Abstract
AIM
To investigate the independent pathogenic role of high serum gamma-glutamyl transferase (GGT) levels, sociodemographic data, dietary and environmental risk factors for visual disability (VD).
METHODS
This was a case-control study, run in 200 black Congolese patients managed in Saint Joseph Hospital Ophthalmology Division from Kinshasa town. Logistic regression model was used to identify determinants of VD (n=58) among sex, age, cigarette smoking, alcohol abuse, rural-urban migration, education levels, aging ≥60 years, intake of red Beans, Safou fruit and Taro leaves, lipid profile, residence, socioeconomic status, and GGT.
RESULTS
After adjusting for confounding factors, we identified migration (OR=3.7 95% CI: 1.2-11.3; P=0.023), low education level (OR=3.1 95% CI 1.1-8.5; P=0.026), no intake of Safou fruit (OR=34.2 95% CI 11.5-102; P<0.0001), age ≥ 60 years (OR=2.5 95% CI 1.01-6.5; P=0.049), and serum GGT ≥10 U/L (OR=3.6 95% CI 1.3-9.6; P=0.012) as the significant and independent determinants of VD.
CONCLUSION
VD appears as a major public health problem in Central Africa to be prevented or delayed by control of migration, lifestyle changes, antioxidant supplements, appropriate diet, nutrition education, and blocking of oxidative stress.
Keywords: oxidative stress, serum gamma-glutamyl transferase, intake of tropical food plants, aging, vision loss
INTRODUCTION
In healthy individuals, the eye needs specific nutrients to prevent oxidative stress and maintain normal vision. Aging, a marker of oxidative stress, reduces the levels of these nutrients, and the eyes' ability to remove free radicals.
Serum gamma-glutamyl transferase (GGT), enzyme mostly derived from the liver and present on cell surfaces and in serum, contributes to the extracellular catabolism of glutathione (GSH)[1]. Serum GGT is closely related to visual dysfunction and optic nerve degeneration in animal and human studies[2]. Endeed, serum GGT is closely correlated with fatty liver, and might be a marker of high risk of type 2 diabetes mellitus (T2DM) and metabolic syndrome (MetS)[2]-[5]. GGT is also a marker of intra-abdominal obesity[6] and increase in oxidative stress in combination with ferritin (another marker of oxidative stress)[7]. GGT-mediated cleavage of glutathione (GSH) could initiate a redox-cycling mechanism resulting in the production of the superoxide anion and hydrogen peroxide.
Other reports from the literature showed a positive and significant association between serum GGT and dietary factors in young vs adults with Coronary Artery Risk development[8]. Both oxidative stress and low intake of vegetables are related to T2DM, MetS, atherosclerosis, and diabetic ocular complications[9]. The essential antioxidants capable to keep ocular tissues healthy are exogenous products from diet or dietary supplements. The eye requires on a daily basis to eat a variety of wholesome and nutritious foods. Dark green leafy vegetables and vividly colored fruits are the best sources of nourishing antioxidants for eyes. The traditional diets of rural Congolese people, similar with that of our ancestors is replete of foods rich in vegetables, fibers and fruits. But in both rural and urban Kinshasa Hinterland[10], facing nutrition transition, most people do not get enough eye-protecting nutrients from their diet.
In Kinshasa town characterized by few green spaces and trees umbrellas, they need an active antioxidant system for protection against oxidative stress generated by light exposure, other external oxidants, and free radicals generated with cells during metabolic processes. In Kinshasa city, the capital of Democratic Republic of Congo (DRC), the majority of residents are migrants and facing epidemiologic, demographic (aging, another marker of oxidative stress), and nutrition transition[11]. Their diet is reduced in vegetables and fruits content and it contains large amount of salt, refined sugar, fat, and starches that set off unpredictable blood glucose fluctuations, increasing the risk of metabolic syndrome (Mets), type 2 diabetes mellitus (T2DM) and diabetic complications such as cataract[11],[12].
In Congolese diet, red Beans consumption is popular as a “meat” of poor, while Taro leaves and Safou fruit are uncommonly consumed in urban areas.
Colocasia Antiquorum (Taro leaves) or imperial Taro spreads freely in rich, wet soils; more slowly in dry clay soil. It adds bold color and tropical flair to any garden (Figure 1). This leafy green vegetable, popular in rural areas, is rich in antioxidants[13]. It contains anti-lipid peroxidative activity found in leaf juice. The seasonal consumption of Safou prune/Africa pear (Dacryodes edulis), with variety parvicarpa (small, rounded) and variety edulis (large, elongated and cylindrical), a vividly fruit rich in antioxidant[14],[15] is only for affluent people (Figure 2). In Congolese people, red beans (Figure 3) are well established as a protective intake against MetS[12] and against cataract[11] and other causes of visual disability.
Figure 1. Taro leaves (Colocasia Antiquorum).

Figure 2. Different species of Safou prune (Dacryodes edulis).
A: D.edulis var.paricarpa.; B: D.edulis var.edulis.
Figure 3. Red beans.

Visual disability (VD) remains a public health problem in general Central African population (Cameroon) and among patients with diabetes mellitus (DM)[16],[17]. Most of the causes of VD among these Africans are preventable[18]. However, the elimination of this preventable disability causes by the year 2020 as recommended by the World Health Organization (WHO) “VISION 2020 initiative, the Right to Sight” is questionable for Central Africa including DRC, our Country. Up to now, no African published data addressed these issues. Therefore, the present study was initiated to investigate the independent pathogenic role of elevated serum GGT, sociodemographic data, dietary and environmental risk factors for VD among urban Congolese people. This was a descriptive, analytic and hospital-based survey conducted in Kinshasa town, DRC, from July to September 2010, from a case-control study. Kinshasa, the largest city (7 million inhabitants) enjoys a tropical climate and constitutes a Hinterland with 4 administrative districts (Mont Amba, Funa, Lukunga, and Tshangu). Permission for the study was obtained from the authorities of St Joseph Hospital in Kinshasa Limete, DRC. The study protocol was approved by the University of Kinshasa Medical Ethics Committee and the study was performed in full compliance with the Declaration of Helsinki II.
SUBJECTS AND METHODS
Subjects
Two hundred black Congolese patients managed in Saint Joseph Hospital Ophthalmology Division from Kinshasa town were included in this study, and these cases were matched for gender and age.
Methods
The structured and standardized questionnaire, administered to each participant during 30 minutes, sought relevant information on age, gender, rural-urban migration, residence, education level, cigarette smoking, alcohol intake, socioeconomic status (SES), ethnicity, and diet. To assess dietary intake, a-24 hour recall of eating Colocasia Antiquorum (Yes or No) and 10-month recall of eating Safou (Dacryodes edulis; Yes or No) was used.
Laboratory data
Blood samples were collected as per the Clinical and Laboratory Standards Institute (CLSI) document[19]. Samples were collected in one heparanised vacutainer (4mL) and one plain vacutainer (4mL) for each participant after a 10-12 hours' overnight fasting. They were immediately analysed at Lomo Central Laboratories, Kinshasa Limete, DRC, for cardiometabolic risk (glucose, HbA1c, Insulin, total cholesterol, triglycerides, LDL-C, HDL-C, non-HDL, VLDL, Total cholesterol/HDL ratio, triglycerides/HDL ratio, LDL/HDL ratio), certain antioxidant markers (uric acid, Albumin), and GGT oxidative stress marker.
Fasting plasma glucose (FPG) levels were measured using glucose-oxidase method. The levels of C-peptide were measured by solid phase two-site enzyme immunoassay (ELISA) based on the direct sandwich technique in which two monoclonal antigenic determinants on the C-peptide molecules (Mercodia AB, Sylveniusgaton 8A, SE 75 450, Uppsala, Sweden). Plasma insulin was also assayed using Mercodia kits. Enzyme activities for serum GGT were determined according to standard laboratory procedures. Serum total cholesterol (TC) for CHOD-PAP Method, triglycerides (TG) for GPO-PAP method, high-density lipoprotein-cholesterol (HDL-C) for IRC method, uric acid, and low-density lipoprotein cholesterol (LDL-C) were measured using enzymatic colorimetric kits (Biomerieux, Marcy l'Etoile, France).
The laboratory tests in LOMO Medical Laboratories were assayed using an automatic analyzer (Hospitex Diagnostics, Florence, Italy). Glycated hemoglobin fractions were measured in fresh anticoagulated blood samples with both migration set-up using a semi-automated multi-parameter instrument (HYDRASYS system, Serbia, Evry, France) and densitometric scanning of unstained gels 9HYDRGEL 7/15 Hba1c) performed on HYRYS Densitometer and gel carrier O and specific HbA1c (NGSP) software (HYRYS densitometer, Serbia, Evry, France). After including a control blood sample into each run of samples, relative concentrations (%) of three fractions in each hemolyzate were yielded by the Densitometer scanning as follows: the most cathodic corresponding to the minor glycated hemoglobin A1c (HbA1c), and the most anodic being the main fraction containing Ao and A2 hemolgobins.
Levels of TBARS were measured from serum samples were measured at the Analytical Chemistry Laboratory of the Walter Sisulu University, Mthatha, South Africa, and expressed as mmol/L[19]. Commercial Cayman's Kits (Cayman Chemical Company, Ann Arbor, MI, USA) were used to measure. For that, samples were run simultaneously with quality control samples of pooled serum nonstudy normal healthy people. If analysis of the quality control TBARS level varied from ±2SD of a mean determined by a total of 60 runs, and between-week runs, the analysis was deemed unacceptable and was repeated. The very low density lipoprotein cholesterol (VLDL-C) was calculated based on the Friedewald equation[20] and excluding participants with TG ≥400mg/dL : VLDL=TG/5.
Eye examination
All participants in this study underwent a routine eye examination. Eye examination of each participant included visual acuity (VA) measurement, ocular alignment and motility, pupil reactivity and function, visual fields, intraocular pressure measurement (mmHg), slit-lamp examination of the cornea, iris, lens and vitreous, and dilated funduscopic evaluation. This fundus examination was detailed and performed at the best possible mydriasis, after dilating the pupils with tropicamide (1%) and phenylephrine (10%), by indirect ophthalmoscopy at the slit lamp (Haag Streit 900) with 90 D lens.
Participants were refracted with the use of standard subjective refraction techniques. VA was measured under similar lighting conditions by an ophthalmologist. VA was measured separately for each eye and was defined according to the lowest line on the chart for which the majority of letters were read correctly, with the full required distance correction as determined by the decimal optometric scale.
Longer DM duration was ≥5 years (the median and quartile value). Cardiometabolic risk was defined by the variations of the mean values of age (aging), BMI, TC, 10-year absolute cardiovascular risk (10-Year ACVR) according to Framingham equation, TC/HDL ratio, Non-HDL (TC-HDL-C), TG/HDL ratio, LDL/HDL ratio, VLDL, HDL-C, LDL-C, TG, WC, SBP, DBP, and HOMA-IR.
The presence of VD included participants with either bilateral blindness or visual impairment. Visual disability (VD) was defined as blindness and visual impairment using the World Health Organization (WHO) definition and the recommendation for the revision of classification[21]. Blindness was defined as VA < 0.1 (<6/60) and visual impairment (low vision) was defined as VA <0.3 ≥0.1 (< 6/18 ≥6/60). Normal vision was defined by a VA between 1.0 and 0.3 (6/6-6/18). Aging was defined as age ≥60 years. Uncontrolled diabetes was defined by fasting plasma glucose ≥126mg/dL at evaluation. Self-reported ethnicity included Kongo tribe from South-West of DRC, Ngala tribe from the North-West, Luba tribe from the Centre and Swahili people from the Eastern part of DRC. According to the 4 administrative districts of Kinshasa town, the environment of residence of participants was defined as rural for Lukunga and Tshangu districts and urban for Funa and Mont Amba districts.
The education level included illiteracy, primary school, high school, and university levels. Low education level was defined as illiteracy and primary school levels. High education level included secondary school (High school) and university levels. Socioeconomic status (SES) included low (lack of income, unemployment) and high SES. Imbalance of Oxidant/Antioxidant Status was defined by increase in mean values of the assayed biomarkers of oxidative stress or their elevated levels ≥ mean values with the presence of VD, and decrease in mean values or their elevated levels < mean values for the antioxidant markers with the presence of DR. The imbalance of Oxidant/Antioxidant status was also defined by the negative correlations between important independent determinants of oxidant and antioxidant markers of VD in all participants.
Statistical Analysis
Qualitative data were reported as frequency and proportions (%). Continuous data were expressed as mean±SD. In univariate analysis, the Chi-square was used to compare proportions, comparisons of means between groups were performed using the Student's t-test. The univariate risk of VD was assessed in calculating Odds ratio (OR) with 95% confidence intervals (95% CI). Multivariate analysis such as logistic regression model, was used to assess the independent effect of selected variables on the presence of VD after adjusting for the effect of other potential confounders.
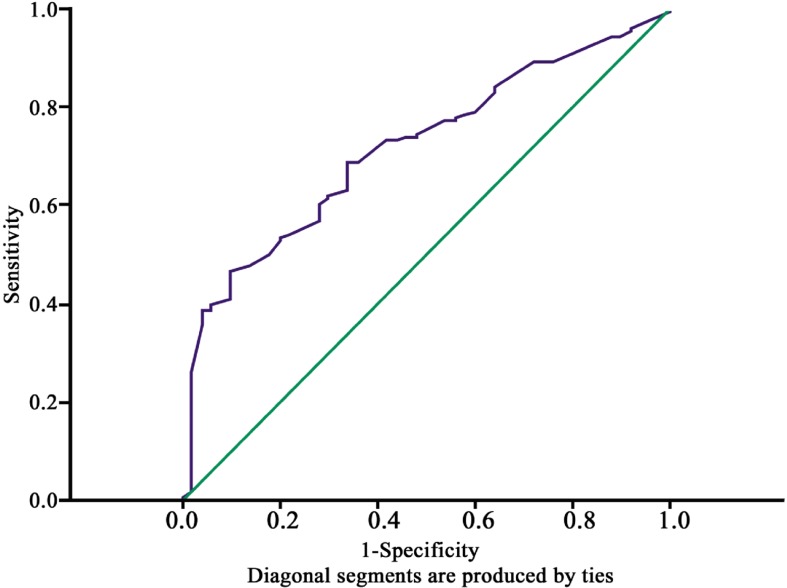
The receiver operating characteristic (ROC) curve for GGT to predict the presence of VD, was plotted (Figure 4). The optimal cutoff point values of serum GGT were calculated by plotting sensitivity against 1-speicifity. Thus, GGT ≥10UI/L was considered as elevated with sensitivity of 70% and specificity of 66%. P<0.05 was considered significant. Data analysis was carried out using the Statistical Package for Social Sciences (SPSS) for Windows version 19 (SPSS Inc., Chicago, IL, USA).
Figure 4. ROC curve of GGT to discriminate presence of visual disability from absence of visual disability: AUC = 0.717 95% CI 0.642-0.793. Standard Error = 0.038; P<0.0001.
RESULTS
Residence, cigarette smoking, SES, ethnicity, lipid profile, TBARS, glucose, diabetes, and alcohol intake did not significantly (P>0.05) influence the presence of visual disability, respectively (Results not shown).
Table 1 showed the significant association between aging, low education level, no intake of Safou fruit, rural-urban migration, no intake of red Beans, elevated GGT, and visual disability, while no intake of Taro leaves and gender did not significantly influence the presence of visual disability.
Table 1. Univariate association between variables and visual disability (VD).
| Variables of interest | Presence of VD |
P | |
| n(%) | OR(95%) | ||
| Age ≥ 60 years | 28(36.8) | 1.8(1.01-3.4) | 0.040 |
| Low education level | 43(33.6) | 1.9(1.02-3.8) | 0.039 |
| No intake of Safou fruit | 40(88.9) | 60.9(21.3-174.3) | <0.0001 |
| Migration | 46(34.6) | 2.4 (1.2-5) | 0.014 |
| No intake of red Beans | 23(43.4) | 1.9(1.01-3.7) | 0.048 |
| No intake of Taro leaves | 43(81.1) | 0.229 | |
| Elevated GGT | 46(38) | 3.4(1.7-7) | <0.0001 |
| Gender | 0.341 | ||
| Males | 31 (34.4) | ||
| Females | 31 (28.2) | ||
After adjusting for gender, red Beans intake, and intake of Taro leaves in multivariate analysis, we identified migration, low education level, no intake of Safou fruit, aging, and elevated GGT as the important, significant and independent determinants of the prevalence of visual disability in these Central Africans (Table 2).
Table 2. Independent role of elevated GGT on VD in Central Africans.
| Independent variables | B coefficient | Standard error | Wald Chi-square | OR (95% CI) | P |
| Migration (yes vs no) | 1.300 | 5.158 | 5.158 | 3.7 (1.2-11.3) | 0.023 |
| Education level (low vs high) | 1.136 | 0.511 | 4.941 | 3.1 (1.1-8.5) | 0.026 |
| Safou fruit (Dacryodes edulis) intake (no vs yes) | 3.533 | 0.557 | 40.191 | 34.2 (11.5-102) | <0.0001 |
| Aging ( ≥60 vs <60) | 0.924 | 0.486 | 3.612 | 2.5(1.01-6.5) | 0.05 |
| Gamma GT (elevated vs normal) | 1.276 | 0.506 | 6.369 | 3.6(1.3-9.6) | 0.012 |
| Constant | -5.081 | 0.836 | 36.893 | <0.0001 |
DISCUSSION
The present study demonstrate a possible implication for oxidative stress, urbanization, poverty, and no intake of fruits-vegetables in the development of visual disability in black dwellers from Kinshasa, DRC, Central Africa. Univariate and multivariate analyses from this study may explain the burden of VD as reported by the literature[11],[17],[18],[22]-[25]. In univariate analysis, advanced age, low education level, no intake of Safou fruit, rural-urban migration, no intake of red Beans, and elevated serum GGT levels were individually and significantly associated with the presence of visual disability. However, in multivariate analysis, no intake of red Beans was no longer a factor associated with the high risk of visual disability. The pathophysiology of visual disability may underlay on possible implication of Oxidant/Antioxidant imbalance through individual action or interaction of aging, migration, low education level, no intake of Safou, and elevated GGT levels. Aging and migration are already known risk factors of metabolic syndrome in Central Africans[12]; metabolic syndrome induces oxidative stress.
Because of epidemiologic transition (emergence of chronic non-communicable diseases such as hypertension, metabolic syndrome, obesity and type 2 diabetes mellitus vs decline in communicable diseases), lifestyle changes with urbanization (physical inactivity = inflammation), demographic transition (longer life span), and advanced nutrition transition[26], these Central Africans may be at higher risk of visual disability.
Indeed, after rural-urban migration, they are exposed to external sources of free radicals from Kinahasa town (one of the African towns mostly polluted by heavy metals and dioxins, WHO, Longo-Mbenza, unpublished data) and ionizing radiation (exposure to lightsun in slums without green spaces).
The study suggests that healthy individual eyes need intake of red Beans[12] and Safou fruit[27], rich in antioxidants and vitamins. No intake of Safou, a seasonal fruit, is explained by its price not affordable by poor people reflected in low education level. Lack of nutrition education may be reflected in low education level. Culturally, African adults do not eat fruits and green vegetables. Aging, however, a condition linked with oxidative stress[28], reduces the amount of nutrients from red Beans and Safou, and the eyes' capability to remove free radicals. The present study showed that serum GGT in its normal range was positively and independently associated with the prevalence of visual disability. This suggest that strong association of serum GGT with vision loss might be explained by a mechanism related to oxidative stress enhanced by antioxidant deficiency from no intake of red Beans and Safou fruits as well as by aging and lifestyle changes[1]-[8]. Reports from the literature indicate that elevated GGT in conditions related to visual disability (anomalies in lens, carotenoids, vitamin E, and type 2 diabetes)[29]-[36]. A subclinical liver dysfunction is not excluded as a risk factor of visual disability. It is known that GGT helps transport vitamin C into cells and is correlated with lifestyles, obesity, dietary carotenoids and other factors associated with oxidative stress[29],[30]. In Germany, elevated serum GGT is a strong risk indicator of all-cause occupational disability[31].
These study findings will impact on the African Curriculum of training ophthalmologists and the current management strategy for vision loss. Training ophthalmologists should also focus on clinical nutrition and oxidative stress. This calls for the implementation of more preventive strategies among these Africans with low education level, low intake of vegetables and fruits.
GGT is not merely a sensitive marker for liver disorders and alcohol abuse, but it may also serve as a risk marker for visual disability which is a great burden and an important public health problem globally[16],[17]. These epidemiological findings encourage researchers to use serum GGT in other oxidative stress-related issues such as aging, diabetic retinopathy, cataracts and glaucoma. It is important to confirm the negative association between serum antioxidants and GGT in Africans as reported in US population[32]. It is necessary to avoid alcohol abuse, cigarette smoking, obesity and cardiovascular diseases which are related with GGT[2]-[8].
The selection of participants in hospital, 24 hour recall of eating Colocasia Antiquorum(Yes or No) and 10-Month recall of eating Safou(Dacryodes edulis: Yes or No) might limit the study to some degree because sampling bias to not be generalized to the general hospital and memory biases. In conclusion, visual disability appears a public health problem in these Central Africans. Findings suggest the possible implications of migration, aging, low education level, inappropriate diet, and oxidative stress in the development of visual disability. Antioxidant supplements, nutrition education, control of rural-urban migration and reducing GGT levels to block oxidative stress are recommended for delayed onset of visual disability in aging people.
Acknowledgments
We thank Mr Simon Stimela Mathabatha in Memoriam, Analytical chemistry Laboratory from Walter Sisulu University, Mthatha, South Africa and the staff of Lomo Laboratories, Kinshasa, Limete, DRC for their assistance with blood samples analyses. We pay tribute to the Division of Ophthalmology, Saint Joseph hospital, Kinshasa, Limete, DRC and all participants for their total support in performing the study.
REFERENCES
- 1.Whitefield JB. Gamma glutamyl transferase. Crit Rev Clin Lab Sci. 2002;38(4):263–355. doi: 10.1080/20014091084227. [DOI] [PubMed] [Google Scholar]
- 2.Perry IJ, Wannamethee SG, Shaper AG. Prospective study of serum gamma-glutamyl transferase and risk of NIDDM. Diab Care. 1998;21(5):732–737. doi: 10.2337/diacare.21.5.732. [DOI] [PubMed] [Google Scholar]
- 3.Nakanishi N, Suzuki K, Tatara K. Serum gamma-glutamyl transferase and risk of metabolic syndrome and type 2 diabetes in middle-aged Japanese men. Diab Care. 2004;27(6):1427–1432. doi: 10.2337/diacare.27.6.1427. [DOI] [PubMed] [Google Scholar]
- 4.Lee DH, Silventoinen K, Jacobs DR, Jr, Jousilahti P, Tuomileto J. Gamma-glutamyl transferase, obesity, and the risk of type 2 diabetes: observational cohort study among 20,158 middle-aged men and women. J Clin Endocrinol Metab. 2004;89(1):5410–5414. doi: 10.1210/jc.2004-0505. [DOI] [PubMed] [Google Scholar]
- 5.Chen T, Ren Y, Liu Y, Lon Y, Zhang X, Yu H, Xu J, Yu T. Serum gamma-glutamyl transferase, ferritin and risk of type 2 diabetes in women from a Chinese minority. Diabetes Research and Clinical Practice. 2010;90(3):352–357. doi: 10.1016/j.diabres.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 6.Stranges S, Dorn JM, Muti P, Freudenheim JL, Farinaro E, Russell M, Nochajski TH, Trevisan M. Body fat distribution, relative weight, and liver enzyme levels: a population-based study. Hepatology. 2004;39(3):754–763. doi: 10.1002/hep.20149. [DOI] [PubMed] [Google Scholar]
- 7.Iciek M, Chwatko G, Rokita H, Bald E, Wlodek L. The effects of modulation of gamma-glutamyl transpeptidase activity in HepG2 cells on thiol homeostasis and caspase-3-activity. Biochim Biophys Acta. 2007;1773(2):201–208. doi: 10.1016/j.bbamcr.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Lee DH, Stffen LM, Jacobs DR., Jr Association between serum gamma-glutamyl transferase and dietary factors : the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Clin Nutr. 2004;79(4):600–605. doi: 10.1093/ajcn/79.4.600. [DOI] [PubMed] [Google Scholar]
- 9.Sundaran RK, Bhaskar A, Vijayalingam S, Viswanathan M, Mohan R, Shanmugasundaram KR. Antioxidant status and lipid peroxidation in type II diabetes mellitus with and without complications. Clin Sci. 1996;90(4):255–260. doi: 10.1042/cs0900255. [DOI] [PubMed] [Google Scholar]
- 10.Kasiam Lasi On'Kin JB, Longo-Mbenza B, Nge Okwe, Kabangu NK, Mpandamadi SD, Wemankoy O, He J. Prevalence and risk factors of diabetes mellitus in Kinshasa Hinterland. Int J Diabetes Metab. 2008;16:97–106. [Google Scholar]
- 11.Mvitu M, Longo-Mbenza B, Tulomba D, Nge A. Regular, high and moderate intake of vegetables rich in antioxidants may reduce cataract risk in Central African type 2 diabetics. Int J Gen Med. 2012;5:489–493. doi: 10.2147/IJGM.S28588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mvitu M, Longo-Mbenza B, Tulomba D, Nge A. Reduced risk of metabolic syndrome due to regular intake of vegetables rich in antioxidants among African type 2 diabetics. Diabetes Metab Syndr ( Clin Res Rev) 2010;4(3):132–136. [Google Scholar]
- 13.Patil BR, Ageely HM. Anti-Lipid peroxidative activity of Colocasia esculenta leaf juice against CCL4 and acetomonophen mediated cell damage. Int J Pharm Appl. 2011;2(3):141–149. [Google Scholar]
- 14.Lindsey K, Motsei M, Jager A. Screening of South African food plants for antioxidant activity. J Food Sci. 2002;67(6):2129–2131. [Google Scholar]
- 15.Ajibesin KK. Dacryodes edulis (G. Don) H.J. Lam: a review on its medicinal, phytochemical and economical properties. Res J Med Plant. 2011;5(1):32–41. [Google Scholar]
- 16.Mvitu Muaka M, Longo-Mbenza B, Kaimbo Wa Kaimbo D. Frequency and the causes of blindness and visual impairment among patients with diabetes mellitus from DR Congo. Mali Med. 2009;24(3):22–26. [PubMed] [Google Scholar]
- 17.Mvitu Muaka M, Longo-Mbenza B. Causes of blindness and visual impairment among Congolese diabetics. Afr Health Sci. 2012 doi: 10.4314/ahs.v12i2.18. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. www.v2020.org. The global initiative for the elimination of avoidable blindness, a joint programme of the World Health Organization (WHO) and the International Agency for the Prevention of Blindness (IAPB)
- 19.Wang J, Thorton JC, Bari S, Williamson B, Gallagher D, Heymsfield SB, Horlick M, Kotler D, Laferrère B, Mayer L, Pi-Sunyer FX, Pierson RN., Jr Comparison of waist circumference measured at four sites. Am J Clin Nutr. 2003;77(2):379–384. doi: 10.1093/ajcn/77.2.379. [DOI] [PubMed] [Google Scholar]
- 20.Armstrong D, Browne R. The analysis of free radicals, lipid peroxides, antioxidant enzymes and compounds related to oxidative stress as applied to the clinical chemistry laboratory. Adv Exp Med Biol. 1994;366:43–58. doi: 10.1007/978-1-4615-1833-4_4. [DOI] [PubMed] [Google Scholar]
- 21.Dandona L, Dandona R. Revision of visual impairment definitions in the International Statistical Classification of Diseases. BMC Med. 2006;4:7. doi: 10.1186/1741-7015-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Longo-Mbenza B, Muaka MM, Mbenza G, Mbungu-Fuele S, Mabwa-Mbalanda , Nzuzi-Babeki V, Mbadi-A-Sungu J. Risk Factors of poor control of HBA1c and high values of HDL in African diabetic patients. Int J Diabetes Metab. 2008;16(1):69–78. [Google Scholar]
- 23.Rotimi C, Daniel H, Zhou J, Obisesan A, Chen G, Chen G, Chen Y, Amoah A, Opoku V, Acheampong J, Agyenim-Boateng K, Eghan BA, Jr, Oli J, Okafor G, Ofoegbu E, Osotimehin B, Abbiyesuku F, Johnson T, Fasanmade O, Doumatey A, Aje T, Collins F, Dunston G. Prevalence and determinants of diabetic retinopathy and cataracts in West African Type 2 Diabetes Patients. Ethn Dis. 2003;13(2 Suppl 2):S110–117. [PubMed] [Google Scholar]
- 24.American Diabetes Association Screening for type 2 diabetes. Diabetes Care. 1992;22(Suppl 1):S20–S23. [Google Scholar]
- 25.Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995;854:1–452. [PubMed] [Google Scholar]
- 26.Longo-Mbenza B, Vangu Ngoma D, Mbungu Fuele S. Low birth weight, metabolic syndrome and their association with the global crisis of 1930-1945, rapidly growing economy and coronary heart disease in Central Africa. Int J Nutr Metab. 2010;2(1):1–10. [Google Scholar]
- 27.Ajayi IA, Adesanwo O. Comparative study of the mineral element and fatty acid composition of dacryodes edulis pulp and seed. World J Agric Sci. 2009;5(3):279–283. [Google Scholar]
- 28.Nutall SL, Dunne F, Kendall MJ, Martin U. Age-independent oxidative stress in elderly patients with non-insulin-dependent diabetes mellitus. QJM. 1999;92(1):33–38. doi: 10.1093/qjmed/92.1.33. [DOI] [PubMed] [Google Scholar]
- 29.Corti A, Franzini M, Casini AF, Paolicchi A, Pompella A. Vitamin C supply to bronchial epithelial cells linked to glutathione availability in elf-a role for secreted gamma-glutamyl transferase? J Cyst Fibros. 2008;7(2):174–178. doi: 10.1016/j.jcf.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Lim JS, Yang JH, Chun BY, Kam S, Jacobs DR, Jr, Lee DH. Is serum gamma-glutamyl transferase inversely associated with serum antioxidants as marker of oxidative stress? Free Radic Biol Med. 2004;37(7):1018–1023. doi: 10.1016/j.freeradbiomed.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 31.Claessen H, Hermann B, Christoph D, Volker A. Gamma-glutamyl transferase and Disability Pension: A Cohort Study of Construction Workers in Germany. Hepatology. 2010;51(2):482–490. doi: 10.1002/hep.23324. [DOI] [PubMed] [Google Scholar]
- 32.Duk-hee L, Blomhoff R, Jacobs DR., Jr Is serum Gamma Glutamyltransferase a Marker of Oxidative Stress? Free Radic Research. 2004;38(6):535–539. doi: 10.1080/10715760410001694026. [DOI] [PubMed] [Google Scholar]
- 33.Wegner A, Khoramnia R. Cataract is a self-defense reaction to protect the retina from oxidative damage. Med Hypotheses. 2011;76(5):741–744. doi: 10.1016/j.mehy.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 34.Ho Chan Cho. The association between serum GGT Concentration and Diabetic Peripheral Polyneuropathy in type 2 Diabetic Patients. Korean Diabetes J. 2010;34(2):111–118. doi: 10.4093/kdj.2010.34.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujita M, Ueno K, Hata A. Association of gamma-glutamyltransferase with incidence of type 2 diabetes in Japan. Exp Biol Med. 2010;235(3):335–341. doi: 10.1258/ebm.2009.009232. [DOI] [PubMed] [Google Scholar]
- 36.Donnelly CA, Seth J, Clayton RM, Phillips CI, Cuthbert J, Prescott RJ. Some blood plasma constituents correlate with human cataract. Br J Ophthalmol. 1995;79(11):1036–1041. doi: 10.1136/bjo.79.11.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]