Abstract
Localization of brain lesions and prevention of damage to vital structures are important in operation of brain pathologies. Despite development of many techniques including angiography, MRI, sonography, and frame base stereotaxy, a more accurate localizing technique is still needed (1, 2). A step forward to achieve this goal is to develop a navigation system. In this manuscript, we explained some clinical applications, advantages, and disadvantages of navigation system and tried to have a short glimpse on its future.
Keywords: Neuronavigation, Neurosurgery, Skull Base
Localization and delineation of extent of lesions are critical for safe maximal resection of brain and spinal cord tumors. Neuronavigation systems have been developed for image-guided neurosurgery to aid in the accurate resection of brain tumors (3, 4). Basic principles of navigated surgery are to see the tip of a pointer in an image space. A relationship between the device space and the image space has to be established [5, 6]. This operation is called registration or calibration of the navigation device. Basically, a transformation matrix (T) has to be calculated to map the coordinates of any point between the image and the device spaces. The aim of transformation matrix is to create a linkage between digital image data and anatomical structure, and therefore, to provide increasing 3-D orientation (7). Today's navigation systems provide approximately 2mm accuracy (8).
Stereoscopic navigation-controlled display of preoperative MRI and intraoperative 3D ultrasound is a new technology for minimally invasive image-guided surgery approaches in planning and guiding neurosurgery. Interactive stereoscopic visualization improves perception and enhances the ability to understand complex 3D anatomy [9, 10]. In 1947, Spiegel and Wycis performed the first stereotactic thalamotomy in humans, using the commissura posterior or pineal body as an internal individual reference system (11, 12). Functional operations with similar frames and techniques were introduced by Talairach in Paris in 1949 (13), by Riechert [14] in Freiburg, Germany in 1952, and by Leksell [3] in Stockholm in 1949 for the treatment of extrapyramidal movement disorders, intractable pain, epilepsy, and psychiatric disorders.
After the development of CT technology by Hounsfield in 1973 (15) and Cormack (16, 17) based on mathematical solutions published by the Viennese mathematician Radon in 1917 [18], stereotactic coordinate based calculation was applicable in the whole intracranial space, enlarging the field of indications to biopsies, interstitial brachytherapy, endoscopy, and localization of tumors for open surgery (4, 19). Till the end of the 1980s, frame-based stereotaxy was the standard method for accurately localizing small intracranial lesions by introducing catheters into the tumors or for determining the tumor volume in space (20, 21, 22). Coordinate transformation of the selected target point between the image and the frame space was established using a localization frame.
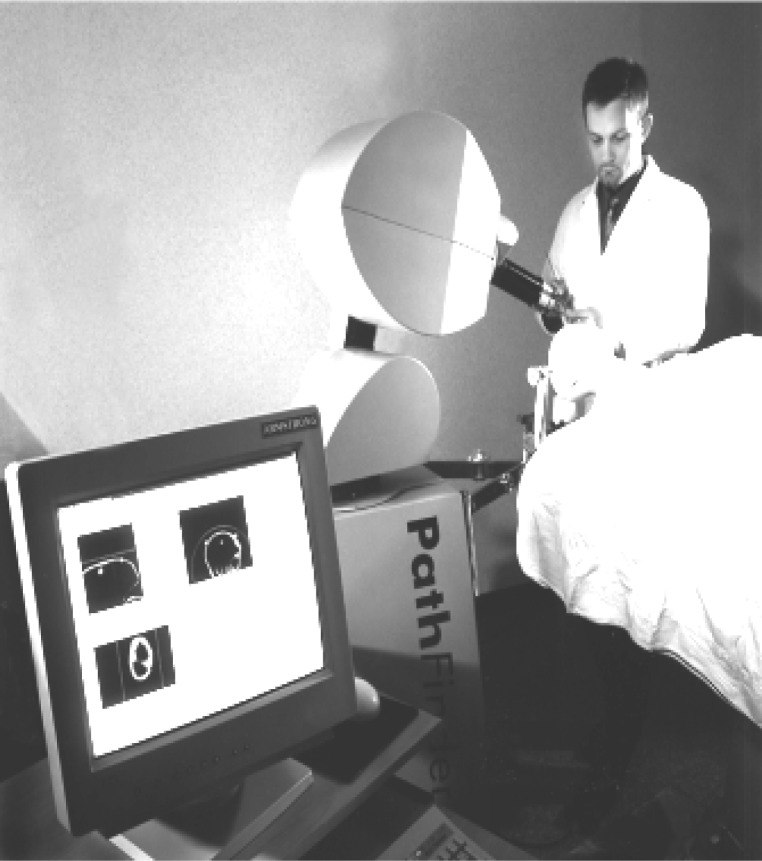
Figure 1.
Arm base navigation
The idea behind using frameless, interactive, computer-aided surgery in navigation systems is to show in real time the position of the tip of an instrument in the corresponding images, without requiring a stereotactic frame for calculation. In Switzerland in 1988, Reinhardt was working on an armless navigation system which used a pointer emitting ultrasound sources (23, 24).
Magnetic sources were also described later by Kato [25], and infrared light-emitting diodes (LEDs) as emitting sources by Zamorano [26]. Additional robotic capabilities were integrated into navigated microscopes by Giorgi (27) and Luber (28).
Figure 2.
Arm less navigation
Clinical applications
The aim of image-guided neurosurgery is to accurately project computed tomography (CT) or magnetic resonance imaging (MRI) data into the operative field for defining anatomical landmarks, pathological structures and tumor margins. To achieve this goal, different image-guided and computer-assisted, so-called "neuronavigation" systems have been developed to provide precise spatial information to neurosurgeons (29). The main clinical utilities in modern neurosurgery are: localization of small intracranial lesions, skull-base surgery, intra cerebral biopsies, intracranial endoscopy, functional neurosurgery and spinal navigation (30). Localization of small intracranial tumors is currently the most frequent application of navigated technology in neurosurgery for adults and children (31, 32).
Safe navigating of the critical anatomy is of prime importance in skull base surgeries. This is particularly the case for endoscopic skull base surgery (ESBS) where the surgeons work within millimeters of neurovascular structures (7, 33).
Navigation can help accurate localization of important anatomic structures such as the carotid artery or cranial nerves, particularly if they are deep in the tumor, as in medial peritoneal wing meningioma or transsphenoidal pituitary surgery [34, 35]. Navigation can also help make approaches through the petrosal bone safer, sparing structures of the inner ear. In orofacial approaches to the C2 vertebra [36], and in tumor recurrences at the skull base with a changed anatomic situation, navigation can help the neurosurgeon to operate accurately. Ear, nose, and throat surgeons also use navigation as an aid for nasal, Para nasal, and otologic interventions. (37, 38, 39)
Endoscopic procedures are mostly performed by the freehand technique. However, navigation can also be incredibly useful in endoscopy and help the surgeon to make more precise planned trajectories; for instance, in cases of narrow access through the foramen of Monro into the third ventricle to prevent damage to the fornix either the navigation is performed with the pointer to mark the burr hole and decide the trajectory, or the endoscope itself is used as a pointer navigation system (40, 41). Inside the third ventricle, Muacevic used navigation in ventriculostomies passes through the narrow passage of the foramen of Monro (42). Schr_der and Gaab (43) also used navigation in aqueductoplasty to choose the best approach to the aqueduct. Furthermore, navigation could provide information about the structures behind the surface of the ventricle wall which is visible by the endoscope. Therefore, the best place can be selected for removal of the specimens or defining the perforation point (44).
Above the third ventricle, fenestration of a cavum vergae can be supported by stereotactic methods to plan an approach, sparing eloquent cortical areas. In addition, in cases of very large cysts around the lateral ventricles, the perforation into the lateral ventricle can be dangerous if the membrane is thick and not translucent. Orientation inside the cyst is difficult if anatomic landmarks are missing. In these cases, the point of perforation into the lateral ventricle can be defined by navigation. The endoscopic image itself can be further digitized and mapped into the image space of the navigation system. The spatial resolution allows performance of distance measurements and, theoretically, also coagulation in the presence of bleeding (45). Zamorano used endoscopic navigation in over 150 tumor cases including biopsies, colloid cyst removals, and tumor extirpations (46). There is also a report on using navigation in pseudo tumor cerebri. In such cases, ventriculoperitoneal shunts were used instead of lumboperitoneal shunts, and favorable outcomes were reported [47, 48]. Moreover, endoscopic navigation has been used for decompression of Superior Orbital Fissure Fracture. (49) Functional neurosurgery, intracranial neurosurgical interventions in the deep brain structures regarding pain, extrapyramidal movement disorders, and particularly epilepsy are the classic indications for applying the frame-based technique. Navigation can also be successfully used in epilepsy surgery for localization and introduction of subdural strip and grid electrodes or for implanting deep-brain electrodes in the hippocampus. Equally interesting is the navigated orientation during ablative surgery in cases of epilepsy, such as with hippocampectomy, to more accurately localize the resection size (50, 51, 52, 53, 54).
Laborde reported the drainage of abscesses guided by navigation (55). It is also possible to use these catheters for local antibiotic therapy. Rohde introduced a catheter by navigation into intracranial bleedings for evacuation and lysis therapy (56). Other authors used the navigated placement of catheters in connection with interstitial radiation therapy (57).
The magnetic-force-based Computed Assisted Neurosurgery System has been used for epilepsy surgery to localize targets accurately in the operative field (58).
It can also be used in the treatment of Parkinson's disease (59). Furthermore, use of neuronavigation and electrophysiology in surgery of subcortically located lesions in the sensorimotor strip have been reported [60]. There are some peculiarities about the anatomy navigation in spinal surgery. The spinal cord is much more flexible, and therefore dependent on the position of the patient. Skin markers are thus not applicable. Registration must be performed after preparation of the vertebrae on their characteristic anatomic landmarks either with a paired-point technique or in combination with surface matching. The dynamic reference frame is fixed on a spinous process inside the operating field to register any displacement close to the working space. The main clinical indication for computer-aided navigation in spinal surgery is the transpedicular insertion of screws in the thoracolumbar region (61, 62, 63, 64).
Advantages and disadvantages
There are some concerns about navigation systems including time consuming calculation and registration, restriction of space and view inside the operating field,and so on. Nevertheless, there are many advantages that can be helpful in the process of operation (65, 66).
An error in the white matter by the navigation device even in the range of 3 mm or 4 mm is still lower than when relying only on neurosurgical knowledge. The neurosurgeon is able to calculate the localization and approach a small lesion accurately, therefore feeling more confident. The corticotomy is associated with less stress, particularly in eloquent areas such as the central region in some pathologies such as low grade gliomas. After opening the Dura, we will not be able to see superficial visible pathology. In such situations, we can find the right sulcus by using navigation device. Thus, another important function of surgical navigation is providing guidance to sub cortical tumors (67, 68, 69). By improved CT/MR imaging, a more precise anatomic localization is possible, and navigation techniques help to make atraumatic openings and approaches (70).
Intra-operative brain deformation (brain shift) limits the accuracy of image-guided neuro-surgery systems (71). Ultrasound imaging as a simple, fast and being real time has become an alternative to MR imaging which is an expensive system for brain shift calculation (9, 72). The main challenges due to speckle noise and artifacts in US images, is to perform an accurate and fast registration of US images with pre-operative MR images (73). There are some ideas that suggest an efficient point based registration method called Coherent Point Drift (CPD), which is implemented and compared to the conventional ICP method. Fusion technique can also be used in this system. It may help lower the cost by allowing previously acquired non stereotactic images such as MRI to be fused to a low-cost stereotactic scan such as CT without contrast. Incorporation of diffusion tensor imaging (DTI) and fiber tracking into the image data set also helps the precision of the system and prevents damage to the eloquent areas. Visualization of certain low-grade tumors may be enhanced by fusing color-encoded fluid-attenuated inversion recovery (FLAIR) images with high-resolution volume MRI. PET, cerebral blood volume or MRS maps may be fused with a stereotactic study to identify the optimal point for brain biopsy (1).
Navigation system also reduces the length of surgery, lowers the incidence of wound infections, and shortens length of hospital stay (74).
In addition, it reduces the risk for neurological morbidity by allowing the surgeon to determine the relationships of the lesion and surgical approach to nearby critical brain structures. Moreover, visualization of critical surface or draining veins may be facilitated using these systems. Accurate, safe intracranial access for the purpose of biopsy by a variety of techniques can be provided by surgical navigation systems (75, 76).
One of the shortcomings of this system is Brain Shift and Local Tissue Deformation. This can be minimized
Methods
As a simple, fast, and being real time, ultrasound imaging is an alternative to MR imaging which is considered an expensive system for brain shift calculation. The main challenges to speckle noise and artifacts in US images is to perform an accurate and fast registration of US images with pre-operative MR images (9).
It is important to ensure that the DRF (dynamic reference frame) is securely affixed, so its relationship to the head cannot be disturbed after the registration procedure. Brain shift is generally straight down toward the center of the earth. Therefore, by orienting the patient's head in a position where a vertical surgical trajectory is possible, the surgeon will only need to compensate for brain shift in one direction (i.e., the brain and tumor are lower than expected) rather than for a complex three-dimensional slide that may occur when operating from a different direction. Diuretics usage should be minimized; and compensating for volume loss by limiting or reversing hyperventilation may be a useful strategy (77).
When only part of the resection involves critical brain, the surgeon should work on that area first while the shift is minimal. En bloc removal of tumors should be performed as much as possible [78]. Surgeons should avoid puncturing any cystic components or entering the ventricles until all critical areas of the tumor boundary have been surgically defined. Placing large cotton balls in the resection cavity can usually expand the cavity to the preoperative dimensions (79).
Future
It is difficult to provide any prognosis for the development and role of navigated surgery in the future, as the computer technology is changing so rapidly. Manwaring reported a magnetic emitting source fixed directly to the patient's skull, producing a nonlinear magnetic field through the brain. Flexible catheters with a magnetic tip could be introduced along these nonlinear magnetic trajectories to the target point. Navigational instruments are presently undergoing a process of evolution with many types developing due to different technical realizations (80).
A presumable future of navigation seems to depend on microsurgical robots. There are some ideas about combining these two innovations to solve the most important shortcoming of neuronavigation: brain shift. This important can be achieved by injecting microsurgical robots through the vessels and synchronizing registration while observing the brain through various aspects from different points. By this root, signals can be transferred to a central computer out of the body. By integrating this information, a 3D map of different points of the brain and its pathologies can be formed. Simultaneously, these robots can play some therapeutic roles. (81, 82, 83)
Conclusion
Navigation system has some limitations in clinical applications and is expensive. Nevertheless, it can be useful for the surgeons and patients. Most reports indicate that these devices are cost-effective, and may reduce surgical morbidity, and enhance outcome.
References
- 1.Archip N, Clatz O, Whalen S, Kacher D, Fedorov A, Kot A, et al. Non-rigid alignment of pre-operative MRI, fMRI, and DT-MRI with intra-operative MRI for enhanced visualization and navigation in image-guided neurosurgery. Neuroimage. 2007;35:609–624. doi: 10.1016/j.neuroimage.2006.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kato A, Yoshimine T, Hayakawa T, Tomita Y, Ikeda T, Mitomo M, et al. [Computer assisted neurosurgery: development of a frameless and armless navigation system (CNS navigator)] No Shinkei Geka. 1991;19:137–142. [PubMed] [Google Scholar]
- 3.Leksell L. A stereotactic apparatus for intracranial surgery. Acta Chir Scand. 1949;99:229–233. [Google Scholar]
- 4.Apuzzo ML, Sabshin JK. Computed tomographic guidance stereotaxis in the management of intracranial mass lesions. Neurosurgery. 1983;12:277–285. doi: 10.1227/00006123-198303000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Grunert P, Darabi K, Espinosa J, Filippi R. Computer-aided navigation in neurosurgery. Neurosurg Rev. 2003;26:73–99. doi: 10.1007/s10143-003-0262-0. discussion 100-101. [DOI] [PubMed] [Google Scholar]
- 6.Wirtz CR, Bonsanto MM, Knauth M, Tronnier VM, Albert FK, Staubert A, et al. Intraoperative magnetic resonance imaging to update interactive navigation in neurosurgery: method and preliminary experience. Comput Aided Surg. 1997;2:172–179. [PubMed] [Google Scholar]
- 7.Mirota DJ, Wang H, Taylor RH, Ishii M, Gallia GL, Hager GD. A system for video-based navigation for endoscopic endonasal skull base surgery. IEEE Trans Med Imaging. 2012;31:963–976. doi: 10.1109/TMI.2011.2176500. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe Y, Hayashi Y, Fujii M, Kimura M, Sugiura A, Tsuzaka M, et al. Development of automatic navigation measuring system using template-matching software in image guided neurosurgery] Nihon Hoshasen Gijutsu Gakkai Zasshi. 2010;66:131–136. doi: 10.6009/jjrt.66.131. [DOI] [PubMed] [Google Scholar]
- 9.Farnia P, Ahmadian A, Khoshnevisan A, Jaberzadeh ansari A, Dadashi Serej N, Fathi Kazerooni A. An Efficient Point Based Registration of Intra-Operative Ultrasound Images with MR Images for Computation of Brain Shift; a Phantom Study. 33rd Annual International IEEE EMBS Conference. Scheduled for presentation during the Poster Session “Image Processing: Filtering, Enhancement, Segmentation, Registration, Classification, Compression, and Coding”. (SaP24); Saturday, September 3, 2011; America Ballroom Westin; pp. 30–17:00. [Google Scholar]
- 10.Suess O, Kombos T, Kurth R, Suess S, Mularski S, Hammersen S, et al. Intracranial image-guided neurosurgery: experience with a new electromagnetic navigation system. Acta Neurochir (Wien) 2001;143:927–934. doi: 10.1007/s007010170023. [DOI] [PubMed] [Google Scholar]
- 11.Spiegel EA, Wycis HT, Marks M, Lee AJ. Stereotaxic Apparatus for Operations on the Human Brain. Science. 1947;106:349–350. doi: 10.1126/science.106.2754.349. [DOI] [PubMed] [Google Scholar]
- 12.Spiegel EA. Stereoencephalotomy (Thalamotomy and related procedures) Part I-Methods and Stereotaxic Atlas of the Human Brain. Monographs in Biology and Medicine; Grune and Stratton; 1952. [Google Scholar]
- 13.Talairach J, David , Corredor H, Krasina T, David M, Monnier M, De Ajuriaguerra J. Recherches sur la coagulation therapeutique des structures sous-corticales chez l'homme. Rev Neurol. 1949;81:4–24. [Google Scholar]
- 14.Riechert T, Wolff M. [A new directive apparatus for the coagulation of the ganglion Gasseri and other intracerebral procedures] Acta Neurochir (Wien) 1952;2:405–407. doi: 10.1007/BF01405830. [DOI] [PubMed] [Google Scholar]
- 15.Hounsfield GN. Computerized transverse axial scanning (tomography). 1. Description of system. Br J Radiol. 1973;46:1016–1022. doi: 10.1259/0007-1285-46-552-1016. [DOI] [PubMed] [Google Scholar]
- 16.Cormack AM. Representation of a function by its line integrals, with some radiological applications. J Appl Phys. 1963;34:2722–2727. [Google Scholar]
- 17.Cormack AM. Representation of a function by its line integrals with some radiological applications II. J Appl Phys. 1964;35:195–207. [Google Scholar]
- 18.Radon J. Uber die Bestimmung von Functionen durch ihre Integralwerte langs gewisser Mannigfaltigkeiten. Akad. Wiss. 1917;69:262–277. [Google Scholar]
- 19.Heilbrun MP. Computed tomography-guided stereotactic systems. Clin Neurosurg. 1983;31:564–581. doi: 10.1093/neurosurgery/31.cn_suppl_1.564. [DOI] [PubMed] [Google Scholar]
- 20.Boecher-Schwarz HG, Grunert P, Guenthner M, Kessel G, Mueller-Forell W. Stereotactically guided cavernous malformation surgery. Minim Invasive Neurosurg. 1996;39:50–55. doi: 10.1055/s-2008-1052216. [DOI] [PubMed] [Google Scholar]
- 21.Ebeling U, Steinmetz H, Huang Y, Kahn T. Topography and identification of the inferior precentral sulcus in MR imaging. AJNR Am J Neuroradiol. 1989;10:937–942. [PMC free article] [PubMed] [Google Scholar]
- 22.Kelly PJ, Kall BA, Goerss S. Transposition of volumetric information derived from computed tomography scanning into stereotactic space. Surg Neurol. 1984;21:465–471. doi: 10.1016/0090-3019(84)90452-x. [DOI] [PubMed] [Google Scholar]
- 23.Reinges MH, Spetzger U, Rohde V, Adams L, Gilsbach JM. Experience with a new multifunctional articulated instrument holder in minimally invasive navigated neurosurgery. Minim Invasive Neurosurg. 1998;41:149–151. doi: 10.1055/s-2008-1052032. [DOI] [PubMed] [Google Scholar]
- 24.Reinhardt H, Meyer H, Amrein E. A computer-assisted device for the intraoperative CT-correlated localization of brain tumors. Eur Surg Res. 1988;20:51–58. doi: 10.1159/000128741. [DOI] [PubMed] [Google Scholar]
- 25.Kato A, Yoshimine T, Hayakawa T, Tomita Y, Ikeda T, Mitomo M, et al. A frameless, armless navigational system for computer-assisted neurosurgery. Technical note. J Neurosurg. 1991;74:845–849. doi: 10.3171/jns.1991.74.5.0845. [DOI] [PubMed] [Google Scholar]
- 26.Zamorano LJ, Nolte L, Kadi AM, Jiang Z. Interactive intraoperative localization using an infrared-based system. Neurol Res. 1993;15:290–298. doi: 10.1080/01616412.1993.11740150. [DOI] [PubMed] [Google Scholar]
- 27.Giorgi C, Eisenberg H, Costi G, Gallo E, Garibotto G, Casolino DS. Robot-assisted microscope for neurosurgery. J Image Guid Surg. 1995;1:158–163. doi: 10.1002/(SICI)1522-712X(1995)1:3<158::AID-IGS5>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 28.Luber J, Mackevics A. Multiple coordinate manipulator (MKM). A computer assisted microscope. In: Lemke H, Inamura K, Jaffe CC, editors. Computer Assisted Radiology. Berlin: Springer-Verlag; 1995. [Google Scholar]
- 29.Fengqiang L, Jiadong Q, Yi L. Computer-assisted stereotactic neurosurgery with framework neurosurgery navigation. Clin Neurol Neurosurg. 2008;110:696–700. doi: 10.1016/j.clineuro.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 30.Barnett GH, Kormos DW, Steiner CP, Weisenberger J. Intraoperative localization using an armless, frameless stereotactic wand. Technical note. J Neurosurg. 1993;78:510–514. doi: 10.3171/jns.1993.78.3.0510. [DOI] [PubMed] [Google Scholar]
- 31.Golfinos JG, Fitzpatrick BC, Smith LR, Spetzler RF. Clinical use of a frameless stereotactic arm: results of 325 cases. J Neurosurg. 1995;83:197–205. doi: 10.3171/jns.1995.83.2.0197. [DOI] [PubMed] [Google Scholar]
- 32.Grunert P, Muller-Forell W, Darabi K, Reisch R, Busert C, Hopf N, et al. Basic principles and clinical applications of neuronavigation and intraoperative computed tomography. Comput Aided Surg. 1998;3:166–173. doi: 10.1002/(SICI)1097-0150(1998)3:4<166::AID-IGS6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 33.Westermann B, Hauser R. Non-invasive 3D patient registration for image-guided skull base surgery. Comput Graph. 1996;20:793–799. [Google Scholar]
- 34.Schul C, Wassmann H, Skopp GB, Marinov M, Wolfer J, Schuierer G, et al. Surgical management of intraosseous skull base tumors with aid of Operating Arm System. Comput Aided Surg. 1998;3:312–319. doi: 10.1002/(SICI)1097-0150(1998)3:6<312::AID-IGS5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 35.Carrau RL, Snyderman CH, Curtin HD, Janecka IP, Stechison M, Weissman JL. Computer-assisted intraoperative navigation during skull base surgery. Am J Otolaryngol. 1996;17:95–101. doi: 10.1016/s0196-0709(96)90003-4. [DOI] [PubMed] [Google Scholar]
- 36.Dyer PV, Patel N, Pell GM, Cummins B, Sandeman DR. The ISG viewing wand: an application to atlanto-axial cervical surgery using the Le Fort I maxillary osteotomy. Br J Oral Maxillofac Surg. 1995;33:370–374. doi: 10.1016/0266-4356(95)90138-8. [DOI] [PubMed] [Google Scholar]
- 37.Hassfeld S, Zoller J, Albert FK, Wirtz CR, Knauth M, Muhling J. Preoperative planning, intraoperative navigation in skull base surgery. J Craniomaxillofac Surg. 1998;26:220–225. doi: 10.1016/s1010-5182(98)80017-6. [DOI] [PubMed] [Google Scholar]
- 38.Hauser R, Westermann B, Probst R. A non-invasive patient registration and reference system for interactive intraoperative localization in intranasal sinus surgery. Proc Inst Mech Eng H. 1997;211:327–334. doi: 10.1243/0954411971534458. [DOI] [PubMed] [Google Scholar]
- 39.Selesnick SH, Kacker A. Image-guided surgical navigation in otology and neurotology. Am J Otol. 1999;20:688–693. discussion 693-687. [PubMed] [Google Scholar]
- 40.Grunert P, Perneczky A, Resch K. Endoscopic procedures through the foramen interventriculare of Monro under stereotactical conditions. Minim Invasive Neurosurg. 1994;37:2–8. doi: 10.1055/s-2008-1053440. [DOI] [PubMed] [Google Scholar]
- 41.Grunert P, Hopf N, Perneczky A. Frame-based and frameless endoscopic procedures in the third ventricle. Stereotact Funct Neurosurg. 1997;68:80–89. doi: 10.1159/000099907. [DOI] [PubMed] [Google Scholar]
- 42.Muacevic A, Muller A. Image-guided endoscopic ventriculostomy with a new frameless armless neuronavigation system. Comput Aided Surg. 1999;4:87–92. doi: 10.1002/(SICI)1097-0150(1999)4:2<87::AID-IGS3>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 43.Schroeder HW, Gaab MR. Neurosurgery. 1999. Endoscopic aqueductoplasty: technique and results; pp. 508–515. discussion 515-508. [DOI] [PubMed] [Google Scholar]
- 44.Hopf NJ, Grunert P, Darabi K, Busert C, Bettag M. Frameless neuronavigation applied to endoscopic neurosurgery. Minim Invasive Neurosurg. 1999;42:187–193. doi: 10.1055/s-2008-1053396. [DOI] [PubMed] [Google Scholar]
- 45.Konen W, Scholz M, Tombrock S. The VN project: endoscopic image processing for neurosurgery. Comput Aided Surg. 1998;3:144–148. doi: 10.1002/(SICI)1097-0150(1998)3:3<144::AID-IGS6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 46.Zamorano L, Jiang C, Chavantes C, Diaz FG. Stereotactic and interactive image-guided neuroendoscopy. In: Alexander E, Maciunas RJ, editors. Advanced neurosurgical navigation. New York: Thieme; 1999. [Google Scholar]
- 47.Kandasamy J, Hayhurst C, Clark S, Jenkinson MD, Byrne P, Karabatsou K, et al. Electromagnetic stereotactic ventriculoperitoneal csf shunting for idiopathic intracranial hypertension: a successful step forward? World Neurosurg. 2011;75:155–160. doi: 10.1016/j.wneu.2010.10.025. discussion 132-153. [DOI] [PubMed] [Google Scholar]
- 48.Azeem SS, Origitano TC. Ventricular catheter placement with a frameless neuronavigational system: a 1-year experience. Neurosurgery. 2007;60:243–247. doi: 10.1227/01.NEU.0000255387.03088.53. discussion 247-248. [DOI] [PubMed] [Google Scholar]
- 49.Gasco J, Hooten K, Ridley RW, Rangel-Castilla L, Adewumi A, Nauta HJ, et al. Neuronavigation-guided endoscopic decompression of superior orbital fissure fracture: case report and literature reviewm. Skull Base. 2009;19:241–246. doi: 10.1055/s-0028-1114297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nguyen JP, Lefaucheur JP, Decq P, Uchiyama T, Carpentier A, Fontaine D, et al. Chronic motor cortex stimulation in the treatment of central and neuropathic pain. Correlations between clinical, electrophysiological and anatomical data. Pain. 1999;82:245–251. doi: 10.1016/S0304-3959(99)00062-7. [DOI] [PubMed] [Google Scholar]
- 51.Olivier A, Germano IM, Cukiert A, Peters T. Frameless stereotaxy for surgery of the epilepsies: preliminary experience. Technical note. J Neurosurg. 1994;81:629–633. doi: 10.3171/jns.1994.81.4.0629. [DOI] [PubMed] [Google Scholar]
- 52.Tanaka T, Olivier A, Hashizume K, Hodozuka A, Nakai H. Image-guided epilepsy surgery. Neurol Med Chir (Tokyo) 1999;39:895–900. doi: 10.2176/nmc.39.895. [DOI] [PubMed] [Google Scholar]
- 53.Van Roost D, Schaller C, Meyer B, Schramm J. Can neuronavigation contribute to standardization of selective amygdalohippocampectomy? Stereotact Funct Neurosurg. 1997;69:239–242. doi: 10.1159/000099881. [DOI] [PubMed] [Google Scholar]
- 54.Wurm G, Wies W, Schnizer M, Trenkler J, Holl K. Advanced surgical approach for selective amygdalohippocampectomy through neuronavigation. Neurosurgery. 2000;46:1377–1382. doi: 10.1097/00006123-200006000-00016. discussion 1382-1373. [DOI] [PubMed] [Google Scholar]
- 55.Laborde G, Klimek L, Harders A, Gilsbach J. Frameless stereotactic drainage of intracranial abscesses. Surg Neurol. 1993;40:16–21. doi: 10.1016/0090-3019(93)90163-u. [DOI] [PubMed] [Google Scholar]
- 56.Rohde V, Rohde I, Reinges MH, Mayfrank L, Gilsbach JM. Frameless stereotactically guided catheter placement and fibrinolytic therapy for spontaneous intracerebral hematomas: technical aspects and initial clinical results. Minim Invasive Neurosurg. 2000;43:9–17. doi: 10.1055/s-2000-8411. [DOI] [PubMed] [Google Scholar]
- 57.Bale RJ, Freysinger W, Martin A, Vogele M, Auer T, Eichberger P, et al. First experiences with computer-assisted frameless stereotactic interstitial brachytherapy (CASIB)] Strahlenther Onkol. 1998;174:473–477. doi: 10.1007/BF03038626. [DOI] [PubMed] [Google Scholar]
- 58.Hirabayashi H, Chitoku S, Hoshida T, Sakaki T. Accuracy and availability of the computed assisted neurosurgery navigation system during epilepsy surgery. Stereotact Funct Neurosurg. 1999;72:117–124. doi: 10.1159/000029710. [DOI] [PubMed] [Google Scholar]
- 59.Tseng CS, Chung CW, Chen HH, Wang SS, Tseng HM. Development of a robotic navigation system for neurosurgery. Stud Health Technol Inform. 1999;62:358–359. [PubMed] [Google Scholar]
- 60.Eisner W, Burtscher J, Bale R, Sweeney R, Koppelstatter F, Golaszewski S, et al. Use of neuronavigation and electrophysiology in surgery of subcortically located lesions in the sensorimotor strip. J Neurol Neurosurg Psychiatry. 2002;72:378–381. doi: 10.1136/jnnp.72.3.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bolger C, Wigfield C. Image-guided surgery: applications to the cervical and thoracic spine and a review of the first 120 procedures. J Neurosurg. 2000;92:175–180. doi: 10.3171/spi.2000.92.2.0175. [DOI] [PubMed] [Google Scholar]
- 62.Bandela JR, Jacob RP, Arreola M, Griglock TM, Bova F, Yang M. World Neurosurg. 2011. Use of CT-Based Intraoperative Spinal Navigation: Management of Radiation Exposure to Operator, Staff, and Patients. [DOI] [PubMed] [Google Scholar]
- 63.Sonntag VK. World Neurosurg. 2011. Pedicle Screw Fixation Made Safer by the Use of Computed Tomography-Guided Intraoperative Navigation. [DOI] [PubMed] [Google Scholar]
- 64.Tian W, Weng C, Liu B, Li Q, Hu L, Li ZY, et al. Posterior fixation and fusion of unstable Hangman's fracture by using intraoperative three-dimensional fluoroscopy-based navigation. Eur Spine J. 2012;21:863–871. doi: 10.1007/s00586-011-2085-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leksell L. The stereotaxic method and radiosurgery of the brain. Acta Chir Scand. 1951;102:316–319. [PubMed] [Google Scholar]
- 66.Archip N, Clatz O, Whalen S, Kacher D, Fedorov A, Kot A, et al. Non-rigid alignment of pre-operative MRI, fMRI, and DT-MRI with intra-operative MRI for enhanced visualization and navigation in image-guided neurosurgery. Neuroimage. 2007;35:609–624. doi: 10.1016/j.neuroimage.2006.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reinhardt HF, Horstmann GA, Gratzl O. Sonic stereometry in microsurgical procedures for deep-seated brain tumors and vascular malformations. Neurosurgery. 1993;32:51–57. doi: 10.1227/00006123-199301000-00008. discussion 57. [DOI] [PubMed] [Google Scholar]
- 68.Reinhardt HF, Trippel M, Westermann B, Horstmann GA, Gratzl O. Computer assisted brain surgery for small lesions in the central sensorimotor region. Acta Neurochir (Wien) 1996;138:200–205. doi: 10.1007/BF01411361. [DOI] [PubMed] [Google Scholar]
- 69.Wagner W, Tschiltschke W, Niendorf WR, Schroeder HW, Gaab MR. Infrared-based neuronavigation and cortical motor stimulation in the management of central-region tumors. Stereotact Funct Neurosurg. 1997;68:112–116. doi: 10.1159/000099911. [DOI] [PubMed] [Google Scholar]
- 70.Guthrie BL, Adler JR., Jr Computer-assisted preoperative planning, interactive surgery, and frameless stereotaxy. Clin Neurosurg. 1992;38:112–131. [PubMed] [Google Scholar]
- 71.Mitsui T, Fujii M, Tsuzaka M, Hayashi Y, Asahina Y, Wakabayashi T. Skin shift and its effect on navigation accuracy in image-guided neurosurgery. Radiol Phys Technol. 2011;4:37–42. doi: 10.1007/s12194-010-0103-0. [DOI] [PubMed] [Google Scholar]
- 72.Moiyadi A, Shetty P. Objective assessment of utility of intraoperative ultrasound in resection of central nervous system tumors: A cost-effective tool for intraoperative navigation in neurosurgery. J Neurosci Rural Pract. 2011;2:4–11. doi: 10.4103/0976-3147.80077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hernes TA, Ommedal S, Lie T, Lindseth F, Lango T, Unsgaard G. Stereoscopic navigation-controlled display of preoperative MRI and intraoperative 3D ultrasound in planning and guidance of neurosurgery: new technology for minimally invasive image-guided surgery approaches. Minim Invasive Neurosurg. 2003;46:129–137. doi: 10.1055/s-2003-40736. [DOI] [PubMed] [Google Scholar]
- 74.Wirtz CR, Tronnier VM, Bonsanto MM, Hassfeld S, Knauth M, Kunze S. [Neuronavigation. Methods and prospects] Nervenarzt. 1998;69:1029–1036. doi: 10.1007/s001150050380. [DOI] [PubMed] [Google Scholar]
- 75.Wadley J, Dorward N, Kitchen N, Thomas D. Pre-operative planning and intra-operative guidance in modern neurosurgery: a review of 300 cases. Ann R Coll Surg Engl. 1999;81:217–225. [PMC free article] [PubMed] [Google Scholar]
- 76.Spetzger U, Laborde G, Gilsbach JM. Frameless neuronavigation in modern neurosurgery. Minim Invasive Neurosurg. 1995;38:163–166. doi: 10.1055/s-2008-1053478. [DOI] [PubMed] [Google Scholar]
- 77.Eljamel MS. Accuracy, efficacy, and clinical applications of the Radionics Operating Arm System. Comput Aided Surg. 1997;2:292–297. doi: 10.1002/(SICI)1097-0150(1997)2:5<292::AID-IGS5>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 78.Gumprecht HK, Widenka DC, Lumenta CB. BrainLab VectorVision Neuronavigation System: technology and clinical experiences in 131 cases. Neurosurgery. 1999;44:97–104. doi: 10.1097/00006123-199901000-00056. discussion 104-105. [DOI] [PubMed] [Google Scholar]
- 79.Maciunas RJ, Galloway RLJR, Fitzpatrick JM, Mandava VR, Edwards CA, Allen GS. A universal system for interactive image-directed neurosurgery. Stereotact Funct Neurosurg. 1992;58:108–113. doi: 10.1159/000098982. [DOI] [PubMed] [Google Scholar]
- 80.Manwaring KH. 1999. Neuronavigation using magnetic fields. Workshop on endoscopy and navigation.Greifswald (personal communication) [Google Scholar]
- 81.Ferrarini L, Verbist BM, Olofsen H, Vanpoucke F, Frijns JH, Reiber JH, et al. Autonomous virtual mobile robot for three-dimensional medical image exploration: application to micro-CT cochlear images. Artif Intell Med. 2008;43:1–15. doi: 10.1016/j.artmed.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 82.Brett PN, Taylor RP, Proops D, Coulson C, Reid A, Griffiths MV. A surgical robot for cochleostomy. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:1229–1232. doi: 10.1109/IEMBS.2007.4352519. [DOI] [PubMed] [Google Scholar]
- 83.Goh P, Krishnan SM. Micromachines in endoscopy. Baillieres Best Pract Res Clin Gastroenterol. 1999;13:49–58. doi: 10.1053/bega.1999.0007. [DOI] [PubMed] [Google Scholar]