). Based on the ability of natural zircon to retain Pb, Gentry et al. (39) suggested that materials, like zircon, could effectively retain radioactive waste. This early suggestion was prescient but did not evaluate the extent of solid-solution of actinides in zircon and did not consider the much greater radiation damage that would occur in such a radioactive waste form (40).
Structure.
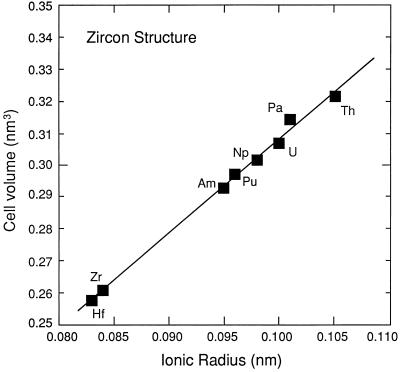
The zircon structure consists of triangular dodecahedral ZrO8 groups that form edge-sharing chains parallel to the a axis and SiO4 tetrahedral monomers that form edge-sharing chains with alternating ZrO8 groups parallel to the c axis (25, 26). U and Th replace the Zr in low concentrations; however, compositions of ASiO4, in which A4+ = Zr, Hf, Th, Pa, U, Np, Pu, and Am, have been synthesized (27). The regular increase in the unit cell volume with the increasing ionic radius of the A-site cation confirms the homologous topologies of these structures (Fig. 2). Four of these compositions, hafnon (HfSiO4), zircon, coffinite (USiO4), and thorite (ThSiO4), occur naturally. Structure refinements (27) and structural analyses (41, 42) suggest complete miscibility between ZrSiO4 and HfSiO4, but there are miscibility gaps on the ZrSiO4–USiO4–ThSiO4 joins (43). Zircon with 9.2 atom percent plutonium (8.1% Pu238; 1.1% Pu239) substituting for Zr has been synthesized (44). This is equal to a waste loading of 10 wt % Pu, but the maximum extent of the solubility of Pu in zircon has not been determined. That a pure, endmember composition, PuSiO4, has been synthesized (27) suggests extensive substitution of Pu for Zr is possible (42).
Figure 2.
Variation in unit cell volume of actinide silicates, ASiO4, with the zircon structure as a function of ionic radius (VIIIA4+). Data are from Keller (27) after Speer (41).
The zircon structure is part of a larger class of ABO4 structure types (silicates and phosphates) and is closely related to the structure of monazite, CePO4, (another durable phase commonly used in geologic age-dating). Numerous A-site compositions (La, Pr, Nd, Sm, Eu, Gd, Tb, Tm, Yb, Lu, Sc, and Y) with the silicate and phosphate zircon and monazite structure-types have been synthesized, and their structures have been refined. These synthesized materials have provided the basis for detailed optical-absorption spectroscopy and electronic paramagnetic resonance studies of U, Pu, Cm, Np, and Gd incorporation into the zircon-structure type (45, 46).
Geochronology.
Recent progress in the utilization of zircon in age dating has come from the use of the sensitive high-resolution ion microprobe (SHRIMP), a method that allows the measurement of isotopic ratios on areas as small as 20–30 μm, thus providing age dates on separate zones within single crystals of zircon. Detrital zircons in a quartzite at Mount Narryer, Western Australia have been dated at 4,100–4,300 million years ago (Ma), the oldest terrestrial minerals yet found (47). In Western Australia, the Jack Hills contain slightly younger (3,900–4,270 Ma) detrital zircons (48). The zircons in Australia are individual, recycled grains in a younger (3,500 Ma) sequence of metamorphosed sedimentary rocks, as are similarly dated zircons from the Sino-Korean craton in northeast China (≥3,800 Ma) (49). The oldest so-called intact crust is found in the early Archean (3,800–3,960 Ma) granitoids in northwestern Canada (50) and western Greenland (51). The zircons formed at the same time as the first crustal rocks on Earth. The oldest zircons in the solar system are found as rare inclusions in meteorites and were dated at 4,560 Ma (52). There are many hundreds of papers that can be cited to illustrate the use of zircon in dating very old rocks, but of greater importance are that these field studies establish the effects of geologic processes on the durability of zircon either under surface conditions of sedimentary transport (29) or at much higher temperatures, e.g., granulite facies metamorphism (53), extremely high metamorphic pressures (54–57), or meteorite impact (58).
The most recent and dramatic example of the extraordinary ability of zircon to retain its U-Pb systematics is from studies of zircons from the Chicxulub impact structure of the Yucatan Peninsula (58–60). The shocked zircons were exhumed from the Chicxulub basement rock during meteorite impact and dispersed in the fine dust of the impact cloud. Discordancies in the U-Pb systematics (e.g., Pb loss) are proportional to the extent of impact-induced shock textures, and isotopic resetting is consistent with partial lead loss at the time of impact (65 Ma), thus providing convincing support for the meteorite impact origin of the Chicxulub crater and its being the source of ejected material found at the Cretaceous-Tertiary (K-T) boundary in North America. Uranium–lead dating studies of shocked zircons in the fine-grained ejecta deposited in areas as wide apart as Colorado, Saskatchewan, and Haiti at the Cretaceous-Tertiary boundary have a predominant age of 545 Ma, in agreement with dates for shocked zircons from the Chicxulub crater (59–60). Only a material of remarkable durability, such as zircon, could preserve the isotopic signature of an event of such extreme conditions (60).
Alteration and Disturbed U/Pb Systematics.
Any process that can disturb the U/Pb isotopic systematics of zircon crystals has an important effect on whether the radiometrically determined dates are concordant or discordant; thus, there is an extensive literature that describes this type of alteration (30–32, 61). Discordant ages are common, mainly because of Pb-loss and less frequently because of U-loss. The discordant ages are usually attributed to episodic loss of Pb or U during thermal events (61) and are enhanced by (i) physical degradation caused by microfracturing that is the result of the volume expansion associated with α-decay damage that results in an increase of surface area (57, 62, 63); and (ii) chemical alteration caused by radiation-induced amorphization that creates damaged, aperiod domains in which Pb-diffusion is enhanced and for which bulk leach rates are increased. Thus, the discordant ages are often the result of the accumulation of highly damaged regions resulting from α-decay damage; however, improved U/Pb dates may be obtained by removing the radiation-damaged regions either by physical abrasion or etching techniques (64). Differential etching experiments (using 48% HF) have shown by scanning electron microscopy that the removal of radiation damaged zones improved the concordance of U/Pb age dates in the remaining, unaltered material (32). Additionally, under extreme geologic conditions, e.g., zircons subjected to deformation in shear zones and altered by hydrothermal solutions, disturbed U/Pb systematics are clearly documented. Detailed experimental studies (65) have shown that experimentally induced U/Pb isotopic discordance in zircon is a complex function of zircon durability and the annealing of the radiation damage. Recent studies have investigated the incorporation of Pb into zircon (66) and the mechanisms by which Pb may be lost (67). The loss of Pb by diffusion is an extremely slow process (at 1,000°C, the diffusivity is 10−25 m2⋅s−1), and the low diffusivities confirm Pb closure temperatures in excess of 900°C and imply that U/Th/Pb isotopic ages are unlikely to be reset by thermal events alone except under the most extreme geologic conditions, i.e., partial melting or granulite-grade metamorphism (67). In summary, there are abundant data from geochronologic studies to show that natural zircons can quantitatively retain Pb for billions of years in the absence of episodic, thermally induced Pb loss (40). The minor alteration of zircon over long periods of time and under rather extreme conditions stands in contrast to the observations of other potential ceramic nuclear waste forms such as pyrochlore (68) for which the data of the type summarized above are simply not presently available.
Diffusion of Tetravalent Cations and Rare Earth Elements.
Even though diffusion rates for Pb are low, the diffusivities of U and Th at 1,100°C are orders of magnitude lower (69), suggesting complete containment of actinides for billions of years. The principal process by which this might change is α-decay radiation damage (40). Elements may preferentially segregate into aperiodic, damaged regions that have inherently higher solubilities than crystalline ceramics (70), and the annealing kinetics of recrystallization of damaged domains may substantially change the response of a material to the damage accumulation process (71).
The development of a waste form for weapons 239Pu requires a knowledge of diffusion rates of the actinides, as well as elements with high neutron-capture cross-sections to control criticality (e.g., Hf and Gd). Natural zircons can contain up to several thousand parts per million rare earths, including up to 500 ppm of Gd, and zircon exhibits nearly complete solid solution with hafnon, HfSiO4. Thus, neutron absorbing nuclides may be incorporated into the zircon structure; however, the neutron absorber must remain within atomic scale proximity of the fissile radionuclide (239Pu or 235U). Recent determinations of diffusion rates for tetravalent cations (Th, U and Hf) give diffusivities in the order of 10−22-10−20 m2⋅s−1 in the temperature range of 1,400–1650°C (72). Based on similarities in ionic radii, Pu4+ is expected to have a similar diffusivity, although slightly faster because of its smaller ionic radius, which means that it is essentially immobile under all but the most extreme geologic conditions. This is why fine-scale chemical zoning and isotopic signatures are preserved in the inherited cores of zircon crystals that have experienced protracted thermal events in their past history. In contrast, the rare-earth elements (REE) diffuse at rates 4–5 orders of magnitude faster than the tetravalent cations (73). The diffusion rates vary among the REEs in a systematic manner as a function of ionic radius; thus, for Gd, the estimated activation energy and diffusivity (1,000°C) are 189 kcal/mol and 3.2 × 10−26 m2⋅s−1, respectively. Again, the closure temperature of zircon for REEs is quite high (>1,000°C) for all but the smallest grains. Other phases that have been suggested as waste form phases, e.g., titanite and apatite, have closure temperatures many hundreds of degrees lower than those of zircon.
Dissolution Studies.
Studies of natural zircons under extreme laboratory conditions have confirmed the loss of U, Th, and Pb (30, 31, 65). The lead loss can be the result of grain boundary or volume diffusion (in which there is no dissolution of the zircon), or the bulk dissolution of zircon. However, at lower temperatures (<80°C) and near neutral pH values, i.e., conditions more pertinent to nuclear waste disposal, zircon is extremely insoluble. There are much less leach data in the literature than necessary for a full evaluation of zircon as a waste form. Crystalline zircon is stable to such an extent that the equilibrium concentrations of Zr and Si are in the order of 10−9 moles/liter (0.1 parts per billion) at 25°C (74). Dissolution of amorphous zircon followed a first order reaction based on Si concentrations. Zr concentrations remained <0.05 ppm, the instrument detection limit, because of precipitation of ZrO2 and ZrSiO4 (74). The leach rate of zircon increases with α-decay damage on the order of one to two orders of magnitude (75). Comparing the dissolution rate for a metamict zircon (74), r = 10−7 moles/m2/day at 80°C, pH 5, with the long-term rate of a nuclear waste borosilicate glass, r = 10−5 moles/m2/day (which equals 10−3 g/m2/day) in water at 98°C (76), shows that the dissolution rate of amorphous zircon is still considerably lower than that of glass in stagnant, silica-saturated solutions. In an open system (e.g., moving groundwater for which solubility limits are not reached), the leach rate for zircon (74) used in this comparison does not increase; however, the leach rate of borosilicate glass may increase by three orders of magnitude until reaching the final rate of reaction (21).
Thus, one of the main advantages of zircon may be its high durability in an open system in which groundwaters are present because this allows considerably greater flexibility in disposal strategy (e.g., deep borehole). For this reason, it is essential to determine the maximum forward rate of the dissolution reaction. This is particularly challenging because the dissolution rate for zircon is low, and precipitation of zirconia removes Zr from the leaching solution. Recently, a high-temperature Soxhlet extractor was designed to measure the forward rate of dissolution of zircon in the range of 120–250°C. The measured rates were 4.1 × 10−4 g/m2/day at 250°C, 1.7 × 10−4 g/m2/day at 200°C, and 7.1 × 10−5 g/m2/day at 120°C. The rate extrapolated to 90°C is 4.6 × 10−5 g/m2/day; therefore, in an open system, in the absence of a solubility-limiting phase, a 100-μm crystal of zircon would require 150,000 years for complete dissolution (77, 78). Under static conditions, the dissolution rate is substantially reduced. The experimental results are consistent with the high chemical durability of zircon in a wide range of geologic environments.
Physical Properties.
The mechanical properties of zircon change with increasing α-decay event dose (79) and as a result of implantation by Pb ions (540 keV) up to fluences of 3.3 × 1011 to 5 × 1015 ions/cm2 [a dose range that spans the crystalline-to-amorphous transition (80)]. The α-decay-induced softening leads to a decrease in hardness (40%) and the bulk elastic modulus (70%) (81), but there is an increase in fracture toughness probably caused by crack-tip blunting by the aperiodic domains (79). The ion beam irradiation results in softening (70%) and a decrease in modulus (42%). The principal effect of the changes in mechanical properties is the formation of a pronounced fracture system (62) caused by differential volume expansion in zones of different α-decay doses. A model has been developed that describes the radial and concentric fracture sets that are characteristic of zircon: principally, a function of the degree of damage (e.g., the amorphous fraction), the zone thicknesses, and the confining pressure (82). This type of analysis is required for evaluating the development of microfractures as a function of radiation damage, particularly for disposal in a deep borehole.
Radiation Damage.
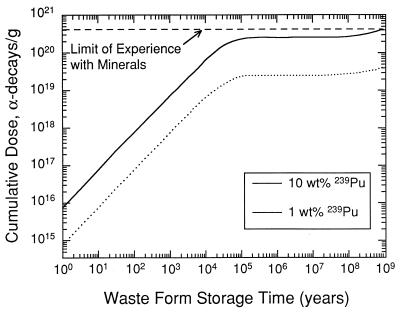
Radiation damage resulting from the α-decay of 239Pu and its daughter products (e.g., 235U) has an important effect on the physical and chemical durability of actinide-bearing zircons. Depending on the waste loading, significant doses (>1018 α-decay events/g) accumulate, and crystalline phases become aperiodic in relatively short periods of time (103 years) (Fig. 3) (83, 84). Note that natural zircons can accumulate relatively high α-decay doses (horizontal line in Fig. 3), and this allows the comparison of data resulting from accelerated irradiation techniques (either actinide doping with short lived α-decay nuclides such as 238Pu or 244Cm that have reached α-decay doses of 1019 α-decay event/g or ion beam irradiation experiments).
Figure 3.
Cumulative α-decay dose as a function of waste form storage time for a ceramic, e.g., zircon, containing 1 and 10 wt % loading of 239Pu (as in ref. 85). The dashed horizontal line indicates maximum doses reached in natural zircon from decay of uranium and thorium and daughter products.
In an α-decay event, the α-particle dissipates most of its energy [4.5–5.8 million electronvolts (MeV) for actinides] (1 eV = 1.602 × 10−19 J) by ionization processes over a range of 16–22 μm but undergoes enough elastic collisions along its path to produce several hundred isolated atomic displacements. The largest number of displacements occurs near the end of the α-particle range. The more massive but lower-energy α-recoil (86 keV 235U recoil from decay of 239Pu) dissipates nearly all of its energy in elastic collisions over a very short range, 30–40 nm, causing ≈1,000 atomic displacements. The density of energy deposited into the cascade is high (up to 1 eV/atom) and occurs over an extremely short time (<10−12 s). Thus, a single α-decay event generates ≈1,400 atomic displacements, which is significantly greater than the 0.1 displacements generated per β-decay event. Clearly, α-decay from incorporated actinides will have a profound effect on the structure of a crystalline solid. The cumulative effect of dose will be time- and temperature-dependent because of annealing and recrystallization of damaged areas.
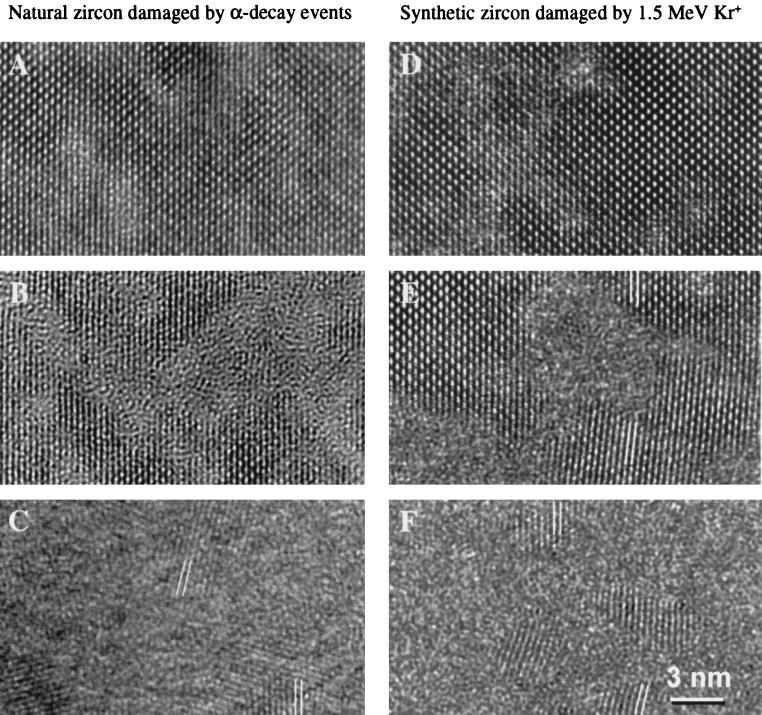
Studies of radiation effects in zircon have a long history (86–91). Zircon undergoes a radiation-induced transformation from the periodic-to-aperiodic state (metamict state) at doses over the range of 1018-1019 α-decay events/g [which equals 0.2–0.6 displacements per atom (dpa)] with a density decrease and a corresponding volume expansion of 18%. Previous studies include analysis of natural zircons that have accumulated α-decay event damage up to doses of nearly 0.7 dpa over 550 million years (63, 87, 88), 238Pu-doped zircons (half-life = 87.7 years) up to doses of 0.7 dpa in 6.5 years (44, 91, 92), and heavy ion beam irradiation using 2 MeV He+, 0.8 MeV Ne+, 1.5 MeV Ar+, 0.7–1.5 MeV Kr+, and 1.5 MeV Xe+ up to doses of 0.2–2.3 dpa in times of <1 hour (91, 93). All three types of damage experiments include detailed studies of annealing kinetics. The natural zircon studies (e.g., of samples that are 550 million years old) and the experimental results for ion beam irradiation experiments of <1-hour duration have a range of dose rates >108. In situ ion beam irradiation combined with high resolution transmission electron microscopy have demonstrated that the damage microstructures and the ingrowth of damage with increasing dose can be simulated by using heavy ion irradiation (Fig. 4). Thus, there is a firm basis for predicting the microstructure of the radiation-damaged zircon as a function of dose, temperature, and type of radiation (91, 93, 94). Systematic studies have been completed for monazite- and zircon-structure orthophosphates of a wide variety of A-site end-member compositions (95, 96) and orthosilicates (97–99). Zircon, hafnon (HfSiO4), thorite (tetragonal ThSiO4), and huttonite (monoclinic ThSiO4) become amorphous in a two-stage process with increasing temperature when irradiated with 800 keV Kr+ or Xe+ ions in the temperature range of 20–1,100 K. The temperature above which amorphization does not occur (i.e., the temperature at which the rate of simultaneous annealing is equal to the rate of damage accumulation) increased in the order huttonite, zircon, hafnon, and thorite. When irradiated with heavy ions, all four orthosilicates may decompose into crystalline oxides: ZrO2, HfO2, or ThO2 plus amorphous SiO2 (98). Ion beam irradiation studies of synthetic and natural zircons and monazites (with impurities) revealed that impurities lower the dose required for amorphization and correspondingly increase the temperature above which a material cannot be amorphized. This suggests that impurities increase susceptibility to amorphization and inhibit annealing, particularly when coupled charge balance substitutions are required (96, 99). Details of structural rearrangements during damage accumulation and annealing have been obtained by extended x-ray absorption fine structure spectroscopy (EXAFS) studies of metamict zircons (89, 100). Metamictization is accompanied by major atomic reorganization: loss of well defined medium range order, disruption of the immediate environment of Zr, a decrease of the average Zr-coordination number, and tilting and distortion of SiO4 polyhedral. A two-stage thermal annealing process was observed that, at lower temperatures (400–500°C), resulted in the formation of minor Zr-rich domains (100). Finally, transmutation effects (239Pu decays to 235U) are important in crystalline materials because they may lead to phase instability. In the case of zircon, the solubility of U in zircon is only known approximately (4 ± 2 mole percent) (43); thus, for higher concentrations of U, one may expect the formation of USiO4, also with the zircon structure, and UO2.
Figure 4.
Comparison of radiation damage in natural (A–C) and 1.5 MeV Kr+-irradiated synthetic (D–F) zircon. (A) 5 × 1013 α-decay events/mg (0.003 dpa). (B) 1.8 × 1015 α/mg (0.091 dpa). (C) 6.4 × 1015 α/mg (0.32 dpa). (D) 5 × 1013 Kr+/cm2 (0.057 dpa). (E) 1.5 × 1014 Kr+/cm2 (0.17 dpa). (F) 3 × 1014 Kr+/cm2 (0.34 dpa). Complete amorphization in both was observed after 0.5–0.55 dpa. Figure courtesy of L. M. Wang (91).
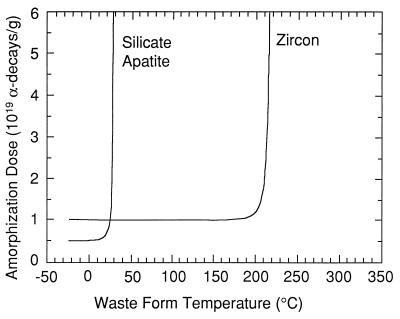
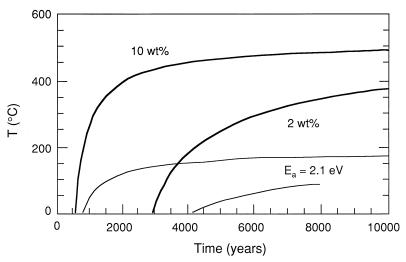
There are few crystalline ceramics for which such a wide variety of data on radiation damage are available. Based on these data, for a waste loading of 10 wt % of 239Pu under ambient conditions, the zircon will reach the saturation dose of damage (1.2 × 1019 α-decay events/g or 0.8 dpa) in <2,000 years (Fig. 3); thus, the properties of zircon must be considered in light of its radiation-damaged, aperiodic state. Based on the extensive database, it is possible to model the damage accumulation (e.g., percent amorphous fraction) as a function of dose and temperature for zircon and compare the results to those of other phases (e.g., apatite) (85). Fig. 5 is a plot of the critical amorphization dose for zircon as compared with a silicate apatite (data for both phases are based on ion beam irradiation experiments). Above 225°C, zircon with a 10 wt % loading of 239Pu will remain in the crystalline state because of thermal annealing. In contrast, silicate apatite anneals readily and will remain in the crystalline state at ambient temperatures. Such models are very sensitive to the activation energies for thermal annealing (101), and more recent work has suggested that temperatures as high as 400°C will be required to maintain the crystallinity of zircon (98). The efficacy of such an approach is that modeled results can be confirmed by comparison to naturally occurring zircons of known thermal history, and the modeled results are in good agreement with the amorphous fraction accumulation determined for natural zircons (unless there has been episodic thermal annealing) (101). The same type of analysis allows one to calculate minimum storage temperatures required to maintain the crystallinity of any actinide-bearing waste form (Fig. 6). At present, sufficient data for this type of analysis are only available for zircon.
Figure 5.
Critical amorphization dose vs. storage temperature for silicate apatite and zircon containing 10 wt % 239Pu (85).
Figure 6.
Minimum storage temperature of zircon containing 10 and 2 wt % 239Pu, respectively, vs. storage time to ensure complete crystallinity of zircon. Such modeled calculations are very sensitive to the activation energy. The dark solid line is for an activation energy of 3.6 eV; the lower, thinner line if for an activation energy of 2.1 eV (101).
Stored energy values have been carefully determined (102) by transposed temperature drop calorimetry over the range of the periodic-to-aperiodic transition on a suite of zircons from Sri Lanka (550 million years old). The energy released during annealing varies sigmoidally as a function of α-decay event dose reaching a saturation value of 322 ± 16 J/g at doses >5 × 1018 α-decay events/g. This is greater than values typical of nuclear waste glasses, which are generally <150 J/g and saturate at a dose of 1018 α-decay events/g; however, sudden release of this energy is not anticipated to cause a significant rise in temperature for either the glass or zircon. The magnitude of the enthalpy of annealing suggests that the radiation damage is pervasive on the scale of fractions of nanometers, perhaps leading to the formation of microdomains of amorphous SiO2-rich and ZrO2-rich regions in the metamict state. This suggestion is consistent with observations made by secondary ion mass spectrometry (SIMS) and high resolution transmission electron microscopy (HRTEM) (70) and extended x-ray absorption fine structure spectroscopy (EXAFS) of annealed zircons (100).
Summary
There can be little doubt that a phase such as zircon provides a demonstrable case for its long-term chemical and mechanical durability as an actinide waste form. The database for zircon is unique and extensive, providing ample opportunity to confirm experimental and modeled results against actual behavior in a wide range of geologic environments. If the issue is one of long-term verification of materials performance, no other comparable database exists for an actinide-bearing phase, as there is for zircon.
Are there other mineralogic candidates? Most definitely yes, and prominent among them are monazite and the polymorphs of zirconia. I believe that we have only just scratched the surface of mineralogic applications to issues related to nuclear waste disposal. Are there other mineralogic applications? Much can be learned about the alteration of UO2 in spent nuclear fuel by studying the alteration products of uraninite, UO2 + x (102, 103).
Acknowledgments
I have benefited greatly from collaborations with Werner Lutze, Lumin Wang, and Bill Weber, and most importantly with students, Gregory Lumpkin and Al Meldrum, in the development of the ideas presented in this paper. Lynn Boatner’s enthusiasm for this work has kept me in the game. I thank John Hanchar for very useful discussions of diffusion in zircon. This work has been sustained by support by Basic Energy Research Sciences of the Department of Energy (DE-FG02-97ER45656).
ABBREVIATIONS
- HLW
high-level waste
- Ma
million years ago
- MeV
million electronvolts
- dpa
displacements per atom
- REE
rare-earth element
References
- 1.Linking Legacies Connecting the Cold War Nuclear Weapons Production Processes to Their Environmental Consequences (1997) Department of Energy, Office of Environmental Management 0319, Washington, DC.
- 2.Ahearne J F. Phys Today. 1997;50:24–29. [Google Scholar]
- 3.Crowley K D. Phys Today. 1997;50:32–39. [Google Scholar]
- 4.Richardson J A. Proc Inst Mech Eng Part A. 1997;211:381–392. [Google Scholar]
- 5.Committee on International Security and Arms Control, National Academy of Sciences. Management and Disposition of Excess Weapons Plutonium. Washington, DC: National Academy Press; 1994. [Google Scholar]
- 6.Panelon Reactor-Related Options for the Disposition of Excess Weapons Plutonium, National Research Council. Management and Disposition of Excess Weapons Plutonium: Reactor-Related Options. Washington, DC: National Academy Press; 1995. [Google Scholar]
- 7.von Hippel F N. Nature (London) 1998;394:415–416. [Google Scholar]
- 8.Stoll W. Mat Res Soc Bull. 1998;23:6–16. [Google Scholar]
- 9.Oversby V M, McPheeters C C, Degueldre C, Paratte J M. J Nucl Mat. 1997;245:17–26. [Google Scholar]
- 10.Mark J C. Sci Global Security. 1993;4:111–128. [Google Scholar]
- 11.Hedin A. SKB Technical Report 97–13. Stockholm: Swedish Nuclear Fuel and Waste Management Co.; 1997. [Google Scholar]
- 12.Langmuir D. Aqueous Environmental Geochemistry. Englewood Cliffs, NJ: Prentice–Hall; 1997. [Google Scholar]
- 13.Hatch L P. Am Sci. 1953;41:410–421. [Google Scholar]
- 14.Roy R. In: Scientific Basis for Nuclear Waste Management. McCarthy G J, editor. Vol. 1. New York: Plenum; 1979. pp. 1–20. [Google Scholar]
- 15.Haaker R F, Ewing R C. Naturally Occurring Crystalline Phases: Analogues for Radioactive Waste Forms. Richland, WA: Pacific Northwest Laboratory; 1981. [Google Scholar]
- 16.Ringwood A E. Safe Disposal of High Level Nuclear Reactor Wastes: A New Strategy. Canberra, Australia: Australian National Univ. Press; 1978. [Google Scholar]
- 17.Ringwood A E, Kesson S E, Ware N G, Hibberson W, Major A. Nature (London) 1979;278:219–223. [Google Scholar]
- 18.Kerr R A. Science. 1979;204:289–291. doi: 10.1126/science.204.4390.289. [DOI] [PubMed] [Google Scholar]
- 19.Carter L J. Science. 1979;205:287–289. doi: 10.1126/science.205.4403.287. [DOI] [PubMed] [Google Scholar]
- 20.Garmon L. Sci News. 1981;120:396–399. [Google Scholar]
- 21.Lutze W, Ewing R C, editors. Radioactive Waste Forms for the Future. Amsterdam: North–Holland; 1988. [Google Scholar]
- 22.Ewing R C, Weber W J, Lutze W. In: Disposal of Weapon Plutonium Approaches and Prospects. Merz E R, Walter C E, editors. Dordrecht, The Netherlands: Kluwer; 1995. pp. 65–83. [Google Scholar]
- 23.Hutton C O. Geol Soc Am Bull. 1950;61:635–716. [Google Scholar]
- 24.Cochran S G, Dunlop W H, Edmunds T A, MacLean L M, Gould T H. Fissile Material Disposition Program Final Immobilization Form Assessment and Recommendation. Livermore, CA: Lawrence Livermore National Laboratory; 1997. [Google Scholar]
- 25.Robinson K, Gibbs G V, Ribbe P H. Am Mineral. 1971;56:782–790. [Google Scholar]
- 26.Taylor M, Ewing R C. Acta Crystallogr B. 1978;34:1074–1079. [Google Scholar]
- 27.von Keller C. Nukleonik. 1963;5:41–48. [Google Scholar]
- 28.Hanchar J M, Miller C F. Chem Geol. 1993;110:1–13. [Google Scholar]
- 29.Riggs N R, Lehman T M, Gehrels G E, Dickinson W R. Science. 1996;273:97–100. doi: 10.1126/science.273.5271.97. [DOI] [PubMed] [Google Scholar]
- 30.Pidgeon R T, O’Neil J R, Silver L T. Science. 1966;154:1538–1540. doi: 10.1126/science.154.3756.1538. [DOI] [PubMed] [Google Scholar]
- 31.Craig H. Science. 1968;159:447. doi: 10.1126/science.159.3813.447. [DOI] [PubMed] [Google Scholar]
- 32.Krogh T E, Davis G L. Year Book Carnegie Inst Washington. 1975;74:619–623. [Google Scholar]
- 33.Suzuki K. Geochem J. 1987;21:173–182. [Google Scholar]
- 34.Harker A B, Flintoff J F. J Am Ceramic Soc. 1990;73:1901–1906. [Google Scholar]
- 35.Anderson E B, Burakov B E, Pazukhin E M. Radiochim Acta. 1993;60:149–151. [Google Scholar]
- 36.Burakov B E. Proc SAFE WASTE 93. 1993;2:19–28. [Google Scholar]
- 37.Anderson E B, Burakov B E, Vasiliev V G. Proc SAFE WASTE 93. 1993;2:29–33. [Google Scholar]
- 38.Ewing R C, Lutze W, Weber W J. J Mat Res. 1995;10:243–246. [Google Scholar]
- 39.Gentry R V, Sworski T J, McKown H S, Smith D H, Eby R E, Christie W H. Science. 1982;216:296–298. doi: 10.1126/science.216.4543.296. [DOI] [PubMed] [Google Scholar]
- 40.Ludwig K R, Zartman R E, Goldich S S. Science. 1984;223:835. doi: 10.1126/science.223.4638.835. [DOI] [PubMed] [Google Scholar]
- 41.Speer J A. In: Reviews in Mineralogy, Orthosilicates. Ribbe P H, editor. Washington, DC: Mineralogical Soc. Am.; 1982. pp. 67–135. [Google Scholar]
- 42.Speer J A, Cooper B J. Am Mineral. 1982;67:804–808. [Google Scholar]
- 43.Mumpton F A, Roy R. Geochim Cosmochim Acta. 1961;21:217–238. [Google Scholar]
- 44.Weber W J. Radiat Effects Defects Solids. 1991;115:341–349. [Google Scholar]
- 45.Poirot I S, Kot W K, Edelstein N M, Abraham M M, Finch C B, Boatner L A. Phys Rev B. 1989;39:6388–6394. doi: 10.1103/physrevb.39.6388. [DOI] [PubMed] [Google Scholar]
- 46.Poirot I, Kot W, Shalimoff G, Edelstein N, Abraham M M, Finch C B, Boatner L A. Phys Rev B. 1988;37:3255–3264. doi: 10.1103/physrevb.37.3255. [DOI] [PubMed] [Google Scholar]
- 47.Froude D O, Ireland T R, Kinny P D, Williams I S, Compston W, Williams I R, Myers J S. Nature (London) 1983;304:616–618. [Google Scholar]
- 48.Maas R, Kinny P D, Williams I S, Froude D O, Compston W. Geochim Cosmochim Acta. 1992;56:1281–1300. [Google Scholar]
- 49.Liu D Y, Nutman A P, Compston W, Wu J S, Shen Q H. Geology. 1992;20:339–342. [Google Scholar]
- 50.Bowring S A, Williams I S, Compston W. Geology. 1989;17:971–975. [Google Scholar]
- 51.Nutman A P, Friend C R, Kinny P D, McGregor V R. Geology. 1993;21:415–418. [Google Scholar]
- 52.Ireland T R, Wlotzka F. Earth Planet Sci Lett. 1992;109:1–10. [Google Scholar]
- 53.Roberts M P, Finger F. Geology. 1997;25:319–322. [Google Scholar]
- 54.Deutsch A, Schärer U. Geochim Cosmochim Acta. 1990;54:3427–3434. [Google Scholar]
- 55.Claoué-Long J C, Sobolev N V, Shatsky V S, Sobolev A V. Geology. 1991;19:710–713. [Google Scholar]
- 56.Wayne D M, Sinha A K. Contrib Mineral Petrol. 1988;98:109–121. [Google Scholar]
- 57.Wayne D M, Sinha A K. J Geol. 1992;100:593–603. [Google Scholar]
- 58.Bohor B F, Betterton W J, Krogh T E. Earth Planet Sci Lett. 1993;119:419–424. [Google Scholar]
- 59.Krogh T E, Kamo S L, Sharpton V L, Marin L E, Hildebrand A R. Nature (London) 1993;366:731–734. [Google Scholar]
- 60.Kamo S L, Krogh T E. Geology. 1995;23:281–284. [Google Scholar]
- 61.Davis G L, Hart S R, Tilton G R. Earth Planet Sci Lett. 1968;5:27–34. [Google Scholar]
- 62.Chakoumakos B C, Murakami T, Lumpkin G R, Ewing R C. Science. 1987;236:1556–1559. doi: 10.1126/science.236.4808.1556. [DOI] [PubMed] [Google Scholar]
- 63.Holland H D, Gottfried D. Acta Crystallogr. 1955;8:291–300. [Google Scholar]
- 64.Krogh T. Geotimes. 1995;Nov:20–22. [Google Scholar]
- 65.Sinha K A, Wayne D M, Hewitt D A. Geochim Cosmochim Acta. 1992;56:3551–3560. [Google Scholar]
- 66.Watson E B, Cherniak D J, Hanchar J M, Harrison T M, Wark D A. Chem Geol. 1997;141:19–31. [Google Scholar]
- 67.Lee J K W. In: Defects and Processes in the Solid State: Geoscience Applications, The McLaren Volume. Boland J N, FitzGerald J D, editors. Amsterdam: Elsevier Science; 1993. pp. 423–446. [Google Scholar]
- 68.Lumpkin G R, Ewing R C. Am Mineral. 1996;81:1237–1248. [Google Scholar]
- 69.Cherniak D J, Watson E B. Proc. Annu. Meet. Geol. Soc. Am. abstr.; 1998. [Google Scholar]
- 70.McLaren A C, FitzGerald J D, Williams I S. Geochim Cosmochim Acta. 1994;58:993–1005. [Google Scholar]
- 71.Cherniak D J, Lanford W A, Ryerson F J. Geochim Cosmochim Acta. 1991;55:1663–1673. [Google Scholar]
- 72.Cherniak D J, Hanchar J M, Watson E B. Contrib Mineral Petrol. 1997;127:383–390. [Google Scholar]
- 73.Cherniak D J, Hanchar J M, Watson E B. Chem Geol. 1997;134:289–301. [Google Scholar]
- 74.Tole M P. Geochim Cosmochim Acta. 1985;49:453–458. [Google Scholar]
- 75.Ewing R C, Haaker R F, Lutze W. In: Scientific Basis for Nuclear Waste Management V. Lutze W, editor. Pittsburgh: Materials Research Soc.; 1982. pp. 389–397. [Google Scholar]
- 76.Werme L O, Björner I K, Bart G, Zwicky H-U, Grambow B, Lutze W, Ewing R C, Magrabi C. J Mat Res. 1990;5:1130–1146. [Google Scholar]
- 77.Helean K B. M.S. thesis. Albuquerque: Univ. of New Mexico; 1997. [Google Scholar]
- 78.Helean, K. B., Lutze, W. & Ewing, R. C. (1999) Proc. 100th Annu. Meet. Am. Ceramic Soc., in press.
- 79.Chakoumakos B C, Oliver W C, Lumpkin G R, Ewing R C. Radiat Effects Defects Solids. 1991;118:393–403. [Google Scholar]
- 80.Oliver W C, McCallum J C, Chakoumakos B C, Boatner L A. Radiat Effects Defects Solids. 1994;132:131–141. [Google Scholar]
- 81.Ozkan H. J Appl Phys. 1976;47:4772–4779. [Google Scholar]
- 82.Lee J K W, Tromp J. J Geophys Res. 1995;100:17,753–17,770. [Google Scholar]
- 83.Ewing R C, Weber W J, Clinard F W., Jr Prog Nucl Energy. 1995;29:63–127. [Google Scholar]
- 84.Weber W J, Ewing R C, Catlow C R A, Diaz de la Rubia T, Hobbs L W, Kinoshita C, Matzke H, Motta A T, Nastasi M, Salje E K H, et al. J Mat Res. 1998;13:1434–1484. [Google Scholar]
- 85.Weber W J, Ewing R C, Meldrum A. J Nucl Mat. 1997;250:147–155. [Google Scholar]
- 86.Ewing R C. Nucl Instr Methods Phys Res B. 1994;91:22–29. [Google Scholar]
- 87.Murakami T, Chakoumakos B C, Ewing R C, Lumpkin G R, Weber W J. Am Mineral. 1991;76:1510–1532. [Google Scholar]
- 88.Nasdala L, Pidgeon R T, Wolf D. Geochim Cosmochim Acta. 1996;60:1091–1097. [Google Scholar]
- 89.Farges F, Calas G. Am Mineral. 1991;76:60–73. [Google Scholar]
- 90.Woodhead J A, Rossman G R, Silver L T. Am Mineral. 1991;76:74–82. [Google Scholar]
- 91.Weber W J, Ewing R C, Wang L M. J Mat Res. 1994;9:688–698. [Google Scholar]
- 92.Weber W J, Maupin G D. Nucl Instr Methods Phys Res B. 1988;32:512–515. [Google Scholar]
- 93.Wang L M, Ewing R C, Weber W J, Eby R K. In: Beam-Solid Interactions: Fundamentals and Applications. Nastasi M, Harriott L R, Herbots N, Averback R S, editors. Pittsburgh: Materials Research Soc.; 1993. pp. 451–456. [Google Scholar]
- 94.Ewing R C. In: Scientific Basis for Nuclear Waste Management XVI. Interrante C G, Pabalan R T, editors. Pittsburgh: Materials Research Soc.; 1993. pp. 559–568. [Google Scholar]
- 95.Meldrum A, Boatner L A, Wang L M, Ewing R C. Nucl Instr Methods Phys Res B. 1997;127/128:160–165. [Google Scholar]
- 96.Meldrum A, Boatner L A, Ewing R C. Phys Rev B. 1997;56:13805–13814. [Google Scholar]
- 97.Meldrum, A., Boatner, L. A., Zinkle, S. J., Wang, S. Wang, L. M. & Ewing, R. C. (1999) Can. Mineral., in press.
- 98.Meldrum A, Zinkle S J, Boatner L A, Ewing R C. Nature (London) 1998;395:56–58. [Google Scholar]
- 99.Meldrum, A., Zinkle, S. J., Boatner, L. A. & Ewing, R. C. (1999) Phys. Rev. B, in press.
- 100.Farges F. Phys Chem Miner. 1994;20:504–514. [Google Scholar]
- 101.Meldrum A, Boatner L A, Weber W J, Ewing R C. Geochim Cosmochim Acta. 1998;62:2509–2520. [Google Scholar]
- 102.Ellsworth S, Navrotsky A, Ewing R C. Phys Chem Miner. 1994;21:140–149. [Google Scholar]
- 103.Janeczek J, Ewing R C, Oversby V M, Werme L. J Nucl Mat. 1996;238:121–130. [Google Scholar]
- 104.Burns P C, Finch R J, Hawthorne F C, Miller M L, Ewing R C. J Nucl Mat. 1997;249:199–206. [Google Scholar]