Abstract
Parkinson's disease (PD) is a progressive neurodegenerative disorder which leads to the selective loss of dopaminergic neurons. This causes a decrease in the important neurotransmitter dopamine (DA), which is essential for coordinated movement. Previous studies have implicated the monoamine oxidase metabolite of DA, 3,4-dihydroxphenylacetaldehyde (DOPAL), in the pathogenesis of PD and have shown it to be a reactive intermediate capable of protein modification. DOPAL also has demonstrated the ability to cause mitochondrial dysfunction and lead to significant inhibition of the rate-limiting enzyme in DA synthesis, tyrosine hydroxylase (TH). The current study was undertaken to investigate four analogs of DOPAL, including a novel nitrile analog, to determine how the structure of DOPAL is related to its toxicity and inhibition of TH. Both mitochondrial function and inhibition of TH in cell lysate were investigated. Furthermore, a novel whole cell assay was designed to determine the consequence to enzyme action when DOPAL levels were elevated. The results presented here demonstrate that changes to DOPAL structure lead to a decrease in toxicity and inhibition of enzyme activity as compared to the parent compound. Furthermore, the production of superoxide anion but not hydrogen peroxide increased in the presence of elevated DOPAL. These results reveal the toxicity of DOPAL and demonstrate that both the catechol and aldehyde are required to potently inhibit TH activity.
Keywords: Structure-activity relationship, tyrosine hydroxylase, Parkinson's disease, enzyme inhibition, mitochondrial dysfunction
1. INTRODUCTION
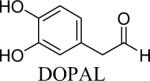
Parkinson's disease (PD) is a neurodegenerative disorder which affects over a million people in the United States. It is marked by the selective loss of dopaminergic neurons in the substantia nigra, leading to a decrease in the important neurotransmitter, dopamine (DA), which necessary for the initiation and execution of coordinated movement (Andersen, 2004; Fleming et al., 2005). Currently, the cause of pathogenesis in PD is unknown, but there is significant evidence that the aldehyde metabolite of DA plays a role in the disease. DA is metabolized by monoamine oxidase (MAO) to 3,4-dihydroxyphenylacetaldehyde (DOPAL), which is further metabolized by aldehyde dehydrogenase (ALDH) to form the acid product 3,4-dihydroxyphenylacetic acid (DOPAC), or in the minor pathway, by aldehyde reductase (AR), to the alcohol product, 3,4-dihydroxyphenylethanol (DOPET). Studies have demonstrated that normal physiologic levels of DOPAL are between 2–3 μM; however, when slightly elevated (~6 μM), significant cell death, protein modification and enzyme inhibition, and an increase in oxidative stress have been shown to occur (Burke, 2003; Burke et al., 2003; Mexas et al., 2011; Rees et al., 2007; Wey et al., 2012).
Previously, our lab has established that DOPAL modifies and inhibits tyrosine hydroxylase (TH, E.C. 1.14.16.2), leading to a decrease in the production of L-DOPA (Mexas et al., 2011). TH catalyzes the rate-limiting step in DA synthesis, oxidizing tyrosine to L-DOPA, which is further metabolized to DA (Elsworth and Roth, 1997; Nagatsu et al., 1964). As previous results indicate, inhibition of TH leads to a detrimental decrease in L-DOPA production. Studies have shown that L-DOPA has neurotrophic properties, and loss of this neurochemical may lead to a decrease in cell viability (Datla et al., 2001; Mena et al., 1997; Murer et al., 1998). In addition, significant mitochondrial dysfunction was exhibited when dopaminergic PC6-3 cells were treated with only 10 μM DOPAL for 2 hrs, further indicating the ability of DOPAL to compromise cell viability (Mexas et al., 2011).
DOPAL is known to modify proteins and is toxic to cells (Helander and Tottmar, 1989; Nilsson and Tottmar, 1987; Rees et al., 2009; Ungar et al., 1973); however, the mechanism of TH inhibition and cytotoxicity are unknown. Rees et al. demonstrated the reaction of DOPAL with N-acetyl-Lys to be dependent upon both the catechol and aldehyde. Changes in the structure of DOPAL lead to a decreased reaction rate with Lys residues (Rees et al., 2009). Based on this knowledge, it is hypothesized that the potent cellular toxicity and inhibition of TH by DOPAL is dependent on the presence of both the catechol and aldehyde. The current study was undertaken to elucidate the structural features of DOPAL necessary for cytotoxicity and enzyme inhibition. It was of interest to fully investigate how structural changes to DOPAL would affect the inhibition of the important enzyme TH, as well as the mitochondrial dysfunction demonstrated by elevation in DOPAL levels. Mitochondrial function was assessed in the presence of elevated DOPAL analogs, and inhibition of TH in cell lysate was determined. A novel whole cell assay using dopaminergic PC6-3 cells was developed to study the inhibition of TH by DOPAL and the analogs intracellularly. Furthermore, the production of reactive oxygen species (ROS) was also determined in the presence of elevated DOPAL. Four analogs of DOPAL, including the DOPAL metabolite 3-hydroxy-4-methoxyphenylacetaldehyde (MOPAL) were employed to reveal the relationship between structure and activity for DOPAL. The work presented here demonstrates DOPAL to be toxic to dopaminergic cells and a potent inhibitor of TH, with structural changes leading to a significant decrease in inhibition and mitochondrial dysfunction. Furthermore, for the first time, the inhibition of TH by DOPAL was examined in PC6-3 cells, with results showing decreased TH activity in the presence of elevated cellular DOPAL.
2. RESULTS
2.1. Structure-Activity Studies of DOPAL Analogs
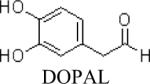
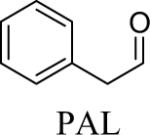
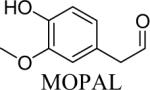
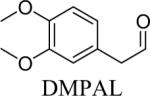
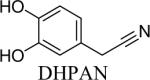
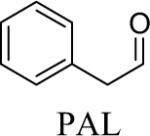
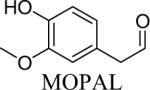
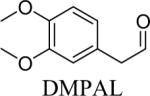
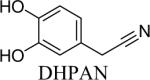
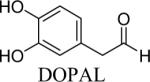
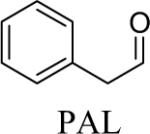
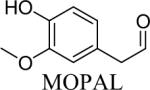
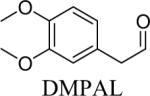
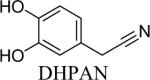
Shown in Table 1 are the structures of each analog, the important feature of DOPAL studied, and the relative protein reactivity as determined by NBT staining. Analogs include removal of the catechol in phenylacetaldehyde (PAL), addition of a single methyl group on the catechol (MOPAL), complete masking of the catechol in DMPAL, and finally, a new analog in which the aldehyde is replaced by a nitrile group to study the importance of the aldehyde in toxicity and inhibition (DHPAN). The results of the NBT studies demonstrate decreased relative protein reactivity when the analogues are used as compared to DOPAL. Of note, the nitrile analogue, DHPAN, showed no significant staining by NBT, indicating it was not significantly modifying BSA compared to DOPAL. Furthermore, PAL displayed 10 times less reactivity and MOPAL and DMPAL show 5 times less reactivity.
Table 1.
Comparison of DOPAL analogs and their relative protein reactivity
| Compound | Reactive Group | Relative Protein Reactivity (Rees et al. 2009) |
|---|---|---|
|
Catechol and aldehyde | 1 |
|
Aldehyde | 0.1a |
|
Aldehyde and monomethyl catechol | 0.2a |
|
Aldehyde and dimethyl catechol | 0.2a |
|
Catechol | NDb |
NaCNBH3 required for protein reactivity and adduct stability.
As described in the methods section 2.4, no protein reactivity with BSA was determined as compared to DOPAL using NBT staining.
2.2. Mitochondrial Dysfunction and DOPAL Analogs
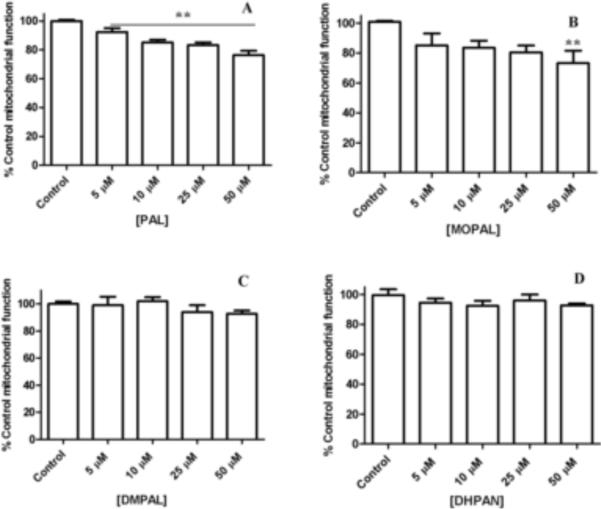
It has been shown that DOPAL leads to significant cell death as compared to controls after just 2 h at the low concentration of 10 μM (Mexas et al., 2011), and the IC50 for DOPAL was calculated to be ~65 μM. The MTT assay was used to assess mitochondrial function in the presence of the DOPAL analogues. PAL and MOPAL treatment yielded some mitochondrial dysfunction as compared to control cells (Figures 1A and B, respectively). Although PAL treatment demonstrated ~15% loss of mitochondrial viability at 10 μM, this is less than that observed for DOPAL at the same concentration (~22%, (Mexas et al., 2011)). Furthermore, while both PAL and MOPAL caused significant mitochondrial dysfunction at 50 μM when compared with control cells (~25%), this is considerably less toxic than DOPAL at 50 μM (~50%, (Mexas et al., 2011)). DMPAL and DHPAN caused no significant loss of mitochondrial viability as compared to controls, with mitochondrial function showing no change even at higher analog concentrations (Figures 1C and D, respectively). These results demonstrate the importance of both the catechol and aldehyde in the toxicity of DOPAL-incubated cells, and show that even slight structural modifications, as in the case of MOPAL, lead to significant changes to the toxic properties of the molecule.
Figure 1.
MTT assay results for PAL (A), MOPAL (B), DMPAL (C), DHPAN (D), respectively. The MTT assay determined mitochondrial dysfunction in the presence of increasing concentrations of PAL, MOPAL, DMPAL, and DHPAN (5, 10, 25, 50 μM) after a 2 h incubation. PAL and MOPAL exhibit some concentration-dependent dysfunction (A and B, respectively), while DMPAL and DHPAN exhibit no changes to mitochondrial function (C and D, respectively). All values shown represent the mean ± SEMs (n = 3). **indicates significance from control cells (i.e. no DOPAL or analogs present).
2.3. Inhibition of TH in Cell Lysate
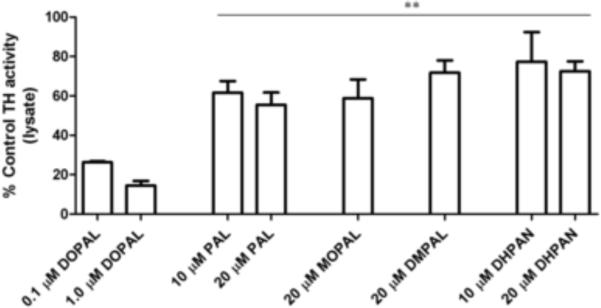
Dopaminergic PC6-3 cell lysate was used to assess TH activity in the presence of analogues first to directly compare to previously published results (Mexas et al., 2011). HPLC analysis of L-DOPA production showed that DOPAL is highly inhibitory of TH activity (Figure 2), leading to almost 95% inhibition at 1.0 μM. These results were modified from previously published results in Mexas et al. in order to demonstrate the potent inhibition. The DOPAL analogs exhibit significantly decreased inhibition of TH activity. 20 μM PAL and MOPAL exhibit only ~50% inhibition of L-DOPA production when compared to controls, and DMPAL and DHPAN lead to only 30–40% inhibition when 10 μM was incubated with lysate. These data show that TH inhibition by DOPAL is also affected by structural changes.
Figure 2.
DOPAL and analogue inhibition of TH in cell lysate. Results expressed in terms of %TH activity as compared to the control (i.e. production of L-DOPA over 2 h). In the case of PAL and DHPAN, 10 and 20 μM were investigated. Only 20 μM was used for MOPAL and DMPAL. All values shown represent the mean ± SEMs (n = 3). **indicates significantly less TH inhibition as compared to DOPAL inhibition in lysate (p 0.05).
2.4. Inhibition of TH in PC6-3 Cells
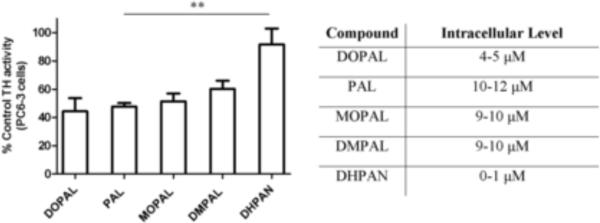
Previously, there have been no studies investigating the functional consequence of DOPAL protein modification in dopaminergic PC6-3 cells. When 5 μM DOPAL was placed exogenously on cells, intracellular concentrations were found to be ~4–5 μM using HPLC analysis (Figure 3, table inset) and TH inhibition was ~60%. A final concentration 20 μM of each analogue was used in cell experiments, and PAL and MOPAL exhibited μ50% inhibition when intracellular concentrations were 9–12 μM. Furthermore, DMPAL-treated cells exhibited only ~40% inhibition when levels were ~9 μM, intracellular. Interestingly, cells incubated with DHPAN showed no significant TH inhibition, and levels of the analogue within the cell were found to be 1 μM or less.
Figure 3.
Inhibition of TH in dopaminergic PC6-3 cells by DOPAL and analogues. Results are expressed in terms of %TH activity as compared to control cells (i.e. no DOPAL or analogs present). 20 μM was used for PAL, MOPAL, DMPAL, and DHPAN. 5 μM was used for DOPAL. Values in table inset are indicative of intracellular concentrations of DOPAL or analogs after HPLC analysis. All values shown represent the mean ± SEMs (n = 3). **indicates significantly less inhibition as compared to DOPAL inhibition in PC6-3 cells (p 0.05).
A summary of the structure-activity relationship studies is in Table 2, where the toxicity and inhibition of TH in both lysate and cells is presented. It can be seen that the structure of DOPAL, specifically the presence of the catechol and aldehyde, leads to significant changes in toxicity and inhibition of TH. These properties are greatly decreased when DOPAL is modified. DHPAN in particular exhibits significantly decreased toxicity and intracellular TH inhibition compared to DOPAL.
Table 2.
Comparison of analog toxicity, and inhibition of TH activity
| Compound | Toxicity at 25 μM (% Cell viability) | TH inhibition (lysate, 20 μM) | TH inhibition (PC6-3 cells) |
|---|---|---|---|
|
70a | ~95%b | ~56% 5 μMd |
|
83 (IC50 >> 1000 μM) | ~44% | ~53% 20 μMd |
|
80 (IC50 > 1000 μM) | ~42% | ~49% 20 μMd |
|
NSc | ~28% | ~35% 20 μMd |
|
NSc | ~27% | NSc 20 μMd |
Based on MTT results from data in (Mexas et al. 2011)
See reference (Mexas et al. 2011).
NS = no significant toxicity or inhibition of TH activity.
Concentration of DOPAL or analogue placed exogenously in PC6-3 cell experiments.
Furthermore, Table 3 summarizes the calculated IC50 values for the inhibition of TH by DOPAL and the analogues. It can be seen that DOPAL exhibits potent inhibition of TH activity in both lysate and PC6-3 cells as compared to the analogues, indicating changes to the structure of DOPAL lead to a great change in the concentration necessary to inhibit the important enzyme TH.
Table 3.
Comparison of IC50 values for inhibition of TH in cell lysate and PC6-3 cells.
| Compound | TH inhibition in cell lysate IC50 | TH inhibition in PC6-3 cells IC50a |
|---|---|---|
|
0.04 μM | ~5 μM |
|
20.1 μM | ~20 μM |
|
30.0 μMb | ~20 μM |
|
30.3 μMb | ~20 μM |
|
37.6 μM | >20 μM |
IC50 values are estimates.
IC50 value based on % control L-DOPA production in cell lysate when 20 or 30 μM MOPAL or DMPAL used (30 μM data not shown).
These results also demonstrate DOPAL inhibits TH within the cell when levels are just slightly elevated from normal, physiologic concentrations of 2–3 μM. These data have implications for the onset and progression of PD which is further discussed below.
2.5 DOPAL Leads to Oxidative Stress
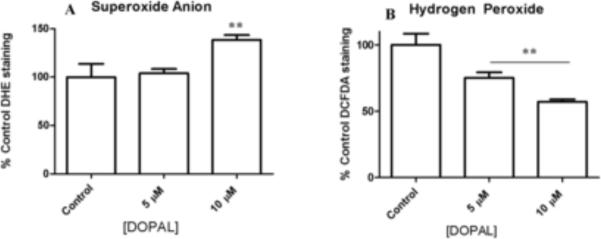
As previously published, low concentrations of DOPAL (10 μM) lead to significant mitochondrial dysfunction and cell death (Mexas et al., 2011). In order to determine possible mechanisms of cell death, the production of ROS was determined using flow cytometry. The data in Figure 4A show an increase in superoxide anion as levels of DOPAL increase, with 10 μM DOPAL leading to superoxide anion concentrations significantly different from control cells after only a 1h DOPAL incubation. These results indicate oxidative stress in the form of superoxide anion production occurs in the cell when DOPAL is elevated.
Figure 4.
Flow cytometry analysis of PC6-3 cells incubated with DOPAL for 1 h to determine levels of superoxide anion and hydrogen peroxide. DHE (A) and DCF-DA (B) were used to probe for superoxide anion and hydrogen peroxide, respectively. Cells were preincubated with each dye for 20 min, and then DOPAL was placed on cells for 1 h prior to flow cytometry analysis. All values shown represent the mean ± SEMs (n = 3). **indicates significantly more ROS production as compared to control cells where no DOPAL was present (p 0.05).
In contrast, cells treated with 5 and 10 μM DOPAL exhibited a decreasing trend in hydrogen peroxide production, and there was significantly less hydrogen peroxide than controls cells (Figure 4B).
3. DISCUSSION
DOPAL, the endogenously produced metabolite of DA, is known to exhibit toxic effects in cells and lead to the potent inhibition of TH in both cell lysate and dopaminergic cells (Mexas et al., 2011). Furthermore, it has been shown that modification of DOPAL structure leads to decreased reactivity with Lys residues, indicating protein reactivity is dependent upon both the catechol and the aldehyde (Rees et al., 2009). This study furthers such results by investigating the importance of these functional groups in the inhibition of a known protein target of DOPAL modification, the enzyme TH. This work also demonstrates the importance of DOPAL structure for cytotoxicity (i.e., mitochondrial dysfunction) and investigates the production of ROS in the presence of elevated DOPAL.
The following order for toxicity and potency in regards to TH inhibition was found: DOPAL ⪢ PAL=MOPAL > DMPAL=DHPAN. While PAL and MOPAL demonstrated some toxicity it was significantly less compared to DOPAL at the same concentrations. For example, PAL treatment yielded ~15% loss of mitochondrial viability as compared to controls at 25 μM, while DOPAL exhibited almost 50% toxicity at this same concentration (Mexas et al., 2011). Furthermore, DMPAL and DHPAN showed no significant toxicity.
The analogues demonstrated significantly less inhibition of TH in both cell lysate and PC6-3 cells as compared to DOPAL. While PAL and MOPAL exhibited similar TH inhibition in PC6-3 cells, a 2-fold higher level was required as compared to DOPAL intracellular concentration. It is important to note that the use of MDA to decrease ALDH and ALR activity did not adversely affect the cell or TH activity as results in our lab and others have previously demonstrated (data not shown, (Stone et al., 1986)).
For the first time, the importance of the aldehyde was studied using an analogue in which a nitrile group replaces the carbonyl (i.e., DHPAN). Results indicate that DHPAN inhibits TH in cell lysate, but such a finding was not observed for PC6-3 cells treated with the nitrile analogue. Intracellular concentrations of DHPAN were very low, indicating that DHPAN did not enter the cell (via transport or diffusion) or was rapidly effluxed. Given the structural similarity of DOPAL and DHPAN, these results have implications for the uptake and trafficking of DOPAL in a cell.
While trafficking of DA and DOPAC is established (Elsworth and Roth, 1997; Lamensdorf et al., 2000; Leviel), it is currently unknown how DOPAL movement is mediated in the cell, although passive diffusion might be expected. When PC6-3 cells are treated exogenously with DOPAL, the acid and alcohol metabolites (i.e., DOPAC and DOPET, respectively) are observed in the extracellular media (Jinsmaa et al., 2009), indicating that DOPAL is taken up into the cell and metabolized. Such a finding is in stark contrast to that for the structurally analogous DHPAN. It is possible that DOPAL uses the DA-transporter, and replacement of the aldehyde with the nitrile group leads to a decrease in recognition of the compound as compared to DOPAL (Mattammal et al., 1995). Furthermore, it is conceivable there is an undiscovered mechanism for DOPAL transport, which facilitates the movement of DOPAL in and out of the cell. In this case, the nitrile analog would be unrecognized by the transporter and would not be able to enter the cell.
It is important to note that the charge state of these analogues at physiologic pH would most likely be neutral; therefore, the issue of charge state would not significantly affect the transport in and out of the cell. The pKa values for substituted catechols, such as DOPAL and analogues, are predicted to be 8.8 or above, and therefore, DOPAL and analogues would be mostly uncharged at pH 7.4 (Falkenburger and Schulz, 2006). Studies are in progress to determine how DOPAL transport is mediated in the cell.
MOPAL, a DOPAL-metabolite generated via catechol-O-methyl transferase activity (Eisenhofer et al., 1995) exhibited toxicity and inhibition of TH. While the toxicity and TH inhibition via MOPAL is significantly less than that for DOPAL, these results indicate the potential for other metabolites of DA to exhibit harmful cellular consequences. It is important to note though, that while MOPAL is generated simultaneously with DOPAL (Eisenhofer et al., 1995) and therefore has the ability to compete with DOPAL for protein binding sites, the results here, as well as previously published data show DOPAL is significantly more protein reactive (Burke, 2003; Mexas et al., 2011; Rees et al., 2009). Furthermore, it is important to note that while the other analogues used in this study (i.e. PAL, DMPAL, and DHPAN) do not occur naturally, they provided important information regarding how the structure of DOPAL leads to potent TH inhibition and mitochondrial dysfunction.
Flow cytometry results in Figures 4A and B demonstrated that increased DOPAL in cells leads to higher levels of superoxide anion (4A) but decreased hydrogen peroxide (4B). While initially unexpected, due to the data showing that elevated DOPAL leads to increased cellular oxidative stress (Burke et al., 2003; Kristal et al., 2001), these results support the hypothesis that DOPAL is inhibiting cellular TH. Previous studies by Kulagina et al. demonstrated that inhibition of TH by the known inhibitor, alpha-methyl-tyrosine, led to a reduction in levels of rat brain hydrogen peroxide due to a decrease in DA metabolism by MAO (Kulagina and Michael, 2003). Our results demonstrate significant inhibition of TH (both in cell lysate and intracellular) by DOPAL leading to a decrease in L-DOPA production, and in PC6-3 cell experiments, a significant decrease in DA production versus controls. Therefore, a decrease in DA metabolism by MAO is predicted, leading to lower levels of the byproduct, hydrogen peroxide. Such findings and predictions agree very well with the flow cytometry results shown in Figure 4B. It could be argued that DOPAL is inhibiting the aromatic amino acid decarboxylase (AADC) step converting L-DOPA to DA; however, it is important to note that while L-DOPA production is inhibited ~60% in PC6-3 cells by DOPAL (Figure 3), the DA concentration only decreased by ~40% as compared to control cells (data not shown). Significant inhibition of both TH and AADC would be predicted to lead to almost complete loss of DA production, instead of only ~40%.
Overall, these results demonstrate that the catechol as well as the aldehyde are necessary to facilitate the harmful cellular effects of DOPAL as cytotoxicity and cellular TH inhibition are greatly diminished when either functional group is modified. Such findings are in line with previous studies demonstrating DA and DOPAL metabolites (i.e., DOPAC, DOPET) exhibit far less toxicity to dopaminergic cells than DOPAL (Burke, 2003). For the first time, the functional consequence of elevated DOPAL in dopaminergic PC6-3 cells was investigated, with results demonstrating significant inhibition of TH when levels were only slightly above normal. As shown in Figure 3, an intracellular concentration of 4–5μM DOPAL in PC6-3 cells leads to ~60% inhibition of TH activity, which is an important finding to note given that normal, physiologic levels of DOPAL have been measured at 2–3 μM (Burke et al., 2003). In addition, others have shown that slight elevations (to ~6 μM) lead to dopaminergic cell death over time (Burke, 2003; Kristal et al., 2001; Mattammal et al., 1995). Taking into account previous and current findings, slight elevations in the intracellular level of DOPAL are predicted to be detrimental to dopaminergic cell viability and protein/enzyme function. While it could be argued that inhibition of TH by DOPAL would lead to decreased DA and therefore DOPAL production, it is important to note that levels of DOPAL can be affected by a number of pathways. There is evidence that MAO activity increases with age, and products of lipid peroxidation can lead to inhibition of ALDH and AR (Andersen, 2004; Jinsmaa et al., 2009; Oreland and Gottfries, 1986). Both situations would lead to an increase in DOPAL production. Furthermore, recent evidence indicates vesicle leakage or decreased vesicular monoamine transporter activity would lead to elevated cytosolic DA, which in turn would cause DOPAL levels to increase (Taylor et al., 2011). These studies demonstrate that elevated DOPAL leads to not only mitochondrial dysfunction, but inhibition of the important enzyme TH, which is essential to the production of DA. Furthermore, studies have demonstrated that elevated DOPAL leads to protein aggregation, another hallmark of PD, indicating another role DOPAL may play in the onset and progression of the disease.
Several questions remain regarding the mechanism via which DOPAL inhibits TH and causes cytotoxicity. While both the aldehyde and catechol influence TH inhibition and cytotoxicity, neither functional group is absolutely required, as demonstrated via the DOPAL analogs. It is conceivable the catechol of DOPAL oxidizes to a quinone, yielding a highly reactive product (Anderson et al., 2011; Kuhn et al., 1999; LaVoie et al., 2005; Xu et al., 1998). Currently, work is underway to map sites of TH modification by DOPAL and elucidate sites of adduction. In summary, the present work demonstrates the significance of the DOPAL structure for its cytotoxicity and TH inhibition. Using dopaminergic PC6-3 cells, elevation of cellular DOPAL levels (4–5 μM) led to inhibition of TH, indicating DOPAL is not only toxic in cells, but capable of modifying and inhibiting important cellular proteins. Structure-activity studies of DOPAL analogs, including a novel nitrile analog, revealed decreased toxicity and ability to inhibit TH activity. New evidence involving the use of flow cytometry to investigate reactive oxygen species production indicated that DOPAL leads to oxidative stress, and provided further confirmation that inhibition of TH occurs when DOPAL is elevated. These results further support the idea that DOPAL is a bifunctional electrophile which requires both the catechol and aldehyde in order to cause potent cellular toxicity. Furthermore, protein modification appears to be hindered upon the loss of either component, indicating DOPAL requires both in its mechanism of protein adduction and toxicity. These results provide both confirmation and insight into the importance of the DOPAL structure in regards to harmful cellular consequences relevant to dopaminergic cells and PD.
4. Experimental Procedure
4.1. Materials
DOPAL was biosynthesized as previously described using enzyme-catalyzed conversion of DA to DOPAL by rat liver MAO (Nilsson and Tottmar, 1987), and the concentration was determined via an ALDH assay (Ungar et al., 1973) and HPLC analysis as described below. Tyrosine, L-DOPA, DA, DOPAC, phenylacetaldehyde, and all other chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise noted. Malondialdehyde (MDA) was obtained by heating 1,1,3,3-tetraethoxypropane in an aqueous solution containing HCl (Esterbauer et al., 1991).
4.2. Cell Culture
PC6-3 cells were cultured in RPMI 1640 medium supplemented with heat-inactived 10% horse serum, 5% fetal bovine serum, penicillin (10 IU/mL) and streptomycin (10 μg/mL). PC6-3 cells assume a neuronal cell phenotype when given nerve growth factor, and act in a similar manner to dopaminergic neurons (Pittman et al., 1993; Seegal et al., 1989; Shafer and Atchison, 1991; Strack, 2002), and are an accepted model of a dopaminergic neuron (Hirata et al., 1998; Kitazawa et al., 2001). PC6-3 cells are a rat pheochromocytoma subline of PC-12 cells and were chosen for a variety of other reasons; including, a decreased tendency to aggregate and less background cell death as compared to the parent cell line. Also, cells were grown in a 10 cm tissue culture dish at 37 °C in 5% CO2 for 3 days. Cells were then seeded into six-well plates (1 × 105) and were incubated at 37 °C in 5% CO2 for 3 days prior to the addition of nerve growth factor (NGF, 50 ng/mL) to stimulate cell differentiation. PC6-3 cells were then incubated in the same conditions for 4 days prior to use. For experiments involving treatment with the four DOPAL analogs, cellular media was removed in order to eliminate any interaction of the compounds with serum proteins and replaced with HEPES-buffered saline (HBS) containing 115 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 0.8 mM MgSO4, 5.5 mM glucose, 1 mM NaH2PO4, and 15 mM HEPES (pH 7.4).
4.3 Synthesis of DOPAL Analogs
3,4-Dimethoxyphenylacetaldehyde (DMPAL) and 3-methoxy-4-hydroxyphenylacetaldehyde (MOPAL) were synthesized as previously described (Rees et al., 2009). Briefly, DMPAL was obtained using an alkene oxidation of eugenol methyl ether (0.096 μL, 5.5 mmol) with 3.5% mol RuCl3, and 4 mol equivalents of NaIO4 (0.4798 g, 5 eq) for 3 hrs in 6:1 acetonitrile:water (20% yield). NMR values were as follows: 1H (CDCl3): δ 3.6 (d, 2H), 5.93 (s, 2H), 6.65–6.82 (m, 3H), 9.71 (t, 1H) (Yang and Zhang, 2001). MOPAL was biosynthesized using a procedure similar to the production of DOPAL, with rat liver MAO being used to convert 3-methoxytyramine to MOPAL (Rees et al., 2009).
3,4-Dihydroxyphenylacetonitrile (DHPAN) was synthesized via the deprotection of 3,4-(methylenedioxy)-phenylacetonitrile (0.109 g) by 1 equivalent of boron tribromide in methylene chloride at −80°C under N2. After stirring for 8 minutes, the solution was allowed to slowly warm to room temperature, and it was left to stir overnight at room temperature under N2 (19% yield). NMR values were as follows: 1H (DMSO-d6): 3.67 (d, 2H), 6.65–6.68 (m, 3H), 8.99 (s, 1H), 9.1 (s, 1H) (Mzengeza, 1988; Nguyen et al., 2009).
4.4 Relative Protein Reactivity
1.1.1 Relative protein reactivity of each compound was determined using BSA
DOPAL, MOPAL, PAL, and DMPAL reactivity was calculated previously (Rees et al., 2009). In order to determine the reactivity for DHPAN, BSA (1 mg/mL) was incubated with either DOPAL (as a positive control, 100 μM), or DHPAN (100 μM) for 4 h at 37°C. Samples (9 μg) were run on SDS-PAGE and transferred to a nitrocellulose membrane (20 V, 40 min). As previously shown, nitroblue tetrazolium (NBT) will stain proteins containing catechol adducts (Akagawa et al., 2006; Paz et al., 1991). The membrane was placed in 0.24 mM NBT in 2 mM potassium glycine buffer (pH 10) and allowed to incubate over night at 4°C. Integration of band density was performed using the NIH program Image J, version 1.37.
2.5 Tyrosine Hydroxylase Isolation and Activity in Cell Lysate and PC6-3 Cells
As previously described in Mexas, et al., cell lysate was collected from PC6-3 cells and assayed for TH activity. Lysate was stored at −80 °C until assays were performed; TH activity is stable in these conditions. (Laschinski et al., 1986). Activity was measured using HPLC to follow the production of L-DOPA over a 2 h time course. Cell lysate experiments were performed for DOPAL analogs in order to directly compare previous data obtained with DOPAL. Furthermore, this was a starting point from which to investigate the effect of the analogs on TH activity without the complication of trafficking or possible metabolism. As previously reported, cell lysate (0.2 mg/mL) was incubated with tyrosine (100 μM), tetrahydrobiopterin (cofactor, 0.25 mM), and the addition of either 10 or 20 μM PAL, MOPAL, DMPAL, or DHPAN. Time points were taken at 0, 30, 60, 90, and 120 min and placed in 5% (v/v) perchloric acid to stop the reaction. All assays were done in 10 mM sodium phosphate buffer (pH 6.8, optimal for TH activity (Gahn and Roskoski, 1993)).
To study the effect of DOPAL and analogs on TH in a whole cell environment, NGF-differentiated dopaminergic PC6-3 cells were preincubated with HBS for 15 min and then 10 μM tyrosine, 5 μM malondialdehyde (MDA, to inhibit ALDH and AR, (Jinsmaa et al., 2009)), and 5 μM of DOPAL were added and incubated for 2 hs. In analog studies, 20 μM of PAL, DMPAL, MOPAL, or DHPAN were added in place of DOPAL. At time points of 0, 30, 60, 90, and 120 min, the supernatant was removed and cells were lysed using 300 μL of 0.1% triton-X in potassium phosphate (pH 7.4). Both supernatant and lysis were analyzed using HPLC as described below for L-DOPA production (and the change in analog or DOPAL over time). It is important to note that MDA does not inhibit or alter TH activity (data not shown), which has also been demonstrated in previous studies (Stone et al., 1986).
4.6 HPLC Analysis of L-DOPA Production
HPLC analysis of L-DOPA production in assays was performed as previously described (Mexas et al., 2011). Briefly, an Agilent 1200 Series Capillary HPLC system with a photodiode array detector measuring absorbance at 202 and 280 nm was used. 15 μL of sample was injected and separated using a Phenomenex Luna C18 column. The mobile phase consisted of 97% 0.1% trifluoroacetic acid (v/v) in HPLC-grade water (A), and 3% acetonitrile (B) for DOPAL studies. For studies using PAL, DMPAL, MOPAL, and DHPAN a gradient was used as follows: 0–12 min: 3% B, 12–13 min: 15% B, 15–20 min: 15% B, 20–35 min: 3% B. Retention times were determined to be: 6.8 (DA), 7.5 (L-DOPA), 10.5 (tyrosine), 11.2 (DOPAL), 22 (PAL), 18 (MOPAL), 21 (DMPAL), and 25 min (DHPAN). Conversion of peak area into concentration units was achieved using a calibration curve of standards.
4.7 Cytotoxicity Assay
To determine the effect on mitochondrial function of the DOPAL analogs in PC6-3 cell cultures, the MTT reduction assay was used as previously described (Mexas et al., 2011). NGF differentiated cells were treated with DOPAL analogs at 0, 5, 10, 25, and 50 μM and incubated for 2 h at 37°C in 5% CO2. MTT reagent was then added (0.5 mg/mL) to each well, and incubated for 2 hrs. The purple crystals were dissolved in DMSO and the formazan product absorbance at 570 nm was read using a Molecular Devices Spectra-Max plate reader. Absorbance values for the analogs were compared to controls.
4.8 Flow Cytometry Analysis
PC6-3 cells were cultured and maintained as described above. Four days after the addition of NGF, cells were washed with HBS (pH 7.4) and pretreated with 2'-7'-Dichlorodihydrofluorescein diacetate (DCF-DA, 50 μM) and dihydroethidium (DHE, 10 μM) at 37°C for 20 min in the dark. Supernatant was then removed, and new HBS was added. DOPAL (5, 10 μM) was added to the cells and incubated for 1 h at 37°C. Cells were then removed from the plate by decanting the supernatant. After spinning for 5 min at 4600 x g, supernatant was removed and cells were resuspended in 1 mL phosphate buffered saline (PBS) in order to wash. Cells were once again pelleted via centrifugation, and then resuspended in 300 μL HBS. Prior to analysis by flow cytometry, cells were filtered using a 70 μm Falcon filter and 4.8 μL Hoechst 33250 stain was added. A Becton Dickeninson LSR II flow cytometer was used with lasers set to 530 and 610 nm.
4.9 Statistical Analysis
All linear regression and statistical analysis were performed using the software GraphPad Prism 5.0 (Graph Pad Software, San Diego, CA). Data for cells treated with DOPAL or the analogs were compared to the controls and significant differences (p < 0.05) were determined using an ANOVA with a Tukey post-test. Data for TH activity and L-DOPA formation was determined using a linear regression. Standard curves were generated for all analogs and used to convert peak area into concentration in whole PC6-3 cell studies.
Highlights
Structure-activity studies were performed on analogues of 3,4-dihydroxyphenylacetaldehyde
This intermediate is a metabolite of dopamine and linked to Parkinson's disease
Tyrosine hydroxylase is potently inhibited by this metabolite
This metabolite leads to high toxicity in dopaminergic cells
Studies show both the catechol and aldehyde are necessary for toxicity and inhibition
Acknowledgements
This work was supported by NIH R01 ES15507 (J.A.D.). We sincerely thank Laurie L. Eckert and Natalie Simmons for providing MOPAL for analog studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest. The authors declare that there are no conflicts of interest.
5. REFERENCES
- Akagawa M, Ishii Y, Ishii T, Shibata T, Yotsu-Yamashita M, Suyama K, Uchida K. Metal-catalyzed oxidation of protein-bound dopamine. Biochemistry. 2006;45:15120–8. doi: 10.1021/bi0614434. [DOI] [PubMed] [Google Scholar]
- Andersen JK. Oxidative stress in neurodegeneration: cause or consequence? Nat Med. 2004;10(Suppl):S18–25. doi: 10.1038/nrn1434. [DOI] [PubMed] [Google Scholar]
- Anderson DG, Mariappan SV, Buettner GR, Doorn JA. Oxidation of 3,4-dihydroxyphenylacetaldehyde, a toxic dopaminergic metabolite, to a semiquinone radical and an ortho-quinone. J Biol Chem. 2011;286:26978–86. doi: 10.1074/jbc.M111.249532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke WJ. 3,4-dihydroxyphenylacetaldehyde: a potential target for neuroprotective therapy in Parkinson's disease. Curr Drug Targets CNS Neurol Disord. 2003;2:143–8. doi: 10.2174/1568007033482913. [DOI] [PubMed] [Google Scholar]
- Burke WJ, Li SW, Williams EA, Nonneman R, Zahm DS. 3,4-Dihydroxyphenylacetaldehyde is the toxic dopamine metabolite in vivo: implications for Parkinson's disease pathogenesis. Brain Res. 2003;989:205–13. doi: 10.1016/s0006-8993(03)03354-7. [DOI] [PubMed] [Google Scholar]
- Datla KP, Blunt SB, Dexter DT. Chronic L-DOPA administration is not toxic to the remaining dopaminergic nigrostriatal neurons, but instead may promote their functional recovery, in rats with partial 6-OHDA or FeCl(3) nigrostriatal lesions. Mov Disord. 2001;16:424–34. doi: 10.1002/mds.1091. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Aneman A, Hooper D, Holmes C, Goldstein DS, Friberg P. Production and metabolism of dopamine and norepinephrine in mesenteric organs and liver of swine. Am J Physiol. 1995;268:G641–9. doi: 10.1152/ajpgi.1995.268.4.G641. [DOI] [PubMed] [Google Scholar]
- Elsworth JD, Roth RH. Dopamine synthesis, uptake, metabolism, and receptors: relevance to gene therapy of Parkinson's disease. Exp Neurol. 1997;144:4–9. doi: 10.1006/exnr.1996.6379. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- Falkenburger BH, Schulz JB. Limitations of cellular models in Parkinson's disease research. J Neural Transm Suppl. 2006:261–8. doi: 10.1007/978-3-211-45295-0_40. [DOI] [PubMed] [Google Scholar]
- Fleming SM, Fernagut PO, Chesselet MF. Genetic mouse models of parkinsonism: strengths and limitations. NeuroRx. 2005;2:495–503. doi: 10.1602/neurorx.2.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahn LG, Roskoski R., Jr. Tyrosine hydroxylase activity and extrinsic fluorescence changes produced by polyanions. Biochem J. 1993;295(Pt 1):189–94. doi: 10.1042/bj2950189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helander A, Tottmar O. Reactions of biogenic aldehydes with hemoglobin. Alcohol. 1989;6:71–5. doi: 10.1016/0741-8329(89)90076-1. [DOI] [PubMed] [Google Scholar]
- Hirata Y, Adachi K, Kiuchi K. Activation of JNK pathway and induction of apoptosis by manganese in PC12 cells. J Neurochem. 1998;71:1607–15. doi: 10.1046/j.1471-4159.1998.71041607.x. [DOI] [PubMed] [Google Scholar]
- Jinsmaa Y, Florang VR, Rees JN, Anderson DG, Strack S, Doorn JA. Products of oxidative stress inhibit aldehyde oxidation and reduction pathways in dopamine catabolism yielding elevated levels of a reactive intermediate. Chem Res Toxicol. 2009;22:835–41. doi: 10.1021/tx800405v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa M, Anantharam V, Kanthasamy AG. Dieldrin-induced oxidative stress and neurochemical changes contribute to apoptopic cell death in dopaminergic cells. Free Radic Biol Med. 2001;31:1473–85. doi: 10.1016/s0891-5849(01)00726-2. [DOI] [PubMed] [Google Scholar]
- Kristal BS, Conway AD, Brown AM, Jain JC, Ulluci PA, Li SW, Burke WJ. Selective dopaminergic vulnerability: 3,4-dihydroxyphenylacetaldehyde targets mitochondria. Free Radic Biol Med. 2001;30:924–31. doi: 10.1016/s0891-5849(01)00484-1. [DOI] [PubMed] [Google Scholar]
- Kuhn DM, Arthur RE, Jr., Thomas DM, Elferink LA. Tyrosine hydroxylase is inactivated by catechol-quinones and converted to a redox-cycling quinoprotein: possible relevance to Parkinson's disease. J Neurochem. 1999;73:1309–17. doi: 10.1046/j.1471-4159.1999.0731309.x. [DOI] [PubMed] [Google Scholar]
- Kulagina NV, Michael AC. Monitoring hydrogen peroxide in the extracellular space of the brain with amperometric microsensors. Anal Chem. 2003;75:4875–81. doi: 10.1021/ac034573g. [DOI] [PubMed] [Google Scholar]
- Lamensdorf I, Hrycyna C, He LP, Nechushtan A, Tjurmina O, Harvey-White J, Eisenhofer G, Rojas E, Kopin IJ. Acidic dopamine metabolites are actively extruded from PC12 cells by a novel sulfonylurea-sensitive transporter. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:654–64. doi: 10.1007/s002100000246. [DOI] [PubMed] [Google Scholar]
- Laschinski G, Kittner B, Brautigam M. Direct inhibition of tyrosine hydroxylase from PC-12 cells by catechol derivatives. Naunyn Schmiedebergs Arch Pharmacol. 1986;332:346–50. doi: 10.1007/BF00500085. [DOI] [PubMed] [Google Scholar]
- LaVoie MJ, Ostaszewski BL, Weihofen A, Schlossmacher MG, Selkoe DJ. Dopamine covalently modifies and functionally inactivates parkin. Nat Med. 2005;11:1214–21. doi: 10.1038/nm1314. [DOI] [PubMed] [Google Scholar]
- Leviel V. Dopamine release mediated by the dopamine transporter, facts and consequences. J Neurochem. 118:475–89. doi: 10.1111/j.1471-4159.2011.07335.x. [DOI] [PubMed] [Google Scholar]
- Mattammal MB, Haring JH, Chung HD, Raghu G, Strong R. An endogenous dopaminergic neurotoxin: implication for Parkinson's disease. Neurodegeneration. 1995;4:271–81. doi: 10.1016/1055-8330(95)90016-0. [DOI] [PubMed] [Google Scholar]
- Mena MA, Davila V, Sulzer D. Neurotrophic effects of L-DOPA in postnatal midbrain dopamine neuron/cortical astrocyte cocultures. J Neurochem. 1997;69:1398–408. doi: 10.1046/j.1471-4159.1997.69041398.x. [DOI] [PubMed] [Google Scholar]
- Mexas LM, Florang VR, Doorn JA. Inhibition and covalent modification of tyrosine hydroxylase by 3,4-dihydroxyphenylacetaldehyde, a toxic dopamine metabolite. Neurotoxicology. 2011;32:471–7. doi: 10.1016/j.neuro.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murer MG, Dziewczapolski G, Menalled LB, Garcia MC, Agid Y, Gershanik O, Raisman-Vozari R. Chronic levodopa is not toxic for remaining dopamine neurons, but instead promotes their recovery, in rats with moderate nigrostriatal lesions. Ann Neurol. 1998;43:561–75. doi: 10.1002/ana.410430504. [DOI] [PubMed] [Google Scholar]
- Mzengeza S, Whitney RA. Asymmetric Induction in Nitrone Cycloadditions. J. Org. Chem. 1988;53:4074–4081. [Google Scholar]
- Nagatsu T, Levitt M, Udenfriend S. Conversion of L-tyrosine to 3,4-dihydroxyphenylalanine by cell-free preparations of brain and sympathetically innervated tissues. Biochem Biophys Res Commun. 1964;14:543–9. doi: 10.1016/0006-291x(64)90266-9. [DOI] [PubMed] [Google Scholar]
- Nguyen NH, Cougnon C, Gohier F. Deprotection of arenediazonium tetrafluoroborate ethers with BBr3. J Org Chem. 2009;74:3955–7. doi: 10.1021/jo8027906. [DOI] [PubMed] [Google Scholar]
- Nilsson GE, Tottmar O. Biogenic aldehydes in brain: on their preparation and reactions with rat brain tissue. J Neurochem. 1987;48:1566–72. doi: 10.1111/j.1471-4159.1987.tb05702.x. [DOI] [PubMed] [Google Scholar]
- Oreland L, Gottfries CG. Brain and brain monoamine oxidase in aging and in dementia of Alzheimer's type. Prog Neuropsychopharmacol Biol Psychiatry. 1986;10:533–40. doi: 10.1016/0278-5846(86)90023-0. [DOI] [PubMed] [Google Scholar]
- Paz MA, Fluckiger R, Boak A, Kagan HM, Gallop PM. Specific detection of quinoproteins by redox-cycling staining. J Biol Chem. 1991;266:689–92. [PubMed] [Google Scholar]
- Pittman RN, Wang S, DiBenedetto AJ, Mills JC. A system for characterizing cellular and molecular events in programmed neuronal cell death. J Neurosci. 1993;13:3669–80. doi: 10.1523/JNEUROSCI.13-09-03669.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees JN, Florang VR, Anderson DG, Doorn JA. Lipid peroxidation products inhibit dopamine catabolism yielding aberrant levels of a reactive intermediate. Chem Res Toxicol. 2007;20:1536–42. doi: 10.1021/tx700248y. [DOI] [PubMed] [Google Scholar]
- Rees JN, Florang VR, Eckert LL, Doorn JA. Protein reactivity of 3,4-dihydroxyphenylacetaldehyde, a toxic dopamine metabolite, is dependent on both the aldehyde and the catechol. Chem Res Toxicol. 2009;22:1256–63. doi: 10.1021/tx9000557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seegal RF, Brosch K, Bush B, Ritz M, Shain W. Effects of Aroclor 1254 on dopamine and norepinephrine concentrations in pheochromocytoma (PC-12) cells. Neurotoxicology. 1989;10:757–64. [PubMed] [Google Scholar]
- Shafer TJ, Atchison WD. Transmitter, ion channel and receptor properties of pheochromocytoma (PC12) cells: a model for neurotoxicological studies. Neurotoxicology. 1991;12:473–92. [PubMed] [Google Scholar]
- Stone DM, Stahl DC, Hanson GR, Gibb JW. The effects of 3,4-methylenedioxymethamphetamine (MDMA) and 3,4-methylenedioxyamphetamine (MDA) on monoaminergic systems in the rat brain. Eur J Pharmacol. 1986;128:41–8. doi: 10.1016/0014-2999(86)90555-8. [DOI] [PubMed] [Google Scholar]
- Strack S. Overexpression of the protein phosphatase 2A regulatory subunit Bgamma promotes neuronal differentiation by activating the MAP kinase (MAPK) cascade. J Biol Chem. 2002;277:41525–32. doi: 10.1074/jbc.M203767200. [DOI] [PubMed] [Google Scholar]
- Taylor TN, Caudle WM, Miller GW. VMAT2-Deficient Mice Display Nigral and Extranigral Pathology and Motor and Nonmotor Symptoms of Parkinson's Disease. Parkinsons Dis. 2011;2011:124165. doi: 10.4061/2011/124165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungar F, Tabakoff B, Alivisatos SG. Inhibition of binding of aldehydes of biogenic amines in tissues. Biochem Pharmacol. 1973;22:1905–13. doi: 10.1016/0006-2952(73)90050-6. [DOI] [PubMed] [Google Scholar]
- Wey MC, Fernandez E, Martinez PA, Sullivan P, Goldstein DS, Strong R. Neurodegeneration and motor dysfunction in mice lacking cytosolic and mitochondrial aldehyde dehydrogenases: implications for Parkinson's disease. PLoS One. 2012;7:e31522. doi: 10.1371/journal.pone.0031522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Stokes AH, Roskoski R, Jr., Vrana KE. Dopamine, in the presence of tyrosinase, covalently modifies and inactivates tyrosine hydroxylase. J Neurosci Res. 1998;54:691–7. doi: 10.1002/(SICI)1097-4547(19981201)54:5<691::AID-JNR14>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Yang D, Zhang C. Ruthenium-catalyzed oxidative cleavage of olefins to aldehydes. J Org Chem. 2001;66:4814–8. doi: 10.1021/jo010122p. [DOI] [PubMed] [Google Scholar]