Abstract
In vivo, the enzyme 11β-hydroxysteroid dehydrogenase type 2 influences ligand access to the mineralocorticoid receptor. Ablation of the encoding gene, HSD11B2, causes the hypertensive syndrome of Apparent Mineralocorticoid Excess. Studies in humans and experimental animals have linked reduced 11β-hydroxysteroid dehydrogenase type 2 activity and salt-sensitivity of blood pressure. In the present study, renal mechanisms underpinning salt-sensitivity were investigated in Hsd11b2+/− mice fed low, standard and high sodium diets.
In wild-type mice, there was a strong correlation between dietary sodium content and fractional sodium excretion, but not blood pressure. High sodium feeding abolished amiloride-sensitive sodium reabsorption, consistent with down-regulation of the epithelial sodium channel. In Hsd11b2+/− mice, the natriuretic response to increased dietary sodium content was blunted and epithelial sodium channel activity persisted. High sodium diet also reduced renal blood flow and increased blood pressure in Hsd11b2+/− mice. Aldosterone was modulated by dietary sodium in both genotypes and salt-sensitivity in Hsd11b2+/− mice was associated with increased plasma corticosterone levels. Chronic administration of an epithelial sodium channel blocker or a glucocorticoid receptor antagonist prevented salt-sensitivity in Hsd11b2+/− mice, whereas mineralocorticoid receptor blockade with spironolactone did not.
This study shows that reduced 11β-hydroxysteroid dehydrogenase type 2 causes salt-sensitivity of blood pressure due to impaired renal natriuretic capacity. This reflects deregulation of epithelial sodium channels and increased renal vascular resistance. The phenotype is not caused by illicit activation of mineralocorticoid receptors by glucocorticoids, but by direct activation of glucocorticoid receptors.
Keywords: glucocorticoid receptor, RU486, spironolactone, renin-angiotensin system
Introduction
Hypertension remains a significant public health burden worldwide, being a major risk factor for cardiovascular mortality and chronic kidney disease1. Although specific causes of hypertension are often difficult to resolve, salt-sensitivity of blood pressure (BP) is a contributory mechanism in a number of patient subgroups2. Salt-sensitivity is also an independent risk factor for adverse cardiovascular events in normotensive individuals3 and is a negative prognostic indicator for clinical progression towards hypertension, microalbuminuria, and endothelial dysfunction4. The underlying mechanisms of salt-sensitivity are not well defined but subclinical renal impairment reducing the natriuretic efficiency of the kidney may be contributory. Abnormal modulation of the renin-angiotensin-aldosterone system (RAAS) by dietary salt has been linked to salt-sensitivity and cardio-renal damage in both patients5 and in experimental models6. Mineralocorticoid receptor (MR) blockade is cardio-protective, even when aldosterone levels are low or normal7, and pathophysiological activation of MR by alternative ligands has been found in rodent models of salt-sensitive hypertension8,9.
Cross-talk at the receptor level between the RAAS and hypothalamic-pituitary-adrenal (HPA) axis is normally prevented by 11β-hydroxysteroid dehydrogenase type 2 (11βHSD2). This enzyme protects MR directly by restricting the local availability of active glucocorticoids10, and may also confer indirect protection by locking glucocorticoid-occupied MR in a transcriptionally inactive state11. Null mutations in the encoding gene (HSD11B2) cause the syndrome of Apparent Mineralocorticoid Excess (AME; OMIM +218030); an autosomal recessive disorder presenting with hypertension, severe hypokalaemia and low aldosterone. Hsd11b2 null (Hsd11b2−/−) mice have a similar phenotype to AME patients: unregulated activation of MR by glucocorticoids appears to be causative12 and hypertension is associated with transient activation of the epithelial sodium channel (ENaC) in the aldosterone sensitive distal nephron (ASDN)13.
A type 2 variant of AME (OMIM 207765) presents in adults as essential hypertension with mild abnormalities in steroid metabolism14,15. With a strong correlation between the severity of the AME phenotype and the underlying HSD11B2 mutation14, HSD11B2 is an attractive candidate gene for salt-sensitivity. Indeed, HSD11B2 polymorphisms associated with either raised BP per se or salt-sensitivity of BP have been found in several populations16-19, although not all studies report a positive correlation20,21.
We recently demonstrated a causal link between 11βHSD2 activity and salt-sensitivity in Hsd11b2 heterozygote null (Hsd11b2+/−) mice22. These mice were found to have salt-sensitive BP and electrolyte abnormalities consistent with mineralocorticoid excess. AME is classically considered a renal disease and we have therefore analyzed renal sodium handling in Hsd11b2+/− mice. We find that sodium excretion is abnormally modulated by dietary salt in these mice, dependent upon a dysregulation of ENaC. Our studies also point towards a major role for the glucocorticoid receptor (GR), and not the MR, in the salt-sensitive phenotype.
Methods
Experiments on age-matched cohorts of Hsd11b2+/+ and Hsd11b2+/− mice were performed under a UK Home Office Licence, following ethical review by the University.
Renal Clearance
Renal function and BP was measured in anesthetized Hsd11b2+/− and Hsd11b2+/+ mice, maintained on either a low (LS; 0.03%; n=7/8), standard (SS; 0.25%; n=8/8) or high sodium (HS; 2.5%; n=11/9) diet. After baseline measurements, amiloride (2 mg/kg; IV) was injected to measure ENaC activity. (online Data Supplement, http://hyper.ahajournals.org).
Chronic Inhibitor Administration
Renal function was measured mice maintained on a HS diet, receiving one of three co-treatments: spironolactone (n=6/8), RU486 (n= 9/10) or benzamil (n=7/6).
Sodium Balance in Conscious Mice
Mice were housed continuously in metabolic cages. Cumulative sodium balance was measured over cycles of 3 days. Mice were first fed SS diet, after which benzamil or vehicle was administered. After another balance period, HS diet was given for 3 days, this being the period of sodium retention22.
Quantitative PCR
mRNA abundance was measured using a Universal Probe Library kit (Roche, UK) and primers designed for the following targets: nr3c1, nr3c2, scnn1a, scnn1b and sgk1. (See Table S1)
Statistics
All data are presented as means ± standard error. Statistical comparisons were made using Prism 5 (GraphPad Software, USA).
Results
Salt-sensitive BP and renal hemodynamics in Hsd11b2+/− mice
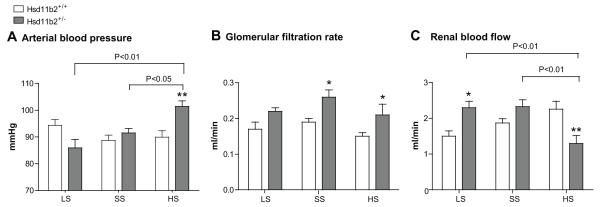
BP was measured in groups of Hsd11b2+/+ and Hsd11b2+/− mice maintained on LS, SS or HS diet. On LS and HS diet, BP was comparable between genotypes. HS diet caused a significant increase in BP in Hsd11b2+/− mice (Figure 1A). Overall, there was a significant correlation between MABP and dietary sodium content in Hsd11b2+/− mice (Pearson r= 0.67; P<0.001); no such relationship was observed in Hsd11b2+/+ mice.
Figure 1.
A) Arterial blood pressure; B) glomerular filtration rate; and C) renal blood flow in Hsd11b2+/+ (open bars) and Hsd11b2+/− (grey bars) mice after 3 weeks on either low sodium (LS; n=7/8), standard sodium (SS; n=8/8) or high sodium (HS; 11/9) diet. Data are means±SEM. Comparisons were made using ANOVA with Bonferroni post hoc test. *P<0.05; **P<0.01.
Glomerular filtration rate (GFR) was higher in Hsd11b2+/− mice than controls but there was no relationship between this and dietary salt (Figure 1B). Renal blood flow (RBF) was higher in Hsd11b2+/− mice than Hsd11b2+/+on LS and SS diets (Figure 1C). Dietary salt loading decreased RBF in Hsd11b2+/− mice: filtration fraction (FF) and renal vascular resistance (RVR) were both significantly elevated (Table 1).
Table 1.
Renal and plasma data. Hsd11b2+/+ and Hsd11b2+/− mice were maintained on a low sodium (LS; n=7/8), standard sodium (SS; n=8/8) or high sodium (HS; n=11/9) diet. Data are means ± SEM. Statistical comparisons were made using ANOVA with Bonferroni post hoc test. **P<0.01; aP<0.001 versus wild-type.
| Parameter |
Hsd11b2+/+ LS |
Hsd11b2+/− LS |
Hsd11b2+/+ SS |
Hsd11b2+/− SS |
Hsd11b2+/+ HS |
Hsd11b2+/− HS |
|
|---|---|---|---|---|---|---|---|
| FF* | (%) | 30.0 ± 6.1 | 22.2 ±3.8 | 23.8 ± 1.7 | 25.7 ± 1.8 | 16.8 ± 0.8 | 42.1 ± 4.1** |
| RVR† | (mmHg/ml.min−1) | 67.2 ± 7.0 | 37.8 ± 2.9 | 48.8 ± 3.3 | 41.1 ± 3.5 | 42.4 ± 4.2 | 93.8 ± 18.7** |
| ENa‡ | (μmol/min) | 0.05±0.02 | 0.06±0.01 | 0.26±0.07 | 0.07±0.03* | 0.35±0.03 | 0.19±0.04** |
| EK§ | (μmol/min) | 0.3 ± 0.06 | 0.24 ± 0.04 | 0.28 ± 0.03 | 0.44 ± 0.03* | 0.17 ± 0.03 | 0.20 ± 0.07 |
| PNa|| | (mmol/l) | 152.6 ± 3.5 | 149.0 ± 0.4 | 146.0 ± 0.6 | 147.0 ± 0.9 | 149.1 ± 1.2 | 149.8 ± 0.9 |
| Pk¶ | (mmol/l) | 4.41 ± 0.34 | 4.02 ± 0.19 | 4.25 ± 0.08 | 4.55 ± 0.14 | 4.83 ± 0.34 | 3.72 ± 0.11** |
| PCort# | (nmol/l) | 515 ± 78 | 425 ± 21 | 294 ± 24 | 330 ± 34 | 429 ± 44 | 698 ± 48** |
| Paldo** | (pmol/l) | 1615 ±81 | 809 ± 103** | 537 ± 164 | 421 ± 94 | 332±59 | 39±0.6a |
Filtration fraction,
renal vascular resistance,
urinary excretion of sodium and
potassium and plasma concentrations of
sodium,
potassium,
corticosterone and
aldosterone.
Hsd11b2+/− mice have impaired fractional sodium excretion
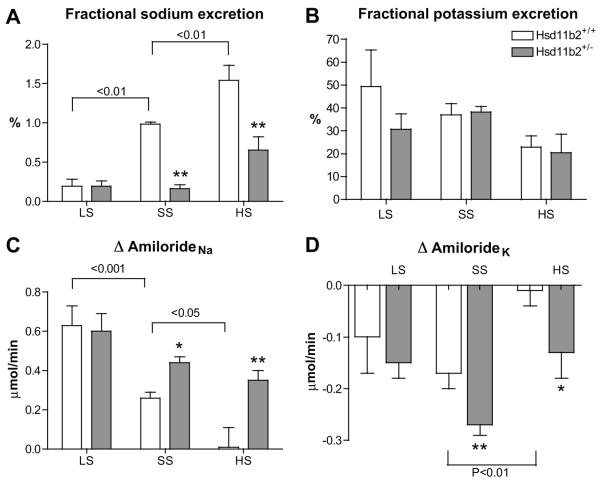
Sodium excretion was lower in Hsd11b2+/− mice than wild-types on both SS and HS diets (Table 1) and fractional sodium excretion (FENa) was calculated to assess tubular function. In Hsd11b2+/+ mice, there was an appropriate increase in FENa with increasing dietary sodium (Figure 2A. Pearson r= 0.67; P<0.01). This relationship was blunted in Hsd11b2+/− mice and on SS or HS diet, FENa was significantly lower than in Hsd11b2+/+ mice (P<0.01). Although Hsd11b2+/− mice were not able to adapt their renal sodium excretion to their dietary sodium load as effectively as Hsd11b2+/+ mice, plasma sodium concentration was not affected (Table 1).
Figure 2.
A) Fractional sodium excretion; B) fractional potassium excretion; C) amiloride-sensitive sodium reabsorption (ΔamilorideNa) and D) amiloride sensitive potassium secretion (Δamiloridek) in Hsd11b2+/+ (open bars) and Hsd11b2+/+ (grey bars) mice after 3 weeks on either low sodium (LS; n=7/8), standard sodium (SS; n=8/8) or high sodium (HS; n=11/9) diet. Data are means±SEM. Comparisons were made using ANOVA with Bonferroni post hoc test. *P<0.05; **P<0.01.
Potassium excretion was elevated in Hsd11b2+/− mice, but only on a SS diet (Table 1) and fractional potassium excretion (FEK) was comparable across all dietary regimens (Figure 2B).
Amiloride-sensitive sodium reabsorption in Hsd11b2+/− mice
The natriuretic effect of amiloride (ΔamilorideNa) was used to quantify ENaC-mediated sodium reabsorption. In Hsd11b2+/+ mice (Figure 2C), there was an inverse relationship between dietary sodium content and ΔamilorideNa (Pearson r=−0.74, P<0.001), consistent with down-regulation of functional ENaC following increased sodium intake.
In Hsd11b2+/− mice, the inverse relationship was blunted (Pearson r=−0.42; P<0.05). Critically, amiloride evoked a significant natriuresis on HS diet, suggesting that Hsd11b2+/− mice fail to regulate their ENaC activity appropriately in relation to sodium intake. This does not reflect altered gene transcription: mRNA abundance for ENaC subunits was not different between genotypes. Sgk1 expression was increased, which would promote ENaC retention in the apical membrane (see Figure S1) and maintain an electrophysiological driving force for potassium secretion via ROMK. Indeed, despite hypokalemia, the potassium sparing effect of amiloride was sustained (Figure 2D) in Hsd11b2+/− mice on HS diet, whereas wild-type mice maintain potassium homeostasis through processes independent of ENaC activity.
The effect of the specific ENaC antagonist, benzamil, on sodium balance was assessed in conscious mice. In untreated Hsd11b2+/− mice, the transition to HS diet caused a positive sodium balance (Hsd11b2+/− =104±15 versus Hsd11b2+/+= −6.9±30.5 μmoles, P<0.05). In benzamil-treated mice, sodium balance remained neutral during this transition (Hsd11b2+/−=28.87±68 versus Hsd11b2+/+= 17.46±2.9 μmoles, NS).
Role of GR and MR
In the current study, increased sodium intake reduced aldosterone in both genotypes (Table 1). Nevertheless, Hsd11b2+/− mice had significantly lower levels than Hsd11b2+/+ mice suggesting tonic suppression of the RAAS. It is therefore unlikely that aldosterone excess is responsible for the increased ENaC activity observed in Hsd11b2+/− mice.
Corticosterone was similar between genotypes on LS and SS diets (Table 1) but was elevated in Hsd11b2+/− mice on HS diet. To militate against confounding effects of anesthesia, corticosterone was measured in unrestrained conscious mice before and after HS feeding. The sodium-induced increase in corticosterone was confirmed in the PM measurement, prior to the active phase: AM corticosterone was not different between genotypes. (Figure S2).
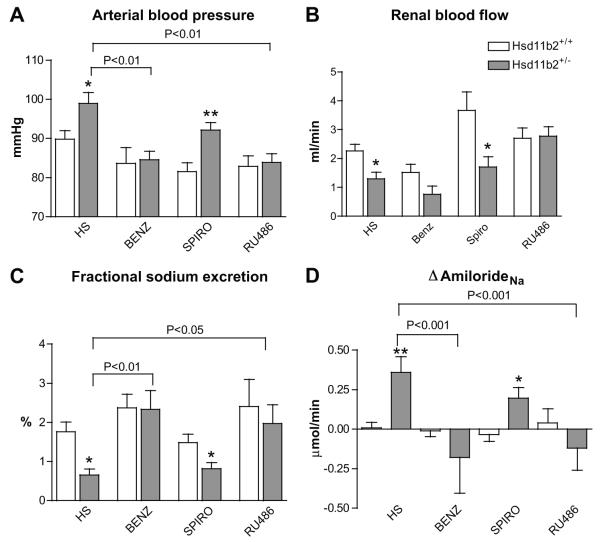
Since activation of MR and/or GR by glucocorticoids was likely to be causative to the salt-induced phenotype, the renal expression of both was measured: MR expression was similar in both genotypes but GR expression was higher in Hsd11b2+/− mice (See Figure S3). To resolve the mechanisms for salt-sensitivity, Hsd11b2+/+ and Hsd11b2+/− mice on a HS diet were treated chronically with i) the ENaC blocker benzamil, ii) the MR antagonist spironolactone, or iii) the GR antagonist RU486. The salt-sensitive phenotype was ameliorated by either benzamil or RU486 treatment, but not by spironolactone treatment (Figure 3A). The sodium-induced reduction in RBF was also ameliorated by benzamil treatment, and RBF was actually increased by RU486 treatment (Figure 3B). Spironolactone did not improve FENa (Figure 3C) and ΔamilorideNa remained high. In contrast, both benzamil and RU486 treatment restored FENa in Hsd11b2+/− mice to Hsd11b2+/+ levels and abolished genotype differences in ΔamilorideNa (Figure 3D).
Figure 3.
A) Arterial blood pressure; B) renal blood flow; C) fractional sodium excretion; and D) amiloride-sensitive sodium reabsorption (ΔamilorideNa) in Hsd11b2+/+ (open bars) and Hsd11b2+/− (grey bars) mice after 3 weeks on a high sodium (HS) diet, or with HS diet accompany by either benzamil (Benz; n=7/6), spironolactone (Spiro; n=6/8) or RU486 treatment (n=9/10). Data are means±SEM. Comparisons were made using ANOVA with Bonferroni post hoc test. *P<0.05; **P<0.01.
Discussion
In Hsd11b2+/− mice BP is directly influenced by dietary sodium intake. This strong salt-sensitivity of BP was not observed on the parental C57BL/6J background, and thus the HSD11B2 locus is a plausible candidate gene for salt-sensitivity in humans. As gene defects associated with monogenic BP disorders affect the renal handling of sodium23, we hypothesized that salt-sensitivity in Hsd11b2+/− mice may also reflect abnormal renal function. Data obtained across a regimen encompassing dietary sodium restriction and sodium loading supported this hypothesis, indicating that abnormal renal sodium homeostasis is driven by activation of GR, and not MR, in this model.
Renal sodium handling is the major determinate of long-term BP control and Hsd11b2+/− mice had lower FENa than Hsd11b2+/+. Elevated tubular reabsorption is likely to be the major factor for impaired natriuresis and salt-sensitive BP in Hsd11b2+/− mice. Since 11βHSD2 governs ligand access to MR in vivo, we focused on the classical MR target, ENaC24. Hsd11b2+/− mice failed to down-regulate amiloride-sensitive sodium reabsorption with increasing dietary sodium, and impaired natriuresis therefore reflects an inability to regulate ENaC activity appropriately for sodium intake. We recognize that amiloride can also inhibit other sodium transport proteins, notably the sodium-hydrogen exchanger 3 (NHE3) in the proximal tubule25. Two lines of evidence argue against a major role of NHE3 here. Firstly, the potassium-sparing effect of amiloride localizes the natriuretic effect to the ASDN26. Secondly, chronic administration of the selective amiloride analogue benzamil attenuated the salt-sensitive phenotype. Our data plausibly suggest that dysregulated ENaC activity is a key mechanism for salt-sensitivity in this model. This effect is not underpinned by increased transcription of the rate-limiting αENaC subunit. The higher abundance of sgk1 in salt-loaded heterozygote mice indicates that the dominant effect is to sustain the channel assembly in the apical membrane of the principal cell24.
Control by MR of ENaC and its regulatory proteins is well documented24. In the current study, the physiological ligand aldosterone was appropriately modulated by dietary sodium in both groups of mice. However, there was a tonic suppression of the RAAS in Hsd11b2+/− mice across all dietary regimens, consistent with MR activation by glucocorticoids following reduced 11βHSD2. To investigate the contribution of MR, mice fed HS diet were chronically treated with spironolactone. MR antagonism caused a small reduction in BP in both groups of mice, but the pressure differential between the genotypes persisted, as we have previously reported22. Critically, spironolactone treatment did not normalize ENaC-mediated sodium reabsorption in Hsd11b2+/− mice. The lack of sensitivity to spironolactone may reflect the increased abundance of GR, relative to MR and it is possible that a higher dose would uncover an MR-mediated effect. Nevertheless, the current dose was previously found to be effective against similar concentrations of glucocorticoid8 and although spironolactone treatment can improve both the hypertension and hypokalemia observed in AME27, it is of variable benefit in long term treatment28.
We found that chronic GR blockade normalized ENaC activity and increased RBF. RU486 also prevented the salt-induced rise in BP, consistent with our previous findings22. Quantitatively, the effect of RU486 was similar to that of chronic benzamil administration. Together, these data indicate that the cluster of ENaC-related phenotypes in the Hsd11b2+/− mice are mediated via GR, not MR. Regulation of ENaC by GR-dependent pathways has been documented in renal cell lines29,30, in dexamethasone-treated adrenalectomised rats31 and in a mouse model of Cushing syndrome8. A recent study immunolocalized both MR and GR to 11βHSD2-expressing cells in the rat ASDN, demonstrating that physiological variations in circulating aldosterone regulated the translocation of GR and not MR between the nucleus and the cytoplasm32. This challenges the conventional view of ASDN regulation by corticosteroids, but is consistent with our data and suggests a mechanism for salt-sensitivity in this model.
The infusion of the dexamethasone can increase the abundance of renal αENaC mRNA and protein expression. This does not automatically equate to an increased physiological activity for ENaC33 and in Hsd11b2+/− mice, ENaC transcription was comparable to controls. Additional ENaC regulatory pathways may be critical, and our data in Hsd11b2+/− mice, as well as other models4, suggests that dietary salt and/or activation of the HPA axis plays an important role. A realistic point of convergence under these circumstances may be WNK4, which exerts a negative regulatory effect upon ENaC activity34. WNK4 is physiologically regulated by dietary sodium status35, and by glucocorticoids via a negative GRE in the promotor region of the gene36. In addition, β2-receptor activation has been demonstrated to induce salt-sensitive hypertension in mice due to GR-mediated inhibition of WNK4 expression37. Conversely, Cre-lox technology has suggested that GR expression in the ASDN is not critical to dexamethasone-induced hypertension when salt intake is normal38. However, mice lacking GR in the ASDN had elevated BP prior to dexamethasone treatment and the effect of a HS diet was not assessed in this study.
HS diet also increased RVR in Hsd11b2+/− mice, which would reduce natriuretic capacity, particularly if the medullary vasa recta were constricted. 11βHSD2 is expressed in both arteriole smooth muscle39 and the vascular endothelium40. On a mixed MF1 background, Hsd11b2−/− mice had endothelial dysfunction and enhanced vasoconstriction to norepinephrine41. This may relate to the genetic background; vascular function was normal on a congenic C57Bl/6J strain13. Nevertheless, we cannot discount a vascular component of salt-sensitive BP in Hsd11b2+/− mice. ENaC in the vascular endothelium is stimulated by aldosterone excess and high sodium42 and this is associated with reduced nitric oxide release43. Notably, in Hsd11b2+/− mice, chronic ENaC blockade prevented the salt-induced increase in RVR.
Perspectives
Failure to regulate ENaC activity with sodium status underpins salt-sensitivity in Hsd11b2+/− mice. This is a GR, rather than MR-mediated phenotype. HS feeding increased corticosterone in Hsd11b2+/− mice and it is notable that salt-sensitive individuals display both an enhanced stress-induced activation of the HPA axis44 and attenuated glucocorticoid clearance45. Since renal enzyme activity is not influenced by dietary sodium in Hsd11b2+/− mice22, impaired peripheral metabolism cannot fully explain this phenomenon and we suggest that dietary salt activates the HPA axis. Indeed, 11βHSD2 is also expressed in cardiovascular control centers of the brain influencing sympathetic outflow46. Central mechanisms are therefore likely to contribute to salt-sensitive BP, at least in the stable phase when sodium balance is restored.
Supplementary Material
Novelty and Significance.
- What is new?
- Reduced activity of a steroid-processing enzyme impairs the efficiency of salt excretion by the kidney
- The protein causing sodium retention was identified and a new role for regulation of sodium transport in the kidney by steroid hormones revealed.
- High intake of salt in the diet increases glucocorticoids in the blood, which increases salt retention by the kidney. This causes blood pressure to rise
- What is relevant?
- In some people, blood pressure rises with high salt intake and this increases the risk for developing hypertension, cardiovascular and renal disease.
- High levels of glucocorticoids are common in stress and metabolic disorders and could reduce the excretion of salt by the kidney
- The mechanisms for salt retention could therefore be potential targets for antihypertensive therapy
Summary: Reduced activity of a glucocorticoid-metabolizing enzyme in the kidney and brain causes increased levels of steroid in the blood, impairs the ability of the kidney to excrete sodium and causes blood pressure to rise.
Acknowledgments
Sources of Funding
EC received an MRC Capacity Building PhD studentship. This work was supported by Wellcome Trust Intermediate (MB) and Principal (JM) Fellowships and by a British Heart Foundation Centre of Research Excellence Award.
Footnotes
Conflict of interest/Disclosures: NONE
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Couser WG, Riella MC. Cardiovascular disease: World Kidney Day 2011: protect your kidneys, save your heart. Nat Rev Nephrol. 2011;7:130–132. doi: 10.1038/nrneph.2011.11. [DOI] [PubMed] [Google Scholar]
- 2.Haddy FJ. Role of dietary salt in hypertension. Life Sci. 2006;79:1585–1592. doi: 10.1016/j.lfs.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 3.Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension. 2001;37:429–432. doi: 10.1161/01.hyp.37.2.429. [DOI] [PubMed] [Google Scholar]
- 4.Sanders PW. Vascular consequences of dietary salt intake. Am J Physiol Renal Physiol. 2009;297:F237–F243. doi: 10.1152/ajprenal.00027.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waanders F, de Vries LV, van Goor H, Hillebrands JL, Laverman GD, Bakker SJ, Navis G. Aldosterone, from (patho)physiology to treatment in cardiovascular and renal damage. Curr Vasc Pharmacol. 2011;9:594–605. doi: 10.2174/157016111796642689. [DOI] [PubMed] [Google Scholar]
- 6.Nishiyama A, Yao L, Nagai Y, Miyata K, Yoshizumi M, Kagami S, Kondo S, Kiyomoto H, Shokoji T, Kimura S, Kohno M, Abe Y. Possible contributions of reactive oxygen species and mitogen-activated protein kinase to renal injury in aldosterone/salt-induced hypertensive rats. Hypertension. 2004;43:841–848. doi: 10.1161/01.HYP.0000118519.66430.22. [DOI] [PubMed] [Google Scholar]
- 7.Usukura M, Zhu A, Yoneda T, Karashima S, Yagi K, Yamagishi M, Takeda Y. Effects of a high-salt diet on adipocyte glucocorticoid receptor and 11β-hydroxysteroid dehydrogenase 1 in salt-sensitive hypertensive rats. Steroids. 2009;74:978–982. doi: 10.1016/j.steroids.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Bailey MA, Mullins JJ, Kenyon CJ. Mineralocorticoid and glucocorticoid receptors stimulate epithelial sodium channel activity in a mouse model of Cushing syndrome. Hypertension. 2009;54:890–896. doi: 10.1161/HYPERTENSIONAHA.109.134973. [DOI] [PubMed] [Google Scholar]
- 9.Shibata S, Mu S, Kawarazaki H, Muraoka K, Ishizawa K, Yoshida S, Kawarazaki W, Takeuchi M, Ayuzawa N, Miyoshi J, Takai Y, Ishikawa A, Shimosawa T, Ando K, Nagase M, Fujita T. Rac1 GTPase in rodent kidneys is essential for salt-sensitive hypertension via a mineralocorticoid receptor-dependent pathway. J Clin Invest. 2011;12:3233–3243. doi: 10.1172/JCI43124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Funder JW, Pearce PT, Smith R, Smith AI. Mineralocorticoid action: target tissue specificity is enzyme, not receptor, mediated. Science. 1988;242:583–585. doi: 10.1126/science.2845584. [DOI] [PubMed] [Google Scholar]
- 11.Funder JW. Aldosterone and mineralocorticoid receptors in the cardiovascular system. Prog Cardiovasc Dis. 2010;52:393–400. doi: 10.1016/j.pcad.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Kotelevtsev Y, Brown RW, Fleming S, Kenyon C, Edwards CR, Seckl JR, Mullins JJ. Hypertension in mice lacking 11β-hydroxysteroid dehydrogenase type 2. J Clin Invest. 1999;103:683–689. doi: 10.1172/JCI4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bailey MA, Paterson JM, Hadoke PW, Wrobel N, Bellamy CO, Brownstein DG, Seckl JR, Mullins JJ. A switch in the mechanism of hypertension in the syndrome of apparent mineralocorticoid excess. J Am Soc Nephrol. 2008;19:47–58. doi: 10.1681/ASN.2007040401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dave-Sharma S, Wilson RC, Harbison MD, Newfield R, Azar MR, Krozowski ZS, Funder JW, Shackleton CH, Bradlow HL, Wei JQ, Hertecant J, Moran A, Neiberger RE, Balfe JW, Fattah A, Daneman D, Akkurt HI, De Santis C, New MI. Examination of genotype and phenotype relationships in 14 patients with apparent mineralocorticoid excess. J Clin Endo Metab. 1998;83:2244–2254. doi: 10.1210/jcem.83.7.4986. [DOI] [PubMed] [Google Scholar]
- 15.Ulick S, Tedde R, Mantero F. Pathogenesis of the type 2 variant of the syndrome of apparent mineralocorticoid excess. J Clin Endo Metab. 1990;70:200–206. doi: 10.1210/jcem-70-1-200. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal AK, Giacchetti G, Lavery G, Nikkila H, Palermo M, Ricketts M, McTernan C, Bianchi G, Manunta P, Strazzullo P, Mantero F, White PC, Stewart PM. CA-Repeat polymorphism in intron 1 of HSD11B2: effects on gene expression and salt sensitivity. Hypertension. 2000;36:187–194. doi: 10.1161/01.hyp.36.2.187. [DOI] [PubMed] [Google Scholar]
- 17.Alikhani-Koupaei R, Fouladkou F, Fustier P, Cenni B, Sharma AM, Deter HC, Frey BM, Frey FJ. Identification of polymorphisms in the human 11β-hydroxysteroid dehydrogenase type 2 gene promoter: functional characterization and relevance for salt sensitivity. FASEB J. 2007;21:3618–3628. doi: 10.1096/fj.07-8140com. [DOI] [PubMed] [Google Scholar]
- 18.Bocchi B, Kenouch S, Lamarre-Cliche M, Muffat-Joly M, Capron MH, Fiet J, Morineau G, Azizi M, Bonvalet JP, Farman N. Impaired 11β-hydroxysteroid dehydrogenase type 2 activity in sweat gland ducts in human essential hypertension. Hypertension. 2004;43:803–808. doi: 10.1161/01.HYP.0000121362.64182.ad. [DOI] [PubMed] [Google Scholar]
- 19.Poch E, Gonzalez D, Giner V, Bragulat E, Coca A, de La Sierra A. Molecular basis of salt sensitivity in human hypertension. Evaluation of renin-angiotensin-aldosterone system gene polymorphisms. Hypertension. 2001;38:1204–1209. doi: 10.1161/hy1101.099479. [DOI] [PubMed] [Google Scholar]
- 20.Kerstens MN, Navis G, Dullaart RP. Salt sensitivity and 11β-hydroxysteroid dehydrogenase type 2 activity. Am J Hypertens. 2004;17:283–284. doi: 10.1016/j.amjhyper.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 21.Melander O, Frandsen E, Groop L, Hulthen UL. No evidence of a relation between 11β-hydroxysteroid dehydrogenase type 2 activity and salt sensitivity. Am J Hypertens. 2003;16:729–733. doi: 10.1016/s0895-7061(03)00981-6. [DOI] [PubMed] [Google Scholar]
- 22.Bailey MA, Craigie E, Livingstone DE, Kotelevtsev YV, Al-Dujaili EA, Kenyon CJ, Mullins JJ. Hsd11b2 haploinsufficiency in mice causes salt sensitivity of blood pressure. Hypertension. 2011;57:515–520. doi: 10.1161/HYPERTENSIONAHA.110.163782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mullins LJ, Bailey MA, Mullins JJ. Hypertension, kidney, and transgenics: a fresh perspective. Physiol Rev. 2006;86:709–746. doi: 10.1152/physrev.00016.2005. [DOI] [PubMed] [Google Scholar]
- 24.Soundararajan R, Pearce D, Ziera T. The role of the ENaC-regulatory complex in aldosterone-mediated sodium transport. Mol Cell Endocrinol. 2012;350:242–247. doi: 10.1016/j.mce.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yun CH, Tse CM, Nath SK, Levine SA, Brant SR, Donowitz M. Mammalian Na+/H+ exchanger gene family: structure and function studies. Am J Physiol. 1995;269:G1–G11. doi: 10.1152/ajpgi.1995.269.1.G1. [DOI] [PubMed] [Google Scholar]
- 26.Kleyman TR, Cragoe EJ., Jr. The mechanism of action of amiloride. Semin Nephrol. 1988;8:242–248. [PubMed] [Google Scholar]
- 27.Morineau G, Sulmont V, Salomon R, Fiquet-Kempf B, Jeunemaitre X, Nicod J, Ferrari P. Apparent Mineralocorticoid Excess: Report of Six New Cases and Extensive Personal Experience. J Am Soc Nephrol. 2006;17:3176–3184. doi: 10.1681/ASN.2006060570. [DOI] [PubMed] [Google Scholar]
- 28.Quinkler M, Stewart PM. Hypertension and the cortisol-cortisone shuttle. J Clin Endo Metab. 2003;88:2384–2392. doi: 10.1210/jc.2003-030138. [DOI] [PubMed] [Google Scholar]
- 29.Gaeggeler HP, Gonzalez-Rodriguez E, Jaeger NF, Loffing-Cueni D, Norregaard R, Loffing J, Horisberger JD, Rossier BC. Mineralocorticoid versus glucocorticoid receptor occupancy mediating aldosterone-stimulated sodium transport in a novel renal cell line. J Am Soc Nephrol. 2005;16:878–891. doi: 10.1681/ASN.2004121110. [DOI] [PubMed] [Google Scholar]
- 30.Naray-Fejes-Toth A, Fejes-Toth G. Glucocorticoid receptors mediate mineralocorticoid-like effects in cultured collecting duct cells. Am J Physiol. 1990;259:F672–F678. doi: 10.1152/ajprenal.1990.259.4.F672. [DOI] [PubMed] [Google Scholar]
- 31.Muller OG, Parnova RG, Centeno G, Rossier BC, Firsov D, Horisberger JD. Mineralocorticoid effects in the kidney: correlation between alphaENaC, GILZ, and Sgk-1 mRNA expression and urinary excretion of Na+ and K+ J Am Soc Nephrol. 2003;14:1107–1115. doi: 10.1097/01.asn.0000061777.67332.77. [DOI] [PubMed] [Google Scholar]
- 32.Ackermann D, Gresko N, Carrel M, Loffing-Cueni D, Habermehl D, Gomez-Sanchez C, Rossier BC, Loffing J. In vivo nuclear translocation of mineralocorticoid and glucocorticoid receptors in rat kidney: differential effect of corticosteroids along the distal tubule. Am J Physiol Renal Physiol. 2010;299:F1473–1485. doi: 10.1152/ajprenal.00437.2010. [DOI] [PubMed] [Google Scholar]
- 33.Frindt G, Palmer LG. Regulation of epithelial Na+ channels by adrenal steroids: Mineralocorticoid and glucocorticoid effects. Am J Physiol Renal Physiol. 2012;302:F20–F26. doi: 10.1152/ajprenal.00480.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ring AM, Cheng SX, Leng Q, Kahle KT, Rinehart J, Lalioti MD, Volkman HM, Wilson FH, Hebert SC, Lifton RP. WNK4 regulates activity of the epithelial Na+ channel in vitro and in vivo. Proc Natl Acad Sci. 2007;104:4020–4024. doi: 10.1073/pnas.0611727104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Reilly M, Marshall E, Macgillivray T, Mittal M, Xue W, Kenyon CJ, Brown RW. Dietary electrolyte-driven responses in the renal WNK kinase pathway in vivo. J Am Soc Nephrol. 2006;17:2402–2413. doi: 10.1681/ASN.2005111197. [DOI] [PubMed] [Google Scholar]
- 36.Li C, Li Y, Liu H, Sun Z, Lu J, Zhao Y. Glucocorticoid repression of human with-no-lysine (K) kinase-4 gene expression is mediated by the negative response elements in the promoter. J Mol Endocrinol. 2008;40:3–12. doi: 10.1677/JME-07-0049. [DOI] [PubMed] [Google Scholar]
- 37.Mu S, Shimosawa T, Ogura S, Wang H, Uetake Y, Kawakami-Mori F, Marumo T, Yatomi Y, Geller DS, Tanaka H, Fujita T. Epigenetic modulation of the renal beta-adrenergic-WNK4 pathway in salt-sensitive hypertension. Nat Med. 2011;17:573–580. doi: 10.1038/nm.2337. [DOI] [PubMed] [Google Scholar]
- 38.Goodwin JE, Zhang J, Velazquez H, Geller DS. The glucocorticoid receptor in the distal nephron is not necessary for the development or maintenance of dexamethasone-induced hypertension. Biochem Biophys Res Commun. 2010;394:266–271. doi: 10.1016/j.bbrc.2010.02.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hatakeyama H, Inaba S, Takeda R, Miyamori I. 11β-hydroxysteroid dehydrogenase in human vascular cells. Kidney Int. 2000;57:1352–1357. doi: 10.1046/j.1523-1755.2000.00974.x. [DOI] [PubMed] [Google Scholar]
- 40.Gong R, Morris DJ, Brem AS. Variable expression of 11β Hydroxysteroid dehydrogenase isoforms in vascular endothelial cells. Steroids. 2008;73:1187–1196. doi: 10.1016/j.steroids.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 41.Hadoke PW, Christy C, Kotelevtsev YV, Williams BC, Kenyon CJ, Seckl JR, Mullins JJ, Walker BR. Endothelial cell dysfunction in mice after transgenic knockout of type 2, but not type 1, 11β-hydroxysteroid dehydrogenase. Circulation. 2001;104:2832–2837. doi: 10.1161/hc4801.100077. [DOI] [PubMed] [Google Scholar]
- 42.Kusche-Vihrog K, Sobczak K, Bangel N, Wilhelmi M, Nechyporuk-Zloy V, Schwab A, Schillers H, Oberleithner H. Aldosterone and amiloride alter ENaC abundance in vascular endothelium. Pflugers Arch. 2008;455:849–857. doi: 10.1007/s00424-007-0341-0. [DOI] [PubMed] [Google Scholar]
- 43.Oberleithner H, Riethmuller C, Schillers H, MacGregor GA, de Wardener HE, Hausberg M. Plasma sodium stiffens vascular endothelium and reduces nitric oxide release. Proc Natl Acad Sci. 2007;104:16281–16286. doi: 10.1073/pnas.0707791104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weber CS, Thayer JF, Rudat M, Sharma AM, Perschel FH, Buchholz K, Deter HC. Salt-sensitive men show reduced heart rate variability, lower norepinephrine and enhanced cortisol during mental stress. J Hum Hypertens. 2008;22:423–431. doi: 10.1038/jhh.2008.11. [DOI] [PubMed] [Google Scholar]
- 45.Kerstens MN, van der Kleij FG, Boonstra AH, Sluiter WJ, Koerts J, Navis G, Dullaart RP. Salt loading affects cortisol metabolism in normotensive subjects: relationships with salt sensitivity. J Clin Endo Metab. 2003;88:4180–4185. doi: 10.1210/jc.2002-021625. [DOI] [PubMed] [Google Scholar]
- 46.Geerling JC, Loewy AD. Aldosterone-sensitive neurons in the nucleus of the solitary tract: efferent projections. J Comp Neurol. 2006;497:223–250. doi: 10.1002/cne.20993. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.