Abstract
AIM
To investigate the expression of nucleotide oligomerization domain 2 (NOD2) in the immortalized human corneal epithelial cell line (THCE), and its role in the innate immune response triggered by inactive Aspergillus fumigatus (Af) conidia.
METHODS
The normal THCE cells were investigated as controls. After incubation with inactive Af conidia for 0.5, 2, 4, 6, and 8 hours, THCE cells were harvested, mRNA expression of NOD2 and receptor interacting protein 2 (RIP2) was detected by RT-PCR. Intracellular proteins including NOD2, NF-κB and proinflammatory cytokines such as TNF-α, IL-8, IL-6 in the cell supernatant were analyzed by ELISA.
RESULTS
Our data indicate that NOD2 expressed in the normal THCE cells. After triggered by the inactive Af conidia, the expression of NOD2, RIP2 mRNA and the secretion of NOD2, NF-κB, TNF-α, IL-8, IL-6 both increased in a time-depended manner, and reached the peak point at 4, 6, 6, 4, 6, 6, 4 hours, respectively. And after pretreated with NOD2 neutralizing antibody, the expression of RIP2, NF-κB, TNF-α, IL-8 both decreased dramatically at the peak point, while the secretion of IL-6 changed little.
CONCLUSION
The results of this study suggest that NOD2 exists and expresses in the THCE cells, and contributes to the innate immune responses triggered by inactive Af conidia by induction of proinflammatory cytokines such as TNF-α and IL-8 through the NF-κB pathway.
Keywords: nucleotide oligomerization domain 2, corneal epithelial cell, Aspergillus fumigatus, innate immune response
INTRODUCTION
Fungal keratitis (FK) is an opportunistic infection of the cornea caused by pathogenic fungi, resulting in a high blindness rate. It will happen when the defensive barrier of the cornea is damaged, just because of eye injury, long time use of antibiotics or corticosteroids and the decreased resistance of the body, etc. Fusarium and Aspergillus fumigatus (Af) are the two main pathogenic fungi in China, and the incidence rate of FK increases gradually[1]. As the first barrier of the cornea against the pathogenic microorganisms, the corneal epithelial cells play an irreplaceable role in the innate immune response. However, the mechanism of the host corneal epithelium in this response is still unknown.
Nucleotide oligomerization domain 2 (NOD2) is a member of nucleotide oligomerization domain-like receptors (NLRs), which is a specialized group of intracellular proteins that play a critical role in the regulation of the host innate immune response[2]. As an intracellular pattern recognition receptor (PRR), NOD2 is a multi-domain protein composed of a variable N-terminal effector region consisting of caspase recruitment domain (CARD), a centrally located NOD (also referred as the NACHT cassette) that is critical for activation, and C-terminal leucine-rich repeats (LRR) that senses pathogen-associated molecular patterns (PAMPs)[3]. When activated by the ligands, NOD2 directly recruits receptor interacting protein 2 (RIP2) through CARD-CARD interactions, and participates in the inflammatory response through the NF-κB pathway[4]. Recent studies have found that NOD2 plays a critical role in the immune response of many organs, such as intestine[5],[6], liver[7] and lung[8],[9]. The mechanisms of these immune responses have got more and more attentions.
Recent studies have demonstrated that NOD2 can recognize the PAMPs in the Af and induce the following immune response through the NF-κB pathway, inducing the expression of inflammatory cytokines[9], also NOD2 has been found in the eyes of healthy murine and canine, especially in the corneal epithelium[10],[11]. Therefore, we hypothesize that NOD2 expressed by corneal epithelial cells may interact with PAMPs in the Af, contributing to the induction of innate immune response to the pathogen. In this study, we tested our hypothesis in vitro on an immortalized corneal epithelial cell line-THCE.
MATERIALS AND METHODS
Materials
Delbeccon's modified Eagle's medium (DMEM) was purchased from HyClone (Utah, USA); Fetal Bovine Serum (FBS) was purchased from Gibco (San Diego, California , USA); 0.25% trypsin-EDTA solution was purchased from Solarbio (Beijing, China); Dimethyl sulfoxide (DMSO) was purchased from Sigma-Aldrich (St Louis, Missouri, USA); Sabouroud medium was purchased from Babio biotech (Jinan, China); Monoclonal antibody to NOD2 was purchased from Santa Cruz Biotechnology (Santa Cruz, California, USA); RNAiso Plus and reverse transcriptase polymerase chain reaction (RT-PCR) kits were purchased from TaKaRa (Dalian, China); Enzyme-linked immunosorbent assay (ELISA) kits was purchased from R&D (Minnealpolis, Minnesota, USA).
Methods
Generation of Af conidia
Af strain CPCC 800061 was purchased from Center for culture collection of pharmaceutical microorganisms (CPCC) and grown on Sabouraud dextrose agar (DIFCO) at 28°C for 5 days. Conidia were harvested from Af mycelium as described previously[12]. Washed conidia were harvested by centrifugation and killed by treatment with 70% ethanol for 30 minutes. Killed conidia were washed three times in sterile phosphate buffere saline (PBS). Cell numbers and the wet weight of the pellets were determined and adjusted to 50mg/mL sterile PBS[13].
Cell culture
THCE cells were kindly provided by Ocular Surface Laboratory of Zhongshan Ophthalmic Center and cultured in DMEM with 15% FBS, 100U/mL penicillin G and 100µg/mL streptomycin sulfate at 37°C in a humidified atmosphere containing 5% CO2. The medium was replaced every 2 days.
THCE cell suspensions of 1×105/mL were seeded onto 6-well tissue culture plates and grew to 70%-80% confluence. Before challengement THCE cells were incubated in serum-free medium for 12 hours. Then some THCE cells were added with serum-free medium as controls, and others were added with inactive Af conidia. After 0.5, 2, 4, 6, 8 hours' incubation, THCE cells were harvested and the culture medium was collected for detection.
Semiquantitative RT-PCR
Total RNA was isolated from THCE cells with RNAiso plus reagent and quantified by spectrophotometry. RNA (1µg) was used for first-strand cDNA synthesis according to the protocol for a reverse transcription system. Then cDNA (5µL) was used for PCR in 50µL reaction volume following the manufacturer's instructions. The accession number, primer sequence, and PCR amplification product size for each gene were shown in Table 1. PCR cycling conditions were as follows: an initial denaturation step of 95°C for 5 minutes, followed by 35 cycles of 95°C for 30 seconds, 60°C for 30 seconds, 72°C for 60 seconds for NOD2, RIP2 and β-actin, and 72°C for 7 minutes. PCR products were separated on 2% agarose gels containing ethidium bromide (0.5g/mL), then visualized and photographed by the UVP gel imaging system (VILBER LOURM, USA). Band intensity was quantitated using quantity one software.
Table 1. Primer list used for RT-PCR.
| Gene | Accession number | Primer(5′-3′) | Product size (bp) |
| NOD2 | NM_022162.1 | Forward | 412 |
| CACCTCAATGACGATGCGGACAC | |||
| Reverse | |||
| TCACCACCTTGCGGGCATTCTT | |||
| RIP2 | NM_003821.5 | Forward | 388 |
| CACAGTTGGGATAGCACCATTTC | |||
| Reverse | |||
| GGTAAGGCTGAAGACCCATTTG | |||
| β-actin | A005 | Forward | 205 |
| TGACGTGGACATCCGCAAAG | |||
| A006 | Reverse | ||
| CTGGAAGGTGGACAGCGAGG |
Elisa
Intracellular total proteins were isolated from THCE cells for the detection of NOD2 and NF-κB, while the culture supernatants were collected and centrifuged to remove cellular debris for the detection of TNF-α, IL-8 and IL-6. The 96-well plate was divided into standard group and sample group. Biotin (10µL) was added to the sample group firstly, then standard solution (50µL) and sample (50µL) were added to the plate and a detection antibody (100µL) was added to the plate, too. After incubation at 37°C for 60 minutes, the plate was washed for 5 times, then chromogenic agents A (50µL) and B (50µL) were added to the plate. After another incubation at 37°C for 15 minutes without light, a stop solution (50µL) was added and optical density was measured directly with an appliskan multimode microplate reader (Thermo Scientific, USA) at a test wavelength of 450nm.
NOD2 functional blocking analysis
THCE cell suspensions of 1×105/mL were seeded onto 6-well tissue culture plates and grew to 70%-80% confluence. Then THCE cells were preincubated with anti-human NOD2 (200µg/mL) monoclonal antibody for 30 minutes[14]. Some THCE cells were added with serum-free medium as controls, and others were added with inactive Af conidia. After 4, 6 hours' incubation, THCE cells were harvested and the culture medium was collected for detection.
Statistical Analysis
All experiments were done in six groups and the results were expressed as mean± standard deviation (SD). Statistical comparison was made by independent Student's t-test or by one-way analysis of variance (ANOVA) using SPSS 18.0 software. P<0.05 was set as the level of statistical significance for all tests.
RESULTS
Morphous Observation of Af Conidia and THCE Cells
After 24-30 hours' culture, Af began to form conidia, and the mature conidia grew from light yellow to grass-green, grey-green and black. Purified Af conidia was made into smear and observed under the light microscope, which looked like single transparent spherical or cluster-like aggregation (Figure 1A).
Figure 1. Microscopic of Af conidia and THCE cells.
A: Smear of purified Af conidia observed under light microscope (40×); B and C: THCE cells observed under inverted microscope (100×), at 6 hours and 48 hours respectively.
Anabiotic THCE cells adhere after 2 hours' incubation, extended at 6 hours, division growth from 12 hours, and grew to 70%-80% confluence at 48-72 hours. The cells looked like typical sheets of closely packed epithelial cell-like morphology under the inverted microscope (Figure 1B, C).
Af Conidia Induce Differential Gene Expression In THCE Cells
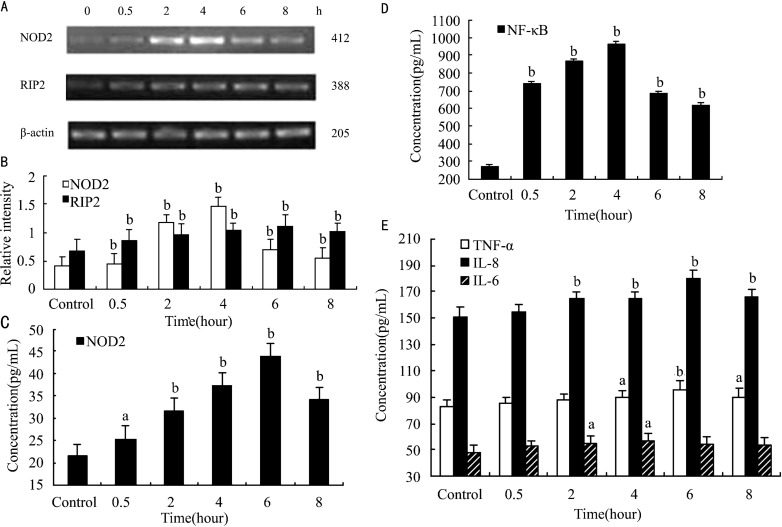
RIP2 is known as a downstream factor of NOD2. We demonstrated that NOD2 and RIP2 express in the normal THCE cells. The relative intensity of NOD2 and RIP2 in normal THCE cells were (0.405±0.170) and (0.666±0.207), respectively. The semiquantitative RT-PCR analysis revealed that Af conidia induced the expressions of NOD2 and RIP2 in a time-depended manner. Increased levels of NOD2 and RIP2 were detectable as early as 0.5 hour after stimulation with Af conidia (P<0.01; P<0.01). Af conidia upregulated the mRNA levels of NOD2 and RIP2 with a significant increase at 4 hour and 6 hour, respectively (NOD2: 1.454±0.167, P<0.01; RIP2: 1.115±0.203, P<0.01; Figure 2B).
Figure 2. Af conidia induced expressions of NOD2, RIP2, NF-κB, TNF-α, IL-8 and IL-6 in THCE cells.
A and B: The synthesis of NOD2 and RIP2 mRNA in THCE cells stimulated by inactive Af conidia, measured by relative intensity; C, D and E: The concentration of NOD2, NF-κB, TNF-α, IL-8 and IL-6 in THCE cells stimulated by inactive Af conidia, measured by ELISA at 0.5, 2, 4, 6, 8 hours. Values represent means±SD. aP<0.05, bP<0.01 vs control, n=6.
Af Conidia Induce NOD2 Synthesis and NF-κB Activation in THCE Cells
The expression of NOD2 and NF-κB in normal THCE cells were (21.565±2.606)pg/mL and (269.412±13.636)pg/mL. To investigate whether Af induces NOD2 synthesis and NF-κB activation, we treated THCE cells with Af conidia and determined NOD2 and NF-κB expression. Thus we demonstrated that Af conidia stimulated the expression of NOD2 and NF-κB in a time-depended manner. Increased levels of NOD2 and NF-κB were detectable as early as 0.5 hour after stimulation with Af conidia (P<0.05, P<0.01). Af conidia upregulated the protein levels of NOD2 and NF-κB with a significant increase at 6 and 4 hours, respectively (NOD2: 43.995±2.743pg/mL, P<0.01, Figure 2C; NF-κB: 965.000±14.174pg/mL, P<0.01, Figure 2D). Especially, the expression of NF-κB kept at a high level after 0.5 hour.
Af Conidia Induce TNF-α, IL-8 and IL-6 Production in THCE Cells
The secretion of TNF-α, IL-8 and IL-6 in normal THCE cells were (82.922±4.825)pg/mL, (151.000±6.87)pg/mL and (48.065±5.271)pg/mL, respectively. The foregoing results suggest that Af conidia induce NOD2, RIP2 synthesis and NF-κB activation. To assess the biological relevance of induced signaling pathways, we determined the effects of Af conidia on the production of proinflammatory cytokines. Thus we demonstrated that Af conidia stimulated the secretion of TNF-α, IL-8 and IL-6 in a time-depended manner. Increased levels of TNF-α, IL-8 and IL-6 were detectable as early as 4 hours, 2 hours, 2 hours after stimulation with Af conidia (P<0.05, P<0.01, P<0.05). Af conidia upregulated the secretion levels of TNF-α, IL-8 and IL-6 with a significant increase at 6 hour, 6 hour and 4 hour, respectively (TNF-α: 95.961±6.111pg/mL, P<0.01; IL-8: 179.854±6.352pg/mL, P<0.01; IL-6: 56.741±5.342pg/mL, P<0.05) (Figure 2E).
Af Conidia-Induced Secretion of TNF-α and IL-8 Is NOD2-Dependent
In order to investigate the role of Af conidia stimulated downstream signaling pathways on the secretion of proinflammatory cytokines in THCE cells, specific inhibitor was used. The normal THCE cells with or without preincubating with NOD2 neutralizing antibody had few difference in the levels of RIP2, NF-κB, TNF-α, IL-8 and IL-6. However, after stimulated by Af conidia, THCE cells preincubated with NOD2 neutralizing antibody had a significant decrease in the levels of RIP2, NF-κB, TNF-α, IL-8 at 4 hours and 6 hours than THCE cells without preincubating with NOD2 neutralizing antibody (RIP2: 0.820±0.164 vs 1.115±0.203, P<0.01, Figure 3B; NF-κB: 719.412±16.941 vs 965.000±14.174pg/mL, P<0.01, Figure 3C; TNF-α: 88.118±2.035 vs 95.961±6.111pg/mL, P<0.05, Figure 3D; IL-8: 166.833±2.398 vs 179.854±6.352pg/mL, P<0.01, Figure 3E). Unlike these four factors, the secretion of IL-6 had few difference between THCE cells with or without preincubating with NOD2 neutralizing antibody (54.653±2.691 vs 56.741±5.342pg/mL, P=0.419, Figure 3F). Thus, these data demonstrated that Af conidia were capable of directly activating THCE cells to secrete proinflammatory cytokines by a NOD2 mediated mechanism.
Figure 3. Decreased expressions of RIP2, NF-κB, TNF-α and IL-8 in stimulated THCE cells preincubated with NOD2 neutralizing antibody.
A and B: The synthesis of RIP2 mRNA in stimulated THCE cells preincubated with NOD2 neutralizing antibody decreased significantly than in stimulated THCE cells without NOD2 antibody, measured using relative intensity; C to E: The concentration of NF-κB, TNF-α and IL-8 decreased in blocked THCE cells, measured by ELISA; F: The concentration of IL-6 changed little in blocked THCE cells. Values represent means±SD. aP<0.05, bP<0.01 vs unblocking, n=6.
DISCUSSION
Corneal epithelial cells act as the first barrier that protects the cornea from infecting by pathogenic microorganisms. There are no blood vessels, few Langerhan's cells and other full-time immune cells in cornea[14]. Thus the ability of corneal epithelial cells to recognize pathogenic microorganisms and start the innate immune response is important to the occurrence, development and prognosis of corneal inflammation. Therefore, we stimulated the THCE cells with inactive Af conidia and investigated if NOD2 participates in this innate immune response. In this study, we found the expression of NOD2 mRNA and proteins in the normal THCE cells cultured in vitro, which was uniform with the studies investigated in the eyes of healthy murine and canine[10],[11]. After stimulated by the inactive Af conidia, there was a significant increase of NOD2 mRNA and proteins in THCE cells, 3.59- and 2.04-fold than the normal THCE cells at the peak point, respectively. These data indicated that NOD2 is very possible to participate in the recognition of Af conidia and the following immune response.
After activated by the ligands, NOD2 directly recruits RIP2 through CARD-CARD interactions[4]. Recent studies have demonstrated that RIP2 null cell or mice was unable to activite NF-κB signaling downstream of NOD2 ligand stimulation, but after exogenous RIP2 was added, the activity of NF-κB recovered[15],[16]. These results indicate that RIP2 is an essential factor for NOD2 activating NF-κB. In our study, as the expression of NOD2 mRNA and proteins increased, the synthesis of RIP2 mRNA and secretion of NF-κB proteins increased significantly in a time-depended manner after stimulated by inactive Af conidia, 1.67- and 3.58-fold than the normal THCE cells at the peak point, respectively. This indicates that THCE cells can recognize Af conidia and activate RIP2 and NF-κB, which is uniform with the study in the A549 and THP-1 cell lines[9]. Furthermore, when preincubated with NOD2 neutralizing antibody, the synthesis of RIP2 mRNA and secretion of NF-κB proteins decreased a lot after stimulated as before, 26.46% and 25.45% at the peak point than the stimulated THCE cells without NOD2 antibody. These data indicate that NOD2 in THCE cells can recognize Af conidia and start the innate immune response through the RIP2- NF-κB signaling pathway. However, though pretreatment with NOD2 neutralizing antibody, the expression of RIP2 and NF-κB still increased than the normal THCE cells without stimulating by inactive Af conidia. This indicates that there are other receptors participating in this innate immune response, such as TLR2 and TLR4 we know at present[14], and they regulate the response together. The mechanisms of the interactions among these receptors are still unknown and should be investigated in future.
Recent studies have demonstrated that the expression of TNF-α increased and induced the infiltration of inflammatory cells in the aspergillosis infection[17]; IL-8 is the major mediator of polymorphonuclear neutrophil (PMNs) influx in ocular infection[18]; TNF-α and IL-8 are the major cytokines that recruit PMNs into the infected cornea[19]; IL-6 is an essential protective factor in the corneal inflammation[20]. In our study, the secretion of TNF-α, IL-8 and IL-6 in the THCE culture supernatant increased after stimulated by inactive Af conidia. This indicates that corneal epithelial cells can recognize fungi antigen and induce the increase of inflammatory cytokines to defend fungi and recruit lots of PMNs, which can start stronger immune response to clear pathological fungi. However, tissue injuries are inevitable in the response. Furthermore, when preincubated with NOD2 neutralizing antibody, as the expression of RIP2 mRNA and NF-κB proteins decreased significantly, the secretion of TNF-α and IL-8 decreased after stimulated than the stimulated THCE cells without NOD2 antibody, while the secretion of IL-6 changed little. These data indicated that the NOD2-RIP2- NF-κB signaling pathways played an important role in the innate immune response of THCE cells stimulated by inactive Af conidia, inducing the secretion of TNF-α and IL-8, which can defend fungi at an early stage and start the following immune response. However, when preincubated with NOD2 neutralizing antibody, the secretion of TNF-α and IL-8 still increased than the normal THCE cells without stimulating by inactive Af conidia, indicating that there are other receptors and signaling pathways participating in the Af keratitis, and they regulate the immune responses together. Meanwhile this signaling pathways had little effect on the secretion of IL-6, indicating that the increasing secretion of IL-6 in this immune response was regulated by other signaling pathways.
In summary, NOD2 exists and expresses in THCE cells, and contributes to the innate immune responses triggered by inactive Af conidia by induction of proinflammatory cytokines such as TNF-α and IL-8 through the NF-κB pathway, affecting the occurrence and development of corneal infection. However, we conjecture that there are other receptors and signaling pathways participating in the Af keratitis, and they regulate the immune responses together. We have already found surfactant protein D[21], NS-398[22] participating in the Af keratitis, but they are not enough. Therefore, investigating the mechanism of these pathways in Af keratitis may be promising for the prevention and treatment of keratitis.
Footnotes
Foundation items: National Natural Science Foundation of China (No. 30672285); Qingdao Municipal Science and Technology Commission, China (No. 10-3-3-10-NSH)
REFERENCES
- 1.Du ZD, Liang T, Zhao GQ. Epidemiological investigationg of pathogenic fungus in fungal keratitis. Qingdao Daxue Yixueyuan Xuebao. 2007;43(2):174–175. [Google Scholar]
- 2.Luigi F, Neil W, Kyle V, Gabriel N. Function of nod-like teceptors in microbial recognition and host defense. Immunol Rev. 2009;227(1):106–128. doi: 10.1111/j.1600-065X.2008.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strober W, Murray PJ, Kitani A, Watanabe T. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat Rev Immunol. 2006;6(1):9–20. doi: 10.1038/nri1747. [DOI] [PubMed] [Google Scholar]
- 4.Ogura Y, Inohara N, Benito A, Chen FF, Yamaoka S, Nunez G. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. Biol Chem. 2001;276(7):4812–4818. doi: 10.1074/jbc.M008072200. [DOI] [PubMed] [Google Scholar]
- 5.Miceli-Richard C, Lesage S, Rybojad M, Prieur AM, Manouvrier-Hanu S, Häfner R, Chamaillard M, Zouali H, Thomas G, Hugot JP. CARD15 mutations in Blau syndrome. Nat Genet. 2001;29(1):19–20. doi: 10.1038/ng720. [DOI] [PubMed] [Google Scholar]
- 6.Kanazawa N, Okafuji I, Kambe N, Nishikomori R, Nakata-Hizume M, Nagai S, Fuji A, Yuasa T, Manki A, Sakurai Y, Nakajima M, Kobayashi H, Fujiwara I, Tsutsumi H, Utani A, Nishigori C, Heike T, Nakahata T, Miyachi Y. Early-onset sarcoidosis and CARD15 mutations with constitutive nuclear factor-kappaB activation: common genetic etiology with Blau syndrome. Blood. 2005;105(3):1195–1197. doi: 10.1182/blood-2004-07-2972. [DOI] [PubMed] [Google Scholar]
- 7.Zheng LJ, Fang L, Mao YK, Meng HB, He YM. NOD2 expression in liver of rats with obstructive jaundice. Tongji Daxue Xuebao. 2007;28(4):34–37. [Google Scholar]
- 8.Opitz B, Püschel A, Schmeck B, Hocke AC, Rosseau S, Hammerschmidt S, Schumann RR, Suttorp N, Hippenstiel S. Nucleotide-binding oligomerization domain proteins are innate immune receptors for internalized Streptococcus pneumoniae. Biol Chem. 2004;279(35):36426–36432. doi: 10.1074/jbc.M403861200. [DOI] [PubMed] [Google Scholar]
- 9.Zhang HJ, Qu JM, Shao CZ, Zhang J, He LX, Yuan ZH. Aspergillus fumigatus conidia upregulates NOD2 protein expression both in vitro and in vivo. Acta Pharmacol Sin. 2008;29(6):1202–1208. doi: 10.1111/j.1745-7254.2008.00860.x. [DOI] [PubMed] [Google Scholar]
- 10.Rodríguez-Martínez S, Cancino-Díaz ME, Jiménez-Zamudio L, García-Latorre E, Cancino-Díaz JC. TLRs and NODs mRNA expression pattern in healthy mouse eye. Br J Ophthalmol. 2005;89(7):904–910. doi: 10.1136/bjo.2004.056218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emma S, Rachael S, Sandra S. Immunohistochemical detection of NOD1 and NOD2 in the healthy murine and canine eye. Veterinary Ophthalmology. 2009;12(4):269–275. doi: 10.1111/j.1463-5224.2009.00698.x. [DOI] [PubMed] [Google Scholar]
- 12.Rohde M, Schwienbacher M, Nikolaus T, Heesemann J, Ebel F. Detection of early phase specific surface appendages during germination of Aspergillus fumigatus conidia. FEMS Microbiol Lett. 2002;206(1):99–105. doi: 10.1111/j.1574-6968.2002.tb10993.x. [DOI] [PubMed] [Google Scholar]
- 13.Meier A, Kirschning CJ, Nikolaus T, Wagner H, Heesemann J, Ebel F. Toll-like receptor (TLR) 2 and TLR4 are essential for Aspergillus-induced activation of murine macrophages. Cell Microbiol. 2003;5(8):561–570. doi: 10.1046/j.1462-5822.2003.00301.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhao J, Wu XY. Triggering of toll-like receptors 2 and 4 by Aspergillus fumigatus conidia in immortalized human corneal epithelial cells to induce inflammatory cytokines. Chin Med J. 2008;121(5):450–454. [PubMed] [Google Scholar]
- 15.Kobayashi K, Inohara N, Hernandez LD, Galán JE, Núñez G, Janeway CA, Medzhitov R, Flavell RA. RICK/Rip2/CAR-DIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature 2002. 6877;416:194–199. doi: 10.1038/416194a. [DOI] [PubMed] [Google Scholar]
- 16.Park JH, Kim YG, McDonald C, Kanneganti TD, Hasegawa M, Body-Malapel M, Inohara N, Núñez G. RICK/RIP2 mediates innate immune responses induced through Nod1 and Nod2 but not TLRs. Immunol. 2007;178(4):2380–2386. doi: 10.4049/jimmunol.178.4.2380. [DOI] [PubMed] [Google Scholar]
- 17.Peeters D, Peters IR, Clercx C, Day MJ. Quantification of mRNA encoding cytokines and chemokines in nasal biopsies from dogs with sino-nasal aspergillosis. Vet Microbiol. 2006;114(3-4):318–326. doi: 10.1016/j.vetmic.2005.11.065. [DOI] [PubMed] [Google Scholar]
- 18.Kernacki KA, Barrett RP, Hobden JA, Hazlett LD. Macrophage inflammatory protein-2 is a mediator of polymorphonuclear neutrophil influx in ocular bacterial infection. Immunol. 2000;164(2):1037–1045. doi: 10.4049/jimmunol.164.2.1037. [DOI] [PubMed] [Google Scholar]
- 19.Hazlett LD. Corneal response to Pseudomonas aeruginosa infection. Prog Retin Eye Res. 2004;23(1):1–30. doi: 10.1016/j.preteyeres.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Cole N, Bao S, Stpleton F, Thakur A, Husband AJ, Beagley KW, Willcox MD. Pseudomonas aeruginosa keratitis in IL-6-deficient mice. Int Arch Allergy Immunol. 2003;130(2):165–172. doi: 10.1159/000069006. [DOI] [PubMed] [Google Scholar]
- 21.Che CY, Jia WY, Xu Q, Li N, Hu LT, Jiang N, Lin J, Wang Q, Zhao GQ. The roles of surfactant protein D during Aspergillus fumigatus infection in human corneal epithelial cells. Int J Ophthalmol. 2012;5(1):13–17. doi: 10.3980/j.issn.2222-3959.2012.01.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li N, Che CY, Hu LT, Lin J, Wang Q, Zhao GQ. Effects of COX-2 inhibitor NS-398 on IL-10 expression in rat fungal keratitis. Int J Ophthalmol. 2011;4(2):116–120. doi: 10.3980/j.issn.2222-3959.2011.02.11. [DOI] [PMC free article] [PubMed] [Google Scholar]