Abstract
During Xenopus laevis metamorphosis, Sonic hedgehog (Shh) is directly induced by thyroid hormone (TH) at the transcription level as one of the earliest events in intestinal remodeling. However, the regulation of other components of this signaling pathway remains to be analyzed. Here, we analyzed the spatiotemporal expression of Patched (Ptc)-1, Smoothened (Smo), Gli1, Gli2 and Gli3 during natural and TH-induced intestinal remodeling. We show that all of the genes examined are transiently up-regulated in the mesenchymal tissues during intestinal metamorphosis. Interestingly, in the presence of protein synthesis inhibitors, Gli2 but not the others was induced by TH, suggesting that Gli2 is a direct TH response gene, while the others are likely indirect ones. Furthermore, we demonstrate by the organ culture experiment that overexpression of Shh enhances the expression of Ptc-1, Smo and Glis even in the absence of TH, indicating that Shh regulates its own pathway components during intestinal remodeling.
Keywords: Patched (Ptc), Smoothened (Smo), Sonic hedgehog (Shh), Gli transcription factor, Xenopus laevis, metamorphosis, intestinal remodeling
INTRODUCTION
Members of the Hedgehog (Hh) family of secreted proteins direct an enormous variety of developmental events by acting as intercellular signals. The best characterized Hh signaling protein to date, Sonic hedgehog (Shh) is expressed in various organs and plays a key role in the regulation of cell proliferation and cell-fate determination (Roberts et al., 1995; Tabin and McMahon, 1997; Sukegawa et al., 2000). Shh binds to a 12-transmembrane receptor Patched (Ptc) (Marigo et al., 1996; Fuse et al., 1999). This ligand-receptor binding relieves Ptc-mediated inhibition of the activity of Smoothened (Smo), a second multipass membrane protein, resulting in up-regulation of Shh target genes such as bone morphogenetic protein (BMP)-4 (Roberts et al., 1995; Sukegawa et al., 2000; van den Brink, 2007). Ptc is also a transcriptional target of Hh signaling and acts in a negative-feedback loop to restrict the range of Hh action (Ingham, 1998; van den Brink, 2007). Intracellular signaling downstream of Smo is mediated by other proteins such as Fused kinase (Fu) (Lees et al., 2005; van den Brink, 2007; Parkin and Ingham, 2008), leading to the activation of the transcription factors Glis, which in turn activate the transcription of Hh target genes (Sasaki et al., 1997; Ruiz i Altaba, 1998; Ruiz i Altaba, 1999; Sasaki et al., 1999; Villavicencio et al., 2000). A number of Hh response genes have been identified (Katoh and Katoh, 2009). Among them are Ptc-1 and Gli1, suggesting the existence of a feedback loop of this signal pathway (Lee et al., 1997; Ingham, 1998; Takabatake et al., 2000; Agren et al., 2004; van den Brink, 2007; Katoh and Katoh, 2009). On the other hand, the effect of Hh on Gli2 and Gli3 expression is quite variable among different organs or animal species (Ruiz i Altaba, 1998; Borycki et al., 2000; Nguyen et al., 2005; Liu et al., 2007; Katoh and Katoh, 2009; Yakushiji et al., 2009), and the transcriptional control of Smo gene is largely unknown.
Shh is important for the larval-to-adult intestinal remodeling during Xenopus laevis metamorphosis (Stolow and Shi, 1995; Ishizuya-Oka et al., 2006). In the X. laevis intestine during metamorphosis, the larval epithelial cells are removed by apoptosis, and concurrently, adult progenitor/stem cells rapidly proliferate and differentiate into the adult absorptive epithelium analogous to the mammalian one (for reviews, see Shi and Ishizuya-Oka, 1996; Ishizuya-Oka, 2007). As all other processes during metamorphosis, intestinal remodeling is controlled by thyroid hormone (TH) (Dodd and Dodd, 1976; Shi, 1999). A number of TH response genes have been isolated from the X. laevisintestine (Shi and Brown, 1993; Buchholz et al., 2007) in an effort to study the molecular basis of its larval-to-adult remodeling. Shh was thus identified as one of such genes and shown to be a direct target of TH (Stolow and Shi, 1995). We have previously shown that the epithelium-specific expression of Shh coincides well with active proliferation of the adult epithelial progenitor cells during metamorphic climax (Ishizuya-Oka et al., 2001b; Hasebe et al., 2008), and more importantly, that Shh induces the connective tissue-specific expression of BMP-4, which in turn promotes the adult epithelial differentiation (Ishizuya-Oka et al., 2006). Thus, Shh plays a pivotal role in the adult epithelial development. However, how Shh functions in this process remains to be investigated.
In the present study, to determine whether and how the components of the Shh signaling pathway are involved in intestinal metamorphosis, we have analyzed the expression of some of the key players of this signaling pathway, i.e., Ptc-1, Smo, Gli1, Gli2 and Gli3, by real-time RT-PCR and in situ hybridization. We show that their expression is mesenchymal tissue-specific and is up-regulated in the X. laevis intestine during natural and TH-induced metamorphosis. In addition, by the organ culture experiment, we demonstrate that exogenous Shh can enhance the expression of those genes even in the absence of TH. These results suggest that TH induces Shh signaling, which in turn further up-regulates the expression of the pathway components to enhance its function during the larval-to-adult intestinal remodeling.
RESULTS
Up-regulation of Ptc-1, Smo, Gli1, Gli2 and Gli3 in the intestine during natural and TH-induced metamorphosis
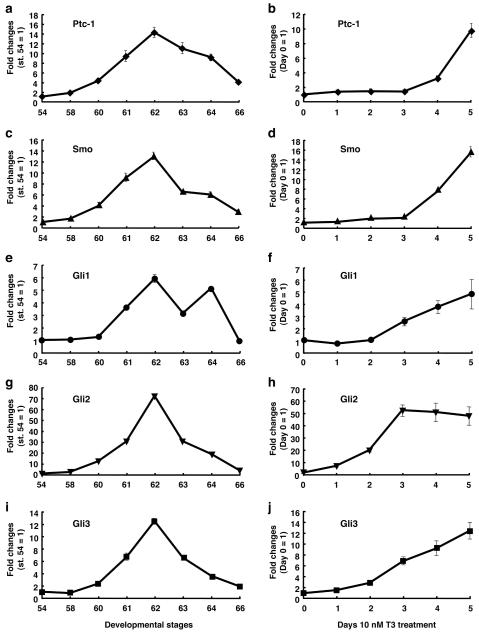
To determine the temporal regulation of Ptc-1, Smo, Gli1, Gli2 and Gli3 expression during the intestinal remodeling, we first carried out real-time RT-PCR using total RNA extracted from the intestine at various metamorphic stages (Fig. 1). Ptc-1 mRNA was expressed at a very low level at stage 54 (premetamorphosis), up-regulated during metamorphic climax, and reached a maximal level at stage 62 (metamorphic climax), when adult epithelial cells are actively proliferating (Ishizuya-Oka and Ueda, 1996). Thereafter, Ptc-1 expression decreased toward stage 66 (the end o f metamorphosis) (Fig. 1a). Similar results were obtained for Smo (Fig. 1c), Gli1 (Fig. 1e), Gli2 (Fig. 1g) and Gli3 (Fig. 1i) mRNAs. These expression profiles coincide well with that of Shh (Hasebe et al., 2008), supporting their role for Shh signal transduction.
Fig. 1.
Expression profiles of Ptc-1, Smo, Gli1, Gli2 and Gli3 in the intestine during natural and TH-induced metamorphosis. Real-time RT-PCR was performed using total RNA prepared from the intestine of animals at indicated developmental stages (a, c, e, g and i) or stage 54 tadpoles after 10 nM T3 treatment (b, d, f, h and j). Levels of Ptc-1 (a, b), Smo (c, d), Gli1 (e, f), Gli2 (g, h) and Gli3 mRNAs (i, j) are shown relative to those of ribosomal protein L8 (rpL8) mRNA, with the values at stage 54 or 0 day treatment set to 1.
As TH can induce precocious intestinal remodeling, if Ptc-1, Smo and Glis participate in Shh signaling, their expression profiles should be reproduced during TH-induced metamorphosis. Thus, we analyzed the expression of these genes in the intestine of premetamorphic tadpoles at stage 54 treated with 10 nM T3 for 1 to 5 days. The expression of Ptc-1 (Fig. 1b) and Smo (Fig. 1d) was up-regulated after 4 days of T3 treatment, and that of Gli1 (Fig. 1f) and Gli3 (Fig. 1j) was up-regulated after 3 days. All of them were markedly increased after 5 days. The late induction of gene expression by T3 has also been observed for BMP-4 (Hasebe et al., 2008), which is known as an indirect TH response gene (Amano and Yoshizato, 1998; Ishizuya-Oka et al., 2001a). In contrast, the expression of Gli2 was highly up-regulated after 1 day of T3 treatment (ca. 10 fold, Fig. 1h), suggesting that Gli2 is one of the direct TH response genes like Shh (Stolow and Shi, 1995; Hasebe et al., 2008).
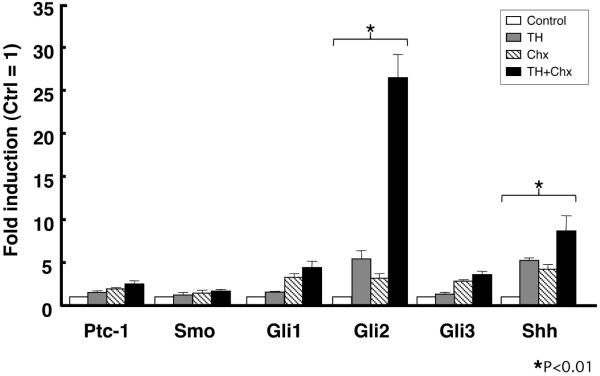
The regulation of direct target genes is mediated by the thyroid hormone receptor (TR) immediately upon TH addition and is thus independent of new protein synthesis. Thus, to examine whether these genes are direct transcriptional targets of TH, protein synthesis inhibitors cycloheximide and anisomycin (Chx) were added to the rearing water of premetamorphic tadpoles at stage 54 for 1 hour before and throughout 50 nM T3 treatment for 6 hours to block protein synthesis (Kanamori and Brown, 1992). Even in the presence of Chx, T3 significantly up-regulated the expression of Gli2 as well as that of Shh, but did not up-regulate that of the other genes (Fig. 2). Treatment with T3 plus Chx resulted in much stronger induction of both Gli2 and Shh than that with T3 alone. This may be due to synergistic effects of T3 and Chx, since protein synthesis inhibitors have been shown to superinduce certain genes (Edwards and Mahadevan, 1992). These results indicate that Ptc-1, Smo, Gli1 and Gli3 are likely indirect TH response genes, whereas Gli2, like Shh, is an immediate early and direct TH response gene.
Fig. 2.
Gli2 is a direct TH response gene. Total RNA was isolated from the intestine of premetamorphic tadpoles at stage 54 treated with DMSO (Control, white bars), 50 nM T3 + DMSO (TH, gray bars), cycloheximide and anisomycin (Chx, shaded bars) and 50 nM T3 + Chx (TH+Chx, black bars) for 6 hours following the pretreatment with DMSO or Chx for 1 hour. mRNA levels of indicated genes were examined by real-time RT-PCR. Control vs TH and Chx vs TH+Chx were analyzed by Student’s T-test, respectively. When both were statistically significant, the gene was determined as the direct TH response gene (asterisk) (Das et al., 2009).
Localization of the expression of Ptc-1, Smo, and Glis in the intestine during natural and TH-induced metamorphosis
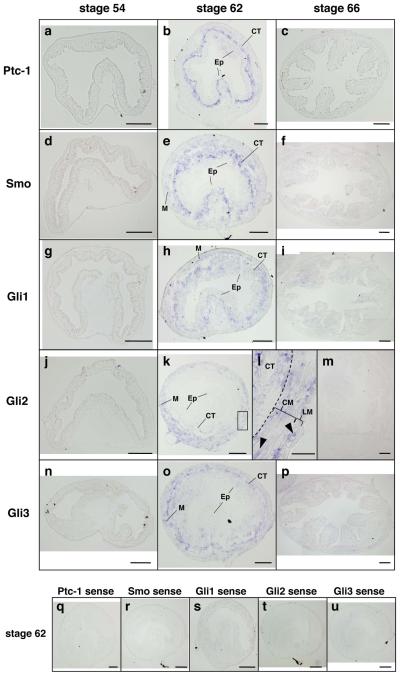
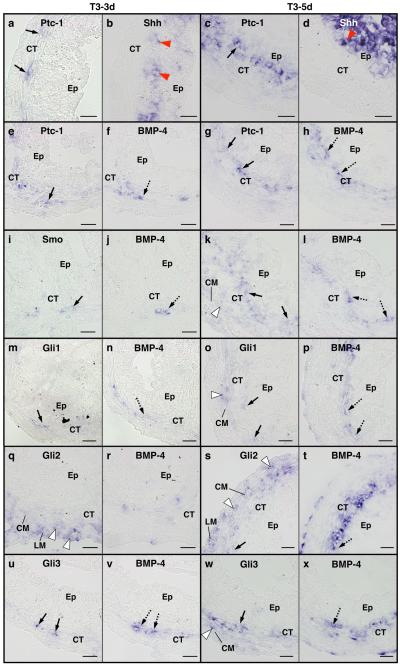
We next examined the spatiotemporal expression of these genes by ISH. The tadpole intestine during pre- and prometamorphosis consists of a monolayer of the larval epithelial cells surrounded by thin layers of the outer longitudinal and inner circular muscles with the intervening connective tissue. In agreement with the RT-PCR analysis shown in Fig. 1, the expression of these mRNAs was low or undetectable in the tadpole intestine during this period (Fig. 3a, d, g, j and n). Then, at stage 62 (early period of metamorphic climax), when the larval epithelium undergoes apoptosis and adult epithelial stem/progenitor cells rapidly proliferate (Ishizuya-Oka and Ueda, 1996), the expression of Ptc-1, Smo, and Glis mRNAs became high in the connective tissue (Fig. 3b, e, h, k and n). In addition, mRNAs of Smo and all Glis were also detected in the muscles (Fig. 3e, h, k, l and o), although their mRNA levels were low except for Gli2, which was expressed in both the circular and longitudinal muscle layers (Fig. 3l). Compared to Ptc-1 and Smo, Glis, especially Gli2 and Gli3, were expressed at much lower levels in the typhlosole (Fig. 3b, e h, k and o), suggesting that Shh signaling is very limited in this region. At stage 66 (the end of metamorphosis), the adult epithelial cells have differentiated into the absorptive epithelium, which acquires a cell-renewal system along the trough-crest axis of epithelial fold, resembling the crypt-villus axis in the adult mammalian intestine (Shi and Ishizuya-Oka, 1996). The expression of all genes examined was down-regulated at this stage (Fig. 3c, f, i, m and p).
Fig. 3.
Localization of Ptc-1, Smo, Gli1, Gli2 and Gli3 mRNAs in the intestine during natural metamorphosis of X. laevis. Cross sections of the intestine at premetamorphic stage 54 (a, d, g, j and n), at metamorphic climax stages 62 (b, e, h, k, l, o and q-u) and at the end of metamorphosis (stage 66) (c, f, i, m and p) were hybridized with antisense Ptc-1 (a-c), Smo (d-f), Gli1 (g-i), Gli2 (j-m) or Gli3 (n-p) probes or their sense probes (q-u). Dark blue deposits indicate the sites of probe binding. Light or dark brown pigments in some pictures are melanin. The expression of all mRNAs becomes high at stage 62. They are expressed in the connective tissue (CT) and muscles (M), but not in the epithelium (Ep). The boxed area in the panel k is magnified to show Gli2 expression in both the circular (CM) and longitudinal (LM) muscle layers (l, arrowheads). A dashed line shows the boundary of the connective tissue and muscle layer. Scale bars: 100 μm (a-k, m-u), 20 μm (l).
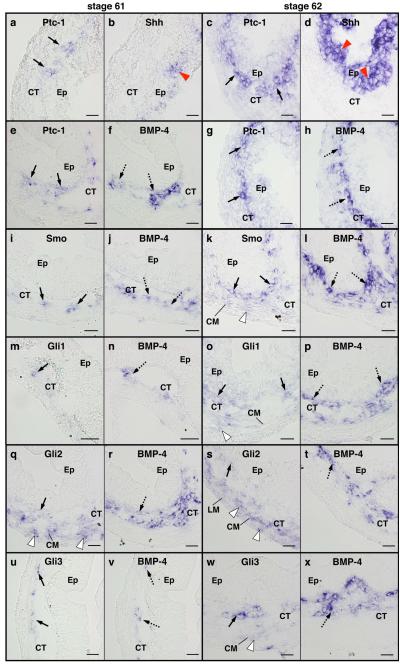
The similarities of their expression patterns to that of BMP-4, which is the only gene demonstrated to be responsive to Shh signaling in the metamorphosing intestine to date (Ishizuya-Oka et al., 2006) and is also transiently up-regulated in the connective tissue (Ishizuya-Oka et al., 2001a), suggest that these factors indeed mediate the Shh signal during metamorphosis. To verify this possibility more precisely, we compared the localization of Ptc-1, Smo and Glis with that of Shh and/or BMP-4 by ISH using adjacent sections of the intestine at stages 61 and 62 (Fig. 4). At stage 61, Ptc-1 mRNA was detected in the connective tissue close to the adult epithelial cells expressing Shh (Fig. 4a, b). Consistent with the high expression of Shh at stage 62, Ptc-1 was highly expressed (Fig. 4c, d). In addition, the expression patterns of Ptc-1 were essentially identical to those of BMP-4 at both stages (Fig. 4e-h). Their mRNAs were detected in a gradient fashion with the highest levels just beneath the epithelial cells and were absent in the muscle layers at stage 62 (Fig. 4g, h), suggesting co-localization of Ptc-1 and BMP-4. Similar results were obtained for Smo (Fig. 4i-l) with strong signals in the connective tissue adjacent to the epithelium, although Smo was very weakly expressed in the circular muscle layer where BMP-4 was not expressed (Fig. 4k). In contrast, the expression patterns of Glis were somewhat different from that of BMP-4. In particular, all Glis were expressed not only in the connective tissue but also in the circular muscle layer. Additionally, Gli2 was weakly expressed in the longitudinal muscle layer. In the connective tissue, most of the cells expressing Gli1 were positive for BMP-4 (Fig. 4m-p). Although some of the cells expressing Gli2 co-expressed BMP-4, the majority of them was detected in the circular muscle layer (Fig. 4q-t). The expression pattern of Gli3 was nearly identical to that of BMP-4 at stage 61 (Fig. 4u, v), but was not at stage 62 (Fig. 4w, x). These results suggest that, although all Glis are likely involved in activating BMP-4 expression in the connective tissue, different Glis may have distinct roles.
Fig. 4.
Comparison of the localization of Ptc-1, Smo, Gli1, Gli2 and Gli3 mRNAs with that of Shh and/or BMP-4 mRNAs in the intestine at metamorphic climax. Cross sections of the intestine at stage 61 (a, b, e, f, i, j, m, n, q, r, u and v) and stage 62 (c, d, g, h, k, l, o, p, s, t, w and x) were hybridized with antisense Ptc-1 (a, c, e and g), Smo (i and k), Gli1 (m and o), Gli2 (q and s) or Gli3 (u and w) probes. Their adjacent sections were hybridized with antisense Shh (b and d) or BMP-4 (f, h, j, l, n, p, r, t, v and x). a-d: Ptc-1 is expressed in the connective tissue (CT, arrows) just beneath the epithelial cells (Ep) expressing Shh (red arrowheads). e-h: Ptc-1 (arrows) is co-expressed with BMP-4 (dashed arrows). i-l: Smo (arrows) is co-expressed with BMP-4 (dashed arrows) in the connective tissue. In addition, Smo is weakly expressed in the circular muscle layer (CM, white arrowhead). m-p: Some cells in the connective tissue co-express Gli1 (arrows) and BMP-4 (dashed arrows). Gli1 is weakly expressed in the circular muscle layer (white arrowhead). q-t: Some cells in the connective tissue co-express Gli2 (arrows) and BMP-4 (dashed arrows). Gli2 is also expressed in both the circular and longitudinal muscle layers (LM) (white arrowheads). u-x: Some cells in the connective tissue co-express Gli3 (arrows) and BMP-4 (dashed arrows). Gli3 is weakly expressed in the circular muscle layer (white arrowhead). Scale bars: 20 μm.
When premetamorphic tadpoles at stage 54 were treated with 10 nM T3, Ptc-1 (Fig. 5a, e, c, g), Smo (Fig. 5i, k), Gli1 (Fig. 5m, o), Gli2 (Fig. 5q, s) and Gli3 (Fig. 5u, w) mRNAs in the intestine became detectable after 3 days of T3 treatment and were strongly expressed after 5 days. Their overall expression patterns as well as their localization relative to those of Shh and BMP-4 mRNAs were essentially identical to those during natural metamorphosis (Compare Fig. 4 with Fig. 5).
Fig. 5.
Comparison of the localization of Ptc-1, Smo, Gli1, Gli2 and Gli3 mRNAs with that of Shh and/or BMP-4 mRNAs in the intestine during TH-induced metamorphosis. Cross sections of the intestine from premetamorphic tadpoles treated with 10 nM T3 for 3 days (a, b, e, f, i, j, m, n, q, r, u and v) and 5 days (c, d, g, h, k, l, o, p, s, t, w and x) were hybridized with antisense Ptc-1 (a, c, e and g), Smo (i and k), Gli1 (m and o), Gli2 (q and s) or Gli3 (u and w) probes. Their adjacent sections were hybridized with antisense Shh (b and d) or BMP-4 (f, h, j, l, n, p, r, t, v and x). a-d: Ptc-1 is expressed in the connective tissue (CT, arrows) just beneath the epithelial cells (Ep) expressing Shh (red arrowheads). e-h: Ptc-1 (arrows) is co-expressed with BMP-4 (dashed arrows). i-l: Smo (arrows) is co-expressed with BMP-4 (dashed arrows) in the connective tissue. In addition, Smo is weakly expressed in the circular muscle layer (CM, white arrowhead). m-p: Some cells in the connective tissue co-express Gli1 (arrows) and BMP-4 (dashed arrows). Gli1 is weakly expressed in the circular muscle layer (white arrowhead). q-t: Some cells in the connective tissue co-express Gli2 (arrow) and BMP-4 (dashed arrow). Gli2 is expressed in both the circular and longitudinal muscle layer (LM) (white arrowheads). u-x: Some cells in the connective tissue co-express Gli3 (arrows) and BMP-4 (dashed arrows). Gli3 was detected also in the circular muscle layer (white arrowhead). Scale bars: 20 μm.
Shh up-regulates the expression of its own pathway
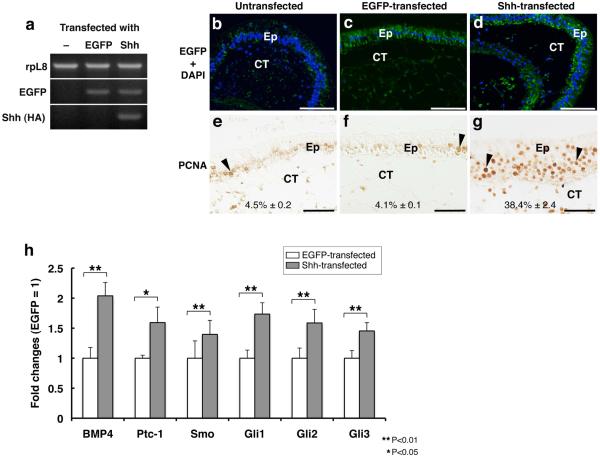
The above results showed that mRNAs of Ptc-1, Smo and Glis were up-regulated in a TH-dependent manner during metamorphosis. Since Shh is known to be able to up-regulate some components of the Shh pathway, it might be possible that Shh, whose expression is directly induced by TH, may up-regulate the expression of these components of the pathway. To examine this possibility, we carried out intestinal organ culture studies in vitro. The intestinal fragments isolated from prometamorphic tadpoles at stage 56/57 were transfected with a plasmid DNA pIRES2_Shh-EGFP to overexpress Shh and the transfection marker EGFP, and were cultured for 3 days in the absence of TH. As a control, pIRES2_EGFP was used. RT-PCR and immunohistochemistry (IHC) using anti-GFP antibody showed that both plasmids were successfully delivered (Fig. 6a-d). Overall morphology of the transfected intestines was normal and essentially the same as that of the untransfected intestine (note that even in the presence of TH, the overall morphology of the intestine is not significantly altered during 3 days of culture, although cell proliferation and larval cell death occur (Ishizuya-Oka et al., 2001b; Ishizuya-Oka et al., 2006)) (Fig. 6b-d). Shh overexpression, however, led to enhanced cell proliferation as observed by IHC using anti-PCNA antibody only in the intestine transfected with Shh (Fig. 6e-g), in agreement with earlier observations (Ishizuya-Oka et al., 2001b). Although this enhanced proliferation was due to the signals from the connective tissue activated by the epithelial Shh, our data indicate that the effect of transfected Shh was comparable to that of exogenously added Shh protein (Ishizuya-Oka et al., 2001b). Importantly, real-time RT-PCR analysis showed that all of the Shh pathway genes were significantly up-regulated in the Shh-transfected intestine (Fig. 6h). However, if the proportion of the mesenchymal cells highly increased in the Shh-transfected intestine by active proliferation, the enhanced expression of those genes could be due to the increase of the number of cells expressing them, but not due to their up-regulation in each cell. To rule out this possibility, we compared the proportion of the mesenchymal cells in the Shh-transfected intestine with that of GFP-transfected one. Since the percentage of the mesenchymal cells was almost the same between the Shh-transfected (51.3% ± 1.2) and GFP-transfected (51.2% ± 0.3) intestines, changes in gene expression were most likely due to the up-regulation in each cell. Thus, these results indicate that Shh can enhance the expression of the Shh pathway genes in the intestine.
Fig. 6.
Overexpression of Shh enhances the expression of genes involved in its signaling pathway. Intestinal fragments from stage 56/57 tadpoles were transfected with pIRES2_EGFP (c, f) or pIRES2_Shh-EGFP (d, g) or remained untreated (b, e), and then were cultured for 3 days in the absence of TH. a: Transfected gene expression was detected by RT-PCR. b-d: Transfected cells were detected by IHC using anti-GFP antibody. Nuclei were stained with DAPI. e-g: Effects of exogenous Shh on cell proliferation were examined by IHC using anti-PCNA antibody. The percentage of nuclei strongly positive for PCNA (arrowheads) in the epithelium was shown as the mean ± standard error in each panel. h: mRNA levels of indicated genes were analyzed by real-time RT-PCR. EGFP-transfected vs Shh-transfected was analyzed by Student’s T-test. All genes were significantly up-regulated by Shh. Ep: epithelium. CT: connective tissue. Scale bars: 100 μm (b-d), 50 μm (e-g).
DISCUSSION
In this study, we have shown that the expression of Ptc-1, Smo, Gli1, Gli2 and Gli3 is transiently up-regulated in the intestine during X. laevis metamorphosis. Their mRNA levels peak at stage 62, when the level of Shh mRNA is highest (Stolow and Shi, 1995; Hasebe et al., 2008). It remains unclear why the up-regulation of Gli2 is much higher than that of the other genes (more than 70 folds). It is possible that at least in the early phase of Shh signaling, Gli2 is the main mediator of Shh signaling in the amphibian remodeling intestine, similar to that shown in the mouse embryonic hair follicle (Mill et al., 2003). In any case, the temporal profiles of their expression suggest that these Shh pathway components are indeed involved in mediating Shh signaling. This is further supported by our ISH data, which have shown that Ptc-1 and Smo are expressed in the connective tissue with the highest levels just beneath the adult epithelium expressing Shh. More importantly, the localization of Ptc-1 and Smo in the connective tissue coincides well with that of BMP-4, which is only one gene known as a target of Shh signaling in the X. laevis remodeling intestine to date (Ishizuya-Oka et al., 2006). Thus, it is very likely that the connective tissue-specific expression of BMP-4 is induced by Shh via Ptc-1 and Smo. These results also indicate that Shh acts in a paracrine fashion in the amphibian intestine as shown in the mammalian intestine (Kolterud et al., 2009).
The downstream transcription factors in the Shh pathway, Glis, have the spatial expression patterns that do not follow closely the pattern of BMP-4 and among each other. However, our ISH data suggest that all Glis are involved in BMP-4 expression. It is very likely that in the connective tissue, the three Glis have overlapping roles to ensure that BMP-4 and other Shh target genes, if any, are activated properly in the remodeling intestine, similar to those reported in several mammalian organs during development (Mo et al., 1997; Motoyama et al., 1998; Park et al., 2000). Aside from the connective tissue, we have also found that all Glis are expressed in the muscle layers. Here, their expression patterns are similar to those of Glis in the mouse intestine, where Gli1 is expressed in the circular muscle layer, while Gli2 is expressed in both the circular and longitudinal muscle layers during organogenesis (Kolterud et al., 2009). Thus, it is suggested that the muscle cells in the metamorphosing intestine are likely responsive to Shh signal. While we could easily detect Gli2 expression in the longitudinal muscle layer, Ptc-1 and Smo were not detectable there under our conditions. It is possible that low levels of Ptc-1 and Smo are present to mediate the Shh signal or that Gli2 may have Shh-independent functions (Hui et al., 1994; Lee et al., 1997; Sasaki et al., 1997; Ruiz i Altaba, 1998; Dennler et al., 2007). Collectively, overall similarities in gene expression patterns of the Shh pathway components between mouse and Xenopus suggest their conserved roles in the development of the intestine.
The participation of the Shh pathway in intestinal remodeling is also substantiated by the similar expression patterns of the genes in the pathway upon the treatment of premetamorphic tadpoles with TH compared to those during natural metamorphosis. Interestingly, our analyses of the TH-treated animals suggest that Ptc-1, Smo, Gli1 and Gli3 are indirect targets of TH, while Gli2 is a direct one, since its induction by TH, just like the induction of Shh, occurs even in the absence of new protein synthesis. Although this could only mean that Shh and Gli2 genes are poised for transcription in response to TH, what makes this possible is the binding of TR to the gene regulatory region, that is, only direct target genes can be regulated in the absence of new protein synthesis. Thus, Shh and Gli2 are considered as the direct targets of TH. To our knowledge, this is the first report showing Gli2 as a direct TH response gene. Although the biological significance is not clear at present, it is possible that Gli2 is required for initial Shh signaling as shown in mouse development (Bai et al., 2002). Thus, Shh-target cells may need Gli2 earlier than any other Gli to properly respond to Shh which is also a direct target of TH. The regulation of the Shh signaling pathway by TH also occurs in mammals. For example, in the rat embryonic and adult brain, TH has recently been shown to regulate the expression of Shh, Ptc and Smo (Desouza et al., 2011). Likewise, in the mammalian intestine, the increase in the circulating levels of TH at weaning coincides well with postnatal intestinal maturation (Sirakov and Plateroti, 2011), when Shh, Ptc, and Glis are shown to be highly expressed (Kolterud et al., 2009). Thus, it is likely that the role of Shh signaling and its regulation by TH is conserved between mammals and amphibians, further supporting the use of frog metamorphosis as a good model to study the mechanisms of intestinal development.
We have demonstrated the ability of exogenous Shh to up-regulate the expression of Ptc-1, Smo and all Glis in the cultured X. laevis intestine. These findings are consistent with the fact that Ptc-1 and Gli1 genes are well known transcriptional targets of Shh signaling in X. laevis embryos and other animals (Lee et al., 1997; Ingham, 1998; Takabatake et al., 2000; Agren et al., 2004; van den Brink, 2007; Katoh and Katoh, 2009). However, the response of Gli2 and Gli3 genes to Shh differs in different organs and animal species. For example, Gli2 is up-regulated by the activated Shh signaling in early embryos of chicken and X. laevis but not in those of mammals (Ruiz i Altaba, 1998; Bai and Joyner, 2001; Nguyen et al., 2005; Liu et al., 2007); Shh down-regulates Gli3 during neurogenesis of X. laevis, but does not in the regenerating limbs of X. laevis and the mammalian skin (Ruiz i Altaba, 1998; Mill et al., 2003; Nguyen et al., 2005; Yakushiji et al., 2009). And, the transcriptional regulation of Smo gene is largely unknown in any organ. Our study indicates that the expression of Smo, Ptc-1 and Gli1-3 is up-regulated by Shh itself during intestinal metamorphosis of X. laevis. Interestingly, the expression of Gli2 is controlled by both TH and Shh, suggesting that both Gli-binding sites to which Gli2 can bind and TH response elements may exist in the regulatory region of the Gli2 gene. Thus, it is likely that during metamorphosis, TH first induces the expression of Shh in the epithelium and Gli2 in the connective tissue and muscle layers. Then, the secreted Shh binds to Ptc-1 in the connective tissue where the intracellular signals are transduced. Gli2 may function as the initial transcriptional activator, which up-regulates other components including Gli2 itself to further enhance Shh signaling for the intestinal transformation. Functional studies such as transgenic expression (Rankin et al., 2009; Rankin et al., 2011) of wild type and mutant components of this pathway in the future should help to test this possibility.
EXPERIMENTAL PROCEDURES
Animal and treatment
Xenopus laevis tadpoles and froglets were obtained and maintained as previously described (Hasebe et al., 2007). The developmental stages of tadpoles were according to Nieuwkoop and Faber (1994). Premetamorphic tadpoles at stage 54 were treated with 10 nM TH (3,5,3′-triiodothyronine or T3) for 1 to 5 days. To inhibit protein synthesis, cycloheximide and anisomycin (Chx) dissolved in DMSO were added to the rearing water of premetamorphic tadpoles at 20 and 25 mg/L, respectively (Kanamori and Brown, 1992). DMSO was used for the control. After 1 hour of Chx treatment, T3 was added at 50 nM, and the tadpoles were treated for another 6 hours. At least 3 tadpoles were analyzed for each stage or day of TH-treatment. Animal rearing and treatment were done according to the guidelines set by Nippon Medical School animal use and care committee.
Real-time reverse transcription-polymerase chain reaction (real-time RT-PCR)
Total RNA from the small intestine of wild type and T3-treated animals was extracted by using RNAiso reagent (Takara Bio, Shiga, Japan) followed by DNase treatment with DNA-free (Ambion, Austin, TX, USA) to remove any DNA contamination. The integrity of RNA was checked based on 18S and 28S ribosomal RNAs by electrophoresis. Total RNA was mixed with RNA-direct SYBR Green Realtime PCR Master Mix (Toyobo, Osaka, Japan), and then quantitative real-time RT-PCR was performed by using Thermal Cycler Dice Real Time System (Model TP800, Takara Bio) or StepOnePlus Real-Time PCR System (Applied Biosystems, Life Technologies, Carlsbad, CA, USA) according to the manufacture’s instructions. The primer pairs used are : 5′-AAACGGCTACCCTTTCCTGTTC-3′ and 5′-GACAATGATTCCAGCAGTCCAAG-3′ for Ptc-1, 5′-GCTGACTTATGCTTGGCACACC-3′ and 5′-CAGAATCCCCATCAACCTGAGC-3′ for Smo, 5′-ACACATTACCAAGAAGCACCG–3′ and 5 ′-CAGCTGGTTTTCCCCTTTAAC-3′ for Gli1, 5′-TGTGATTTAGATGTCCCTGGCG-3′ and 5′-TGCTGCTTTGTAGATTCCGTGC-3′ for Gli2, 5′-ATGACAGGAACCAAATGGATGG-3′ and 5′-GTAAAGTTGGTGCTCTATGAGGTGG-3′ for Gli3. Primer pairs for Shh, BMP-4 and ribosomal protein L8 (rpL8) detection have been described previously (Hasebe et al., 2008). The level of specific mRNA was normalized against the level of rpL8 mRNA (Shi and Liang, 1994) for each sample. Samples were analyzed in duplicate or triplicate for 3 times. The specificity of the amplification was confirmed by the dissociation curve analysis and gel electrophoresis.
Although Gli2 (accession #: NM_001088425) is very similar to its pseudoallele Gli4 (accession #: NM_001087973) (90% homologous at nucleic acid level) and it is sometimes difficult to distinguish these genes even by RT-PCR, sequence analysis of the RT-PCR product showed that Gli2 was exclusively amplified under our RT-PCR conditions.
In situ hybridization (ISH)
A partial cDNA encoding X. laevis Ptc-1 was amplified by SuperScript One-Step RT-PCR system with Platinum Taq (Invitrogen, Carlsbad, CA, USA) with primers 5′-AATTCTAGAACACCACCCAACAAAGAAGC-3′ and 5′-ATACCCGGGTGAGGGTCCTCAAAATGTCC-3′, digested with Xba I and Xma I, inserted into pBSII-KS+ plasmid vector and sequenced (pBSII-KS+_Ptc1-probe).
X. laevis Smo full-length cDNA (accession no.: BC168525) cloned into pCMV-SPORT6 (IMAGE: 6866007) was purchased from Open Biosystems (Huntsville, AL, USA). This plasmid was used to synthesize the full-length probes.
pCS2-fGli1-myc, pCS2-myc-xGli2 and pCS2-fGli3 were kind gifts from Dr. A. Ruiz i Altaba (Lee et al., 1997; Brewster et al., 1998; Ruiz i Altaba, 1999). These plasmids were used to synthesize the full-length probes.
The constructs to synthesize probes for Shh and BMP-4 have been described previously (Hasebe et al., 2008).
All plasmids were linearized to synthesize sense and antisense probes with T3, T7 or SP6 RNA polymerase by using digoxigenin (DIG) RNA Labeling Mix (Roche Applied Science, Indianapolis, IN, USA). Full-length probes were digested by alkaline treatment (40 mM NaHCO3, 60 mM Na2CO3) into ca 200 base-long. Intestinal fragments were isolated from the anterior part of the small intestine just after the bile duct junction in tadpoles at indicated stages as well as T3-treated tadpoles and fixed in MEMFA followed by cryosectioning. Tissue sections were prepared at 7 μm and subjected to ISH. ISH was performed by using sense or antisense probes of Ptc-1, Smo, Gli1, Gli2, Gli3, Shh and BMP-4 (Hasebe et al., 2008) as previously described (Hasebe et al., 2006). Photographs were taken by using a digital CCD color camera (DP70, Olympus, Tokyo, Japan) attached to an optical microscope (BX51, Olympus).
Plasmid DNA constructs for transfection
To generate an expression vector driving Shh, T7Ts_C-Shh-HA (Hasebe et al., 2008) was digested with Bam HI and Xho I. The cDNA encoding Shh fused with HA tag at C-terminus was inserted into pIRES2_EGFP (Clontech, Mountain View, CA, USA) predigested with Bgl II and Sal I. The resultant construct with CMV promoter drives a bicistronic message (pIRES2_Shh-EGFP). pIRES2_EGFP was used as a control.
Transfection and organ culture
The intestinal fragments were isolated from the anterior part of the small intestine of X. laevis tadpoles at stage 56/57, and they were opened lengthwise and cut into small pieces (3-4 mm long). Transfection was performed by using METAFECTENE EASY (Biontex Laboratories GmbH Planegg/Martinsried, Germany) according to the manufacture’s instructions. Briefly, the intestinal fragments were prepared in 500 μL of 1× Marc’s modified ringers (MMR: 100 mM NaCl, 2 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 5 mM HEPES, pH 7.5) and mixed with 100 μL of Lipoplex solution with 5 μg of DNA. Then, the intestinal fragments in the mixture were transferred onto a membrane filter placed in a 6-well plate. After overnight incubation at 26°C, the filter membrane with intestinal fragments was transferred onto a Transwell culture insert (Corning, Lowell, MA, USA) set in a new 6-well plate filled with the culture medium (Ishizuya-Oka and Shimozawa, 1991). The transfected intestines were cultured in the absence of T3 for another 2 days at 26°C. Then, they were collected and prepared for either RT-PCR or immunohistochemistry. For EGFP detection, primers 5′-GCTGACCCTGAAGTTCATCTG-3′ and 5′-GGACTTGAAGAAGTCGTGCTG-3′ were used. For exogenous Shh detection, primers 5′-TGAAGGCATCCACTGGTACTC-3′ and 5′-GTAATCTGGAACATCGTATGG-3′ were used.
Immunohistochemistry (IHC)
The intestinal fragments were fixed in 95% ethanol at 4°C, embedded in paraffin and sectioned at 5 μm. To detect transfected cells, some sections were incubated with the mouse anti-GFP antibody (diluted 1:100; MBL Japan, Nagoya, Japan) followed by incubation with Alexa Fluor 488-conjugated anti-mouse IgG (1:500; Invitrogen), counterstained with 10 μg/ml 4′,6-diamidino-2-phenylindole (DAPI) for 15 min and analyzed by fluorescence microscopy. Other sections were incubated with the mouse anti-proliferating cell nuclear antigen (PCNA) antibody (1:100; Novocastra Laboratories, Newcastle, UK). They were then incubated with peroxidase-labeled streptavidin (Nichirei Biosciences, Tokyo, Japan) followed by 0.02% 3, 3′-diamino-benzidine-4HCl (DAB) and 0.006 % H2O2. The percentage of the nuclei strongly positive for PCNA per all the nuclei in the epithelium was calculated and shown as the mean ± standard error by using at least 3 photographs of each sample. Some sections were stained with hematoxylin. All the nuclei in the entire section were counted, and the percentage of the mesenchymal cells in the intestine was calculated and shown as the mean ± standard error by using 3 photographs of each sample.
ACKNOWLEDGMENTS
We would like to thank to Drs. Masakazu Fujiwara (Nippon Medical School), Itaru Hasunuma (Toho University), Kosuke Kawamura (Waseda University) for providing us technical information, Dr. Kazuhito Takeshima (Nagoya University) for helpful discussions about Gli genes, Dr. Yoshinori Abe (Nippon Medical School) for helpful discussions about Shh signaling, and Dr. Ariel Ruiz i Altaba (University of Geneva) for kind gifts of Gli plasmids. This work was supported in part by JSPS Grants-in-Aid for Scientific Research (C) to A. I-O, and in part by the Intramural Research Program of NICHD, NIH.
Grant Sponsors: JSPS Grants-in-Aid for Scientific Research (C), (grant #: 20570060); Intramural Research Program of NICHD, NIH.
REFERENCES
- Agren M, Kogerman P, Kleman MI, Wessling M, Toftgard R. Expression of the PTCH1 tumor suppressor gene is regulated by alternative promoters and a single functional Gli-binding site. Gene. 2004;330:101–114. doi: 10.1016/j.gene.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Amano T, Yoshizato K. Isolation of genes involved in intestinal remodeling during anuran metamorphosis. Wound Repair Regen. 1998;6:302–313. doi: 10.1046/j.1524-475x.1998.60406.x. [DOI] [PubMed] [Google Scholar]
- Bai CB, Auerbach W, Lee JS, Stephen D, Joyner AL. Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development. 2002;129:4753–4761. doi: 10.1242/dev.129.20.4753. [DOI] [PubMed] [Google Scholar]
- Bai CB, Joyner AL. Gli1 can rescue the in vivo function of Gli2. Development. 2001;128:5161–5172. doi: 10.1242/dev.128.24.5161. [DOI] [PubMed] [Google Scholar]
- Borycki A, Brown AM, Emerson CP., Jr. Shh and Wnt signaling pathways converge to control Gli gene activation in avian somites. Development. 2000;127:2075–2087. doi: 10.1242/dev.127.10.2075. [DOI] [PubMed] [Google Scholar]
- Brewster R, Lee J, Ruiz i, Altaba A. Gli/Zic factors pattern the neural plate by defining domains of cell differentiation. Nature. 1998;393:579–583. doi: 10.1038/31242. [DOI] [PubMed] [Google Scholar]
- Buchholz DR, Heimeier RA, Das B, Washington T, Shi Y-B. Pairing morphology with gene expression in thyroid hormone-induced intestinal remodeling and identification of a core set of TH-induced genes across tadpole tissues. Dev Biol. 2007;303:576–590. doi: 10.1016/j.ydbio.2006.11.037. [DOI] [PubMed] [Google Scholar]
- Das B, Heimeier RA, Buchholz DR, Shi Y-B. Identification of direct thyroid hormone response genes reveals the earliest gene regulation programs during frog metamorphosis. J Biol Chem. 2009;284:34167–34178. doi: 10.1074/jbc.M109.066084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennler S, Andre J, Alexaki I, Li A, Magnaldo T, ten Dijke P, Wang XJ, Verrecchia F, Mauviel A. Induction of sonic hedgehog mediators by transforming growth factor-beta: Smad3-dependent activation of Gli2 and Gli1 expression in vitro and in vivo. Cancer Res. 2007;67:6981–6986. doi: 10.1158/0008-5472.CAN-07-0491. [DOI] [PubMed] [Google Scholar]
- Desouza LA, Sathanoori M, Kapoor R, Rajadhyaksha N, Gonzalez LE, Kottmann AH, Tole S, Vaidya VA. Thyroid hormone regulates the expression of the sonic hedgehog signaling pathway in the embryonic and adult Mammalian brain. Endocrinology. 2011;152:1989–2000. doi: 10.1210/en.2010-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd MHI, Dodd JM. The biology of metamorphosis. In: Lofts B, editor. Physiology of Amphibia. Academic Press; New York: 1976. pp. 467–599. [Google Scholar]
- Edwards DR, Mahadevan LC. Protein synthesis inhibitors differentially superinduce c-fos and c-jun by three distinct mechanisms: lack of evidence for labile repressors. Embo J. 1992;11:2415–2424. doi: 10.1002/j.1460-2075.1992.tb05306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuse N, Maiti T, Wang B, Porter JA, Hall TM, Leahy DJ, Beachy PA. Sonic hedgehog protein signals not as a hydrolytic enzyme but as an apparent ligand for patched. Proc Natl Acad Sci USA. 1999;96:10992–10999. doi: 10.1073/pnas.96.20.10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasebe T, Hartman R, Matsuda H, Shi Y-B. Spatial and temporal expression profiles suggest the involvement of gelatinase A and membrane type 1 matrix metalloproteinase in amphibian metamorphosis. Cell Tissue Res. 2006;324:105–116. doi: 10.1007/s00441-005-0099-7. [DOI] [PubMed] [Google Scholar]
- Hasebe T, Kajita M, Fujimoto K, Yaoita Y, Ishizuya-Oka A. Expression profiles of the duplicated matrix metalloproteinase-9 genes suggest their different roles in apoptosis of larval intestinal epithelial cells during Xenopus laevis metamorphosis. Dev Dyn. 2007;236:2338–2345. doi: 10.1002/dvdy.21252. [DOI] [PubMed] [Google Scholar]
- Hasebe T, Kajita M, Shi Y-B, Ishizuya-Oka A. Thyroid hormone-up-regulated hedgehog interacting protein is involved in larval-to-adult intestinal remodeling by regulating sonic hedgehog signaling pathway in Xenopus laevis. Dev Dyn. 2008;237:3006–3015. doi: 10.1002/dvdy.21698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui CC, Slusarski D, Platt KA, Holmgren R, Joyner AL. Expression of three mouse homologs of the Drosophila segment polarity gene cubitus interruptus, Gli, Gli-2, and Gli-3, in ectoderm- and mesoderm-derived tissues suggests multiple roles during postimplantation development. Dev Biol. 1994;162:402–413. doi: 10.1006/dbio.1994.1097. [DOI] [PubMed] [Google Scholar]
- Ingham PW. Transducing Hedgehog: the story so far. EMBO J. 1998;17:3505–3511. doi: 10.1093/emboj/17.13.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuya-Oka A. Regeneration of the amphibian intestinal epithelium under the control of stem cell niche. Dev Growth Differ. 2007;49:99–107. doi: 10.1111/j.1440-169X.2007.00913.x. [DOI] [PubMed] [Google Scholar]
- Ishizuya-Oka A, Hasebe T, Shimizu K, Suzuki K, Ueda S. Shh/BMP-4 signaling pathway is essential for intestinal epithelial development during Xenopus larval-to-adult remodeling. Dev Dyn. 2006;235:3240–3249. doi: 10.1002/dvdy.20969. [DOI] [PubMed] [Google Scholar]
- Ishizuya-Oka A, Shimozawa A. Induction of metamorphosis by thyroid hormone in anuran small intestine cultured organotypically in vitro. In Vitro Cell Dev Biol. 1991;27A:853–857. doi: 10.1007/BF02630987. [DOI] [PubMed] [Google Scholar]
- Ishizuya-Oka A, Ueda S. Apoptosis and cell proliferation in the Xenopus small intestine during metamorphosis. Cell Tissue Res. 1996;286:467–476. doi: 10.1007/s004410050716. [DOI] [PubMed] [Google Scholar]
- Ishizuya-Oka A, Ueda S, Amano T, Shimizu K, Suzuki K, Ueno N, Yoshizato K. Thyroid-hormone-dependent and fibroblast-specific expression of BMP-4 correlates with adult epithelial development during amphibian intestinal remodeling. Cell Tissue Res. 2001a;303:187–195. doi: 10.1007/s004410000291. [DOI] [PubMed] [Google Scholar]
- Ishizuya-Oka A, Ueda S, Inokuchi T, Amano T, Damjanovski S, Stolow M, Shi Y-B. Thyroid hormone-induced expression of sonic hedgehog correlates with adult epithelial development during remodeling of the Xenopus stomach and intestine. Differentiation. 2001b;69:27–37. doi: 10.1046/j.1432-0436.2001.690103.x. [DOI] [PubMed] [Google Scholar]
- Kanamori A, Brown DD. The regulation of thyroid hormone receptor beta genes by thyroid hormone in Xenopus laevis. J Biol Chem. 1992;267:739–745. [PubMed] [Google Scholar]
- Katoh Y, Katoh M. Hedgehog target genes: mechanisms of carcinogenesis induced by aberrant hedgehog signaling activation. Curr Mol Med. 2009;9:873–886. doi: 10.2174/156652409789105570. [DOI] [PubMed] [Google Scholar]
- Kolterud A, Grosse AS, Zacharias WJ, Walton KD, Kretovich KE, Madison BB, Waghray M, Ferris JE, Hu C, Merchant JL, Dlugosz AA, Kottmann AH, Gumucio DL. Paracrine Hedgehog signaling in stomach and intestine: new roles for hedgehog in gastrointestinal patterning. Gastroenterology. 2009;137:618–628. doi: 10.1053/j.gastro.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Platt KA, Censullo P, Ruiz i, Altaba A. Gli1 is a target of Sonic hedgehog that induces ventral neural tube development. Development. 1997;124:2537–2552. doi: 10.1242/dev.124.13.2537. [DOI] [PubMed] [Google Scholar]
- Lees C, Howie S, Sartor RB, Satsangi J. The hedgehog signalling pathway in the gastrointestinal tract: implications for development, homeostasis, and disease. Gastroenterology. 2005;129:1696–1710. doi: 10.1053/j.gastro.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Liu G, Moro A, Zhang JJ, Cheng W, Qiu W, Kim PC. The role of Shh transcription activator Gli2 in chick cloacal development. Dev Biol. 2007;303:448–460. doi: 10.1016/j.ydbio.2006.10.051. [DOI] [PubMed] [Google Scholar]
- Marigo V, Davey RA, Zuo Y, Cunningham JM, Tabin CJ. Biochemical evidence that patched is the Hedgehog receptor. Nature. 1996;384:176–179. doi: 10.1038/384176a0. [DOI] [PubMed] [Google Scholar]
- Mill P, Mo R, Fu H, Grachtchouk M, Kim PC, Dlugosz AA, Hui CC. Sonic hedgehog-dependent activation of Gli2 is essential for embryonic hair follicle development. Genes Dev. 2003;17:282–294. doi: 10.1101/gad.1038103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo R, Freer AM, Zinyk DL, Crackower MA, Michaud J, Heng HH, Chik KW, Shi XM, Tsui LC, Cheng SH, Joyner AL, Hui C. Specific and redundant functions of Gli2 and Gli3 zinc finger genes in skeletal patterning and development. Development. 1997;124:113–123. doi: 10.1242/dev.124.1.113. [DOI] [PubMed] [Google Scholar]
- Motoyama J, Liu J, Mo R, Ding Q, Post M, Hui CC. Essential function of Gli2 and Gli3 in the formation of lung, trachea and oesophagus. Nat Genet. 1998;20:54–57. doi: 10.1038/1711. [DOI] [PubMed] [Google Scholar]
- Nguyen V, Chokas AL, Stecca B, Ruiz i Altaba A. Cooperative requirement of the Gli proteins in neurogenesis. Development. 2005;132:3267–3279. doi: 10.1242/dev.01905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal table of Xenopus laevis (Daudin) Garland Publishing Inc; New York: 1994. [Google Scholar]
- Park HL, Bai C, Platt KA, Matise MP, Beeghly A, Hui CC, Nakashima M, Joyner AL. Mouse Gli1 mutants are viable but have defects in SHH signaling in combination with a Gli2 mutation. Development. 2000;127:1593–1605. doi: 10.1242/dev.127.8.1593. [DOI] [PubMed] [Google Scholar]
- Parkin CA, Ingham PW. The adventures of Sonic Hedgehog in development and repair. I. Hedgehog signaling in gastrointestinal development and disease. Am J Physiol Gastrointest Liver Physiol. 2008;294:G363–367. doi: 10.1152/ajpgi.00457.2007. [DOI] [PubMed] [Google Scholar]
- Rankin SA, Hasebe T, Zorn AM, Buchholz DR. Improved cre reporter transgenic Xenopus. Dev Dyn. 2009;238:2401–2408. doi: 10.1002/dvdy.22043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin SA, Zorn AM, Buchholz DR. New doxycycline-inducible transgenic lines in Xenopus. Dev Dyn. 2011;240:1467–1474. doi: 10.1002/dvdy.22642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DJ, Johnson RL, Burke AC, Nelson CE, Morgan BA, Tabin C. Sonic hedgehog is an endodermal signal inducing Bmp-4 and Hox genes during induction and regionalization of the chick hindgut. Development. 1995;121:3163–3174. doi: 10.1242/dev.121.10.3163. [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba A. Combinatorial Gli gene function in floor plate and neuronal inductions by Sonic hedgehog. Development. 1998;125:2203–2212. doi: 10.1242/dev.125.12.2203. [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba A. Gli proteins encode context-dependent positive and negative functions: implications for development and disease. Development. 1999;126:3205–3216. doi: 10.1242/dev.126.14.3205. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Hui C, Nakafuku M, Kondoh H. A binding site for Gli proteins is essential for HNF-3beta floor plate enhancer activity in transgenics and can respond to Shh in vitro. Development. 1997;124:1313–1322. doi: 10.1242/dev.124.7.1313. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Nishizaki Y, Hui C, Nakafuku M, Kondoh H. Regulation of Gli2 and Gli3 activities by an amino-terminal repression domain: implication of Gli2 and Gli3 as primary mediators of Shh signaling. Development. 1999;126:3915–3924. doi: 10.1242/dev.126.17.3915. [DOI] [PubMed] [Google Scholar]
- Shi Y-B. Amphibian Metamorphosis: From morphology to molecular biology. John Wiley & Sons, Inc; New York: 1999. [Google Scholar]
- Shi Y-B, Brown DD. The earliest changes in gene expression in tadpole intestine induced by thyroid hormone. J Biol Chem. 1993;268:20312–20317. [PubMed] [Google Scholar]
- Shi Y-B, Ishizuya-Oka A. Biphasic intestinal development in amphibians: embryogenesis and remodeling during metamorphosis. Curr Top Dev Biol. 1996;32:205–235. doi: 10.1016/s0070-2153(08)60429-9. [DOI] [PubMed] [Google Scholar]
- Shi Y-B, Liang VC. Cloning and characterization of the ribosomal protein L8 gene from Xenopus laevis. Biochim Biophys Acta. 1994;1217:227–228. doi: 10.1016/0167-4781(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Sirakov M, Plateroti M. The thyroid hormones and their nuclear receptors in the gut: From developmental biology to cancer. Biochim Biophys Acta. 2011;1812:938–946. doi: 10.1016/j.bbadis.2010.12.020. [DOI] [PubMed] [Google Scholar]
- Stolow MA, Shi Y-B. Xenopus sonic hedgehog as a potential morphogen during embryogenesis and thyroid hormone-dependent metamorphosis. Nucleic Acids Res. 1995;23:2555–2562. doi: 10.1093/nar/23.13.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukegawa A, Narita T, Kameda T, Saitoh K, Nohno T, Iba H, Yasugi S, Fukuda K. The concentric structure of the developing gut is regulated by Sonic hedgehog derived from endodermal epithelium. Development. 2000;127:1971–1980. doi: 10.1242/dev.127.9.1971. [DOI] [PubMed] [Google Scholar]
- Tabin CJ, McMahon AP. Recent advances in hedgehog signalling. Trends Cell Biol. 1997;7:442–446. doi: 10.1016/S0962-8924(97)01159-8. [DOI] [PubMed] [Google Scholar]
- Takabatake T, Takahashi TC, Takabatake Y, Yamada K, Ogawa M, Takeshima K. Distinct expression of two types of Xenopus Patched genes during early embryogenesis and hindlimb development. Mech Dev. 2000;98:99–104. doi: 10.1016/s0925-4773(00)00436-6. [DOI] [PubMed] [Google Scholar]
- van den Brink GR. Hedgehog signaling in development and homeostasis of the gastrointestinal tract. Physiol Rev. 2007;87:1343–1375. doi: 10.1152/physrev.00054.2006. [DOI] [PubMed] [Google Scholar]
- Villavicencio EH, Walterhouse DO, Iannaccone PM. The sonic hedgehog-patched-gli pathway in human development and disease. Am J Hum Genet. 2000;67:1047–1054. doi: 10.1016/s0002-9297(07)62934-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakushiji N, Suzuki M, Satoh A, Ide H, Tamura K. Effects of activation of hedgehog signaling on patterning, growth, and differentiation in Xenopus froglet limb regeneration. Dev Dyn. 2009;238:1887–1896. doi: 10.1002/dvdy.22011. [DOI] [PubMed] [Google Scholar]