Abstract
AIM
To compare the efficacy of prophylactic vitrectomy for acute retinal necrosis syndrome(ARN) with routine treatment in Chinese patients, thereby investigate the necessity of prophylactic vitrectomy for ARN.
METHODS
Thirty patients (37 eyes) were retrospectively included in this study. The eyes were divided into 2 groups by treatment, including routine treatment, which consisted of antiviral medication and vitrectomy after retinal detachment (RD) (n=21), and prophylactic vitrectomy, which consisted of antiviral medication and vitrectomy for the prevention of RD performed during the active inflammatory phase (n=16). The extent of necrosis was determined by fundus photographs at the time of presentation (for eyes with mild vitreous opacity) or the drawings in the operation records. Necrosis of the 37 eyes was divided into 3 grades, including peripheral, middle-peripheral and extensive. The follow-up period ranged from 8 to 57 months. Differences in visual acuity and necrosis between groups were identified using independent samples t-test.
RESULTS
Necrosis was more extensive in the routine treatment group than in the prophylactic vitrectomy group (P<0.05). In the routine treatment group, conservative treatment improved necrosis and prevented RD in 6 eyes (29%). Seven eyes (33%) obtained anatomical success, but retinal redetachment occurred in 8 eyes (57%). There were also 5 eyes (24%) developed ocular hypotony or atrophy. Ten eyes (48%) achieved equal or increased visual acuity. In the prophylactic vitrectomy group, RD occurred in 2 eyes (13%). Twelve eyes (75%) were completely anatomically successful, and 10 eyes underwent silicone oil removal. Only one eye (6%) became ocular hypotony. Fourteen eyes (88%) achieved equal or increased visual acuity. The prophylactic vitrectomy group achieved better vision trends than the routine treatment group (P<0.05). Eyes with peripheral necrosis had better visual outcomes than those with mid-peripheral (P<0.05) or extensive (P<0.05) necrosis. However, there was no significant difference between eyes with mid-peripheral and extensive necrosis (P=0.3008)
CONCLUSION
Prophylactic vitrectomy can prevent RD and improve the prognosis of ARN, making it an option for cases with rapidly progressing necrosis despite antiviral treatment and cases with moderate to extensive necrosis and severe vitreous opacity.
Keywords: acute retinal necrosis, prophylactic vitrectomy, retinal detachment, visual acuity
INTRODUCTION
Acute retinal necrosis syndrome (ARN), caused by the human herpes virus[1]-[3], has devastating consequences for eyes. The clinical features of this sight-threatening disease are typically diffuse whitish necrotic lesions, retinal vasculitis, and various degrees of intraocular inflammation. Antiviral medication and vitrectomy after retinal detachment is the conventional therapy. However, retinal breaks and subsequent rhegmatogenous retinal detachment (RRD) frequently occur[4], causing loss of vision[5]. Prophylactic laser photocoagulation has been advocated to prevent retinal detachment[6], but its use is limited by vitreous opacity and complications such as triggering retinal detachment[7]. Therefore, outcomes remain unsatisfactory.
There are recent reports of performing prophylactic vitrectomy during the inflammatory stage in the UK, Turkey, and Japan[8]-[10] to prevent RRD in cases with ARN. Prophylactic vitrectomy may help to eliminate inflammatory factors and prevent lesions from occurring and extending, thereby reducing the incidence of retinal detachment (RD) secondary to ARN. However, there is no consensus on its efficacy and surgical indications. The aim of this study is to evaluate the efficacy of prophylactic vitrectomy in comparison with routine treatment and assess the necessity of prophylactic vitrectomy in Chinese patients.
SUBJECTS AND METHODS
Subjects
This was a retrospective study of patients who had received treatment for ARN at the Second Xiangya Hospital of Central South University between February 2006 and April 2011. A total of 36 immunocompetent subjects were identified, 6 of which were excluded due to incomplete data. Of the 30 patients included, 21 (70%) were male and 9 (30%) were female. Their mean age was 46 years with a range of 23-66 years. Seven patients (23%) presented with bilateral involvement. The follow-up period ranged from 8 to 57 months (mean of 25 months). This study was approved by the Medicine Human Ethics Committee of the Second Xiangya Hospital of Central South University.
ARN diagnosis was made based on clinical evidence following the American Uveitis Society Diagnostic Criteria (1994)[11]. Viral serological testing at the initial phase or antiviral antibody titer of vitreous samples was performed for all patients. Herpes simplex virus (HSV) 1/2 was the infectious agent in 14 patients, varicella zoster virus (VZV) was present in 13 patients, two cases had Epstein-Barr virus (EBV). The infectious agent was unknown in 1 patient. Supplementary examinations such as B-ultrasound and fundus fluorescein angiography (FFA) were also performed.
Methods
All patients received antiviral treatment immediately after the clinical diagnosis of ARN. The antiviral regime consisted of intravenous acyclovir (15mg/kg, 3 times daily) for 10 days followed by oral acyclovir (200mg, 5 times per day) for over 6 weeks, even after surgery. Systemic dexamethasone, oral aspirin (100mg daily), and topical cycloplegic were also administered. Vitrectomy and silicone oil tamponade was performed on 14 eyes after RD secondary to ARN, and on 16 eyes which respond not well to about one week of antiviral medication.
The eyes were divided into 2 groups according to the treatment received, including routine treatment, which consisted of antiviral medication and vitrectomy after RD (n=21), and prophylactic vitrectomy, which consisted of antiviral medication and vitrectomy for the prevention of RD performed during the active inflammatory phase (n=16).
Vitrectomy was performed using a 20-gauge standard three-port system. Moderate to advanced cataracts were resected during the initial surgery while mild cataracts and transparent lenses were preserved. Vitreous was adequately resected. Membrane peeling was carried out in all cases. Endolaser photocoagulation was applied around the retinal breaks and behind the junction of the normal and affected retina. Silicone oil with a viscosity of 5700cs was injected into the vitreous cavity after gas/fluid exchange. All surgical procedures were successful in achieving retinal reattachment.
The extent of necrosis was determined by fundus photographs at the time of presentation (for eyes with mild vitreous opacity) or the drawings in the operation records. Fundus photography was performed by the same operator using a retinal camera (Topcon TRC 50EX; Topcon Corporation, Tokyo, Japan). Nine color photographs of each eye were taken using up to 80° fields of the fundus before fundus fluorescein angiography. Using Holland's classification for cytomegalovirus retinopathy[12], the necrosis was divided into 3 grades, including peripheral, in which the necrosis extended from the ora serrata to the ampulla of vortex veins, extensive, in which the necrosis extended to the posterior pole (within 2 disc diameters from the fovea or 1 disc diameter from the optic nerve head), and middle-peripheral, in which the extent of necrosis was between the two other grades.
Statistical Analysis
Statistical analysis was performed using SPSS for Windows, version 16.0 (SPSS Inc., Chicago, Illinois, USA). Snellen visual acuity was assessed in this study. Differences in visual acuity and necrosis between groups were identified using independent samples t-test.
RESULTS
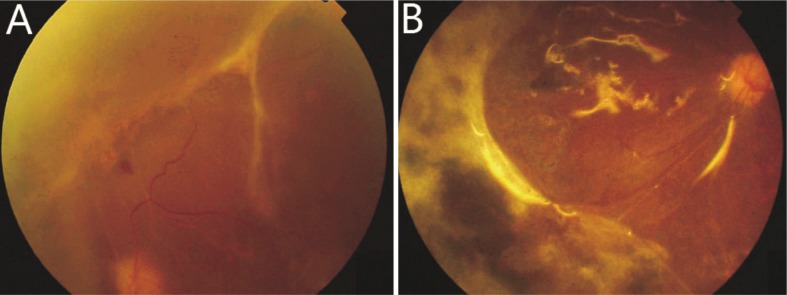
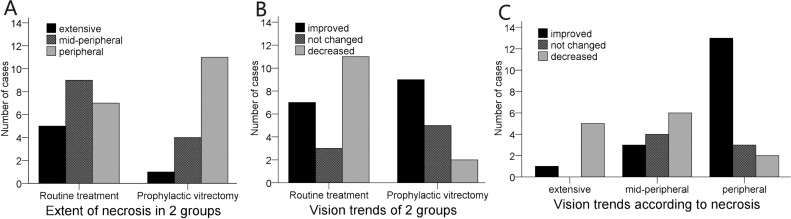
In the routine treatment group, intravitreal injections of acyclovir and prophylactic photocoagulation was performed in eyes presented with mild vitreous opacity at early stage. Necrosis was confined and improved without surgical intervention in 6 eyes. For the other 15 eyes, 2 eyes presented with RD at presentation. Necrosis rapidly progressed and RD developed in 6 eyes. Necrosis improved and inflammation was gradually alleviated, but RD emerged after 2-4 months (Figure 1A) in 7 eyes. The mean duration of symptoms before RD developed in the 15 eyes was 46 days. Vitrectomy and silicone oil tamponade was performed immediately after RD was identified, except one patient who refused any surgical intervention. In the prophylactic vitrectomy group, three eyes with wide range of necrosis and severe vitreous opacity underwent prophylactic vitrectomy soon after presentation. The other 13 eyes in this group did not respond well to one week or more of conservative treatment and also received prophylactic vitrectomy. The duration of symptoms before surgery in this group ranged from 7 to 62 days, with a mean of 24 days. Retinal breaks were found in 5 of the eyes during surgery, among which 3 eyes had partial retinal detachment. It is worth mentioning that one patient who underwent remedial vitrectomy in her left eye developed right eye involvement after 5 months. Prophylactic vitrectomy was subsequently performed on her right eye. Necrosis was more extensive in the routine treatment group than in the prophylactic vitrectomy group (P<0.05, Figure 2A).
Figure 1. Retinal detachment after conservative treatment and after remedial vitrectomy.
Figure 2. Extent of necrosis and vision trends of the 2 groups.
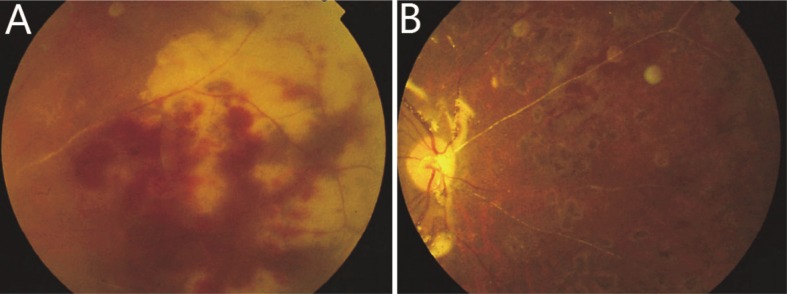
During the follow-up period, proliferative vitreoretinopathy (PVR) arose in most of the 37 eyes and required additional treatments (Table 1). In the routine treatment group, severe proliferation and retinal redetachment occurred in 9 (43%) eyes (Figure 1B), one of which developed a macular hole. Additional surgical procedures, including membrane peeling and silicone oil replacement, were performed on 8 eyes. However, the macular hole remained unclosed by the end of follow-up. Retinal breaks were observed in 2 eyes and supplementary photocoagulation was performed instead of any further surgical procedure. Two eyes (10%) became completely blind due to ocular atrophy, including the patient who refused surgical intervention, for whom retinal break and subsequent RRD occurred in 2 months, and perception of light was lost in the fourth month. There were also 3 eyes (14%) developed ocular hypotony and retained only light perception. In the prophylactic vitrectomy group, necrosis was controlled in all 16 eyes (Figure 3). Membrane proliferation developed in 9 eyes, of which extensive RD recurred in 2 eyes (13%). Membrane peeling and silicone oil replacement was performed in these 2 eyes and one eye with severe fibrous proliferation. Retinal break was observed in 5 eyes and supplementary photocoagulation was performed. Ocular hypotony occurred in 1 eye (6%), which maintained perception of light.
Table 1. Complications and additional treatments in two treatment groups during the follow-up period.
| Groups | PVR | RD | Re-vitrectomy | Secondary glaucoma | Cataract | Phaco | IOL | SO removal | BSK | Optic atrophy | Ocular atrophy /Hypotony | NPL |
| Routine treatment | 18 | 9 | 8 | 1 | 15 | 4 | 1 | 1 | 5 | 13 | 5 | 2 |
| Prophylactic vitrectomy | 9 | 2 | 3 | 4 | 11 | 9 | 5 | 10 | 5 | 5 | 1 | 0 |
| Total | 27 | 11 | 11 | 5 | 26 | 13 | 6 | 11 | 10 | 18 | 6 | 2 |
PVR: proliferative vitreoretinopathy; RD: retinal detachment; Phaco: phacoemulsification; IOL: intraocular lens; SO: silicone oil; BSK: band-shaped keratopathy; NPL: no perception of light.
Figure 3. Retinal necrosis, occlulsive arteritis and retinal hemorrhage before and after prophylactic vitrectomy.
Other complications such as cataracts, optic atrophy, and ocular atrophy also developed. In the routine treatment group, complicated cataract removal was initially performed in one eye and artificial lens implantation was not performed during the follow-up period. Cataracts developed to varying degrees as later complications in 15 eyes. Phacoemulsification was performed in 4 eyes for epiretinal membrane and/or RD. Only one with complete anatomical success underwent posterior chamber intraocular lens implantation. Secondary glaucoma arose in 1 eye and the intraocular pressure returned to normal after silicone oil removal. Cataracts developed in 11 eyes in the prophylactic vitrectomy group. Phacoemulsification was performed in 9 eyes, 5 of which also underwent artificial lens implantation. Secondary glaucoma developed in 4 eyes and intraocular pressure was controlled by short-term treatment with antiglaucoma eye drops.
Silicone oil removal was only performed in cases with satisfactory retinal reattachment. In order to avoid RD, supplementary photocoagulation was applied before the operation. For the 14 eyes received vitrectomy in routine treatment group, one eye (7%) with complete anatomical success underwent silicone oil removal. However, silicone oil could not be removed from 12 eyes (86%) despite variable severity of emulsification due to the high risk of RD. For the prophylactic vitrectomy group, 10 eyes (63%) underwent silicone oil removal and kept anatomical success for 3-15 months.
The initial and final best corrected visual acuity (BCVA) for two groups were listed in Table 2. The prophylactic vitrectomy group achieved better vision trends than the routine treatment group (P<0.05, Figure 2B). Eyes with peripheral necrosis had better visual outcomes than those with mid-peripheral (P<0.05) or extensive (P<0.05) necrosis. However, there was no significant difference between eyes with mid-peripheral and extensive necrosis (P=0.3008, Figure 2C).
Table 2. Initial and final best corrected visual acuity of two treatment groups.
| Group | NPL | LP-20/250 | 20/200-20/63 | 20/50-20/32 | 20/25- |
| Routine treatment (initial) | 0 | 11 | 7 | 3 | 0 |
| Routine treatment (final) | 2 | 12 | 3 | 4 | 0 |
| Prophylactic vitrectomy (initial) | 0 | 4 | 6 | 6 | 0 |
| Prophylactic vitrectomy (final) | 0 | 4 | 7 | 3 | 2 |
NPL, no perception of light; LP, light perception.
DISCUSSION
While antiviral agents as the first line of therapy for necrotizing retinitis[13] can be effective, sensitivity to these agents[14] varies widely among individuals. Cases with severe inflammation are prone to progress and not respond well to medication. Retinal lesions may extend circumferentially towards the posterior pole regardless of adequate antiviral treatment and anti-inflammatory therapy with corticosteroids[15]. Therefore, retinal tears often develop and result in RD even after the resolution of ARN[16]. In our study, RD arose in 18 eyes, even though there was short-term alleviation of necrosis in 7 eyes, within 10-107 days. It has been reported[5],[17] that RD occasionally emerges several months or even years after onset. Conservative treatment improved necrosis and prevented RD in only 6 eyes (29%) in routine treatment group. However, the necrosis in the 6 eyes was mainly located at the peripheral retina and vitreous inflammation was also mild.
Prophylactic photocoagulation has been proposed to prevent RD secondary to ARN[18]. However, examination of fundus is unable to proceed at the severe stage due to dense vitreous debris. Retinal breaks that occur anteriorly at the vitreous base are difficult to be detected and, when observed, cannot get photocoagulation. It is also difficult to obtain laser facula in the area of exudation. Furthermore, prophylactic photocoagulation for managing ARN remains controversial, it has been found to be unhelpful in manipulation of the progression of retinitis[17],[19]. It has also been reported that prophylactic laser photocoagulation does not contribute to a lower detachment rate[20]. In our study, prophylactic photocoagulation was performed in 13 eyes. However, necrosis did not improve and RD developed in all of them. The one patient who chose photocoagulation rather than surgery intervention for RD developed extensive necrosis and became completely blind. Moreover, laser photocoagulation probably triggers retinal traction, possibly resulting in retinal detachment[21], which makes it unfeasible for treatment of ARN.
Since ARN threatens vision at every moment after onset and antiviral medication and prophylactic photocoagulation are frequently unable to reduce the inflammatory processes and prevent retinal detachment, it is necessary to take more active measures in this ophthalmological emergency. Vitrectomy can achieve anatomical success after RD in ARN[21],[22]. When RD occurs, the accompanying occlusive vasculitis leads to circulatory damage in the retinal tissue surrounding necrotic foci and subsequent ischemia[15]. The affected retina becomes seriously impaired and, even when healed, is thinned and atrophic[19]. Postoperative epiretinal proliferation and associated retinal traction remain the most important causes of RD after surgery. Therefore, remedial vitrectomy performed after RD, which shows efficacy in controlling disease activity, is not sufficient for satisfactory prognosis. In our study, nine of the 16 eyes (56%) received remedial vitrectomy developed partial to extensive recurrent RD. Further surgical intervention was needed and the final visual acuity of almost all them was below 20/200. Since vitrectomy for repairing retinal detachment was unable to achieve a satisfactory success rate and visual acuity, the therapeutic strategy for ARN should be to prevent retinal detachment. Prophylactic vitrectomy releases the retinal tissue from traction and contributes to early elimination of inflammatory factors and clearing of necrosis[21], it may play an important role in the treatment of ARN.
This study analyzed the surgical results of ARN patients that underwent different clinical courses. We found that prophylactic vitrectomy significantly improved the anatomical and visual prognoses. Before surgery the majority of the 16 eyes in the prophylactic vitrectomy group displayed poor prognostic signs such as severe vitreous opacity, occlusive vasculitis, and PVR. Though 75% of eyes achieved complete anatomical success, 62% of eyes received silicone oil removal during the follow-up, and no eye became completely blind. For the patient with 2 eyes treated under different strategies, the right eye that received prophylactic vitrectomy obtained better BCVA compared to the left eye. Cases without RD did better after prophylactic vitrectomy compared with those that underwent remedial vitrectomy after RD. Therefore, vision trends instead of visual acuity were also used for comparison. Eighty-seven percent of the eyes in the prophylactic vitrectomy group achieved equal or better BCVA, which was higher than that of the routine treatment group (48%). Even though 6 eyes in the routine treatment group responded well to the conservative treatment did not need surgery, only four of them achieved equal or better BCVA.
Sims et al[20] found no difference in visual outcome according to the extent of necrosis. However, in our study the visual outcome tended to decrease as necrosis spread (Figure 2C). There were 0, 3, and 16 eyes with retinitis of extensive, mid-peripheral, and peripheral stages that achieved useful vision (≥20/200), respectively. There was a higher proportion of eyes (67%) with mid-peripheral or extensive necrosis in the routine treatment group than in the prophylactic vitrectomy group (31%), which inevitably influenced the relatively poor visual prognosis of that group. These data suggest that surgical intervention for marginal or finite lesions result in better vision than for extensive or posterior lesions. Matsuo et al[23] also found that the extent of necrosis plays an important part in visual prognosis.
Examination of ocular fundus is often impeded by severe vitreous opacity and exudations in ARN. It is usually difficult to evaluate the degree of damage of the retina and identify the appropriate treatment. In this study, retinal breaks and/or partial retinal detachment was found in 5 eyes during the prophylactic vitrectomy. Since RD can occur without being identified, remedial vitrectomy may be too late for this rapidly progressing complication. Prophylactic vitrectomy that resects the vitreous and clears lesions at the inflammatory stage contributes to the prevention and treatment of retinal detachment as well as helps to avoid further damage.
The prognosis of ARN mainly depends on the duration of onset to treatment. However, visual outcome in ARN is often poor regardless of prompt diagnosis and treatment[24]. In this study, 51% of eyes were unable to gain useful vision, including 2 eyes that became completely blind. The dissatisfactory outcome urges earlier surgical intervention in severe cases of ARN instead of vitrectomy after vulnerable retina tissue has been destroyed.
This study has some limitations. First, we compared the efficacy of 2 treatment strategies based on the results of 37 eyes from 30 patients. More cases of ARN are needed for greater statistical power. Furthermore, follow-up time varies among the patients. Complications are more likely to develop in cases with a longer follow-up. Finally, the question of whether a quantitative standard of indications for prophylactic vitrectomy can improve prognosis needs to be investigated. Further multicenter, randomized, controlled clinical trials are needed to resolve these issues.
In conclusion, prophylactic vitrectomy contributes to the prevention of retinal detachment and improved prognosis of ARN. For cases with necrosis that rapidly progresses despite antiviral treatment and cases with moderate to extensive necrosis and severe vitreous opacities, prophylactic vitrectomy during the inflammatory stage should be considered.
REFERENCES
- 1.Culbertson WW, Blumenkranz MS, Haines H, Gass DM, Mitchell KB, Norton EW. The acute retinal necrosis syndrome. Part 2: Histopathology and etiology. Ophthalmology. 1982;89(12):1317–1225. doi: 10.1016/s0161-6420(82)34638-2. [DOI] [PubMed] [Google Scholar]
- 2.Culbertson WW, Blumenkranz MS, Pepose JS, Stewart JA, Curtin VT. Varicella zoster virus is a cause of the acute retinal necrosis syndrome. Ophthalmology. 1986;93(5):559–569. doi: 10.1016/s0161-6420(86)33701-1. [DOI] [PubMed] [Google Scholar]
- 3.Tran TH, Rozenberg F, Cassoux N, Rao NA, LeHoang P, Bodaghi B. Polymerase chain reaction analysis of aqueous humour samples in necrotising retinitis. Br J Ophthalmol. 2003;87(1):79–83. doi: 10.1136/bjo.87.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clarkson JG, Blumenkranz MS, Culbertson WW, Flynn HW, Jr, Lewis ML. Retinal detachment following the acute retinal necrosis syndrome. Ophthalmology. 1984;91(12):1665–1668. doi: 10.1016/s0161-6420(84)34107-0. [DOI] [PubMed] [Google Scholar]
- 5.Fisher JP, Lewis ML, Blumenkranz M, Culbertson WW, Flynn HW, Jr, Clarkson JG, Gass JD, Norton EW. The acute retinal necrosis syndrome. Part 1: Clinical manifestations. Ophthalmology. 1982;89(12):1309–1316. doi: 10.1016/s0161-6420(82)34628-x. [DOI] [PubMed] [Google Scholar]
- 6.Sternberg P, Jr, Han DP, Yeo JH, Barr CC, Lewis H, Williams GA, Mieler WF. Photocoagulation to prevent retinal detachment in acute retinal necrosis. Ophthalmology. 1988;95(10):1389–1393. doi: 10.1016/s0161-6420(88)32999-4. [DOI] [PubMed] [Google Scholar]
- 7.Park JJ, Pavesio C. Prophylactic laser photocoagulation for acute retinal necrosis. Does it raise more questions than answers? Br J Ophthalmol. 2008;92(9):1161–1162. doi: 10.1136/bjo.2008.147181. [DOI] [PubMed] [Google Scholar]
- 8.Berker N, Ozdal P, Batman C, Soykan E. Prophylactic vitrectomy in acute retinal necrosis syndrome. Eye (Lond) 2007;21(1):104–106. doi: 10.1038/sj.eye.6702410. [DOI] [PubMed] [Google Scholar]
- 9.Lau CH, Missotten T, Salzmann J, Lightman SL. Acute retinal necrosis features, management, and outcomes. Ophthalmology. 2007;114(4):756–762. doi: 10.1016/j.ophtha.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 10.Ishida T, Sugamoto Y, Sugita S, Mochizuki M. Prophylactic vitrectomy for acute retinal necrosis. Jpn J Ophthalmol. 2009;53(5):486–489. doi: 10.1007/s10384-009-0698-z. [DOI] [PubMed] [Google Scholar]
- 11.Holland GN. Standard diagnostic criteria for the acute retinal necrosis syndrome. Executive Committee of the American Uveitis Society. Am J Ophthalmol. 1994;117(5):663–667. doi: 10.1016/s0002-9394(14)70075-3. [DOI] [PubMed] [Google Scholar]
- 12.Holland GN, Buhles WC, Jr, Mastre B, Kaplan HJ. A controlled retrospective study of ganciclovir treatment for cytomegalovirus retinopathy. Use of a standardized system for the assessment of disease outcome. UCLA CMV Retinopathy. Study Group. Arch Ophthalmol. 1989;107(12):1759–1766. doi: 10.1001/archopht.1989.01070020841024. [DOI] [PubMed] [Google Scholar]
- 13.Blumenkranz MS, Culbertson WW, Clarkson JG, Dix R. Treatment of the acute retinal necrosis syndrome with intravenous acyclovir. Ophthalmology. 1986;93(3):296–300. doi: 10.1016/s0161-6420(86)33740-0. [DOI] [PubMed] [Google Scholar]
- 14.Tam PM, Hooper CY, Lightman S. Antiviral selection in the management of acute retinal necrosis. Clin Ophthalmol. 2010;4:11–20. [PMC free article] [PubMed] [Google Scholar]
- 15.Usui Y, Goto H. Overview and diagnosis of acute retinal necrosis syndrome. Semin Ophthalmol. 2008;23(4):275–283. doi: 10.1080/08820530802111325. [DOI] [PubMed] [Google Scholar]
- 16.Matsuo T. Vitrectomy and silicone oil tamponade as an initial surgery for retinal detachment after acute retinal necrosis syndrome. Ocul Immunol Inflamm. 2005;13(1):91–94. doi: 10.1080/09273940490518838. [DOI] [PubMed] [Google Scholar]
- 17.Duker JS, Blumenkranz MS. Diagnosis and management of the acute retinal necrosis (ARN) syndrome. Surv Ophthalmol. 1991;35(5):327–343. doi: 10.1016/0039-6257(91)90183-g. [DOI] [PubMed] [Google Scholar]
- 18.Meghpara B, Sulkowski G, Kesen MR, Tessler HH, Goldstein DA. Long-term follow-up of acute retinal necrosis. Retina. 2010;30(5):795–800. doi: 10.1097/IAE.0b013e3181c7013c. [DOI] [PubMed] [Google Scholar]
- 19.Lightman S. Acute retinal necrosis. Br J Ophthalmol. 1991;75(8):449. doi: 10.1136/bjo.75.8.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sims JL, Yeoh J, Stawell RJ. Acute retinal necrosis: a case series with clinical features and treatment outcomes. Clin Experiment Ophthalmol. 2009;37(5):473–477. doi: 10.1111/j.1442-9071.2009.02083.x. [DOI] [PubMed] [Google Scholar]
- 21.Kawaguchi T, Spencer DB, Mochizuki M. Therapy for acute retinal necrosis. Semin Ophthalmol. 2008;23(4):285–290. doi: 10.1080/08820530802111192. [DOI] [PubMed] [Google Scholar]
- 22.McDonald HR, Lewis H, Kreiger AE, Sidikaro Y, Heckenlively J. Surgical management of retinal detachment associated with the acute retinal necrosis syndrome. Br J Ophthalmol. 1991;75(8):455–458. doi: 10.1136/bjo.75.8.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuo T, Morimoto K, Matsuo N. Factors associated with poor visual outcome in acute retinal necrosis. Br J Ophthalmol. 1991;75(8):450–454. doi: 10.1136/bjo.75.8.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muthiah MN, Michaelides M, Child CS, Mitchell SM. Acute retinal necrosis: a national population-based study to assess the incidence, methods of diagnosis, treatment strategies and outcomes in the UK. Br J Ophthalmol. 2007;91(11):1452–1455. doi: 10.1136/bjo.2007.114884. [DOI] [PMC free article] [PubMed] [Google Scholar]