Abstract
Objectives
Individuals with mild cognitive impairment (MCI) are at high risk of developing dementia and/or Alzheimer’s disease (AD). Among persons with MCI, depression and anxiety have been associated with an increased risk of incident dementia. We examined whether neuropsychiatric symptoms in MCI increased the risk of incident dementia (all-cause) and incident AD.
Design
Longitudinal cohort study followed annually (median 1.58 years)
Setting
National Alzheimer’s Coordinating Center (NACC) database combining clinical data from 29 Alzheimer’s Disease Centers (ADCs).
Participants
1821 participants with MCI
Measurements
1) Progression to dementia (all-cause) or AD, 2) Neuropsychiatric Inventory Questionnaire (NPI-Q), 3) Geriatric Depression Scale (GDS), 4) Clinical Dementia Rating Global Score and Sum of Boxes (CDR-Sum) 5) Mini-Mental State Exam (MMSE). The association of covariates with risk of incident dementia or AD was evaluated with hazard ratios (HR) determined by Cox proportional-hazards models adjusted for age, ethnicity, CDR-Sum, and MMSE.
Results
527 participants (28.9%) progressed to dementia and 454 (24.9%) to AD. Baseline GDS>0 was associated with increased risk of incident dementia (HR 1.47, 95% CI 1.17, 1.84) and AD (HR 1.45, 95% CI 1.14, 1.83). Baseline NPI>0 was associated with increased risk of incident dementia (HR 1.37. 95% CI 1.12, 1.66) and AD (HR 1.35,95% CI 1.09, 1.66).
Conclusions
Neuropsychiatric symptoms in MCI are associated with significantly increased risk of incident dementia and AD. Neuropsychiatric symptoms may be among the earliest symptoms of preclinical stages of AD and targeting them therapeutically might delay transition to dementia.
Background
Alzheimer’s disease (AD) is a growing public health problem, with an estimated prevalence of 5.3 million in the U.S. alone (33 million worldwide), that is expected to increase to 11 to 16 million by the year 2050 (over 115 million worldwide) (1). There is considerable pathologic and clinical evidence that the neuropathologic processes of AD start years before functional decline (2) and that identification of prodromal states of AD will be crucial to future disease interventions. The most widely studied prodromal state is mild cognitive impairment (MCI), in which patients have subjective complaints and/or objective evidence of memory deficits in the absence of significant functional decline(3). Given that individuals with MCI are a target population for preventive intervention, it is important to identify clinical variables that can be used to predict MCI prognosis. Several variables or biomarkers have been associated with an increased risk of progression from MCI to AD, including cognitive performance(4), CSF or imaging markers of amyloid deposition (5, 6), and magnetic resonance imaging markers of neuronal loss(7).
Neuropsychiatric symptoms (NPS), particularly depression, anxiety, agitation, irritability, and apathy, are more prevalent and severe in AD than in cognitively normal older adults(8). NPS are also more prevalent and severe in MCI than in cognitively intact older adults, but less so than in AD (9) Using the database of the National Alzheimer’s Coordinating Center (NACC) to assess NPS in a large MCI cohort, we recently reported that NPS are common in MCI and associated with executive dysfunction (10).
Given the high prevalence of NPS in AD and MCI, it is possible that NPS are predictors of progression from MCI to AD. There are prior reports linking NPS with increased risk of progression from MCI to AD including depression (11), apathy(12, 13), anxiety (14, 15), and the presence of any NPS (11). The added risk can be substantial: for example, in one study the presence of apathy increased the risk of incident dementia seven-fold (13). Given this evidence we hypothesized that NPS (particularly affective symptoms such as depression) would increase the risk of progression to dementia or AD. To test this hypothesis, we examined the association between NPS and incident dementia and AD in a large well-characterized MCI cohort.
Methods
The National Alzheimer’s Coordinating Center (NACC) is responsible for developing and maintaining a database combining the data collected at the 29 Alzheimer’s Disease Centers (ADCs) funded by the National Institute on Aging. The NACC database has been operational since 2000. In 2002 NACC expanded its efforts into a fully integrated data system, the Uniform Data Set (UDS) (16) which is available to investigators in the field for analytic projects. The UDS is a dataset incorporating the clinical, neuropsychological, and diagnostic results of the ADC assessments and the UDS methods have been previously published (17).
Protocol Approvals, Registrations, and Patient Consents
All participants or their legally authorized representatives signed informed consent prior to participation. The Institutional Review Board overseeing each ADC approved local procedures.
MCI and dementia diagnoses
Participants were assigned a diagnosis at each examination adjudicated by an experienced clinician or an interdisciplinary team (17), based on information from medical, neurologic, and psychiatric history, neuropsychological assessment, and the Clinical Dementia Rating Scale (18). The UDS neuropsychological assessment was used to inform the diagnostic process, but diagnoses were made clinically and not on the basis of strict cutoffs on neuropsychologic tests. The NACC battery includes the Mini-Mental State Exam (19), Wechsler Memory Scale-Revised Logical Memory IA and IIA (20), Digit Span Forward and Backward (20), Category Fluency (animals and vegetables) (21), Boston Naming Test (22), WAIS-R Digit Symbol (23) Trail Making Test Parts A and B (24). Participants were assigned diagnoses of 1) “cognitively normal” if they had normal cognition and lacked evidence of MCI, dementia or other neurologic condition resulting in cognitive impairment, ; 2) “demented” if the participant met standard criteria for dementia of the Alzheimer’s type or for other non-Alzheimer’s dementing disorders; 3) “MCI” if the participant lacked not have normal cognition and was not clinically demented.
Definition of Dementia subtypes
At each visit, participants were first diagnosed as “normal cognition”, “MCI”, or “dementia”. Those participants diagnosed with “dementia” were further subtyped into the specific etiologies “probable” AD, or “possible” AD. For the purposes of improving statistical power we chose to combine “probable” and “possible” AD into “AD”. These two outcomes (dementia and AD) constitute “failure” outcomes in the survival analyses described below. The interdisciplinary team had the option of assigning many other dementia diagnoses of lesser prevalence (i.e., vascular dementia, frontotemporal dementia, progressive supranuclear palsy, multi-system atrophy, etc.). Given the low prevalence of these uncommon dementia diagnoses we did not consider these individually in our analyses, but classified them as “demented”.
Clinical variables
Clinical Dementia Rating (CDR) (18)
CDR is the most widely used rating of global function in dementia and MCI. CDR is performed via a semi-structured interview and it has excellent reliability and validity (25–28). CDR uses a 4 to 5-point scale to characterize six domains of cognitive and functional performance: memory, orientation, judgment, community, hobbies, and personal care. Each domain is rated (0=no impairment, 0.5=questionable impairment, 1=mild impairment, 2=moderate impairment, 3=severe impairment), with the exception of personal care which omits the questionable impairment category; the descriptors in the UDS differ from the original CDR in which 0.5 = very mild dementia, 1 = mild dementia, etc. A global score (CDR-Global) is created using a predefined algorithm (18). The CDR-Sum of Boxes (CDR-Sum) is the sum of individual ratings in each of the six domains, with a range of 0 (no impairment) to 30 (maximum impairment in all domains). Both CDR-Global and CDR-Sum were examined as covariates in the analyses. CDR-Sum has demonstrated sensitivity to functional changes within MCI and AD (29).
Neuropsychiatric Inventory Questionnaire (NPI-Q) (30, 31)
The Neuropsychiatric Inventory (NPI) is a widely used measure of NPS in dementia research that assesses the type and severity of NPS in people with memory disorders. NPI-Q surveys 12 domains: agitation, delusions, hallucinations, depression, euphoria, aberrant motor behavior, apathy, irritability, disinhibition, anxiety, sleep, and eating. It is administered as a structured interview with a knowledgeable informant who can report the patient’s NPS. NPI-Q is a simplified clinical version of the NPI, with two scores reported for each domain: a) presence of symptoms; and b) severity on a 0–3 scale (0= none, 1=mild, 2=moderate, 3=severe). We report severity for each NPI-Q symptom domain, and further calculated the sum referred to below as NPI-Q Total (range 0–36). Unlike the NPI (30) the frequency of symptoms is not assessed on NPI-Q,(31).
Geriatric Depression Scale (GDS), short-form (32)
GDS (short-form) is a 15-item scale in which 15 statements are endorsed “yes” or “no” by the participant. The statements include those for which “yes” reflects a depressed response and those for which “no” reflects a depressed response. The number of depressed responses is summed and range from 0–15. GDS (short-form) has good 11 reliability in older populations (33) and MCI (34)with a cutoff score of 4 providing good sensitivity and specificity for diagnosis of depression (33).
Hachinski Ischemic Score (35)
The Hachinski Ischemic Score was devised to differentiate vascular dementia from degenerative dementia (largely AD). The scale rates eight items associated with vascular dementia including abrupt onset, stepwise progression, emotional incontinence, history of stroke or hypertension, and focal neurologic signs and symptoms. The range is 0–12; scores of 4 or less are consistent with primary degenerative dementias, scores of >=7 with vascular dementias, while scores of 5–6 are considered equivocal.
Statistical Analyses
T-tests (for continuous variables) and χ2 tests (for categorical variables) were used to assess baseline differences between the MCI cases with at least one follow-up and those without a follow-up, as well as those with incident dementia or AD compared to those who did not develop dementia over the follow-up. The analyses of AD excluded non-AD dementias and compared participants who developed AD with those who did not develop any dementia over the follow-up period. Cox proportional hazards models were used to estimate the hazard ratio (HR) for incident dementia and AD. Years at risk were calculated from baseline to the last follow-up (i.e., censoring) or a diagnosis of dementia and AD (i.e., “failure”). To determine which covariates to include in the models, hazard ratios for individual demographic and cognitive variables were calculated including: age in years (continuous variable), race (categorically defined as African-American, Caucasian, other), Hispanic (categorically defined as Hispanic, non-Hispanic, other), education in years (continuous variable), Mini-Mental State Exam score (continuous variable), CDR-Global or CDR-Sum (continuous variables), and Hachinski Ischemic Score (continuous variable). Those covariates with statistically significant HRs were included in subsequent analyses. Neuropsychiatric variables included NPI-Q Total, GDS, and individual NPI-Q symptom domains. Since both NPI-Q Total and GDS were skewed towards zero they were divided into approximately equal tertiles, and additionally categorized as any symptom absent (i.e., NPI-Total = 0) or present (NPI-Total > 0). The a priori threshold for statistical significance was set at p<.05. Holm’s procedure (36) was used to adjust for multiple comparisons. We included CDR-Sum in multivariate models because it is highly correlated with NPI-Q and thus a potential confound. Since CDR-Global and CDR-Sum were highly correlated, we chose to include only CDR-Sum in multivariate models due to its greater range and abovementioned sensitivity to change. The assumption of proportional hazards was tested using the methods of Grambsch and Thernau (37). All analyses were conducted using STATA Version 10.0 (StataCorp, College Station, TX).
Results
Demographics
Of the 3692 NACC participants diagnosed with MCI, 1821 (49%) had at least one follow-up visit and are included in the present analyses. MCI participants with no follow-up visits were younger (mean 74.4 vs. 75.4 years, t=−2.94, df=3690, p=0.003), more likely to be Hispanic (9.4% vs. 6.3%, χ2(1)= 12.08 P= 0.001) or African-American (18.6% vs. 14.4%, χ2(1)= 10.88, p=0.001), and more likely to be female (55.6 % vs. 50.5%, χ2(1) = 9.70, p=0.002) but did not differ on education or MMSE. Demographic variables for the 1821 participants are further described in Table 1. On average, MCI participants were in their mid-70s, had 3 years of college education, and 21% were African-American or Hispanic. The median (25th, 75th percentile) duration of follow-up for all participants was 1.58 (1.08, 2.09) years and mean (SD) number of followup visits was 1.50 (0.62). 527 (28.9%) progressed to dementia after a mean (SD) follow-up of 1.17 (0.33) years. 454 participants (24.9%) progressed to AD after a mean (SD) follow-up of 1.16 (0.32) years. Participants who progressed to dementia were older and less likely to be African-American or Hispanic, had lower baseline MMSE scores, or higher CDR-Global and CDR-Sum scores (Table 1).
TABLE 1. DEMOGRAPHIC VARIABLES.
Means (SDs) are presented for continuous demographic variables, and prevalence (%) reported for dichotomous variables for 1821 NACC participants with MCI and at least one follow up visit. Means of continuous variables compared between participants with and without incident dementia with Student’s t-test, while comparisons of dichotomous variables were made using χ2 variable. p<.05 was used as the threshold for statistical significance.
| Variable | All participants with baseline MCI (N=1821) | Participants with baseline MCI without incident dementia (N= 1294) | Participants with baseline MCI with incident dementia (N= 527) | Statistic |
|---|---|---|---|---|
| Age (yrs) | 75.3 (9.3) | 74.8 (9.0) | 76.5 (9.9) | t=−3.67,df=1819, p<.001 |
| Education (yrs) | 15.2 (6.2) | 15.2 (6.3) | 15.4 (6.1) | t=−.65, df=1819, p=.51 |
| African-American | 13.8% | 16.3% | 9.9% | χ2(1)=12.02, p=.001 |
| Hispanic | 6.3% | 7.2% | 4.0% | χ2(1)=6.61, p=.01 |
| Female | 50.5% | 51.6% | 47.8% | χ2(1)=2.17, p=.14 |
| MMSE | 27.1 (2.5) | 28.7 (9.6) | 27.5 (10.0) | t=2.31, df=1819, p=.02 |
| CDR-Global | .45 (.19) | .43 (.20) | .51 (.17) | t=−8.91, df=1819, p<.001 |
| CDR-Sum | 1.38 (1.19) | 1.1 (1.0) | 2.0 (1.4) | t=−14.1, df=1819, p<.001 |
| Hachinski Ischemic Score* | 1.06 (1.46) | 1.0 (1.4) | .91 (1.1) | t=1.06, df=1792, p=.29 |
Due to missing data, the N for Hachinski ischemic score was 1776 (1276 participants without incident dementia, 520 participants with incident dementia).
At baseline, NPI-Q Total and GDS symptoms were associated with higher CDR-Sum. Participants with baseline NPI-Q Total>0, had a higher baseline CDR-Sum than those with NPI-Q Total = 0(mean (SD) of 1.68 (1.25) vs. 1.00 (.98)). Participants with baseline GDS >0, had a higher baseline CDR-Sum than those with GDS = 0 (mean (SD) of 1.46 (1.17) vs. 1.11 (1.19)). Baseline NPI-Q Total and CDR-Sum were highly correlated (Spearman’s r = 0.38, p<.001).
Clinical variables associated with risk of dementia and AD
527 participants (29.0%) were diagnosed with incident dementia and 454 (24.9%) with incident AD. Older age and lower MMSE were associated with increased risk of incident dementia, while African-American and Hispanic ethnicity were associated with decreased risk of incident dementia (Table 2). Older age, Hispanic ethnicity, and lower MMSE were similarly associated with increased risk of incident AD. Higher baseline CDR-Global, CDR-Sum, and NPI-Q Total were associated with an increased risk of dementia and AD (Table 2). Given these results, baseline CDR-Sum, age, African-American and Hispanic ethnicity, and baseline MMSE were included as covariates in subsequent analyses.
TABLE 2. RISK OF DEMENTIA OUTCOMES: UNIVARIATE ANALYSES OF CLINICAL VARIABLES.
The risk of incident dementia and AD in the 1821 MCI participants with at least one followup was estimated with Cox proportional hazards regression models. The N for MMSE was 1787 due to missing baseline data. Hazard ratios (HRs) with 95% confidence intervals (CI) are reported for the associations of each variable with incident dementia and AD. z-tests were used to test statistical significance.
| Variable | MCI participants who progressed to | |||||
|---|---|---|---|---|---|---|
| Dementia (N=527) | Alzheimer's (N=454) | |||||
| HR | 95% CI | p | HR | 95% CI | p | |
| Age (yrs) | 1.02 | 1.01, 1.03 | <.001* | 1.03 | 1.02, 1.05 | <.001* |
| Education (yrs) | 1.01 | .98, 1.04 | 0.281 | 1.02 | .99, 1.02 | 0.154 |
| African- American | 0.62 | .47, .83 | 0.001* | 0.63 | .45, .86 | 0.004* |
| Hispanic | 0.58 | .37, .89 | 0.013* | 0.71 | .46, 1.10 | 0.127 |
| Female | 0.90 | .76, 1.07 | 0.228 | 0.92 | .76, 1.10 | 0.38 |
| MMSE | 0.87 | .85, .90 | <.001* | 0.85 | .83, .88 | <.001* |
| CDR-Global | 10.06 | 6.19, 16.35 | <.001* | 9.63 | 5.7, 16.4 | <.001* |
| CDR-Sum | 1.49 | 1.42, 1.57 | <.001* | 1.47 | 1.40, 1.56 | <.001* |
| Hachinski Ischemic Score | 1.05 | .99, 1.10 | 0.099 | 0.98 | .92, 1.05 | 0.611 |
The threshold for statistical significance was p<.05 and Holm’s procedure was used to adjust for multiple comparisons. Significant associations are marked with *.
NPI-Q Total and GDS predict dementia incidence
The risk of incident dementia by NPI-Q Total and GDS tertiles is shown in Table 3. Of the original 1821 participants 1787 (98.1%) had complete baseline data for these multivariate analyses (34 lacked baseline MMSE scores). The middle and highest NPI-Q Total tertiles were associated with an increased risk of dementia and AD compared to the lowest tertile, with no evidence of a dose-response for incident dementia or AD (i.e., the highest tertile was associated with a similar risk as the middle tertile). The highest two GDS tertiles were similarly associated with an increased risk of incident dementia but not AD. Baseline NPI-Q Total ≥ 4 and baseline GDS ≥ 4 were not associated with increased incidence of dementia and AD (data not shown).
TABLE 3. RISK OF DEMENTIA OUTCOMES: NPI AND GDS.
The risk of incident dementia and AD in the 1787 MCI participants with complete baseline data and at least one follow-up visitwas estimated with Cox proportional hazards regression models. Hazard ratios (HRs) with 95% confidence intervals (CI) are reported for the associations of NPI and GDS with incident dementia and AD. Data are presented for NPI-Q total and GDS divided into approximately equal tertiles, and additionally for the presence or absence of any NPI-Q or GDS symptoms at baseline. Models are adjusted for baseline CDR-Sum, age, African-American race, Hispanic ethnicity, and MMSE. z-tests were used to test statistical significance.
| Variable | MCI participants who progressed to | |||||||
|---|---|---|---|---|---|---|---|---|
| Dementia (N=527) | Alzheimer's (N=454) | |||||||
| N | Range | HR | 95% CI | p | HR | 95% CI | p | |
| NPI-Q Total (bottom tertile) | 813 (45%) | NPI-Q Total = 0 | 1.00 (reference) | 1.00 | ||||
| NPI-Q Total (middle tertile) | 488 (27%) | NPI-Q Total = 1–2 | 1.43 | 1.15,1.80 | 0.002* | 1.48 | 1.17, 1.88 | 0.001* |
| NPI-Q Total (upper tertile) | 520 (29%) | NPI-Q Total > 3 (3–31) | 1.49 | 1.19, 1.86 | <.001* | 1.36 | 1.07, 1.74 | 0.013* |
| GDS (bottom tertile) | 853 (48%) | GDS = 0–1 | 1.00 | 1.00 | ||||
| GDS (middle tertile) | 508 (29%) | GDS = 2–3 | 1.35 | 1.11, 1.67 | 0.003* | 1.25 | 1.00, 1.56 | 0.049 |
| GDS (upper tertile) | 408 (23%) | GDS >4 (4–88) | 1.37 | 1.10, 1.71 | 0.005* | 1.17 | .92, 1.50 | 0.207 |
No models violated Cox proportional-hazards assum ptions. The threshold for statistical significance was p<.05 and Holm’s procedure was used to adjust for multiple comparisons. Significant associations are marked with *.
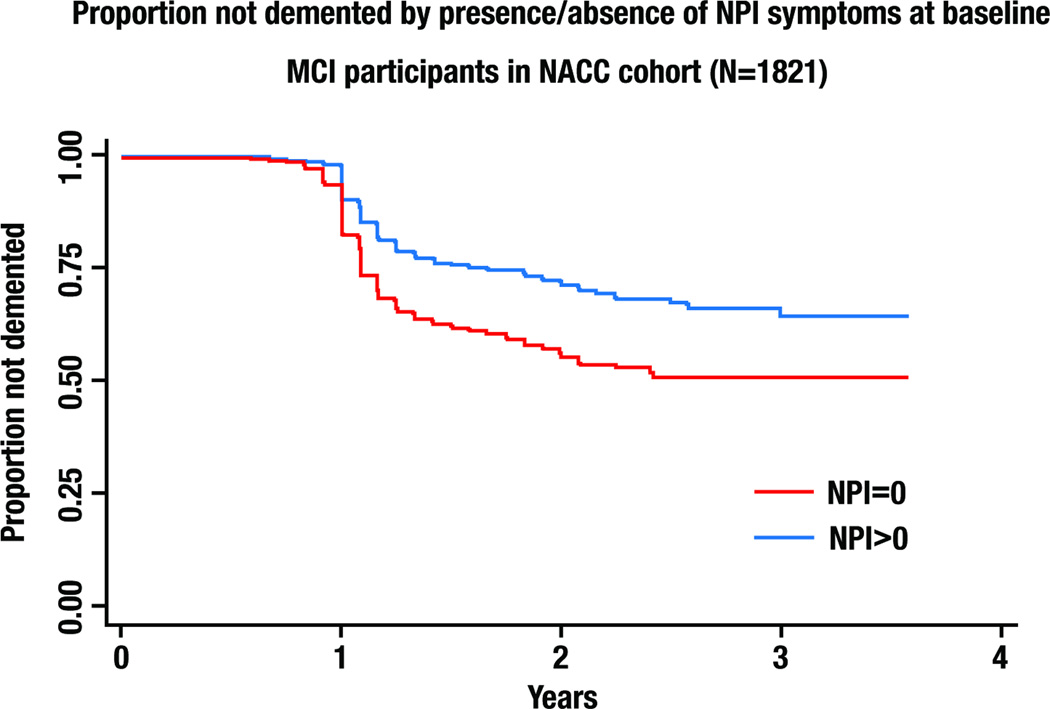
Multivariable models examining NPS (GDS or NPI-Q) as predictors of incident dementia were adjusted for baseline CDR-Sum, age, African-American race, Hispanic ethnicity, and MMSE (Table 4). Higher MMSE, lower CDR-Sum, African-American race and Hispanic ethnicity were associated with a lower risk of incident dementia. The presence of any NPI-Q or GDS symptom was associated with significantly higher risk of incident dementia and AD (Table 4). Figure 1 illustrates visually one of these associations, displaying the Kaplan-Meier survival curve for incident dementia stratified by the presence or absence of NPI-Q >0 vs. NPI-Q=0 at baseline.
TABLE 4. RISK OF DEMENTIA OUTCOMES: MULTIVARIATE MODELS.
The risk of incident dementia and AD in the 1787 MCI participants with complete baseline data and at least one follow-up visit was estimated with Cox proportional hazards regression models. Hazard ratios (HRs) with 95% confidence intervals (CI) are reported for the associations of MCI subtypes with incident dementia and AD. z-tests were used to test statistical significance. No models violated Cox proportional-hazards assumptions.
| Variable | MCI participants who progressed to | |||||
|---|---|---|---|---|---|---|
| Dementia (N=527) | Alzheimer's (N=454) | |||||
| HR | 95% CI | p | HR | 95% CI | p | |
| NPI-Q Total (bottom tertile) | 1.0 (reference) | 1.0 (reference) | ||||
| NPI-Q Total (middle tertile) | 1.41 | 1.12, 1.76 | 0.003* | 1.47 | 1.16, 1.87 | 0.002* |
| NPI-Q Total (upper tertile) | 1.41 | 1.12, 1.77 | 0.003* | 1.35 | 1.05, 1.73 | 0.017* |
| GDS (bottom tertile) | 1.0 (reference) | 1.0 (reference) | ||||
| GDS (middle tertile) | 1.30 | 1.06, 1.60 | 0.012* | 1.21 | .97, 1.50 | 0.099 |
| GDS (upper tertile) | 1.30 | 1.04, 1.62 | 0.023* | 1.11 | 0.87, 1.42 | 0.412 |
| CDR-Sum | 1.38 | 1.29, 1.47 | <.001* | 1.33 | 1.24, 1.42 | <.001* |
| Age (years) | 1.02 | 1.01, 1.03 | <.001* | 1.03 | 1.02, 1.04 | <.001* |
| African-American | 0.74 | 0.55, 0.99 | 0.046* | 0.75 | 0.54, 1.04 | 0.092 |
| Hispanic | 0.34 | 0.21, 0.62 | <.001* | 0.51 | 0.29, 0.90 | 0.021* |
| MMSE | 0.90 | 0.87, 0.93 | <.001* | 0.89 | 0.86, 0.93 | <.001* |
The threshold for statistical significance was p<.05 and Holm’s procedure was used to adjust for multiple comparisons. Significant associations are marked with *.
FIGURE 1. RISK OF INCIDENT DEMENTIA STRATIFIED BY PRESENCE/ABSENCE OF NPI SYMPTOMS AT BASELINE.
Kaplan-Meier survival curves for risk of incident dementia in the 1821 MCI participants with at least one follow-up are displayed, stratified by baseline NPI=0 vs. NPI>=1. Statistics are reported in Table 3.
Analysis of individual NPI-Q domains revealed a substantial prevalence of symptoms (i.e., many domains with NPI-Q >0) but relatively low severity with the range of mean severities being 0.025 to .035 on a scale of 0–3 (Table 5a). We additionally observed significant associations of several domains with incident dementia and AD (Table 5a). However, these associations were no longer observed after CDR-Sum was added as a covariate (Table 5b).
TABLE 5.
| TABLE 5a | ||||||||
|---|---|---|---|---|---|---|---|---|
| RISK OF DEMENTIA OUTCOMES: NPI SYMPTOM DOMAINS (NOT ADJUSTED FOR CDR) | ||||||||
| The risk of incident dementia and AD in the 1787 MCI participants with complete baseline data and at least one followup was estimated with Cox proportional hazards regression models. Hazard ratios (HRs) with 95% confidence intervals (CI) are reported for the associations of the severity of NPI symptom domains with incident dementia and AD. Models are adjusted for baseline age, African-American race, Hispanic ethnicity, and MMSE. z-scores were used to test statistical significance. No models violated Cox proportional-hazards assumptions. | ||||||||
| MCI patients who progressed to | ||||||||
| Dementia (N=527) | Alzheimer’s (N=454) | |||||||
| NPI-Q symptom domain |
Prevalence (%) | Severitym Mean(SD) |
HR | 95% CI | p | HR | 95% CI | p |
| Delusions | 3.4 | .045 (.27) | 1.29 | 1.04, 1.60 | 0.020 | 1.16 | .92, 1.47 | 0.213 |
| Hallucinations | 1.6 | .018 (.16) | 1.24 | .80, 1.94 | 0.330 | 0.91 | .45, 1.82 | 0.788 |
| Agitation | 15.7 | .19 (.51) | 1.28 | 1.11, 1.48 | 0.001* | 1.22 | 1.04, 1.42 | 0.016 |
| Depression | 27.8 | .35 (.65) | 1.23 | 1.09, 1.39 | 0.001* | 1.20 | 1.05, 1.37 | 0.007 |
| Anxiety | 19.4 | .26 (.61) | 1.28 | 1.18, 1.45 | <.001* | 1.28 | 1.12, 1.47 | <.001* |
| Elation | 2.0 | .025 (.20) | 1.35 | .99, 1.85 | 0.060 | 0.91 | .62, 1.31 | 0.601 |
| Apathy | 16.7 | .22 (.56) | 1.38 | 1.22, 1.56 | <.001* | 1.38 | 1.20, 1.57 | <.001* |
| Disinhibition | 8.0 | .10 (.38) | 1.38 | 1.15, 1.67 | 0.001* | 1.15 | .92, 1.43 | 0.226 |
| Irritability | 26.7 | .33 (.63) | 1.26 | 1.10, 1.42 | <.001* | 1.27 | 1.11, 1.46 | <.001* |
| Aberrant motor behavior | 5.0 | .064 (.31) | 1.16 | .92, 1.47 | 0.201 | 1.06 | .81, 1.38 | 0.68 |
| Sleep | 20.0 | .27 (.64) | 1.14 | 1.01, 1.30 | 0.037 | 1.08 | .93, 1.25 | 0.305 |
| Appetite | 11.0 | .15 (.51) | 1.06 | 1.00, 1.34 | 0.053 | 1.11 | .94, 1.32 | 0.230 |
| Delusions | 3.4 | .045 (.27) | 1.29 | 1.04, 1.60 | 0.020 | 1.16 | .92, 1.47 | 0.213 |
| TABLE 5b | ||||||
|---|---|---|---|---|---|---|
| RISK OF DEMENTIA OUTCOMES: NPI SYMPTOM DOMAINS (ADJUSTED FOR CDR) | ||||||
| The risk of incident dementia and AD in the 1787 MCI participants with complete baseline data and at least one followup was estimated with Cox proportional hazards regression models. Hazard ratios (HRs) with 95% confidence intervals (CI) are reported for the associations of the severity of NPI symptom domains with incident dementia and AD. Models are adjusted for baseline CDR Sum, age, African-American race, Hispanic ethnicity, and MMSE. z-tests were used to test statistical significance. No models violated Cox proportional-hazards assumptions. | ||||||
| MCI patients who progressed to | ||||||
| Dementia (N=527) | Alzheimer’s (N=454) | |||||
| NPI-Q symptom domain |
HR | 95% CI | p | HR | 95% CI | p |
| Delusions | 1.17 | .94, 1.46 | 0.156 | 1.09 | .86, 1.39 | 0.456 |
| Hallucinations | 0.96 | .59, 1.59 | 0.887 | 0.64 | .30, 1.38 | 0.254 |
| Agitation | 1.10 | .94, 1.28 | 0.204 | 0.99 | .85, 1.18 | 0.988 |
| Depression | 1.13 | 1.00, 1.28 | 0.050 | 1.06 | .92, 1.21 | 0.422 |
| Anxiety | 1.12 | .98, 1.27 | 0.091 | 1.08 | .93, 1.25 | 0.327 |
| Elation | 1.20 | .88, 1.65 | 0.250 | 0.66 | .45, .96 | 0.030 |
| Apathy | 1.13 | 1.00, 1.28 | 0.056 | 1.16 | 1.01, 1.33 | 0.040 |
| Disinhibition | 1.09 | .89, 1.33 | 0.396 | 0.85 | .67, 1.07 | 0.171 |
| Irritability | 1.11 | .98, 1.26 | 0.105 | 1.15 | 1.00, 1.35 | 0.049 |
| Aberrant motor behavior | 1.03 | .82, 1.30 | 0.805 | 0.98 | .75, 1.28 | 0.883 |
| Sleep | 0.99 | .87, 1.13 | 0.935 | 0.96 | .83, 1.12 | 0.620 |
| Appetite | 0.98 | .83, 1.14 | 0.767 | 0.93 | .77, 1.12 | 0.428 |
| Delusions | 1.17 | .94, 1.46 | 0.156 | 1.09 | .86, 1.39 | 0.456 |
The threshold for statistical significance was p<.05 and Holm’s procedure was used to adjust for multiple comparisons. Significant associations are marked with *.
Discussion
These results support our hypothesis that the presence of any NPS (assessed by NPI-Q) and any depressive symptoms (assessed by GDS) in MCI are associated with a higher risk of incident dementia and AD. The increase in risk was large enough to be clinically significant: participants in the middle and upper tertiles of NPI-Q Total (meaning NPI-Q Total > 0) had >40% increased risk of incident dementia, while participants in the middle and upper tertiles of GDS (meaning GDS > 1) had a 30% increased risk of incident dementia. Similar associations were observed for NPI-Q Total and incident AD, although the associations of GDS with incident AD were not statistically significant. Importantly, these associations were unaffected after controlling for baseline CDR-Sum and MMSE, suggesting that NPS (as measured by NPI-Q) and depressive symptoms (as measured by GDS) in MCI are associated with increased risk of incident dementia independent of baseline cognitive or functional status.
At baseline, the prevalence of NPS were relatively high, but symptom severity was low (Table 5a) (10) and comparable to prior reports (9). After controlling for covariates we did not find significant associations between individual NPS and incident dementia and AD. While GDS was associated with increased risk of incident dementia, HRs were similar for the middle tertile (GDS =2–3) and upper tertile (GDS≥4) suggesting that the increased risk was not limited to participants with clinically significant depressive symptoms.
We observed brisk rates of progression to dementia and AD in these MCI participants with median duration to diagnosis of 1.17 and 1.16 years respectively, equivalent to a 25% annual rate of progression to dementia and 21% to AD. This reflects a relatively high-risk MCI group compared to the 17.2% annual rate reported in the Alzheimer’s Disease Neuroimaging Initiative (38), and is higher than the approximate 15% annual rate of progression observed in population-based MCI samples (39). In Figure 1 it is notable that the survival curves trend steeply downward between 1 and 2 years of followup, and plateau between years 2 and 4. In this cohort, progression to dementia appears to be a relatively early event particularly in participants with NPS, in contrast to the generally accepted idea that MCI progression occurs steadily over many years.
The findings are tempered by methodologic considerations common to observational studies of NPS in MCI and AD. Incident dementia was associated with greater severity of depressive symptoms as quantified on GDS but not on the depression domain of the NPI-Q. These likely resulted from GDS being a more sensitive instrument than NPI-Q for depressive symptoms, as would be expected from a domain-specific instrument (GDS) as opposed to a general NPS instrument (NPI-Q). . Another issue is that the presence of any symptom on NPI-Q increased risk of incident AD and dementia, but the severity of specific NPI-Q domains did not, after controlling for CDR. This may be merely an issue of statistical power, in that no one NPI-Q domain had notably high severity, or the association between NPS and incident dementia and AD may not be domain-specific.
In this cohort participants of African-American ethnicity were at lower risk of incident dementia and AD, while those of Hispanic ethnicity were at lower risk of incident dementia but not AD (Table 2). While these findings may reflect genuine associations they may also reflect “informative” censoring in that participants with poorer outcomes may have been more likely to drop out.
Given the lack of autopsy data or biomarkers we cannot infer that NPS are a prodrome of the AD neuropathological process. There are several other mechanisms beyond the scope of this dataset that could underly these associations including inflammation, nutritional alterations, and vascular pathology. However, our results are consistent with the hypothesis that neuropsychiatric symptoms in MCI even at low severity (“mild behavioral impairment” (40)) may be an early symptom of dementia and AD. Clinicians may want to be especially alert to new NPS symptoms presenting in persons with MCI I.e., neuropsychiatric symptoms may add to cognitive complaints in defining risk factors for progression of MCI to AD. These findings also bring up the interesting question of what biologic mechanisms may be responsible for the association of NPS with incident dementia and AD. One possible explanation is that mood symptoms in AD could be related to decreased monoaminergic innervation and neurotransmission in mood disorders and AD (41) Additionally, neuroinflammatory mechanisms may also underlie mood disorders and AD (42, 43), and there is evidence for monoaminergic regulation of neuroinflammation in AD (44). In mouse models of AD (45) and human AD pathologic specimens (46) loss of monoaminergic innervation is a relatively early event. This loss of monoaminergic innervation might be associated with NPS, which thus might represent a treatment target for early intervention in AD.
Strengths of this study include 1) the large MCI cohort with longitudinal follow-up; 2) use of clinical diagnoses of MCI and dementia by experienced interdisciplinary teams. Limitations include: 1) Delineation of NPS with the NPI-Q. The NPI-Q is a measure administered solely to “knowledgeable informants” (usually caregivers), and assesses only occurrence and severity of NPS but not frequency of symptoms . 2) Limited duration of follow-up averaging about 1.5 years. It is possible that longer-term follow-up would reveal different associations or strength of associations. 3) Assessment of a research, not community-based, sample of participants with MCI. The rate of progression from MCI to dementia has been reported to be much higher in clinical research samples than in community-based samples (47); thus, our data may be more representative of an MCI cohort that is relatively high-risk for progression to dementia, and thus not necessarily generalizable to all MCI. 4) While the clinical measures are standardized there are no measures of interrater or inter-site reliability and no standardized training across the sites. 5) Intercorrelation of covariates. For example, greater NPS were associated with functional deficits, the presence of which are in fact crucial in distinguishing MCI from dementia. 6) The UDS contains only one instrument specific to an NPS domain (GDS for depression) and thus lacks depth in assessment of other important NPS (notably apathy and psychosis). 7) We did not distinguish between MCI subtypes, and the inclusion of participants with nonamnestic MCI could have resulted in underestimation of progression to AD.
In summary, we conclude NPS symptoms in MCI are associated with increased risk of all-cause dementia and of AD, and that depressive symptoms are associated with increased risk of all-cause dementia. The risk added by even mild severity of NPS and depressive symptoms was 30–40% and is a large enough effect to be of potential importance to clinicians. These results suggest that the evaluation of NPS is important in predicting prognosis of MCI, adds to the predictive power of cognitive evaluation, and should be part of a thorough clinical evaluation of MCI. It is possible that the addition of evaluation of NPS to other cognitive and biological predictors of MCI prognosis may allow for identification of MCI patients at high risk of incident dementia who would constitute a target population for future preventive interventions for AD. Targeting NPS may also provide important therapeutic avenues for dementia prevention.
Acknowledgments
Sponsors: National Institute of Aging 1 K08 AG029157-01A1 (to PBR), U01 AG016976 (to the National Alzheimer’s Coordinating Center), National Institute of Mental Health 1U01MH066136 (to CGL), and Stempler Fund for Dementia Research and the Richmond Family Fund for Alzheimer’s and Related Diseases (to BSA).
Disclosures: PBR has received research support from NIA, American Foundation for Aging Research, Merck, Pfizer, Lilly, Elan, and Jannsen. MMM has received research research support from NIA, NINDS, and the George and Cynthia Mitchell Foundation. BSA has received research support from Forrest Research Institute, Richmond Family Foundation for Alzheimer's and Related Diseases, and the Stempler Fund for Dementia Research; loan repayment support from LRP/NIA. ESO has received research support from The Rosalinde and Arthur Gilbert Foundation/American Foundation for Aging Research, NIA, Johns Hopkins Institute for Clinical and Translational Research, Fidelity Foundation, and Hartford Center of Excellence. YEG has received research support from NIMH, NIH, Mayo CTSA, , the Robert Wood Johnson Foundation, and Clarice Smith and Abigail Van Buren Alzheimer’s Disease Research Program.
CGL has received grant support from NIMH, NIA, Associated Jewish Federation of Baltimore, Weinberg Foundation, Forest, Glaxo-Smith-Kline, Eisai, Pfizer, Astra-Zeneca, Lilly, Ortho-McNeil, Bristol-Myers, Novartis, National Football League, and Elan; has served as consultant/advisor to Astra-Zeneca, Glaxo-Smith Kline, Eisai, Novartis, Forest, Supernus, Adlyfe, Takeda, Wyeth, Lundbeck, Merz, Lilly, Pfizer, Genentech, NFL Players Association, and NFL Benefits Office; has received honoraria/travel support from Pfizer, Forest, Glaxo-Smith Kline, and Health Monitor.
Leslie Phillips (NACC)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Dr. Rosenberg had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Previously presented at the Alzheimer’s Research Roundtable, April 29, 2010, Washington DC.
REFERENCES
- 1.Alzheimer's Association: 2009. Alzheimer's disease facts and figures. Alzheimers Dement. 2009;5:234–270. doi: 10.1016/j.jalz.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Riley KP, Snowdon DA, Desrosiers MF, et al. Early life linguistic ability, late life cognitive function, and neuropathology: findings from the Nun Study. Neurobiol Aging. 2005;26:341–347. doi: 10.1016/j.neurobiolaging.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 3.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 4.Dickerson BC, Sperling RA, Hyman BT, et al. Clinical prediction of Alzheimer disease dementia across the spectrum of mild cognitive impairment. Arch Gen Psychiatry. 2007;64:1443–1450. doi: 10.1001/archpsyc.64.12.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okello A, Koivunen J, Edison P, et al. Conversion of amyloid positive and negative MCI to AD over 3 years: an 11C-PIB PET study. Neurology. 2009;73:754–760. doi: 10.1212/WNL.0b013e3181b23564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mattsson N, Zetterberg H, Hansson O, et al. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA. 2009;302:385–393. doi: 10.1001/jama.2009.1064. [DOI] [PubMed] [Google Scholar]
- 7.Leung KK, Barnes J, Ridgway GR, et al. Automated cross-sectional and longitudinal hippocampal volume measurement in mild cognitive impairment and Alzheimer's disease. Neuroimage. 2010 doi: 10.1016/j.neuroimage.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinberg M, Shao H, Zandi P, et al. Point and 5-year period prevalence of neuropsychiatric symptoms in dementia: the Cache County Study. Int J Geriatr Psychiatry. 2008;23:170–177. doi: 10.1002/gps.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geda YE, Roberts RO, Knopman DS, et al. Prevalence of neuropsychiatric symptoms in mild cognitive impairment and normal cognitive aging: population-based study. Arch Gen Psychiatry. 2008;65:1193–1198. doi: 10.1001/archpsyc.65.10.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenberg PB, Mielke MM, Appleby B, et al. Neuropsychiatric symptoms in MCI subtypes: the importance of executive dysfunction. Int J Geriatr Psychiatry. 2010 doi: 10.1002/gps.2535. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teng E, Lu PH, Cummings JL. Neuropsychiatric symptoms are associated with progression from mild cognitive impairment to Alzheimer's disease. Dement Geriatr Cogn Disord. 2007;24:253–259. doi: 10.1159/000107100. [DOI] [PubMed] [Google Scholar]
- 12.Robert PH, Berr C, Volteau M, et al. Importance of lack of interest in patients with mild cognitive impairment. Am J Geriatr Psychiatry. 2008;16:770–776. doi: 10.1097/JGP.0b013e31817e73db. [DOI] [PubMed] [Google Scholar]
- 13.Palmer K, Di Iulio F, Varsi AE, et al. Neuropsychiatric Predictors of Progression from Amnestic-Mild Cognitive Impairment to Alzheimer's Disease: The Role of Depression and Apathy. J Alzheimers Dis. 2010 doi: 10.3233/JAD-2010-1352. [DOI] [PubMed] [Google Scholar]
- 14.Palmer K, Berger AK, Monastero R, et al. Predictors of progression from mild cognitive impairment to Alzheimer disease. Neurology. 2007;68:1596–1602. doi: 10.1212/01.wnl.0000260968.92345.3f. [DOI] [PubMed] [Google Scholar]
- 15.Ramakers IH, Visser PJ, Aalten P, et al. Affective symptoms as predictors of Alzheimer's disease in subjects with mild cognitive impairment: a 10-year follow-up study. Psychol Med. 2010;40:1193–1201. doi: 10.1017/S0033291709991577. [DOI] [PubMed] [Google Scholar]
- 16.Beekly DL, Ramos EM, Lee WW, et al. The National Alzheimer's Coordinating Center (NACC) database: the Uniform Data Set. Alzheimer Dis Assoc Disord. 2007;21:249–258. doi: 10.1097/WAD.0b013e318142774e. [DOI] [PubMed] [Google Scholar]
- 17.Morris JC, Weintraub S, Chui HC, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20:210–216. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- 18.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 19.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 20.Wechsler D, Stone CP. Manual: Wechsler Memory Scale. 1973 [Google Scholar]
- 21.Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 22.Goodglass H, Kaplan E. The Assessment of Aphasia and Related Disorders. 1983 [Google Scholar]
- 23.Wechsler D. Manual: Wechsler Adult Intelligence Scale. 1955 [Google Scholar]
- 24.Armitage SG. An analysis of certain psychological tests used in the evaluation of brain injury. Psych Mono. 1946;60:1–48. [Google Scholar]
- 25.Morris JC, Ernesto C, Schafer K, et al. Clinical dementia rating training and reliability in multicenter studies: the Alzheimer's Disease Cooperative Study experience. Neurology. 1997;48:1508–1510. doi: 10.1212/wnl.48.6.1508. [DOI] [PubMed] [Google Scholar]
- 26.Rockwood K, Strang D, MacKnight C, et al. Interrater reliability of the Clinical Dementia Rating in a multicenter trial. J Am Geriatr Soc. 2000;48:558–559. doi: 10.1111/j.1532-5415.2000.tb05004.x. [DOI] [PubMed] [Google Scholar]
- 27.Fillenbaum GG, Peterson B, Morris JC. Estimating the validity of the clinical Dementia Rating Scale: the CERAD experience. Consortium to Establish a Registry for Alzheimer's Disease. Aging (Milano) 1996;8:379–385. doi: 10.1007/BF03339599. [DOI] [PubMed] [Google Scholar]
- 28.Morris JC. Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr. 1997;9(Suppl 1):173–176. doi: 10.1017/s1041610297004870. discussion 177–8. [DOI] [PubMed] [Google Scholar]
- 29.Pavlik VN, Doody RS, Massman PJ, et al. Influence of premorbid IQ and education on progression of Alzheimer's disease. Dement Geriatr Cogn Disord. 2006;22:367–377. doi: 10.1159/000095640. [DOI] [PubMed] [Google Scholar]
- 30.Cummings JL, Mega M, Gray K, et al. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 31.Kaufer DI, Cummings JL, Ketchel P, et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12:233–239. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]
- 32.Burke WJ, Roccaforte WH, Wengel SP. The short form of the Geriatric Depression Scale: a comparison with the 30-item form. J Geriatr Psychiatry Neurol. 1991;4:173–178. doi: 10.1177/089198879100400310. [DOI] [PubMed] [Google Scholar]
- 33.Korner A, Lauritzen L, Abelskov K, et al. The Geriatric Depression Scale and the Cornell Scale for Depression in Dementia. A validity study. Nord J Psychiatry. 2006;60:360–364. doi: 10.1080/08039480600937066. [DOI] [PubMed] [Google Scholar]
- 34.Debruyne H, Van Buggenhout M, Le Bastard N, et al. Is the geriatric depression scale a reliable screening tool for depressive symptoms in elderly patients with cognitive impairment? Int J Geriatr Psychiatry. 2009;24:556–562. doi: 10.1002/gps.2154. [DOI] [PubMed] [Google Scholar]
- 35.Hachinski VC, Iliff LD, Zilhka E, et al. Cerebral blood flow in dementia. Arch Neurol. 1975;32:632–637. doi: 10.1001/archneur.1975.00490510088009. [DOI] [PubMed] [Google Scholar]
- 36.Holm S. A simple sequentially rejective multiple test procedure. Scand J Statistics. 1979;6:65–70. [Google Scholar]
- 37.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 38.Landau SM, Harvey D, Madison CM, et al. Comparing predictors of conversion and decline in mild cognitive impairment. Neurology. 2010;75:230–238. doi: 10.1212/WNL.0b013e3181e8e8b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tschanz JT, Welsh-Bohmer KA, Lyketsos CG, et al. Conversion to dementia from mild cognitive disorder: the Cache County Study. Neurology. 2006;67:229–234. doi: 10.1212/01.wnl.0000224748.48011.84. [DOI] [PubMed] [Google Scholar]
- 40.Taragano FE, Allegri RF, Krupitzki H, et al. Mild behavioral impairment and risk of dementia: a prospective cohort study of 358 patients. J Clin Psychiatry. 2009;70:584–592. doi: 10.4088/jcp.08m04181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lyness SA, Zarow C, Chui HC. Neuron loss in key cholinergic and aminergic nuclei in Alzheimer disease: a meta-analysis. Neurobiol Aging. 2003;24:1–23. doi: 10.1016/s0197-4580(02)00057-x. [DOI] [PubMed] [Google Scholar]
- 42.Capuron L, Miller AH. Cytokines and psychopathology: lessons from interferon-alpha. Biol Psychiatry. 2004;56:819–824. doi: 10.1016/j.biopsych.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 43.Heneka MT, O'Banion MK, Terwel D, et al. Neuroinflammatory processes in Alzheimer's disease. J Neural Transm. 2010;117:919–947. doi: 10.1007/s00702-010-0438-z. [DOI] [PubMed] [Google Scholar]
- 44.Heneka MT, Nadrigny F, Regen T, et al. Locus ceruleus controls Alzheimer's disease pathology by modulating microglial functions through norepinephrine. Proc Natl Acad Sci U S A. 2010;107:6058–6063. doi: 10.1073/pnas.0909586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Y, Yoo MJ, Savonenko A, et al. Amyloid pathology is associated with progressive monoaminergic neurodegeneration in a transgenic mouse model of Alzheimer's disease. J Neurosci. 2008;28:13805–13814. doi: 10.1523/JNEUROSCI.4218-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grudzien A, Shaw P, Weintraub S, et al. Locus coeruleus neurofibrillary degeneration in aging, mild cognitive impairment and early Alzheimer's disease. Neurobiol Aging. 2007;28:327–335. doi: 10.1016/j.neurobiolaging.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 47.Farias ST, Mungas D, Reed BR, et al. Progression of mild cognitive impairment to dementia in clinic- vs community-based cohorts. Arch Neurol. 2009;66:1151–1157. doi: 10.1001/archneurol.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]