Abstract
AIM
To investigate the effects of bevacizumab and ranibizumab on corneal neovascularization in an alkali burn-induced model of corneal angiogenesis.
METHODS
Fifteen Wistar albino rats were divided randomly into 3 groups after chemical cauterization of the cornea. The first group received a single dose of 0.1mL saline solution as a control group whereas second and third groups received a single dose of 2.5mg bevacizumab or 1mg ranibizumab by subconjunctival injection, respectively. After three weeks, the rat corneas were evaluated by biomicroscopy and corneal photographs were taken. The percentage of neovascularization area, length of the longest new vessel, corneal edema and corneal opacity scores were assessed.
RESULTS
The analysis of digital photographs showed that the percentage of neovascularization area to the total corneal area, the length of the longest new vessel, corneal edema and opacity scores were significantly lower in both study groups compared to the control group (P<0.05). Additionally, the percentage of corneal neovascularization area, the length of the longest new vessel and corneal opacity score were less with bevacizumab than ranibizumab.
CONCLUSION
Subconjunctival bevacizumab and ranibizumab treatments may be effective methods in reducing corneal neovascularization. Furthermore, bevacizumab is more effective than ranibizumab in the inhibition of corneal neovascularization.
Keywords: corneal neovascularisation, bevacizumab, ranibizumab
INTRODUCTION
Inflammatory, infectious, traumatic and degenerative diseases may cause new vessel formation in cornea which normally contains no blood vessels and has a transparent structure[1]. Corneal neovascularization may cause loss of sight by damaging the transparent structure and increase the risk for corneal graft rejection after keratoplasty[2]. Therefore, treatment options inhibiting or decreasing new vessel formation in cornea are needed.
There is a balance between angiogenic and anti-angiogenic factors in cornea which is shifted towards angiogenic factors during formation of corneal neovascularization[1]. Moreover, it has been shown that both vascular endothelial growth factor (VEGF) and VEGF receptor concentrations are increased in human cornea during neovascularization[3]. Recently, it has been shown that VEGF inhibitors are effective in slowing down and reducing the corneal neovascularization[4],[5]. Among these agents, bevacizumab is a recombinant humanized murine monoclonal antibody that binds and inhibits the biological activity of all human VEGF-A isoforms and various studies have shown its effectiveness in the treatment of corneal neovascularization[4]-[6]. Ranibizumab, an isotype monoclonal antibody fragment, is a recombinant humanized immunoglobulin (Ig1) designed for intraocular use which binds to and inhibits the biologic activity of human VEGF-A[7].
In this study, we aimed to compare the effects of bevacizumab and ranibizumab on corneal neovascularization, edema and opacity in an alkali burn induced model of corneal neovascularization.
MATERIALS AND METHODS
Materials
Fifteen Wistar-Albino adult rats, weighing 250g-300g, were randomly allocated to 3 experimental groups each consisting of five rats. The rats were individually placed in plastic cages and temperature-controlled room (22±2)°C in which a 12-12 hour light-dark cycle was maintained (07:00-19:00 hour light). The rats were provided food and water ad libitum. All protocols described in this study were approved by the local ethical committee for animal experimentation of the Cumhuriyet University Medical Faculty. All procedures involving animals were conducted in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Visual Research.
A general anesthesia, combination of 90mg/kg ketamin and 3mg/kg xylazine hydrochloride were injected intraperitoneally. Before alkali burn of corneas, eyes were topically anesthetized with 0.5% proparacain hydrochloride. An alkali burn was created only in the right eye of each subject with a modified model described by Kwon et al[8]. Standard cornea burn was performed by touching central cornea with a 2mm-2.5mm diameter 1mol/L NaOH-soaked cotton applicator for 10 seconds. Corneas and fornices were then washed with isotonic saline solution.
After producing alkali burns in corneas, the first group received 0.1mL saline solution at upper conjuntival area whereas the second and third groups were received 0.1mL, 2.5mg bevacizumab or 0.1mL, 1mg ranibizumab, respectively, through subconjunctival injections.
After 21 days, all subjects were examined by biomicroscopy and anterior segment photos were taken by a Nikon digital camera (D-200 model) mounted on TRC-50IX retina camera (Itabashiku, Tokyo, Japan). Percentage of the neovascularization area was obtained by dividing neovascularization area (pixel) to the total corneal area on a digital computer image analysis program (Topcon Image Net 2000, Itabashiku, Tokyo, Japan). The lengths of the longest new vessel in corneas were assessed.
Corneal edema and opacity were assessed based on biomicroscopic examination by the way described by Yoeruek et al[9]. According to this, presence of corneal edema was scored as follows: grade 1= no edema, grade 2= mild edema, grade 3= very significant edema. Corneal opacity was scored using a scale of 0-4 where grade 0= completely clear; grade 1=slightly hazy, iris and pupils easily visible; grade 2= slightly opaque, iris and pupils still detectable; grade 3=opaque, pupils hardly detectable, and grade 4= completely opaque with no view of the pupils. At the end of the experiment, all the subjects were sacrificed by high dose pentobarbital.
Statistical Analysis
Experimental values were presented as means ± standard deviation and analyzed by Kruskal-Wallis and Mann-Whitney U test using Benferroni correction. P<0.05 was considered statistically significant. All statistical analysis were performed using SPSS ver:16.0.
RESULTS
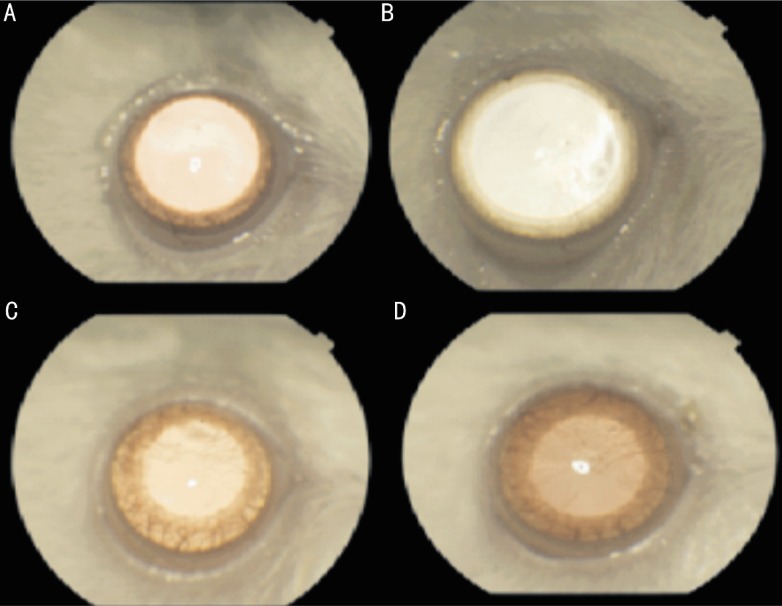
The corneal photographs with neovascularization were shown in Figure 1. The percentage of neovascularization area was (0.59±0.08) in control group, (0.31±0.03) in bevacizumab group and (0.43±0.05) in ranibizumab group. The percentage of neovascularization area was significantly lower in bevacizumab and ranibizumab groups compared with control group (P<0.05). Additionally, the percentage of neovascularization area in bevacizumab group was significantly lower than that of ranibizumab group (P<0.05). The percentage of neovascularisation area of the all groups was shown in Figure 2.
Figure 1. View of rat cornea.
A: Before chemical caterization; B: Bevacizumab group; C: Ranibizumab group; D: Control group.
Figure 2. Percentage of neovascularization areas in control, bevacizumab (2.5mg) and ranibizumab (1mg) groups.
aP<0.05 vs control group; cP<0.05 vs both control and ranibizumab groups.
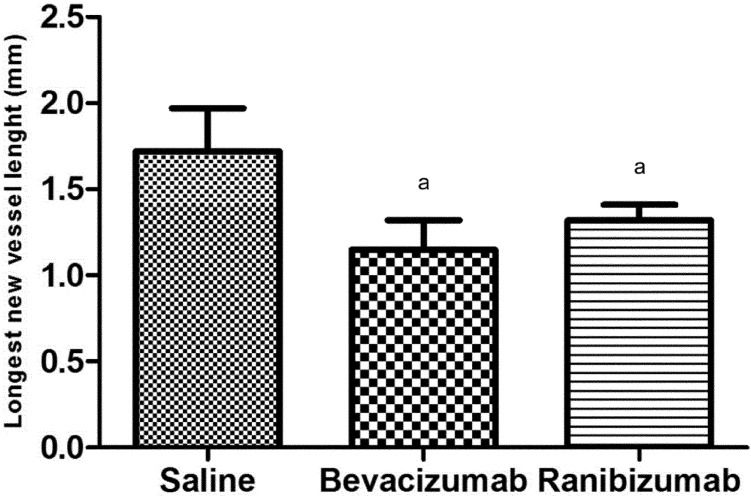
The length of the longest neovascular vessel was (1.72±0.25)mm in control group, (1.15±0.17)mm in bevacizumab group, (1.32±0.39)mm in ranibizumab group. In bevacizumab and ranibizumab groups, the longest neovascular sprout length was significantly shorter than that of control group (P<0.05). Although the longest sprout length of bevacizumab group was shorter than that of ranibizumab group, this difference was not statistically significant. The averages of the longest sprouts in corneas were shown in Figure 3.
Figure 3. The longest new vessel lengths in corneas in control, bevacizumab (2.5mg) and ranibizumab (1mg) groups.
aP<0.05 vs control group.
Corneal edema score was significantly lower in both treatment groups compared with that in control group (P<0.05, Table 1) but there was no significant difference between bevacizumab and ranibizumab groups.
Table 1. Percentage of corneal edema scores of control, bevacizumab (2.5mg) and ranibizumab (1mg) groups.
| Groups | Corneal edema score (%) |
||
| Grade 1 | Grade 2 | Grade 3 | |
| Saline | 0 | 20 | 80 |
| Bevacizumab | 40 | 60 | 0 |
| Ranibizumab | 60 | 40 | 0 |
The lowest corneal opacity score was in bevacizumab group, followed by ranbizumab group and control group (Table 2). Corneal opacity score was significantly lower in bevacizumab and ranbizumab groups compared with control (P<0.05). Although there was no statistical significance, corneal opacity score of bevacizumab group was lower than that of ranibizumab group.
Table 2. Percentage of corneal opacity scores of the control, bevacizumab (2.5mg) and ranibizumab (1mg) groups.
| Groups | Corneal opacity score (%) |
||||
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Saline | 0 | 0 | 20 | 40 | 40 |
| Bevacizumab | 0 | 60 | 40 | 0 | 0 |
| Ranibizumab | 0 | 40 | 40 | 20 | 0 |
DISCUSSION
Corneal neovascularization which may lead to blindness when untreated is very important in the pathogenesis of many corneal diseases. Therefore, effective alternatives preventing and treating corneal neovascularization are required.
Bevacizumab is a recombinant humanized monoclonal IgG1 antibody that specifically binds to and neutralize biological activity of VEGF-A and inhibits neovascularization by binding to all isoforms of VEGF-A[10]. Ranibizumab is a recombinant and humanized monoclonal antibody fragment which decreases vascular permeability by inhibiting angiogenesis through neutralizing all isoforms and degradation products of VEGF-A[11]. Recently, inhibition of vascular endothelial growth factor by anti VEGF monoclonal antibody injection, such as bevacizumab and ranibizumab, has become the first line option in the treatment of age-related macular degeneration[12],[13]. Bevacizumab has been used in different concentrations and lengths, either topically or subconjunctivally, in corneal neovascularization and found to be effective in reducing corneal neovascularization[4],[14]-[16]. Previous studies have investigated the safety of intravitreal injection of bevacizumab in rabbits using electrophysiological testing and histopathological analysis and in another in vitro study it has been reported that bevacizumab could exert a moderate growth inhibition on pig choroidal endothelial cells and high dose bevacizumab may be harmful to a human retinal pigment epithelial cell line, ARPE-19 cells[17],[18]. Besides, some groups have also reported the safety of bevacizumab on retina with studies using murine cells[19]. On the other hand, bevacizumab is specific to human VEGF and as has been clarified by structural analysis, bevacizumab does not bind with murine VEGF 8 because of an amino acid substitution in the bevacizumab-binding site[20]. However, there are limited studies about ranibizumab in corneal neovascularization. Thus, present study was performed to assess and compare efficiencies of subconjunctival bevacizumab and ranibizumab treatments in an alkali burn-induced model of corneal neovascularization.
In an experimental study, Hosseini et al[21] formed two groups to assess efficiency of subconjunctival bevacizumab in a NaOH induced corneal neovascularization model and administered 2.5mg single dose subconjunctival bevacizumab and 2.5mg single dose subconjunctival saline solution, following alkali burn, to the first and second group, respectively. At the end of the three weeks, corneal neovascularization was found to be reduced by 32% in the treatment group compared with control and the length of the longest new vessel was shorter than that of control group. Similarly, in our study, neovascularization was reduced by 28% in the subconjunctival bevacizumab and 21% in the subconjunctival ranibizumab group compared with control group. Besides, the length of the longest new vessel was found to be shorter than that of control group. In another study, Hurmeric et al[22] assessed efficiency of subconjunctival bevacizumab and another anti VEGF agent, Pegaptanip, in an experimental corneal neovascularization model and found that the area of corneal neovascularization was (73.83±6.67)% in the control, (59.84±6.85)% in the bevacizumab and (82.69±5.59)% in the pegaptanip group. In our study, neovascularization area was found to be the lowest in the bevacizumab group. Although there are several studies aimed to find the right dose of bevacizumab to prevent neovascularization[23],[24], studies about the dosage of ranibizumab are limited. Consequently, the efficiency of ranibizumab may increase according to the dose applied.
Yoeruek et al[9] evaluated the corneal neovascularization, edema and opacity of rats in a corneal angiogenesis model induced by alkali burn in early treatment (25mg/mL, topical bevacizumab), the late treatment (25mg/mL topical bevacizumab) and control groups. Corneal neovascularization was significantly lower in bevacizumab treatment groups compared with control group and the corneal opacity of early treatment group was significantly reduced compared with control group. In terms of corneal edema, no difference was found among the groups. In the present study, corneal edema and opacity were significantly lower in bevacizumab and ranibizumab treatment groups compared with control group. Although, corneal opacity score was lower in bevacizumab group compared with ranibizumab group, this difference was not statistically significant. Bevacizumab and ranizumab reduce lymph vessels proliferation beside their blood vessel proliferation suppression. It is not simply related to their anti-angiogenic effects but also to their anti-fibrotic effects [9].
In our study, although we used both bevacizumab and ranibizumab at only single dose and investigated their effects on corneal neovascularization, edema and opacity at single time, as limitations of our study, we showed that subconjuntival bevacizumab and ranibizumab administrations are effective in the treatment of alkali burn induced corneal neovascularization and bevacizumab was more effective than ranibizumab in preventing corneal neovascularization. Further studies using different doses for a longer period are needed to understand long-term effects of bevacizumab and ranibizumab in preventing corneal neovascularisation.
REFERENCES
- 1.Chang JH, Gabison EE, Kato T, Azar DT. Corneal neovascularization. Curr Opin Ophthalmol. 2001;12(4):242–249. doi: 10.1097/00055735-200108000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Dana MR, Schaumberg DA, Kowal VO, Goren MB, Rapuano CJ, Laibson PR, Cohen EJ. Corneal neovascularization after penetrating keratoplasty. Cornea. 1995;14(6):604–609. [PubMed] [Google Scholar]
- 3.Philipp W, Speicher L, Humpel C. Expression of vascular endothelial growth factor and its receptors in inflamed and vascularized human corneas. Invest Ophthalmol Vis Sci. 2000;41(9):2514–2522. [PubMed] [Google Scholar]
- 4.Manzano RP, Peyman GA, Khan P, Carvounis PE, Kivilcim M, Ren M, Lake JC, Chevez-Barrios P. Inhibition of experimental corneal neovascularization by bevacizumab (Avastin) Br J Ophthalmol. 2007;91(6):804–807. doi: 10.1136/bjo.2006.107912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bock F, Onderka J, Dietrich T, Bachmann B, Kruse FE, Paschke M, Zahn G, Cursiefen C. Bevacizumab as a potent inhibitor of inflammatory corneal angiogenesis and lymphangiogenesis. Invest Ophthalmol Vis Sci. 2007;48(6):2545–2552. doi: 10.1167/iovs.06-0570. [DOI] [PubMed] [Google Scholar]
- 6.Ferrara N, Hillan KJ, Novotny W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem Biophys Res Commun. 2005;333(2):328–335. doi: 10.1016/j.bbrc.2005.05.132. [DOI] [PubMed] [Google Scholar]
- 7.Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 8.Kwon YS, Hong HS, Kim JC, Shin JS, Son Y. Inhibitory effect of rapamycin on corneal neovascularization in vitro and in vivo. Invest Ophthalmol Vis Sci. 2005;46(2):454–460. doi: 10.1167/iovs.04-0753. [DOI] [PubMed] [Google Scholar]
- 9.Yoeruek E, Ziemssen F, Henke-Fahle S, Tatar O, Tura A, Grisanti S, Bartz-Schmidt KU, Szurman P. Tu bingen bevacizumab study group: safety, penetration and efficacy of topically applied bevacizumab: evaluation of eyedrops in corneal neovascularization after chemical burn. Acta Ophthalmol. 2008;86(3):322–328. doi: 10.1111/j.1600-0420.2007.01049.x. [DOI] [PubMed] [Google Scholar]
- 10.Usui T, Ishida S, Yamashiro K, Kaji Y, Poulaki V, Moore J, Moore T, Amano S, Horikawa Y, Dart D, Golding M, Shima DT, Adamis AP. VEGF164(165) as the pathological isoform: differential leukocyte and endothelial responses through VEGFR1 and VEGFR2. Invest Ophthalmol Vis Sci. 2004;45(2):368–374. doi: 10.1167/iovs.03-0106. [DOI] [PubMed] [Google Scholar]
- 11.Eng KT, Kertes PJ. Ranibizumab in neovascular age-related macular degeneration. Clin Interv Aging. 2006;1(4):451–466. doi: 10.2147/ciia.2006.1.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T, ANCHOR Study Group Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: Two-year results of the ANCHOR study. Ophthalmology. 2009;116(1):57–65. doi: 10.1016/j.ophtha.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 13.Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY, MARINA Study Group Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 14.Dursun A, Arici MK, Ozec AV, Dursun F, Toker MI, Topalkara A. Application of Topical/Subconjunctival Bevacizumab and Topical Fluorometholone Acetate in Alkali Burn-induced Model of Corneal Angiogenesis. Turk J Ophthalmol. 2010;40(6):318–322. [Google Scholar]
- 15.Bock F, König Y, Kruse F, Baier M, Cursiefen C. Bevacizumab eye drops inhibit corneal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2008;246(2):281–284. doi: 10.1007/s00417-007-0684-4. [DOI] [PubMed] [Google Scholar]
- 16.Ahmed A, Berati H, Nalan A, Aylin S. Effect of bevacizumab on corneal neovascularization in experimental rabbit model. Clin Experiment Ophthalmol. 2009;37(7):730–736. doi: 10.1111/j.1442-9071.2009.02112.x. [DOI] [PubMed] [Google Scholar]
- 17.Feiner L, Barr EE, Shui YB, Holekamp NM, Brantley MA., Jr Safety of intravitreal injection of bevacizumab in rabbit eyes. Retina. 2006;26(8):882–888. doi: 10.1097/01.iae.0000230717.85319.f5. [DOI] [PubMed] [Google Scholar]
- 18.Spitzer MS, Wallenfels-Thilo B, Sierra A, Yoeurek E, Peters S, Henke-Fahle S, Bartz-Schmidth KU, Szurman P. Antiproliferative and cytotoxic properties of bevacizumab on different ocular cells. Br J Ophthalmol. 2006;90(10):1316–1321. doi: 10.1136/bjo.2006.095190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luthra S, Narayanan R, Marques LE, Chwa M, Kim DW, Dong J, Seigel GM, Neekhra A, Gramajo AL, Brown DJ, Kenney MC, Kuppermann BD. Evaluation of in vitro effects of bevacizumab (Avastin) on retinal pigment epithelial, neurosensory retinal, and microvascular endothelial cells. Retina. 2006;26(5):512–518. doi: 10.1097/01.iae.0000222547.35820.52. [DOI] [PubMed] [Google Scholar]
- 20.Fuh G, Wu P, Liang WC, Ultsch M, Lee CV, Moffat B, Wiesmann C. Structure-function studies of two synthetic anti-vascular endothelial growth factor Fabs and comparison with the Avastin Fab. J Biol Chem. 2006;281(10):6625–6631. doi: 10.1074/jbc.M507783200. [DOI] [PubMed] [Google Scholar]
- 21.Hosseini H, Nejabat M, Mehryar M, Yazdchi T, Sedaghat A, Noori F. Bevacizumab inhibits corneal neovascularization in an alkali burn induced model of corneal angiogenesis. Clin Experiment Ophthalmol. 2007;35(8):745–748. doi: 10.1111/j.1442-9071.2007.01572.x. [DOI] [PubMed] [Google Scholar]
- 22.Hurmeric V, Erdurman CF, Mumcuoglu T, Kurt B, Dagli O, Karaca U, Durukan HA. Comparison of the Effect of Subkojunctival Bevacizumab and Pegaptanib Sodium in an Experimental Corneal Neovascularisation. Turk J Ophthalmol. 2009;39(5):349–354. [Google Scholar]
- 23.Habot-Wilner Z, Barequet IS, Ivanir Y, Moisseiev J, Rosner M. The inhibitory effect of different concentrations of topical bevacizumab on corneal neovascularization. Acta Ophtalmol. 2010;88(8):862–867. doi: 10.1111/j.1755-3768.2009.01571.x. [DOI] [PubMed] [Google Scholar]
- 24.You IC, Kang IS, Lee SH, Yoon KC. Therapeutic effect of subconjunctival injection of bevacizumab in the treatment of corneal neovascularization. Acta Ophthalmol. 2009;87(6):653–658. doi: 10.1111/j.1755-3768.2008.01399.x. [DOI] [PubMed] [Google Scholar]