Abstract
AIM
To clarify the molecular mechanism of Celecoxib on corneal collagen degradation and corneal ulcer.
METHODS
Rabbit corneal fibroblasts were harvested and suspended in serum-free MEM. Type I collagen, DMEM, collagen reconstitution buffer and corneal fibroblast suspension were mixed on ice. The resultant mixture solidify in an incubator, after which test reagents and plasminogen was overlaid and the cultures were returned to the incubator. The supernatants from collagen gel incubations were collected and the amount of hydroxyproline in the hydrolysate was measured. Immunoblot analysis of MMP1, 3 and TIMP1, 2 was performed. MMP2, 9 was detected by the method of Gelatin zymography. Cytotoxicity Assay was measured.
RESULTS
Celecoxib inhibited corneal collagen degradation in a dose and time manner; Celecoxib inhibited the IL-1ß induced increases in proMMP1, 2, 3, 9 and active MMP1, 2, 3, 9 in a concentration-depended manner. Celecoxib can also inhibit the IL-1ß induced increases in the TIMP1, 2.
CONCLUSION
Celecoxib can inhibit corneal collagen degradation induced by IL-1β, this effect is the consequence of the reduction of MMP1, 2, 3, 9 and TIMP1, 2. The results of the present study provide new insight into Celecoxib in corneal ulcer treatment.
Keywords: Celecoxib, corneal ulcer, IL-1β, collagen degradation
INTRODUCTION
Homeostasis of corneal stroma depend on the balance of matrix remodeling proteins and matrix disrupting proteins[1]. Structural extracellular matrix proteins such as fibronectin and collagen concerned with matrix metalloproteinase(MMPs) regulating ECM degradation[2]-[4]. Members of the MMPs families cleave cellular, extracellular and extracellular matrix substrates modulating tissue structure[5]. Conversion of matrix plasminogen to plasmin by proteases of the plasminogen activator (PA) system promote proMMPs to their active form. MMP production can be induced by varied cytokines, such as the proinflammatory cytokines TNF-a, IL-1,IL-6[6]. Cytokines and chemokines attract PMN to the cornea thus inducing corneal ulcer.IL-1β is critical to regulation of the corneal bacterial inflammatory process[7].
We previously showed that Pseudomonas aeruginosa elastase and IL-1β stimulated corneal collagen degradation by corneal fibroblasts induced by IL-1β[8]. We also manifested female sex hormone inhibited corneal collagen degradation induced by IL-1β[9],[10].As a selective cyclooxygenase-2(COX-2) inhibitor, Celecoxib is a specific nonsteroidal antiinflammatory drugs(NSAID). Celecoxib down-regulates the inflammatory mediators such as IL-1β, IL-2, IL-4, IFNγ, TNF-α, iNOS, COX-2, MMP1, 2, 3, and PGE(2) to a level that inhibits inflammation[11]-[14].
MMP1, 3 lead to a marked increase in COX-2 expression,PGE2 secretion, and MMP9 expression. Celecoxib can block Proteinase-induced MMP9 expression[15],[16]. Inhibition of COX-2 reduces the collagen fibrillogenesis associated with tumor cell infiltration[17]. Celecoxib, has been reported to have COX-2-independent immunomodulatory effects[18].
Previously we showed that the female sex hormone inhibit IL-1β–induced collagen degradation by corneal fibroblasts[9],[10]. In this study, we examined whether Celecoxib inhibited collagen degradation by rabbit corneal fibroblasts in response to IL-1β. We want to clarify the mechanism and potency of COX-2 inhibitor Celecoxib on the treatment of corneal dissolvability disease and corneal ulcer.
MATERIALS AND METHODS
Materials
Minimum essential medium eagle (MEM), Dulbecco's phosphate-buffered saline (DPBS), antibiotic-antimycotic mixture, and trypsin-EDTA were obtained from Weibo Chem Company; Native porcine type 1 collagen (acid solubilized), 5×Dulbecco's modified Eagle's medium (DMEM), and collagen reconstitution buffer were from Nitta Gelatin (Osaka, Japan). Fetal bovine serum (FBS) was from Shanghai Yantuo Biotecnology(Shanghai, China). Bovine plasminogen, protease inhibitor cocktail, and Celecoxib were from Sigma-Aldrich (Beijing, China). Recombinant human IL-1β was obtained from R&D Systems China (Shanghai, China). Mouse monoclonal antibodies to rabbit MMP1, 3 and TIMP1, 2 were obtained from antibodies-online (German). An enhanced chemiluminescence (ECL) kit as well as horseradish peroxidase–conjugated goat polyclonal antibodies to rabbit or mouse immunoglobulin G were from GE Healthcare (Piscataway, NJ). Coomassie brilliant blue and gelatin were obtained from Bio-Rad (Hercules, CA). A cytotoxicity assay (CytoTox 96Non-Radioactive) was from Promega (Beijing, China). All media and reagents used for cell culture were endotoxin minimized.
Methods
Cell isolation
Rabbit corneal fibroblasts were isolated and maintained as described previously[9],[10],[19],[20]. In brief, the enucleated eye was washed with DPBS containing antibiotic-antimycotic mixture, the endothelial layer of the excised cornea was removed mechanically, and the remaining corneal tissue was incubated with dispase (2mg/mL, in MEM) for 1 hour at 37°C. After mechanical removal of epithelial sheet, the remaining tissue was treated with collagenase (2mg/mL, in MEM) at 37°C until a single-cell suspension of corneal fibroblasts was obtained. The isolated corneal fibroblasts were cultured under a humidified atmosphere of 5% CO2 at 37°C in 60mm culture dishes containing MEM supplemented with 10% FBS. Proliferating cells were harvested for experiments at the subconfluent stage after four to seven passages in monolayer culture.
Three-Dimensional culture system
Culture collagen gels were prepared as described[9],[10],[19],[20]. In brief, corneal fibroblasts were harvested by exposure to trypsin-EDTA followed by centrifugation at 15 000×g for 5 minutes, and they were then suspended in serum-free MEM.Acid-solubilized collagen type I(3mg/mL), 5× DMEM, collagen reconstitution buffer [0.05mol/L NaOH, 0.26mol/L Na2CO3, 0.2mol/L HEPES (pH 7.3)], and corneal fibroblast suspension (2.2×106/mL in MEM) were mixed on ice at a volume ratio of 7:2:1:1. The resultant mixture (0.5mL) was added to each well of a 24-well culture plate and allowed to solidify in an incubator containing 50mol/L CO2 at 37°C, after which 0.5mL of serum-free MEM containing test reagents and plasminogen (60µg/mL) was overlaid and the cultures were returned to the incubator for 48 hours.
Assay of collagenolytic activity
Collagen degradation was measured as previously described. In brief, the supernatants from collagen gel incubations were collected, and native collagen fibrils with a molecular size of >100kDa were removed by ultrafiltration. The filtrate was subjected to hydrolysis with 6mol/L HCl for 24 hours at 110°C, and the amount of hydroxyproline in the hydrolysate was determined by measurement of absorbance at 558nm with a spectrophotometer.
Immunoblot analysis
Immunoblot analysis of MMP1 and MMP3 was performed as described previously. In brief, culture supernatants from collagen gel incubations were subjected to SDS polyacrylamide gel electrophoresis on a 10% gel, and the separated proteins were transferred electrophoretically to a nitrocellulose membrane. Nonspecific sites of the membrane were blocked, and it was then incubated with antibodies to MMP1 or to MMP3. Immune complexes were detected with the use of horseradish peroxidase–conjugated secondary antibodies and ECL reagents.
Gelatin zymography
Gelatin zymography was performed as described previously. In brief, culture supernatants from collagen gel incubations were mixed with SDS sample buffer by the ratio of 2:1, and 5µL of the resulting mixture were subjected to SDS polyacrylamide gel electrophoresis in the dark at 4°C on a 10% gel containing 0.1% gelatin. The gel was then washed with 2.5% Triton X-100 for 1 hour before incubation for 18 hours at 37°C in a reaction mixture containing 50mmol/L Tris-HCl (pH 7.5), 5mmol/L CaCl2, and 1% Triton X-100. The gel was finally stained with Coomassie brilliant blue.
Cytotoxicity assay
LDH release was measured by Non-Radioactive Cytotoxicity assay kit. In brief, 2×104 cells were cultured in 10% FBS in 96-well plates for 24 hours. After washing, the cells were secreted by the compounds in serum-free for extra 24 hours. 1g/L triton was taken as positive control. Supernatants and substrate were mixed in assay buffer in a new plate (1:1 v:v, 30 minutes, RT). Stop Solution was added and absorbance was recorded on spectrophotometer at 400nm.
Statistical Analysis
Data are presented as means±SEM and were analyzed with Dunnett's multiple comparison test. P value <0.0001 was considered statistically significant.
RESULTS
Inhibition effect of Celecoxib on IL-1ß induced collagen degradation by corneal fibroblasts
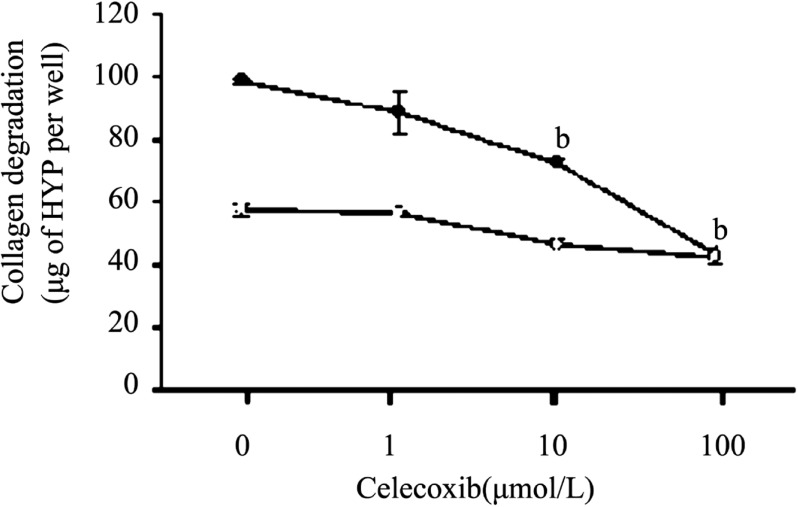
We manifested that proinflammatary factor IL-1ß markedly increased the extent of collagen degradation by cultured corneal fibroblasts[9],[10],[19],[20]. To investigate and analyze the inhibition effect of Celecoxib on collagen degradation resulting from IL-1ß stimulation in three dimensional cultures of rabbit corneal fibroblasts, the cells incubated for 48 hours with Celecoxib (1µmol/L -100µmol/L) resulted in a concentration-depended inhibition of collagen degradation in the presence of IL-1ß (0.1ng/mL, Figure 1).
Figure 1. Dose-dependent inhibition effect by Celecoxib of IL-1ß-induced collagen degradation by corneal fibroblasts.
Rabbit fibroblasts were cultured in collagen gels in the presence of plasminogen, in the absence (open symbols)) or presence (closed symbols) of IL-1ß (0.1ng/mL) and in the presence of the indicated concentrations of Celecoxib. After incubation of the cells for 48 hours, the amount of degraded collagen in the culture supernatants was determined. Data are expressed as micrograms of hydroxyproline (HYP) per well and are means±SEM of values from an experiment that was repeated a total of three times with similar results. bP< 0.001 (Dunnett's test) vs the value for cells cultured with IL-1ß in the absence of Celecoxib.
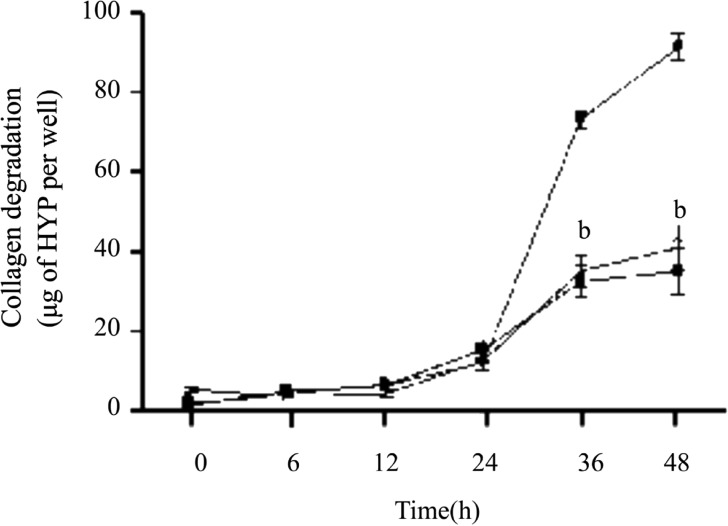
Except the results above, we carried out the time course of collagen degradation by corneal fibroblasts in the absence or presence of IL-1ß (0.1ng/mL) or 10µmol/L Celecoxib. In different time points, the amount of degraded collagen increased gradualy. Compared to the amount of collagen degradation by plasminogen, IL-1ß increased the amount of degraded collagen dramatically at 36 and 48 hours. This effect was inhibited by 10µmol/L Celecoxib at 36 and 48 hours (Figure 2).
Figure 2. Time-dependent inhibition effect of Celecoxib on IL-1ß induced collagen degradation by corneal fibroblasts.
Cells were cultured in collagen gels for the indicated times in the presence of plasminogen 60µg/mL and in the absence (open symbols) or presence of 0.1ng/mL IL-1ß (close symbols), in the absence (circles) or presence (squares) of 10µmol/L Celecoxib, after which the amount of degraded collagen was determined. Data are mean±SEM of values from three experiments. bP< 0.001 (Dunnett test) vs the corresponding value for cells cultured with IL-1 ß and plasminogen.
Effects of Celecoxib on the expression of MMP1, 3
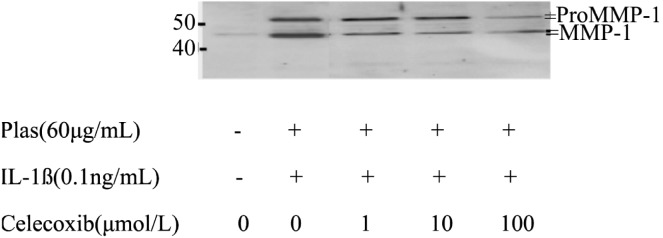
MMP1, 3 expression were detected using the methods of immunoblot analysis. Corneal fibroblasts were cultured in collagen gels for 48 hours in the absence or presence of IL-1ß and in the presence of Celecoxib (1µmol/L-100µmol/L). Coincident with our previous result[9],[10],[19],[20], immunoblot analysis with antibodies to human biotinylated MMP-1 revealed that the culture supernatants of cells incubated without IL-1ß contained relatively small amounts of 61-kDa and 57-kDa immunoreactive proteins corresponding to pro MMP1 as well as of 49-kDa and 45-kDa immunoreactive proteins corresponding to active MMP1. In contrast, large amount of these bands were detected in the culture supernatants of cells incubated with IL-1ß. Celecoxib inhibited the IL-1ß induced increases in proMMP1 and active MMP1 in a dose dependent manner (Figure 3).
Figure 3. Effects of Celecoxib on the expression of proMMP1 and MMP1 by corneal fibroblasts.
Cells were cultured in collagen gels for 48 hours in the presence of plasminogen, in the absence or presence of IL-1ß (0.1ng/mL), and in the presence of the indicated concentrations of Celecoxib. The culture supernatants were then subjected either to immunoblot analysis with antibodies to MMP1. Data are representative of three independent experiments. The positions of bands corresponding to the proMMP1 and MMP1 are indicated on the right, and those of molecular size are shown on the left.
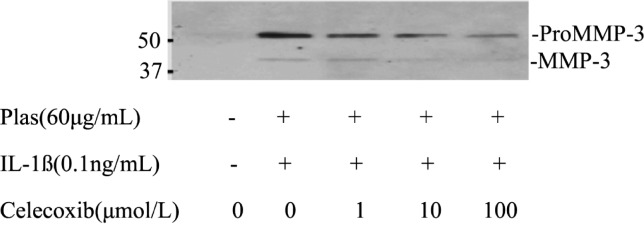
Immunoblot analysis with antibodies to MMP3 detect small amount of proMMP3 in culture supernatants of cells incubated in the absence of IL-1ß. In contrast, 57-kDa and 45-kDa immunoreactive proteins corresponding to proMMP3 and active MMP3, respectively, were apparent in the culture supernatants of cells incubated with IL-1ß. Celecoxib inhibited the IL-1ß induced increases in proMMP3 and active MMP3 in a concentration-depended manner (Figure 4).
Figure 4. Effects of Celecoxib on the expression of proMMP3 and MMP3 by corneal fibroblasts.
Cells were cultured in collagen gels for 48 hours in the presence of plasminogen, in the absence or presence of IL-1ß (0.1ng/mL), and in the presence of the indicated concentrations of Celecoxib.The culture supernatants were then subjected either to immunoblot analysis with antibodies to MMP3. Data are representative of three independent experiments. The positions of bands corresponding to proMMP3 and MMP3 are indicated on the right, and those of molecular size are shown on the left.
Effects of Celecoxib on the expression of MMP2, 9
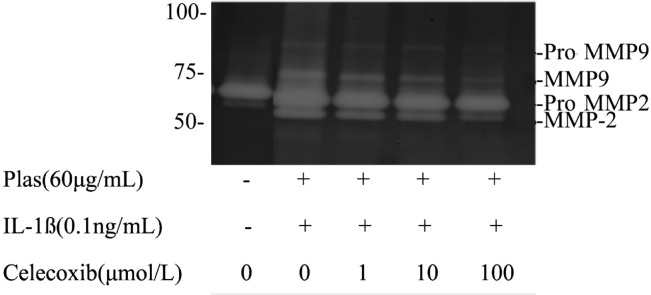
The expressions of MMP2, 9 were detected by gelatin zymography. Culture supernatants of corneal fibroblasts incubated without IL-1ß for 48 hours revealed two major bands of 65kDa and 57kDa corresponding to proMMP2 and active MMP2 and a faint band of 77kDa band corresponding to active MMP9. Cells cultured in the presence of IL-1ß (0.1ng/mL) resulted in an increase in the intensity of the band corresponding to active MMP2 and the appearance of bands at 92 and 77kDa corresponding to proMMP9 and active MMP9 respectively. Celecoxib inhibited the IL-1ß induced increases in the amounts of the proMMP9, active MMP9 and active MMP2 in a concentration-depended manner (Figure 5).
Figure 5. Effects of Celecoxib on the expression MMP2, 9 by corneal fibroblasts.
Cells were cultured in collagen gels for 48 hours in the presence of plasminogen and in the absence or presence of IL-1ß (0.1ng/mL) and the indicated concentrations of Celecoxib. Culture supernatants were then subjected to gelatin zymography. The positions of bands corresponding to pro-MMP2 and pro-MMP9 and active MMP2, 9 are indicated on the right, and those of molecular size are shown on the left. Data are representative of three independent experiments.
Effects of Celecoxib on the expression of TIMP1,2
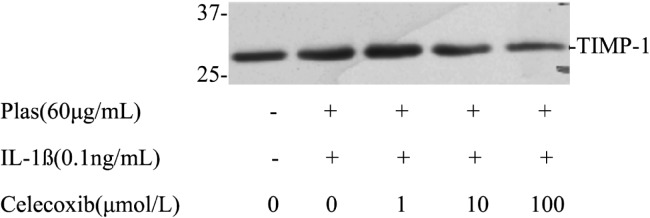
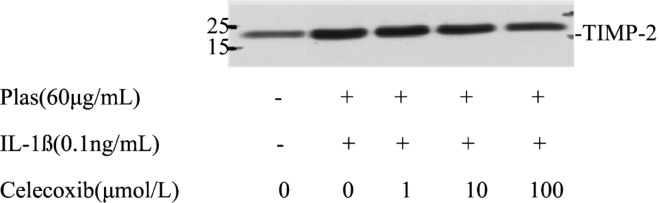
Further analysis emphasized the effects of Celecoxib on the expressions of TIMPs secreted by corneal fibroblasts. Immunoblot analysis with antibodies to TIMP1 revealed that the culture supernatant of cells maintained in collagen gels for 48 hours in the presence or absence of plsminogen with or without Celecoxib contained a 28kDa immunoreactive protein corresponding to TIMP1 (Figure 6). TIMP1 protein levels increased in the presence of IL-1ß (0.1ng/mL) and plasminogen. The increased secretion of TIMP1 was inhibited by Celecoxib in a dose-depended manner. Celecoxib also inhibited the expression of TIMP2 in a dose dependent manner, which corresponding to 21kDa immunoreactive protein (Figure 7).
Figure 6. Effects of Celecoxib on the expression of TIMP1 by corneal fibroblasts.
Cells were cultured in collagen gels for 48 hours in the presence of plasminogen, in the absence or presence of IL-1ß(0.1ng/mL), and in the presence of the indicated concentrations of Celecoxib. The culture supernatants were then subjected to immunoblot analysis with antibodies to TIMP1. Data are representative of three independent experiments. The positions of bands corresponding to the TIMP1 are indicated on the right, and those of molecular size are shown on the left.
Figure 7. Effects of Celecoxib on the expression of TIMP2 by corneal fibroblasts.
Cells were cultured in collagen gels for 48 hours in the presence of plasminogen, in the absence or presence of IL-1ß (0.1ng/mL), and in the presence of the indicated concentrations of Celecoxib. The culture supernatants were then subjected to immunoblot analysis with antibodies to TIMP2. Data are representative of three independent experiments. The positions of bands corresponding to the TIMP2 are indicated on the right, and those of molecular size are shown on the left.
LDH detection
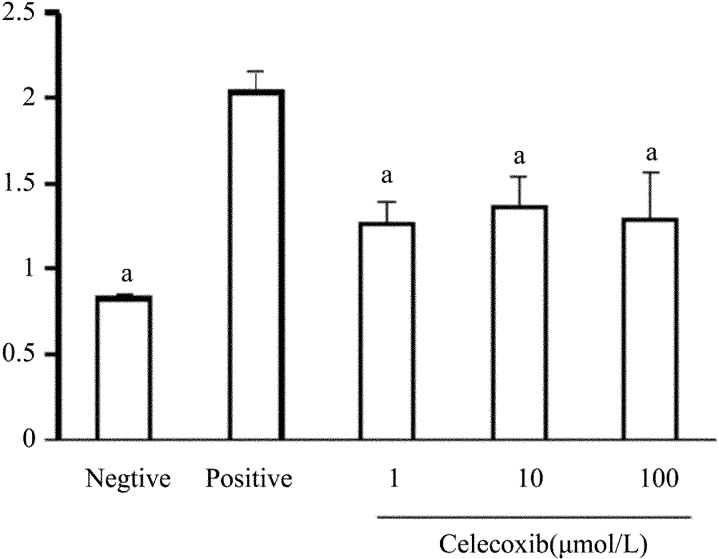
Measurement of LDH release revealed that Celecoxib at 1, 10, 100µmol/L had no cytotoxic effect on corneal fibroblasts (Figure 8).
Figure 8. Lack of a cytotoxic effect of Celecoxib on corneal fibroblasts.
Cells were incubated for 24 hours in MEM and in the absence (negative control) or presence of 10µmol/L or 100µmol/L Celecoxib, after which the culture supernatants were assayed for LDH activity with a colorimetric assay. The amount of LDH released from cells by 0.1% Triton was determined as a positive control. Data are means±SEM from three independent experiments. aP < 0.05 vs 0.1% Triton (Dunnett's test).
DISCUSSION
The present study examined whether Celecoxib can inhibit the degradation of the extracellular matrices during corneal inflammation. We observed that Celecoxib inhibited the amount of collagen degradation of rabbit corneal fibroblasts induced by IL-1β in concentration- and time-depended manner. Celecoxib can suppress the synthesis and activation of MMP1, 2, 3, 9 in corneal fibroblasts exposed to IL-1β in a concentration-depended manner. MMPs play an important role in degradation of the matrix which allows the inflammatory cell such as leucocytes and mononuclear assembling into the infected tissue site[21]. IL-1β can alter MMPs expression involving in leukocyte influx which has a close relationship to the pathogen and resident cells in the inflammation process[22]. Medication of anti IL-1β treatment to bacterial corneal infection mice resulted in a significant reduction in expression of MMP2, 9, TIMP1, 2[23]. We proved that Celecoxib inhibited MMPs expression induced by IL-1β thus inhibiting the corneal collagen degradation.
Celecoxib at 100nmol/L reduced the IL-1β induced of MMP1, 3, iNOS and NO in human articular chondrocytes[24].Celecoxib could inhibit PGE2 production and MMP-2 secretion of Tca8113 cell[25]. Selective cyclooxygenase-2 inhibitor can inhibit gingival tissue MMP8 expression[26]. COX-1 is constitutively expressed in many tissues, whereas COX-2 is induced by inflammatory mediators. COX-2 was expressed in the epithelium, endothelium, and stromal cells of the inflamed cornea[27]. COX-2 can be induced by proinflammatory factors such as IL-1β and TNF. PGE produced in inflammatory tissues by COX-2, play a main role in ocular inflammation[28],[29].
We found that Celexicob inhibited the expressions of TIMP1,2 not as anticipated. Tissue inhibitors of MMPs (TIMPs) and plasminogen activator inhibitor (PAI-1) are produced in relation to urokinase (uPA) and MMP1, 9 in cornea injury. There is an imbalance between the expression of this proteolytic enzyme and its inhibitors, which may contribute to changes in the wound healing process and ultimately lead to corneal ulcer development[30].
Celecoxib reduced type II collagen and MMP1, 3 induced by IL-1β in human osteoarticular cartilage[31]. Celecoxib can inhibit corneal collagen degradation induced by IL-1β, this effect was the consequence of the reduction of MMP1, 2, 3, 9 and TIMP1, 2. Celecoxib showed no evident cytotoxicity on corneal fibroblasts. The results of the present study provide new insight into Celecoxib in corneal ulcer treatment.
Footnotes
Foundation items: Research Fund of the Bethune B Plan of Jilin University, 2012; Research Fund of Jilin Provincial Science and Technology (international cooperation item, 20120726)
REFERENCES
- 1.Wilkins-Port CE, Ye Q, Mazurkiewicz JE, Higgins PJ. TGF-beta1 + EGF-initiated invasive potential in transformed human keratinocytes is coupled to a plasmin/MMP-10/MMP-1-dependent collagen remodeling axis: role for PAI-1. Cancer Res. 2009;69(9):4081–4091. doi: 10.1158/0008-5472.CAN-09-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vollberg TM, Sr, George MD, Jetten AM. Induction of extracellular matrix gene expression in normal human keratinocytes by transforming growth factor his altered by cellular differentiation. Exp Cell Res. 1991;193(1):93–100. doi: 10.1016/0014-4827(91)90542-3. [DOI] [PubMed] [Google Scholar]
- 3.Shang T, Chen Z, Pflugfelder SC, Li DQ. TGF-h1 stimulates production of gelatinase (MMP-9), collagenases (MMP-1, -13) and stromelysins (MMP-3,-10, -11) by human corneal epithelial cells. Exp Eye Res. 2004;79(2):263–274. doi: 10.1016/j.exer.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Lijnen HR. Plasmin and matrix metalloproteinases in vascular remodeling. Thromb Haemost. 2001;86(1):324–333. [PubMed] [Google Scholar]
- 5.Lijnen HR. Matrix metalloproteinases and cellular fibrinolytic activity. Biochemistry (Moscow) 2002;67(1):92–98. doi: 10.1023/a:1013908332232. [DOI] [PubMed] [Google Scholar]
- 6.Hui W, Litherland GJ, Jefferson M, Barter MJ, Elias MS, Cawston TE, Rowan AD, Young DA. Lithium protects cartilage from cytokine-mediated degradation by reducing collagen-degrading MMP production via inhibition of the P38 mitogen-activated protein kinase pathway. Rheumatology. 2010;49(11):2043–2053. doi: 10.1093/rheumatology/keq217. [DOI] [PubMed] [Google Scholar]
- 7.Thakur A, Barrett RP, McClellan S, Hazlett LD. Regulation of Pseudomonas aeruginosa corneal infection in IL-1 beta converting enzyme (ICE, caspase-1) deficient mice. Curr Eye Res. 2004;29(4-5):225–233. doi: 10.1080/02713680490516710. [DOI] [PubMed] [Google Scholar]
- 8.Nagano T, Hao JL, Nakamura M, Kumagai N, Abe M, Nakazawa T, Nishida T. Stimulatory effect of pseudomonal elastase on collagen degradation by cultured keratocytes. Invest Ophthalmol Vis Sci. 2001;42(6):1247–1253. [PubMed] [Google Scholar]
- 9.Zhou H, Kimura K, Orita T, Nishida T, Sonoda KH. Inhibition by medroxyprogesterone acetate of interleukin-1β-induced collagen degradation by corneal fibroblasts. Invest Ophthalmol Vis Sci. 2012 doi: 10.1167/iovs.11-8822. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Zhou H, Kimura K, Orita T, Nishida T, Sonoda KH. Inhibition by female sex hormones of collagen degradation by corneal fibroblasts. Mol Vis. 2011;17:3415–3422. [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y, Zhang W, Zhang X, Qi Y, Huang D, Zhang Y. Arsenic trioxide inhibits invasion/migration in SGC-7901 cells by activating the reactive oxygen species-dependent cyclooxygenase-2/matrix metalloproteinase-2 pathway. Exp Biol Med (Maywood) 2011;236(5):592–597. doi: 10.1258/ebm.2011.010276. [DOI] [PubMed] [Google Scholar]
- 12.Huh JE, Hong JM, Baek YH, Lee JD, Choi DY, Park DS. Anti-inflammatory and anti-nociceptive effect of Betula platyphylla var. japonica in human interleukin-1β-stimulated fibroblast-like synoviocytes and in experimental animal models. J Ethnopharmacol. 2011;135(1):126–134. doi: 10.1016/j.jep.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Cha HS, Ahn KS, Jeon CH, Kim J, Koh EM. Inhibitory effect of cyclo-oxygenase-2 inhibitor on the production of matrix metalloproteinases in rheumatoid fibroblast-like synoviocytes. Rheumatol Int. 2004;24(4):207–211. doi: 10.1007/s00296-003-0359-3. [DOI] [PubMed] [Google Scholar]
- 14.Vaish V, Sanyal SN. Chemopreventive effects of NSAIDs on cytokines and transcription factors during the early stages of colorectal cancer. Pharmacol Rep. 2011;63(5):1210–1221. doi: 10.1016/s1734-1140(11)70641-7. [DOI] [PubMed] [Google Scholar]
- 15.Steenport M, Khan KM, Du B, Barnhard SE, Dannenberg AJ, Falcone DJ. Matrix metalloproteinase (MMP)-1 and MMP-3 induce macrophage MMP-9: evidence for the role of TNF-alpha and cyclooxygenase-2. J Immunol. 2009;183(12):8119–8127. doi: 10.4049/jimmunol.0901925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Figueiredo MJ, de Melo Soares D, Martins JM, de Resende Machado R, Sorgi CA, Faccioli LH, de Melo MC, Malvar Ddo C, Souza GE. Febrile response induced by cecal ligation and puncture (CLP) in rats: involvement of prostaglandin E(2) and cytokines. Med Microbiol Immunol. 2012;201(2):219–229. doi: 10.1007/s00430-011-0225-y. [DOI] [PubMed] [Google Scholar]
- 17.Lyons TR, O'Brien J, Borges VF, Conklin MW, Keely PJ, Eliceiri KW, Marusyk A, Tan AC, Schedin P. Postpartum mammary gland involution drives progression of ductal carcinoma in situ through collagen and COX-2. Nat Med. 2011;17(9):1109–1115. doi: 10.1038/nm.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiba A, Mizuno M, Tomi C, Tajima R, Alloza I, di Penta A, Yamamura T, Vandenbroeck K, Miyake S. A 4-trifluoromethyl analogue of celecoxib inhibits arthritis by suppressing innate immune cell activation. Arthritis Res Ther. 2012;14(1):R9. doi: 10.1186/ar3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hao JL, Nagano T, Nakamura M, Kumagai N, Mishima H, Nishida T. Effect of galardin on collagen degradation by Pseudomonas aeruginosa. Exp Eye Res. 1999;69(6):595–601. doi: 10.1006/exer.1999.0755. [DOI] [PubMed] [Google Scholar]
- 20.Hao JL, Nagano T, Nakamura M, Kumagai N, Mishima H, Nishida T. Galardin inhibits collagen degradation by rabbit keratocytes by inhibiting the activation of pro-matrix metalloproteinases. Exp Eye Res. 1999;68(5):565–572. doi: 10.1006/exer.1998.0637. [DOI] [PubMed] [Google Scholar]
- 21.Berger EA, McClellan SA, Barrett RP, Hazlett LD. Testican-1 promotes resistance against pseudomonas aeruginosa–induced keratitis through regulation of MMP-2 expression and activation. Invest Ophthalmol Vis Sci. 2011;52(8):5339–5346. doi: 10.1167/iovs.10-6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wright KM, Friedland JS. Regulation of monocyte chemokine and MMP-9 secretion by proinflammatory cytokines in tuberculous osteomyelitis. J Leukoc Biol. 2004;75(6):1086–1092. doi: 10.1189/jlb.0903433. [DOI] [PubMed] [Google Scholar]
- 23.Xue ML, Wakefield D, Willcox MD, Lloyd AR, Di Girolamo N, Cole N, Thakur A. Regulation of MMPs and TIMPs by IL-1beta during corneal ulceration and infection. Invest Ophthalmol Vis Sci. 2003;44(5):2020–2025. doi: 10.1167/iovs.02-0565. [DOI] [PubMed] [Google Scholar]
- 24.Tsutsumi R, Ito H, Hiramitsu T, Nishitani K, Akiyoshi M, Kitaori T, Yasuda T, Nakamura T. Celecoxib inhibits production of MMP and NO via down-regulation of NF-kappaB and JNK in a PGE2 independent manner in human articular chondrocytes. Rheumatol Int. 2008;28(8):727–736. doi: 10.1007/s00296-007-0511-6. [DOI] [PubMed] [Google Scholar]
- 25.Li WZ, Huo QJ, Wang XY, Xu F. Inhibitive effect of Celecoxib on the adhesion and invasion of human tongue squamous carcinoma cells to extracellular matrix via down regulation of MMP-2 expression. Prostaglandins Other Lipid Mediat. 2010;93(3-4):113–119. doi: 10.1016/j.prostaglandins.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Vardar-Sengul S, Buduneli E, Turkoglu O, Buduneli N, Atilla G, Wahlgren J, Sorsa T, Baylas H. The effects of selective COX-2 inhibitor/celecoxib and omega-3 fatty acid on matrix metalloproteinases, TIMP-1, and laminin-5gamma2-chain immunolocalization in experimental periodontitis. J Periodontol. 2008;79(10):1934–1941. doi: 10.1902/jop.2008.080001. [DOI] [PubMed] [Google Scholar]
- 27.Sellers RS, Silverman L, Khan KN. Cyclooxygenase-2 expression in the cornea of dogs with keratitis. Vet Pathol. 2004;41(2):116–1121. doi: 10.1354/vp.41-2-116. [DOI] [PubMed] [Google Scholar]
- 28.Bazan HE, Tao Y, DeCoster MA, Bazan NG. Platelet-activating factor induces cyclooxygenase-2 gene expression in corneal epithelium. Requirement of calcium in the signal transduction pathway. Invest Ophthalmol Vis Sci. 1997;38(12):2492–2501. [PubMed] [Google Scholar]
- 29.Bhattacherjee P. The role of arachidonate metabolites in ocular inflammation. Prog Clin Biol Res. 1989;312:211–227. [PubMed] [Google Scholar]
- 30.Ottino P, Taheri F, Bazan HE. Platelet-activating factor induces the gene expression of TIMP-1, -2, and PAI-1: imbalance between the gene expression of MMP-9 and TIMP-1 and -2. Exp Eye Res. 2002;74(3):393–402. doi: 10.1006/exer.2001.1135. [DOI] [PubMed] [Google Scholar]
- 31.Kim JH, Huh JE, Baek YH, Lee JD, Choi DY, Park DS. Effect of phellodendron amurense in protecting human osteoarthritic cartilage and chondrocytes. J Ethnopharmacol. 2011;134(2):234–242. doi: 10.1016/j.jep.2010.12.005. [DOI] [PubMed] [Google Scholar]