Abstract
Background
The enteric nervous system (ENS) develops from neural crest-derived cells that migrate along the intestine to form two plexuses of neurons and glia. While the major features of ENS development are conserved across species, minor differences exist, especially in the colorectum. Given the embryologic and disease-related importance of the distal ENS, the aim of this study was to characterize the migration and differentiation of enteric neural crest cells (ENCCs) in the colorectum of avian embryos.
Results
Using normal chick embryos and vagal neural tube transplants from GFP-transgenic chick embryos, we find ENCCs entering the colon at embryonic day (E) 6.5, with colonization complete by E8. Undifferentiated ENCCs at the wavefront express HNK-1, N-cadherin, Sox10, p75, and L1CAM. By E7, differentiation begins in the proximal colon, with L1CAM and Sox10 becoming restricted to neuronal and glial lineages, respectively. By E8, multiple markers of differentiation are expressed along the entire colorectum.
Conclusions
Our results establish the pattern of ENCC migration and differentiation in the chick colorectum, demonstrate the conservation of marker expression across species, highlight a range of markers, including neuronal cell adhesion molecules, which label cells at the wavefront, and provide a framework for future studies in avian ENS development.
Keywords: enteric nervous system, Hirschsprung’s disease, gut development, chick
Introduction
The enteric nervous system (ENS) is principally derived from vagal-level neural crest cells that migrate rostrocaudally along the entire length of the gastrointestinal tract, giving rise to neurons and glial cells in two ganglionated plexuses (reviewed in Burns, 2005). A smaller contribution of enteric neural crest-derived cells (ENCCs) from the sacral level enters the distal end of the gut and contributes neurons and glia to the colorectum in a caudorostral direction (Burns and Le Douarin, 1998; Wang et al, 2011). Incomplete migration of ENCCs along the gut leads to Hirschsprung’s disease in humans, a congenital disorder characterized by the absence of enteric ganglia along variable lengths of the distal colorectum (Kenny et al, 2010). The colorectal ENS deserves special attention both because of its clinical importance in enteric neuropathies, such as Hirschsprung’s disease, as well as its distinct embryologic development. First, the colorectum is the only portion of the gut colonized by both vagal- and sacral-derived ENCCs, with each population migrating in opposite directions. Second, in the mouse colorectum, the submucosal plexus develops only after birth (McKeown et al, 2001). In avians, the myenteric plexus develops before the submucosal plexus throughout the small intestine, while the reverse is true in the colon (Burns and Le Douarin 1998; Conner et al, 2003; Doyle et al, 2004). Finally, the colorectum fails to be colonized by ENCCs when the number of neural crest-derived cells is experimentally reduced in avians (Yntema and Hammond, 1954; Peters-van der Sanden et al, 1993; Simpson et al, 2007; Barlow et al, 2008) or in certain mouse mutants that specificallyaffect colorectal ENS formation (Jain et al, 2004; Asai et al, 2006; Druckenbrod and Epstein 2009; Uesaka and Enomoto, 2010).
Among the various animal models used to study ENCC development, the avian embryo has played a pivotal role (Goldstein and Nagy, 2008), being the first model to reveal the neural crest origins of the ENS (Yntemma and Hammond, 1954) as well as its specific origins from the vagal and sacral levels (Le Douarin and Teillet 1973). The accessibility of avian embryos throughout development and the variety of embryologic methodologies available, including inter- and intra-species tissue grafting, retroviral-mediated gene transfer, electroporation, and embryo culture, continue to make this a valuable model system for ENS investigations. The aim of this study was to characterize the migration and immunophenotype of the ENCC wavefront as it progresses along the chick colorectum and to establish a spatial and temporal map of colorectal ENS development, ENCC marker profile, and differentiation. Using intra-species chick chimeras in which the vagal neural tube is derived from a GFP-expressing transgenic chicken and transplanted into a normal chick embryo, we were able to visualize the wavefront of ENCC migration and to use both known and novel markers to characterize its immunophenotype. The results of this study establish the normal pattern of migration and differentiation of ENCCs in the avian colorectum and identify useful markers for ENS studies in the avian embryo.
Results
Vagal crest-derived ENCCs arrive at the level of the ceca at E6 (Hamberger-Hamilton stage HH28), with cells at the wavefront expressing Sox10, HNK-1, N-cadherin, p75, and L1CAM (Fig. 1). Background mesenchymal staining is particularly strong in the ceca at this stage, as shown by immunohistochemistry with HNK-1, N-cadherin, and p75 antibodies (Fig. 1B,C,D). Sox10 and L1CAM staining, which do not exhibit this same background, show that ENCCs enter the ceca at their tips and migrate as a wave rostrocaudally across the ceca and terminal midgut toward the colorectum. The pattern and timing of colonization across the cecal level are similar to those described previously (Allan and Newgreen, 1980).
Figure 1. ENCCs reach the cecal level at E6 (HH28).
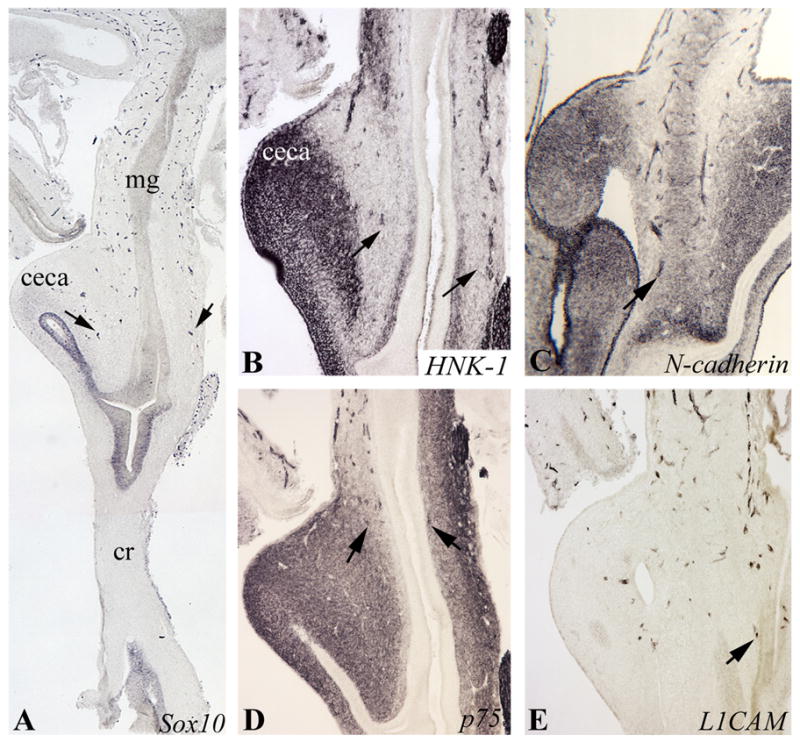
Serial longitudinal sections of the midgut and colorectum at E6 (HH28) were stained with Sox10 (A), HNK-1 (B), N-cadherin (C), p75 (D), and L1CAM (E). In all cases, wavefront cells (arrows) are found at the cecal level, just prior to entering the colon. Background mesenchymal staining, strongest in the ceca, is present with HNK-1, N-cadherin, and p75. mg, midgut; cr, colorectum
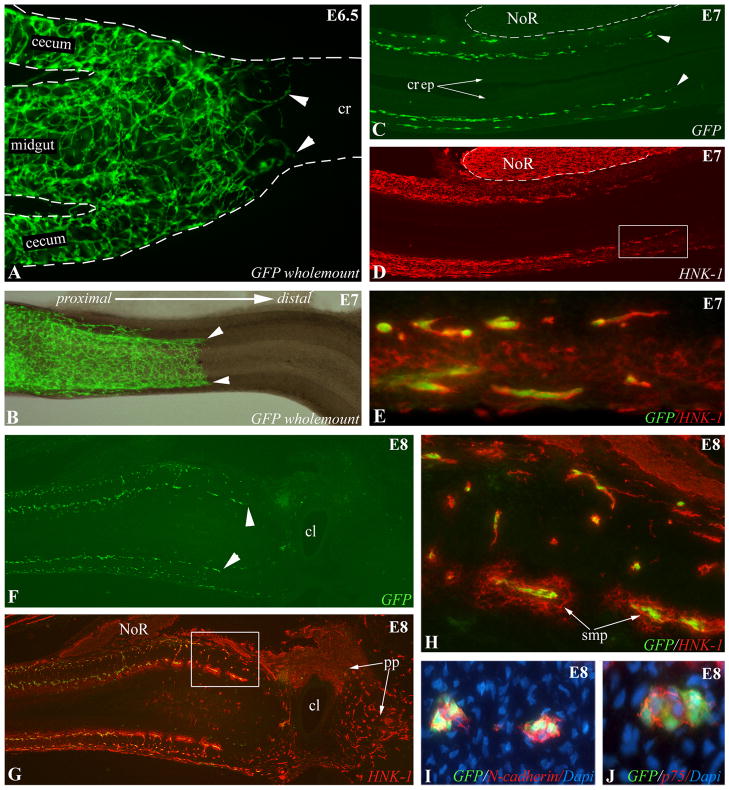
To clearly identify vagal-derived ENCCs in the colorectum, and to definitively label the migratory wavefront, a GFP-labeled ENS was created by transplanting the vagal neural tube adjacent to somites 2–6 from transgenic GFP E1.5 (HH10) chick embryos into age-matched control chick embryos. Strongly fluorescent GFP+ ENCCs were apparent in freshly dissected, unfixed tissue, and these cells could also be labeled with anti-GFP antibody. At E6.5 (HH29), the wavefront of ENCCs is seen just past the ceca and entering the proximal colon (Fig. 2A), while at E7 (HH30) the wavefront has reached the mid-colorectum (Fig. 2B–D). ENCCs appear to migrate in chains (Fig. 2A), as previously described in mouse hindgut (Young et al, 2004) and in chick and mouse midgut (Druckenbrod and Epstein, 2007). The nerve of Remak (NoR) is GFP-negative as this structure is derived from the sacral neural crest (Fig. 2C). The pattern of HNK-1 immunoreactivity, a surface marker of neural crest cells, is similar to that observed with GFP, but demonstrates the typical background mesenchymal staining associated with HNK-1 at this stage (Fig. 2D). All GFP+ cells at the wavefront express HNK-1, confirming that this antibody labels the leading edge of migrating ENCCs (Fig. 2E,H). At E8 (HH32), GFP+ ENCCs have reached the level of the cloaca (Fig. 2F). As expected, neural crest-derived cells in the pelvic plexus and NoR are HNK-1+ and GFP-negative because of their sacral neural crest origin (Fig. 2G,H). Immuofluorescence reveals that the cell adhesion molecule, N-cadherin (Fig. 2I), and the low-affinity neurotrophic receptor, p75 (Fig. 2J), are both strongly expressed by GFP+ cells throughout the colorectal ENS, including at the wavefront of migration. By E7 (HH30), the proximal half of the colorectum has been colonized by ENCCs that express p75, N-cadherin, and L1CAM (Fig. 3). Multiple combinations of double-immunohistochemistry confirm that the same population of migrating ENCCs co-expresses p75, Sox10, N-cadherin, HNK-1, and L1CAM (Fig. 3). While neural crest-derived cells have migrated halfway along the colorectum, neuronal (Fig. 3I) and glial (Fig. 3J) differentiation is just beginning at the proximal end (Fig. 3I,J).
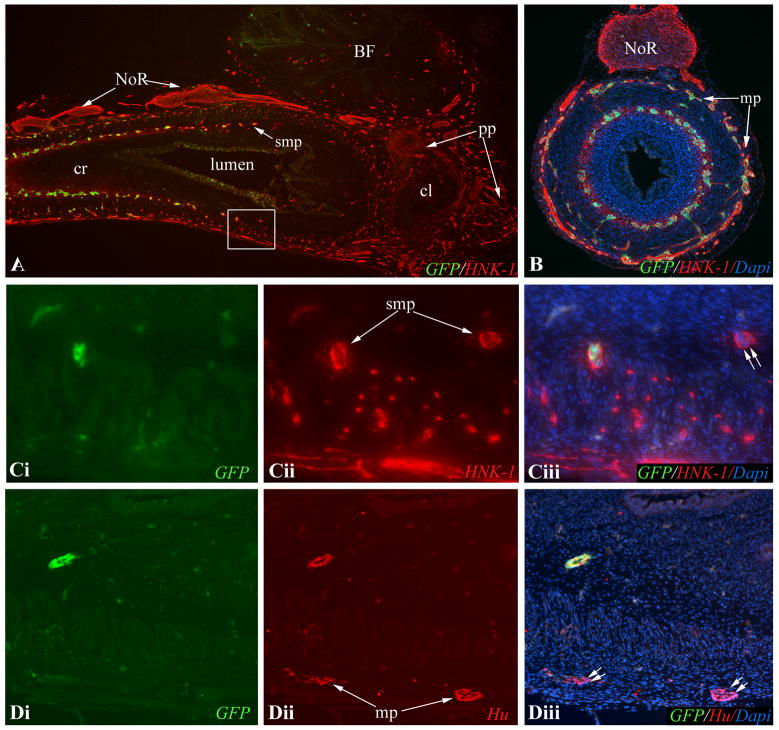
Figure 2. Vagal neural tube chimeras define the wavefront of ENCC migration.
(A) Wholemount E6.5 (HH29) intestine shows GFP+ vagal crest-derived ENCCs just past the cecal level. At E7 (HH30), the GFP+ ENCC wavefront is in the mid-colorectum on wholemount (B) and longitudinal section (C). HNK-1 labeling shows a similar pattern (D, boxed area magnified in E), confirmed by co-labeling of all GFP+ wavefront ENCCs with HNK-1 (E). At E8 (HH32), GFP expression on longitudinal section shows that ENCCs have reached the cloaca (F, arrowheads mark submucosal ENCCs). Co-labeling with HNK-1 at this stage (G, boxed area magnified in H) shows HNK-1 labeling all GFP+ ENCCs. GFP- ENCCs are sacral crest-derived and form the pelvic plexus and NoR (G). Wavefront ENCCs also express N-cadherin (I) and p75 (J). In each panel, the proximal end of the gut is on the left. cl, cloaca; cr, colorectum; cr ep, colorectal epithelium; NoR, nerve of Remak; pp, pelvic plexus; smp, submucosal plexus
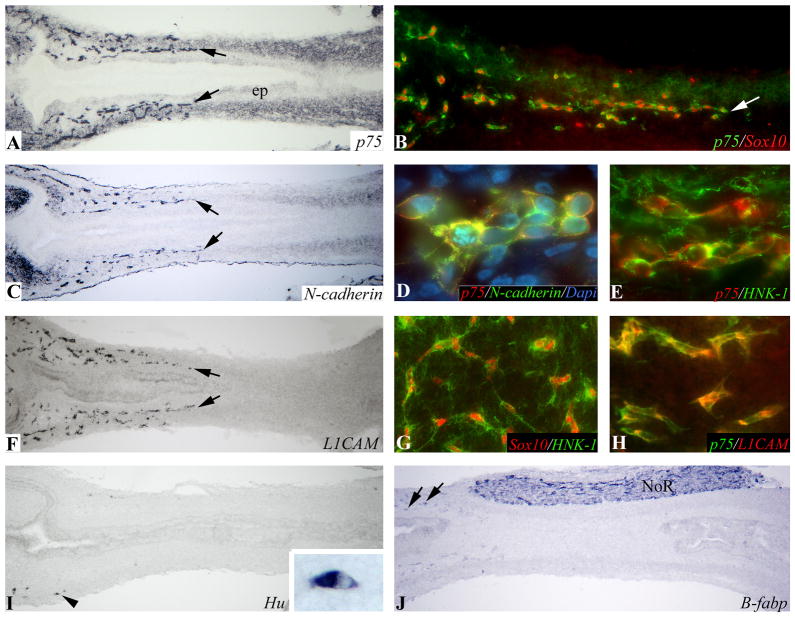
Figure 3. Migratory wavefront at mid-colorectum at E7 (HH30).
p75 (A), N-cadherin (C), and L1CAM (F) labeling of serial longitudinal sections identify the ENCC wavefront (arrows) at the mid-colorectum. Double-labeling with p75 and Sox10 (B), N-cadherin (D), HNK-1 (E), and L1CAM (H) show that these antibodies all label cells at the wavefront. Wavefront ENCCs also co-express nuclear Sox10 and HNK-1 (G). Neuronal (I) and glial (J) differentiation is present only in occasional cells in the proximal colon (arrows). Arrowhead in (I) marks the wavefront of Hu differentiation and is enlarged in the inset. ep, epithelium; NoR, nerve of Remak
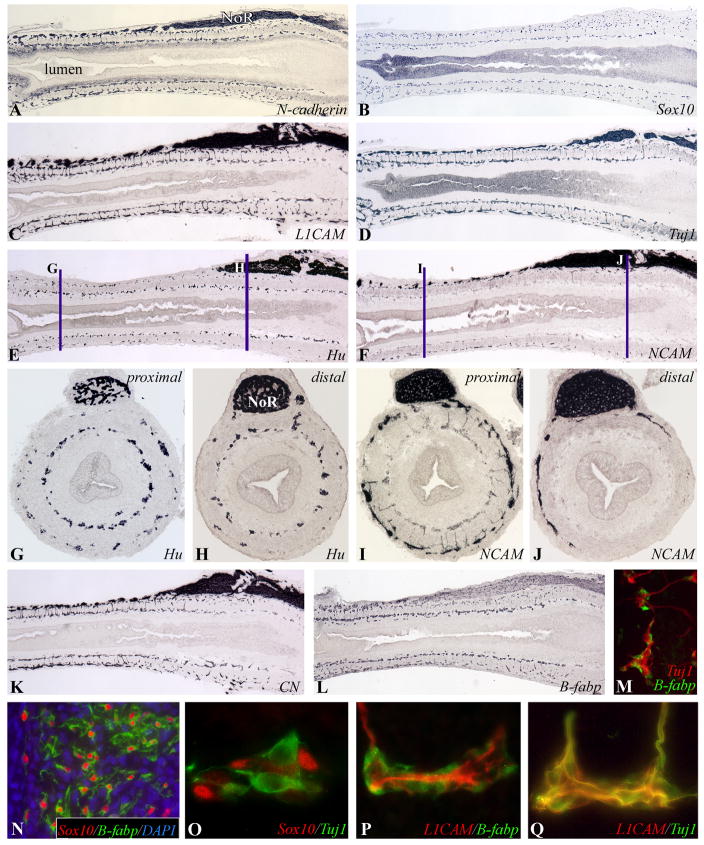
Colorectal colonization is complete by E8 (HH32). At this stage, both submucosal and myenteric plexuses are populated to the level of the cloaca, as shown by N-cadherin, Sox10, and L1CAM immunoreactivity (Fig. 4A–C). Neuronal differentiation has advanced significantly since E7 (HH30), with cells immunoreactive to Tuj1 (class III β-tubulin; Fig. 4D) and CN (chick neurite marker; Fig. 4K) present along the length of the colorectum. CN and Tuj1 both mark the same population of early enteric neurons (data not shown). Hu (neuron-specific family of RNA binding proteins; Fig. 4E) is also expressed along the entire colorectum, but is more advanced in the submucosal plexus, as can be seen on longitudinal section (Fig. 4E) and in cross sections from proximal (Fig. 4G) and distal (Fig. 4H) colorectum. NCAM is a later marker of enteric neurons as its expression is limited to the proximal colon at this stage (Fig. 4F,I), with only fibers from the NoR staining distally (Fig. 4J). Neurofilament expression is even more delayed, with no immunoreactivity present in the colorectal wall at this stage, with the exception of expression by NoR fibers (not shown).
Figure 4. Colorectal colonization is complete at E8 (HH32).
Serial longitudinal sections at E8 (HH32) show ENCCs along the entire colorectum forming two plexuses of cells immunoreactive to ENCC markers N-cadherin (A), Sox10 (B), and L1CAM (C). Early neuronal differentiation is marked by Tuj1 (D) and Hu (E). Differentiation is more advanced in the submucosal than myenteric plexus, as shown by proximal (G) and distal (H) cross-sections (marked in (E)). NCAM is a later marker of neuronal differentiation (F), expressed by enteric neurons in the proximal (I), but not distal (J), colorectum at this stage. CN, another neuronal marker, is expressed throughout the colorectum (K), as is B-fabp, which marks glial cells and their precursors (L). B-fabp is only expressed by glial cells, not neurons, at E8 (HH32; M). By E9 (HH35), Sox-10 is expressed only by glial cells, as shown in the NoR (N), and not by enteric neurons (O), while L1CAM is downregulated in glial cells (P) and expressed only by the neurons (Q).
Glial differentiation also advances significantly in the colorectum between E7 (HH30) and E8 (HH32), with a temporal pattern similar to that observed for enteric neurons. While B-fabp expression is limited to the most proximal colon at E7 (HH30; Fig. 3J), it is present along the length of the colorectum at E8 (HH32; Fig. 4L). Double-staining with B-fabp and Hu or Tuj1 shows B-fabp to be specific to glial cells, and not expressed by enteric neurons at this stage (Fig. 4M). Sox10, which initially labels all ENCCs, becomes exclusively expressed by the glial lineage in the colorectum by E9 (HH35; Fig. 4N), and is no longer expressed by enteric neurons at this stage (Fig. 4O). L1CAM, in contrast, is specifically downregulated by enteric glial cells at the same stage (Fig. 4P) and is only expressed by enteric neurons (Fig. 4Q). We find GFAP to be a later marker of glial differentiation. At E8 (HH32), GFAP is only expressed by NoR fibers extending around the colorectum (not shown), becoming expressed by enteric submucosal and myenteric ganglia after E10 (HH36; Fig. 6H).
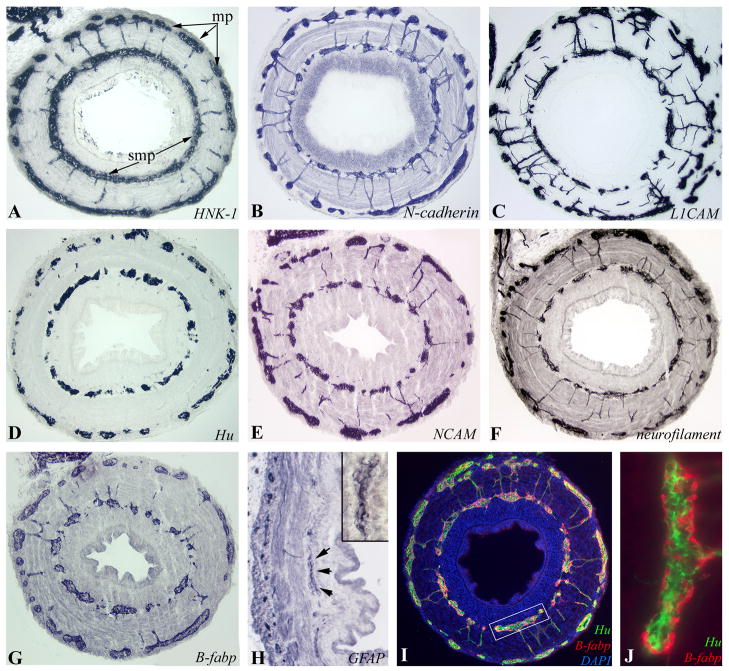
Figure 6. Colorectal ENS at E12-E14 (HH38–40).
Immunohistochemistry was performed on transverse sections of E14 (HH40) mid-colorectum with HNK-1 (A), N-cadherin (B) and L1CAM (C). Differentiated ENCCs express neuronal markers Hu (D), NCAM (E), and neurofilament (F), and glial markers B-fabp (G) and GFAP (H). The submucosal ganglion marked with arrows is magnified in the inset (H). The close relationship between neurons (Hu) and glia (B-fabp) is seen at E12 (HH38; I, box magnified in J).
At E10 (HH36), the colorectum contains both vagal and sacral crest-derived cells. GFP+ vagal crest-derived cells are present along the entire colorectum (Fig. 5A). The boxed area shown in Fig. 5A contains both vagal crest-derived (GFP+/HNK-1+) and sacral crest-derived (GFP-/HNK-1+) ENCCs (Fig. 5C,D), demonstrating mixing of vagal and sacral crest-derived ENCCs in the colorectum. At E14 (HH40; Fig. 6), all ENCCs continue to stain strongly and specifically with antibodies against HNK-1 and N-cadherin. Enteric neurons express L1 CAM, Hu, NCAM, and neurofilament, while glial cells express B-fabp and GFAP. The morphology of ganglia at E12 (HH38) is characterized by the presence of clustered enteric neurons centrally, with glial cells positioned peripherally (Fig. 6J).
Figure 5. E10 (HH36) colorectum contains vagal and sacral crest-derived cells.
HNK-1-immunoreactivity reveals neural crest-derived cells in E10 (HH36) colorectum (A,B). At this stage, GFP+ vagal neural crest cells have colonized the entire colorectum. Neither the NoR nor pelvic plexus express GFP. Boxed area in (A) is shown in (C), with a serial section in (D). Double immunofluorescence with GFP and HNK-1 shows both HNK-1+/GFP+ and HNK-1+/GFP- ENCCs in the submucosal plexus (Ci-iii). In the myenteric plexus, Hu+/GFP-enteric neurons were seen (Di-iii). Double arrows denote GFP- sacral crest-derived ENCCs. BF, bursa of Fabricius; cl, cloaca; cr, colorectum; mp, myenteric plexus; NoR, nerve of Remak; pp, pelvic plexus; smp, submucosal plexus
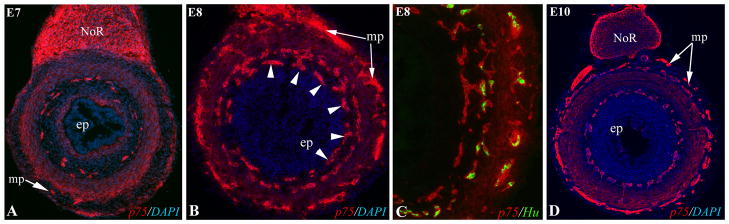
During colorectal ENS development, we observed a concentric ring of undifferentiated ENCCs located in the inner submucosa. This “third plexus” appears to be a transient structure consistently observed in the E8 (HH32) colorectum (Fig. 7B). The cells are located closer to the epithelium and are undifferentiated (Fig. 7C). Only rare Hu+ cells are identified in this plexus, as seen in Fig. 4G. To further confirm the presence of this plexus, we serially sectioned an E8 (HH32) gut from ceca to cloaca and found that 100 of 160 transverse sections contained a p75-immunoreactive inner submucosal plexus. By E10 (HH36), this plexus is no longer present (Fig. 7D).
Figure 7. Transient inner submucosal plexus during colorectal colonization.
During colonization of the colorectum, a transient inner submucosal plexus is present at the migrating wavefront at E7 (HH30; A) and E8 (HH32; B, arrowheads). The ENCCs in this inner plexus remain undifferentiated (p75+/Hu-) (C). By E10 (D), the inner plexus is no longer present and only the normal submucosal plexus is seen. ep, epithelium; mp, myenteric plexus; NoR, nerve of Remak
Discussion
Abnormal colorectal ENS development is the underlying cause of Hirschsprung’s disease, a congenital neurocristopathy that affects 1 in 5000 newborns and is characterized by aganglionosis involving variable lengths of distal colorectum. Understanding the etiology of Hirschsprung’s disease requires a complete understanding of distal ENS development, to which mouse (Burns, 2005; Wang et al, 2011) and chick (Goldstein and Nagy, 2008) embryos have contributed greatly. Work in avian ENS development has been limited by the number of available reagents to identify enteric neurons, glia, and their precursors, and an incomplete characterization of normal colorectal ENS formation. Furthermore, use of HNK-1, the antibody most commonly used to mark avian neural crest cells, is characterized by significant mesenchymal staining in the ceca and colorectum (Luider et al, 1992) at stages during ENCC migration through these regions, limiting its utility. In this study, we have used transgenic GFP chick tissues to permanently label and examine the timing of vagal crest-derived cell migration along the colorectum and have also characterized, by immunohistochemistry, the spatiotemporal pattern of migration and differentiation of these cells. Use of the chickGFP-chick intraspecies grafts has several important advantages over the chick-quail chimeras commonly performed. First, analysis of vagal neural tube transplants from quail to chick embryos is complicated by species differences in rates of embryonic development and ENS formation between these two species, a problem circumvented by the use of intraspecies grafts. Second, GFP expression labels the entire ENCC, including the cell body and its extensions, facilitating examination of ENCC migration and patterning without the need for immunohistochemistry. The markers identified in this study and the patterns of migration and differentiation revealed will enhance future studies on ENS development in this model system.
Development of the colorectal ENS is a highly regulated process conserved across species, although it varies in specific details. The majority of ENCCs in all species examined originate from the vagal neural crest and colonize the gut rostrocaudally, entering the colon at week 7 during human embryogenesis (Fu et al, 2004; Wallace and Burns, 2005), E11.5 in mouse (Young et al., 1998; Druckenbrod and Epstein, 2005), and E6.5 (HH29) in chick, as shown here. Completion of colorectal colonization takes 2.5 days in the mouse (McKeown et al, 2001) as compared to 1.5 days in the chick. Two ganglionated plexuses form in the colorectum of each species, with distinct developmental patterns. In human and mouse colorectum, the myenteric plexus forms first, with submucosal ganglia developing later, by week 14 in human embryos (Fu et al, 2004) and only postanatally in mice (McKeown et al, 2001). In contrast, in chick the colorectal submucosal plexus develops first, although it precedes myenteric plexus development by only a few hours. Both plexuses appear to migrate distally as separate, and nearly parallel, streams, as previously observed (Conner et al, 2003). We cannot, however, rule out the possibility of an additional outward migration of ENCCs from submucosal to myenteric plexus, as has been proposed (Burns and Le Douarin, 1998). A small proportion of the colorectal ENS is comprised of ENCCs originating from the sacral level of the neural tube. In the chick embryo, sacral crest-derived cells entirely comprise the pelvic plexus and nerve of Remak. These cells also contribute to the colorectum as early as E7.5 (Burns and Le Douarin, 1998), entering it either from its distal end (Nagy et al, 2007) or along nerve of Remak fibers (Burns and Le Douarin, 1998). A sacral neural crest contribution also occurs in mice (Kapur, 2000; Wang et al, 2011), but has yet to be demonstrated in humans due to lack of markers unique to sacral ENCCs and difficulty in obtaining tissues at the appropriate stages of development. Development of the zebrafish ENS differs significantly, with ENCCs starting their migration at 36 hours post fertilization (hpf) and completing it by 74 hpf (Wallace et al, 2005). Zebrafish have no submucosal plexus and no sacral neural crest contribution (Burzynski et al, 2009).
Upon entering the chick post-cecal colorectum at E6.5 (HH29), the wavefront of undifferentiated neural crest-derived cells expresses HNK-1, p75, Sox10, N-cadherin, and L1CAM. This panel of markers is very similar to those expressed by migrating ENCCs in the mouse (Young and Newgreen, 2001; Anderson et al, 2006). Although HNK-1 expression has not been detected by immunohistochemistry in fixed mouse tissue (Tucker et al, 1988), it has been successfully used to isolate ENCCs from the embryonic mouse intestine by flow cytometry (Walters et al, 2010). We find these five markers expressed by all vagal crest-derived ENCCs at the wavefront prior to their differentiation. L1CAM and N-cadherin, both neuronal cell adhesion molecules, have also recently been shown to be expressed by migrating ENCCs in the quail midgut (Hackett-Jones et al, 2011).
Neuronal differentiation, which occurs in a rostrocaudal wave along the gut, is first marked in the avian colorectum by expression of the pan-neuronal Hu antibody, which appears in the proximal colon at E7 (HH30), about 12 hours after the arrival of undifferentiated ENCCs. Hu expression first occurs in the submucosal plexus. The wave of differentiation progresses quickly, with Hu expression reaching the distal colorectum in both plexuses by E8 (HH32), similar to the timing described by Fairman et al, 1995. Tuj1 and CN are both also early and specific markers of enteric neuronal differentiation. NCAM is a later marker, present only in the proximal colon at E8, although strongly expressed along the length of the nerve of Remak and its fibers extending into the outer gut mesenchyme at this stage. Neurofilament expression occurs only after E8 in the colorectum. Glial differentiation follows a similar pattern, with B-fabp, a marker of glial cells and their precursors (Kurtz et al, 1994), expressed in the proximal colon at E7 and along the length of the colorectum by E8. GFAP expression is absent in the ganglia at this stage, but can be seen in both plexuses of the colorectum after E10.
During formation of the colorectal ENS, we identified a previously undescribed “third plexus” of ENCCs located in the inner submucosa, close to the epithelium. This plexus is transiently present during development. At E7, and at the E8 wavefront, ENCCs are scattered within the submucosal mesenchyme, as shown in Fig. 7, not yet forming a distinct plexus. By E8, two discrete plexuses of undifferentiated p75+ ENCCs are present in the submucosa. The outer plexus, located along the inner border of the circular muscle, represents the future submucosal plexus, whereas the inner plexus disappears by E10. Interestingly, the inner plexus remains undifferentiated and only rare Hu+ cells are found there. Similar inner submucosal ganglion cells have also been described in human fetal intestine at week 20 (Fu et al, 2004). A possible explanation for the development of a third plexus is offered by Zhang et al, (2010), who use mathematical modeling to suggest that when one plexus reaches maximal cell density, crowding is relieved by movement to another plexus. The transient nature of the inner submucosal plexus in avians may reflect the absence of signals in the mesenchyme required for maintenance of those cells. Additional plexuses have been described in adult large mammals, including human, pig, horse, and cattle, where two to three distinct submucosal plexuses are present at different anatomic levels within the submucous layer (Timmermans et al, 1990; Balemba et al, 1999; Wedel et al, 1999).
Based on the findings of this current study we propose a sequence of ENCC differentiation in the chick colorectum, as presented in Fig. 8. HNK-1, p75, and N-cadherin are expressed by undifferentiated ENCCs and remain expressed in both neuronal and glial lineages after differentiation occurs. L1-CAM, initially expressed by all ENCCs, becomes restricted to the neuronal lineage, while Sox10, which is also initially expressed by all ENCCs, is downregulated in differentiated enteric neurons and becomes limited to the glial precursor population, similar to its expression pattern in mouse (Young et al, 2003). Together, these results confirm the similarities in ENS development between avians and rodents and provide a framework for further studies in avian ENS morphogenesis.
Figure 8. Sequence of colorectal ENCC maturation.
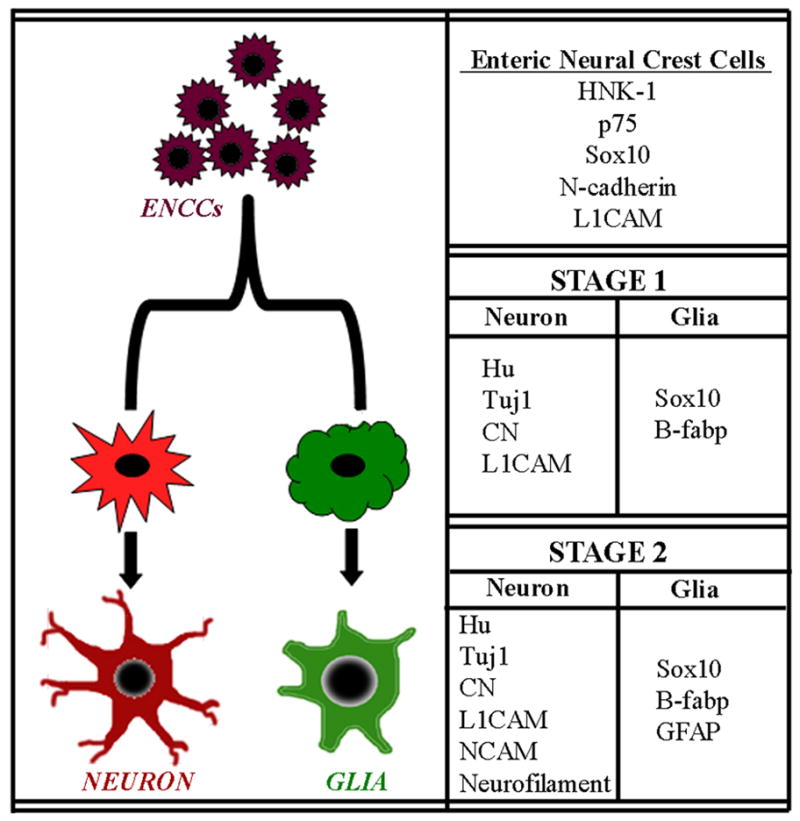
Undifferentiated ENCCs arrive in the proximal colon at E6.5 (HH29) and express HNK-1, p75, Sox10, N-cadherin, and L1CAM at their migratorywavefront. At E7 (HH30) neuronal precursors begin to appear in the proximal colon, expressing Hu, Tuj1, CN, and L1CAM. As neuronal differentiation progresses, NCAM and neurofilament expression occurs. Glial precursors are first marked by Sox10 and B-fabp expression, with GFAP expression coming later.
Experimental Procedures
Animals
Fertilized White Leghorn chicken eggs were obtained from commercial breeders and maintained at 37°C in a humidified incubator. Transgenic GFP-expressing chicken eggs were provided by Prof. Helen Sang, The Roslin Institute, University of Edinburgh (McGrew et al, 2008). Embryos were staged according to Hamburger and Hamilton (HH) tables (Hamburger and Hamilton, 1992) or the number of embryonic days (E).
Immunohistochemistry
Samples were fixed in 4% formaldehyde in phosphate buffered saline (PBS) for 1 hr, rinsed with PBS, and infiltrated with 15% sucrose/PBS overnight at 4°C. The medium was changed to 7.5% gelatin containing 15% sucrose at 37°C for 1–2 hr, and the tissues rapidly frozen at −60°C in isopentane (Sigma). Frozen sections were cut at 10μm, collected on poly-L-lysine–coated slides (Sigma), and stained by immunohistochemistry as previously described (Nagy et al, 2005). The primary antibodies used are listed in Table 1. Frozen sections were incubated with primary antibodies for 45 minutes, followed by biotinylated goat anti-mouse IgG (Vector Labs, Burlingame, CA) and avidin-biotinylated peroxidase complex (Vectastain Elite ABC kit, Vector Labs). Endogenous peroxidase activity was quenched with 3% hydrogen peroxide (Sigma) for 10 minutes. The binding sites of the primary antibodies were visualized by 4-chloro-1-naphthol (Sigma).
Table 1.
Primary antisera used for immunohistochemistry
| Antibody (clone) | Host | Cells identified | Dilution | Source of antibody |
|---|---|---|---|---|
| B-fabp | Rabbit polyclonal | glial cells | 1:300 | kind gift of Thomas Müller, (Kurz et al., 1994) |
| CN | Mouse IgG1 | neurons | 1:10 | kind gift of Hideaki Tanaka, (Tanaka et al., 1990) |
| GFAP | Rabbit polyclonal | glial cells | 1:500 | DAKO |
| HNK-1 | mouse IgM | ENCCs | 1:80 | Neomarkers |
| Hu (clone: 16A11) | Mouse IgG2a | neurons | 1:100 | Invitrogen |
| L1CAM (8D9) | Mouse IgG1 | ENCCs | 1:5 | DSHB |
| N-cadherin (6B3) | Mouse IgG1 | ENCCs | 1:10 | DSHB |
| NCAM (4d) | Mouse IgG1 | neurons | 1:5 | DSHB |
| Neurofilament (4H6) | Mouse IgG | neurons | 1:10 | DSHB |
| p75 | Rabbit polyclonal | ENCCs | 1:10000 | kind gift of Louis Reichardt, (Weskamp and Reichardt, 1991) |
| Sox10 | Mouse IgG | ENCCs and glial cells | 1:50 | kind gift of Michael Wegner, (Schmidt et al., 2003) |
| Tuj1 (B1195) | Mouse IgG2a | neurons | 1:200 | Covance |
For double immunofluorescence staining the sections were incubated with the primary antibodies at room temperature for 45 min. After washing in PBS, secondary antibodies (Alexa Fluor 594 and 488 conjugated anti-mouse IgG, and Alexa Fluor 594 and 488 conjugated anti-mouse IgM, and Alexa Fluor 594 and 488 conjugated anti-rabbit from Invitrogen) were used for 45 min. Cell nuclei were stained by DAPI (Vector Labs). Samples were examined under a Nikon Eclipse 80i microscope and photographed with a Spot camera. Images were compiled using Adobe Photoshop.
GFP-chick chimera
For chickGFP-chick grafting, the neural tube and associated neural crest, adjacent to somites 2–6 inclusive, was microsurgically removed from normal chick embryos at the 9–11 somite stage of development (E1.5) and replaced with equivalent tissue obtained from chickGFP embryos at the same stage of development as previously described for quail-chick grafting (Burns and Le Douarin, 1998). Grafts of this region provide extensive labeling of the ENS, with good embryo survival. Following grafting, eggs were returned to the incubator and allowed to develop up to an additional 5–9 days, such that the wavefront of GFP+ ENCCs entered and colonized the colorectum.
Bullet points.
Undifferentiated ENCCs at the colorectal wavefront express HNK-1, N-cadherin, Sox10, p75, and L1CAM.
ENCCs colonise the colorectum in a rostrocaudal direction during embryonic days 6.5–8.0 and subsequently differentiate rostrocaudally into neurons and glia.
The results highlight a range of markers, including neuronal cell adhesion molecules, which can be used to label cells at the migration wavefront.
Acknowledgments
Grant Information:
AMG is supported by NIH R01DK080914
NN is supported by a Bolyai Fellowship of the Hungarian Academy of Sciences and a Short-Term Fellowship from EMBO.
We thank Louis Reichardt and Thomas Müller for anti-chick p75NTR and B-fabp antibody, and Michael Wegner for Sox10 antibody. Monoclonal antibodies 4d (developed by Urs Rutishauser), 4H6 (developed by Willi Halfter), 8D9 (developed by Vance Lemmon), 6B3 (developed by Karen A. Knudsen) were obtained from the Developmental Studies Hybridoma Bank, developed under the auspices of the NICHD and maintained by the University of Iowa. Fertilized GFP chicken eggs were supplied by Prof. Helen Sang, The Roslin Institute, University of Edinburgh, from projects supported by the Biotechnology and Biological Sciences Research Council (BBSRC) and the Wellcome Trust. AMG is supported by NIH R01DK080914. NN is supported by a Bolyai Fellowship of the Hungarian Academy of Sciences and a Short-Term Fellowship from EMBO.
References
- Allan IJ, Newgreen DF. The origin and differentiation of enteric neurons of the intestine of the fowl embryo. Am J Anat. 1980;157:137–154. doi: 10.1002/aja.1001570203. [DOI] [PubMed] [Google Scholar]
- Anderson RB, Turner KN, Nikonenko AG, Hemperly J, Schachner M, Young HM. The cell adhesion molecule l1 is required for chain migration of neural crest cells in the developing mouse gut. Gastroenterology. 2006;130:1221–1232. doi: 10.1053/j.gastro.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Asai N, Fukuda T, Wu Z, Enomoto A, Pachnis V, Takahashi M, Costantini F. Targeted mutation of serine 697 in the Ret tyrosine kinase causes migration defect of enteric neural crest cells. Development. 2006;133:4507–4516. doi: 10.1242/dev.02616. [DOI] [PubMed] [Google Scholar]
- Balemba OB, Mbassa GK, Semuguruka D, Assey RJ, Kahwa CKB, Hay-Schmidt A, Dantzer V. The topography, architecture, and structure of the enteric nervous system in the jejunum and ileum of cattle. J Anat. 1999;195:1–9. doi: 10.1046/j.1469-7580.1999.19510001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow AJ, Wallace AS, Thapar N, Burns AJ. Critical numbers of neural crest cells are required in the pathways from the neural tube to the foregut to ensure complete enteric nervous system formation. Development. 2008;135:1681–1691. doi: 10.1242/dev.017418. [DOI] [PubMed] [Google Scholar]
- Burns AJ, Le Douarin NM. The sacral neural crest contributes neurons and glia to the post-umbilical gut: spatiotemporal analysis of the development of the enteric nervous system. Development. 1998;125:4335–4347. doi: 10.1242/dev.125.21.4335. [DOI] [PubMed] [Google Scholar]
- Burns AJ. Migration of neural crest-derived enteric nervous system precursor cells to and within the gastrointestinal tract. Int J Dev Biol. 2005;49:143–150. doi: 10.1387/ijdb.041935ab. [DOI] [PubMed] [Google Scholar]
- Burzynski G, Shepherd IT, Enomoto H. Genetic model system studies of the development of the enteric nervous system, gut motility and Hirschsprung’s disease. Neurogastroenterol Motil. 2009;21:113–127. doi: 10.1111/j.1365-2982.2008.01256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner PJ, Focke PJ, Noden DM, Epstein ML. Appearance of neurons and glia with respect to the wavefront during colonization of the avian gut by neural crest cells. Dev Dyn. 2003;226:91–98. doi: 10.1002/dvdy.10219. [DOI] [PubMed] [Google Scholar]
- Doyle AM, Roberts DJ, Goldstein AM. Enteric nervous system patterning in the avian hindgut. Dev Dyn. 2004;229:708–712. doi: 10.1002/dvdy.20011. [DOI] [PubMed] [Google Scholar]
- Druckenbrod NR, Epstein ML. The pattern of neural crest advance in the cecum and colon. Dev Biol. 2005;287:125–133. doi: 10.1016/j.ydbio.2005.08.040. [DOI] [PubMed] [Google Scholar]
- Druckenbrod NR, Epstein ML. Behavior of enteric neural crest-derived cells varies with respect to the migratory wavefront. Dev Dyn. 2007;236:84–92. doi: 10.1002/dvdy.20974. [DOI] [PubMed] [Google Scholar]
- Druckenbrod NR, Epstein ML. Age-dependent changes in the gut environment restrict the invasion of the hindgut by enteric neural progenitors. Development. 2009;136:3195–3203. doi: 10.1242/dev.031302. [DOI] [PubMed] [Google Scholar]
- Fairman CL, Clagett-Dame M, Lennon VA, Epstein ML. Appearance of neurons in the developing chick gut. Dev Dyn. 1995;204:192–201. doi: 10.1002/aja.1002040210. [DOI] [PubMed] [Google Scholar]
- Fu M, Tam PK, Sham MH, Lui VC. Embryonic development of the ganglion plexuses and the concentric layer structure of human gut: a topographical study. Anat Embryol. 2004;208:33–41. doi: 10.1007/s00429-003-0371-0. [DOI] [PubMed] [Google Scholar]
- Goldstein AM, Nagy N. A bird’s eye view of enteric nervous system development: lessons from the avian embryo. Pediatr Res. 2008;64:326–333. doi: 10.1203/PDR.0b013e31818535e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett-Jones EJ, Landman KA, Newgreen DF, Zhang D. On the role of differential adhesion in gangliogenesis in the enteric nervous system. J Theor Biol. 2011;287:148–159. doi: 10.1016/j.jtbi.2011.07.013. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. 1951. Dev Dyn. 1992;195:231–272. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- Jain S, Naughton CK, Yang M, Strickland A, Vij K, Encinas M, Golden J, Gupta A, Heuckeroth R, Johnson EM, Milbrandt J. Mice expressing a dominant-negative Ret mutation phenocopy human Hirschsprung disease and delineate a direct role of Ret in spermatogenesis. Development. 2004;131:5503–5513. doi: 10.1242/dev.01421. [DOI] [PubMed] [Google Scholar]
- Kapur RP. Colonization of the murine hindgut by sacral crest-derived neural precursors: experimental support for an evolutionarily conserved model. Dev Biol. 2000;227:146–155. doi: 10.1006/dbio.2000.9886. [DOI] [PubMed] [Google Scholar]
- Kenny SE, Tam PK, Garcia-Barcelo M. Hirschsprung’s disease. Semin Pediatr Surg. 2010;19:194–200. doi: 10.1053/j.sempedsurg.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Kurtz A, Zimmer A, Schnutgen F, Bruning G, Spener F, Muller T. The expression pattern of a novel gene encoding brain-fatty acid binding protein correlates with neuronal and glial cell development. Development. 1994;120:2637–2649. doi: 10.1242/dev.120.9.2637. [DOI] [PubMed] [Google Scholar]
- Le Douarin N, Teillet M. The migration of neural crest cells to the wall of the digestive tract in avian embryo. J Embryol Exp Morphol. 1973;30:31–48. [PubMed] [Google Scholar]
- Luider TM, Peters-van der Sanden MJ, Molenaar JC, Tibboel D, van der Kamp AW, Meijers C. Characterization of HNK-1 antigens during the formation of the avian enteric nervous system. Development. 1992;115:561–572. doi: 10.1242/dev.115.2.561. [DOI] [PubMed] [Google Scholar]
- McGrew MJ, Sherman A, Lillico SG, Ellard FM, Radcliffe PA, Golhooley HJ, Mitrophanous KA, Cambray N, Wilson V, Sang H. Localised axial progenitor cell populations in the avian tail bud are not committed to a posterior Hox identity. Development. 2008;135:2289–2299. doi: 10.1242/dev.022020. [DOI] [PubMed] [Google Scholar]
- McKeown SJ, Chow CW, Young HM. Development of the submucous plexus in the large intestine of the mouse. Cell Tissue Res. 2001;303:301–305. doi: 10.1007/s004410000303. [DOI] [PubMed] [Google Scholar]
- Nagy N, Bíró E, Takács A, Pólos M, Magyar A, Oláh I. Peripheral blood fibrocytes contribute to the formation of the avian spleen. Dev Dyn. 2005;232:55–66. doi: 10.1002/dvdy.20212. [DOI] [PubMed] [Google Scholar]
- Nagy N, Brewer KC, Mwizerwa O, Goldstein AM. Pelvic plexus contributes ganglion cells to the hindgut enteric nervous system. Dev Dyn. 2007;236:73–83. doi: 10.1002/dvdy.20933. [DOI] [PubMed] [Google Scholar]
- Peters-van der Sanden MJ, Kirby ML, Gittenberger-de Groot A, Tibboel D, Mulder MP, Meijers C. Ablation of various regions within the avian vagal neural crest has differential effects on ganglion formation in the fore-, mid- and hindgut. Dev Dyn. 1993;196:183–194. doi: 10.1002/aja.1001960305. [DOI] [PubMed] [Google Scholar]
- Schmidt K, Glaser G, Wernig A, Wegner M, Rosorius O. Sox8 is a specific marker for muscle satellite cells and inhibits myogenesis. J Biol Chem. 2003;278:29769–29775. doi: 10.1074/jbc.M301539200. [DOI] [PubMed] [Google Scholar]
- Simpson MJ, Zhang DC, Mariani M, Landman KA, Newgreen DF. Cell proliferation drives neural crest cell invasion of the intestine. Dev Biol. 2007;302:553–568. doi: 10.1016/j.ydbio.2006.10.017. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Kinutani M, Agata A, Takashima Y, Obata K. Pathfinding during spinal tract formation in the chick-quail chimera analysed by species-specific monoclonal antibodies. Development. 1990;110:565–571. doi: 10.1242/dev.110.2.565. [DOI] [PubMed] [Google Scholar]
- Timmermans JP, Scheuermann DW, Stach W, Adriaensen D, De Groodt-Lasseel MH. Distinct distribution of CGRP-, encephalin-, galanin-, neuromedin U-, neuropeptide Y-, somatostatin-, substance P-, VIP-, and serotonin-containing neurons in the two submucosal ganglionic neural networks of the porcine small intestine. Cell Tissue Res. 1990;260:367–379. doi: 10.1007/BF00318639. [DOI] [PubMed] [Google Scholar]
- Tucker GC, Delarue M, Zada S, Boucaut JC, Thiery JP. Expression of the HNK-1/NC-1 epitope in early vertebrate neurogenesis. Cell Tissue Res. 1988;251:457–465. doi: 10.1007/BF00215855. [DOI] [PubMed] [Google Scholar]
- Uesaka T, Enomoto H. Neural precursor death is central to the pathogenesis of intestinal aganglionosis in Ret hypomorphic mice. J Neurosci. 2010;30:5211–5218. doi: 10.1523/JNEUROSCI.6244-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace AS, Burns AJ. Development of the enteric nervous system, smooth muscle and interstitial cells of Cajal in the human gastrointestinal tract. Cell Tissue Res. 2005;319:367–382. doi: 10.1007/s00441-004-1023-2. [DOI] [PubMed] [Google Scholar]
- Wallace KN, Akhter S, Smith EM, Lorent K, Pack M. Intestinal growth and differentiation in zebrafish. Mech Dev. 2005;122:157–173. doi: 10.1016/j.mod.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Walters LC, Cantrell VA, Weller KP, Mosher JT, Southard-Smith EM. Genetic background impacts developmental potential of enteric neural crest-derived progenitors in the SoxDom model of Hirschsprung disease. Hum Mol Genet. 2010;19:4353–4372. doi: 10.1093/hmg/ddq357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Chan AKK, Sham MH, Burns AJ, Chan WY. Analysis of the sacral neural crest cell contribution to the hindgut enteric nervous system in the mouse embryo. Gastroenterology. 2011;141:992–1002. doi: 10.1053/j.gastro.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Wedel T, Roblick U, Gleib J, Schiedeck T, Bruch HP, Kuhnel W, Krammer HJ. Organization of the enteric nervous system in the human colon demonstrated by wholemount immunohistochemistry with special reference to the submucous plexus. Ann Anat. 1999;181:327–337. doi: 10.1016/S0940-9602(99)80122-8. [DOI] [PubMed] [Google Scholar]
- Weskamp G, Reichardt LF. Evidence that biological activity of NGF is mediated through a novel subclass of high affinity receptors. Neuron. 1991;6:649–663. doi: 10.1016/0896-6273(91)90067-a. [DOI] [PubMed] [Google Scholar]
- Yntema CL, Hammond WS. The origin of intrinsic ganglia of trunk viscera from vagal neural crest in the chick embryo. J Comp Neurol. 1954;101:515–541. doi: 10.1002/cne.901010212. [DOI] [PubMed] [Google Scholar]
- Young HM, Hearn CJ, Ciampoli D, Southwell BR, Brunet JF, Newgreen DF. A single rostrocaudal colonization of the rodent intestine by enteric neuron precursors is revealed by the expression of Phox2b, Ret, and p75 and by explants grown under the kidney capsule or in organ culture. Dev Biol. 1998;202:67–84. doi: 10.1006/dbio.1998.8987. [DOI] [PubMed] [Google Scholar]
- Young HM, Newgreen D. Enteric neural crest-derived cells: origin, identification, migration, and differentiation. Anat Rec. 2001;262:1–15. doi: 10.1002/1097-0185(20010101)262:1<1::AID-AR1006>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Young HM, Bergner AJ, Muller T. Acquisition of neuronal and glial markers by neural crest-derived cells in the mouse intestine. J Comp Neurol. 2003;456:1–11. doi: 10.1002/cne.10448. [DOI] [PubMed] [Google Scholar]
- Zhang E, Brinas IM, Binder BJ, Landman KA, Newgreen DF. Neural crest regionalization for enteric nervous system formation: Implications for Hirschsprung’s disease and stem cell therapy. Dev Biol. 2010;339:280–294. doi: 10.1016/j.ydbio.2009.12.014. [DOI] [PubMed] [Google Scholar]