Abstract
Introduction
We describe a patient with a prolonged myasthenic crisis refractory to conventional immunomodulatory treatments who was treated with GM-CSF (granulocyte macrophage colony stimulating factor, sargramostim).
Methods
T regulatory cell (Tregs) suppressive function and Foxp3 expression were evaluated before and after treatment with GM-CSF.
Results
Treatment with GM-CSF was associated with clinical improvement, an expansion of the circulating numbers of Foxp3+ cells, an increase in Foxp3 expression levels in Tregs, an early improvement in Treg suppressive capacity for AChR-α induced T cell proliferation, and a subsequent enhancement in Treg suppression of polyclonal T cell proliferation.
Conclusion
Although definitive conclusions cannot be drawn from 1 case, the correlation with similar findings in GM-CSF treated animals with experimental autoimmune myasthenia gravis suggests further exploration of the effects of GM-CSF in myasthenia gravis should be studied in a clinical trial setting.
Keywords: GM-CSF, Myasthenia, regulatory T cells, immunology, Foxp3, sargramostim
INTRODUCTION
Myasthenia gravis (MG) is an autoimmune disease caused by autoantibodies directed against the nicotinic acetylcholine receptor (AChR) at the neuromuscular junction. In vivo administration of granulocyte-macrophage colony-stimulating factor (GM-CSF) has been shown to prevent or attenuate autoimmunity in a number of mouse models of autoimmune disease by expanding dendritic cells and inducing an expansion of regulatory T cells (Tregs).1–4 Treatment of experimental autoimmune myasthenia gravis (EAMG) with GM-CSF has been shown to suppress anti-AChR immune responses, and expand Foxp3+ Tregs with an enhanced ability to selectively suppress AChR-induced T cell proliferation.3,4 We describe a patient with a prolonged myasthenic crisis refractory to conventional immunomodulatory treatments who was treated with GM-CSF and report the subsequent clinical course and the effects of treatment on circulating Treg function.
CASE REPORT
A 77-year-old man presented with several months of diplopia, dysphagia and dyspnea. On examination he had bilateral ptosis, facial diplegia, severe flaccid dysarthria, bilateral tongue weakness and weakness of the neck muscles. His deltoids fatigued after 30 seconds of maintaining outstretched arms. The anti-AChR antibody titer was elevated (11.4 nmol/L). Chest imaging, creatine kinase and thyroid-stimulating hormone levels were normal. His forced vital capacity was 1.5 Liters.
He was admitted to our hospital and started on plasma exchange (PEX) for impending myasthenic crisis, but on hospital day 2 he developed severe respiratory distress and required intubation. In the third week of his hospitalization, he developed diffuse upper extremity weakness despite PEX and prednisone 60mg daily. He was started on tacrolimus 2mg every 12 hours and intravenous immune globulin (IVIg) 2grams/kg in the fourth and fifth weeks, respectively. Muscle strength continued to worsen during the course of IVIg. In the sixth week, tacrolimus and IVIg were discontinued because of pancytopenia (WBC 3.3 thous/µl, Hbg 9.7 g/dl, platelets 110 thousand/µl). Three days after the last IVIg infusion (4/5 doses completed), his muscle strength again declined, and it was decided to administer GM-CSF. The GM-CSF was administered for 2 reasons: 1) conventional treatment failed in this case, and we have prior evidence that GM-CSF is an effective treatment in EAMG,3,4 and 2) it would treat his pancytopenia.
GM-CSF, 750 µg daily was given for 2 days followed by 250 µg daily for 3 days (weeks 6–7). After the fifth dose of GM-CSF, he had an improvement in generalized strength and was eventually weaned from the ventilator. He completed a total of 10 doses of GM-CSF [5 additional daily doses (250 µg) during weeks 7–8] (Figure 1.). A repeat anti-AChR antibody level was 7.8 nmol/L.
Figure 1.
Manual muscle testing. Variation in manual muscle testing (MMT)5 in a patient in myasthenic crisis in response to GM-CSF after failure of conventional immunomodulatory treatments. The higher the MMT score, the weaker the subject. Of note, the patient was taking prednisone 60mg daily. Blood draws for analysis of Treg function were drawn pre-treatment (0), after 5 days of GM-CSF (1), 1 week after completion of 10days of GM-CSF (2), and 4 weeks after completion of GM-CSF (3).
In week 12 he had an episode of mild dysphagia and dysarthria. He improved with re-initiation of PEX and an additional 5 doses of GM-CSF (250 µg), and he was discharged on mycophenolate mofetil 1000mg twice daily, bi-weekly PEX and prednisone 60mg daily. PEX was weaned off over 3 months, followed by a prednisone taper of 5mg monthly to a dose of 15mg daily, followed by further tapering of 2.5 mg monthly until it was discontinued. At the time of this publication, the patient is in pharmacologic remission on mycophenolate mofetil 1000mg twice daily.
MATERIALS AND METHODS
Patient
Initial blood samples were obtained with informed consent under an Institutional Review Board (IRB) approved protocol. Manual muscle testing was performed as previously described.5
Control Subjects
Blood samples were obtained from 14 healthy control subjects after obtaining informed consent under an IRB-approved protocol. Control data is being collected for an ongoing study of Treg function in autoimmune MG.
Collection of peripheral blood mononuclear cells (PBMCs)
Blood samples (80cc) were drawn from the patient and control subjects into heparinized tubes, diluted 1:1 with sterile HBSS at room temperature and centrifuged on Ficoll-Paque gradients (GE Healthcare bioscience). PBMC from the interface were recovered, washed in HBSS, counted in a trypan blue dye and used for the experiments.
Antibodies and Cell culture reagents
Allophycocyanin (APC) conjugated anti-human CD4, Phycoerythrin (PE)-conjugated anti-human CD25, Alexa Fluor 488 (AF488) conjugated anti-human Foxp3, phycoerythrin-Cy-7 (PE-Cy7) conjugated anti-human CD127 and their respective Isotype controls were purchased from eBioscience, CA, USA. RPMI 1640 media supplemented with 1% sodium pyruvate, 1% nonessential amino acids, 2mM L-glutamine, 20mM HEPES, 50 U/ml penicillin and 50 µg/ml streptomycin (all from GIBCO, CA, USA), 10% heat inactivated human AB serum (Invitrogen, CA, USA) were used as culture medium. Anti-human CD3 (Clone OKT3) and CFSE were purchased from eBioscience and Invitrogen, respectively. Two different synthetic peptides representing amino acid sequences (aa195–212: DTPYLDITYHFVMQRLPL and aa257–269: LLVIVELIPSTSS) of the human α-subunit of the nicotinic acetylcholine receptor (AChR) were synthesized and HPLC purified (96%) by the Protein Research Laboratory, University of Illinois at Chicago. Peptides were dissolved in DMSO at high concentration (10mg/ml), aliquoted and stored at −80°C.
Sorting of T cell subsets
PBMCs (1×108) were washed once and suspended in 500µl FACS buffer (PBS + 0.1% BSA + 1mM EDTA) in a 5ml polystyrene round bottom tube. After addition of 5µl/107 cell volume APC-CD4, PE-CD25, and PECy7-CD127 antibodies, the cell suspension was mixed gently and incubated at 4°C for 30 min. Then, cold FACS buffer was added and cells were pelleted. Labeled T cells were sorted by using a MoFlo (Becton Dickinson). The sorted gates for the T cell subsets were set to include only those events exhibiting the CD4-specific fluorescence that were also within the lowest density region of a scatter plot. Based on the CD4 gate, cells were further gated based on CD127 and/or CD25 expression [CD4+CD25highCD127low Regulatory T cells (Treg cells) and CD4+CD25− T effector cells (Teff)]. Cells were collected into 100% human AB serum and washed once with media (RPMI 1640 medium with 5% human AB serum) until they were ready to be plated in suppression assay. Sorted Treg and Teff cells were >96% purity. Isotype controls were run to determine the gating parameters.
FOXP3 expression analysis
For CD4+Foxp3+ T cell analysis, 1×106 PBMCS per sample were resuspended in 100µl FACS buffer in a 5ml round bottom polystyrene tube. After addition of 5µl APC-conjugated anti-human CD4, the cell suspension was mixed gently and incubated at 4°C for 30 min. After incubation, staining buffer was added to each sample, and cells were pelleted and resuspended in 100 µl buffer. Then, Foxp3 staining was conducted as specified below.
Intracellular staining was conducted using the recommended procedure obtained from the manufacturer (eBioscience). Briefly, 25,000 sorted Treg cells (CD4+CD25highCD127low) per sample were washed once with FACS buffer and fixed for 60 min using Foxp3 Fixation/Permiabilization buffer (eBioscience, CA, USA). After wash with added FACS buffer, the cell pellet was resuspended with 100µl FACS buffer. 10 µl Alexa fluor 488-conjugated anti-human FOXP3 was added, and cells were lightly vortexed and incubated at room temperature for 30 min. After incubation, FACS buffer was added to each sample, and cells were pelleted, resuspended in 200 µl buffer, and analyzed on a flow cytometer (CyAn ADP, DakoCytomation). Isotype controls were run to determine the gating parameters.
Suppression Assays
CD4+CD25− T effector cells were resuspended in PBS (0.1% BSA) at 2×106 cells/ml and incubated with CFSE (final concentration: 2 µM) for 10 min at 37 °C. Cells were washed and resuspended in culture medium for 15 min to stabilize the CFSE staining. After a final wash step, cells were resuspended in culture medium at the indicated cell concentrations. Suppression assays were performed as per the method of Venken et al. (2007). Briefly, CFSE labeled CD4+CD25− T cells (responder cells) were cultured in duplicate in 96-well round bottom plates (Falcon, NJ, USA) at 2×104 cells per well in 200µl medium with 1×105 autologous antigen presenting cells (APCs) in the absence or presence equal numbers of CD4+CD25highCD127low/− Tregs (Tresp-Treg ratio 1:0 or 1:1). APCs were prepared from PBMCs by 1-h plastic adherence (to deplete T cells), and the adherent fraction was irradiated with 2000 rad immediately prior to use in the co-culture. Cell cultures were stimulated with 2 µg/ml anti-CD3 (non-specific proliferation) or 3µg/ml AChR peptides (antigen specific proliferation). Cells were also cultured in the presence of non-stimulated irradiated PBMC to assess the background proliferation for each cell type/experiment. After 4 days, cells were harvested, stained for CD4, and the CFSE signal of gated lymphocytes was analyzed by flow cytometry. The suppressive capacity of Tregs towards responder cells in co-culture was expressed as the percentage of proliferation of CFSElow CD4+ T cells. The autofluorescent signal of CFSE unlabeled CD4+CD25hiCD127low T cells (Tresp-Treg ratio 0:1) was subtracted to exclude background levels that would interfere with CFSE diluted cells.
STATISTICAL ANALYSIS
The control proliferation/suppression assay data were subjected to statistical analysis using SPSS software. Differences in percent proliferation with or without Treg co-culture were assessed using the student t-test. The significance was level was set at p<0.05.
RESULTS
Clinical Course
The patient’s clinical course is summarized above and in Figure 1. As shown, blood for analysis of Treg function was drawn pre-treatment (0), after 5 days of GM-CSF (1), one week after completion of 10days of GM-CSF (2), and 4 weeks after completion of GM-CSF (3).
Intensity of FoxP3 expression within isolated Tregs
A diminished expression of FoxP3 was demonstrated within isolated Tregs in the patient prior to GM-CSF treatment [Mean fluorescent intensity (MFI) = 20.33 arbitrary units (AU) MFI vs. 43.145 AU (standard deviation 13.6) in healthy controls]. Treatment with GM-CSF was associated with an increase in the relative numbers of Foxp3-expressing CD4+ cells and an enhanced intensity of Foxp3 expression within these cells (Fig. 2A and 2B).
Figure 2.
Expression of Foxp3 in T cells. A) FACS analysis of the percentage of Foxp3-expressing cells within the CD4+ T cell subset. CD4+ T cells were sorted and isolated as described. The sorted cells were stained with AF488-anti-human Foxp3 and analyzed by flow cytometry. A plot from a healthy control subject is shown in addition to the results from the patient pre-treatment (MG), after 5 days of GM-CSF (MG + GM-CSF-1), 1 week after completion of 10 days of GM-CSF (MG + GM-CSF-2), and 4 weeks after completion of GM-CSF (MG + GMCSF-3). B) The mean fluorescent intensity (MFI) of Foxp3 expression was determined within the isolated CD4+CD25highCD127low Treg cells. The cells were sorted using APC-anti-human CD4, PE- anti-human CD25 and PE-Cy7 anti-human CD127 antibodies. The sorted cells were stained with AF488-anti-human Foxp3 and analyzed by flow cytometry. Results for a healthy control and the patient (pre- and post-treatment) are shown.
Suppressive Function of Tregs
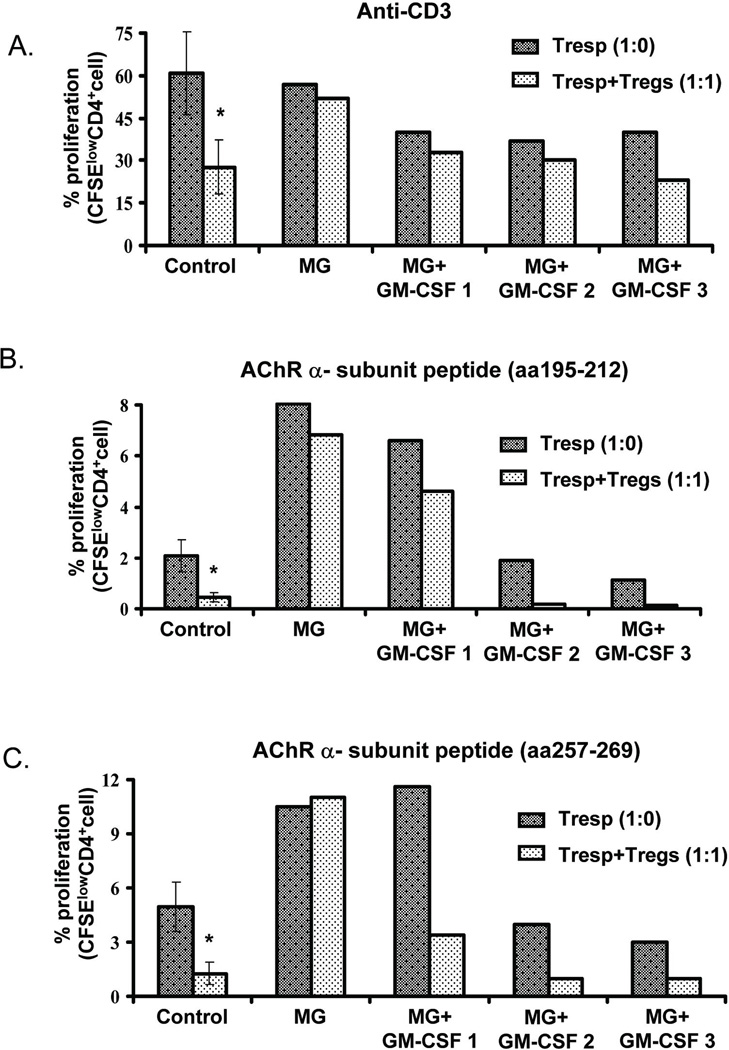
To assess Treg suppressive function, we co-cultured T responder (Tresp) cells with and without isolated Tregs and measured the ability of Tregs to suppress Tresp cell proliferation in response to polyclonal (anti-CD3 antibodies) stimulation. We found a relative defect in Treg suppressive capacity in the patient (compared to controls) prior to treatment, which improved after the third week (Fig. 3A). We also examined the ability of Tregs to suppress specific (AChR)-induced proliferation using 2 AChR-α subunit peptides previously shown to induce proliferation in approximately 70% of patients with MG.6 No significant T cell proliferation in response to either peptide was observed in control subjects, while T cells derived from the patient exhibited a proliferative response which was not suppressed or only minimally suppressed by the addition of Tregs prior to treatment with GM-CSF (Fig. 3B and 3C). Interestingly, the suppressive capacity of these Tregs (to inhibit AChR-α subunit-induced proliferation) improved in response to GM-CSF treatment, (particularly to α subunit amino acid 257–269).
Figure 3.
T regulatory cell proliferation/suppression studies pre and post-treatment with GM-CSF. CD4+CD25high CD127low Treg cells were isolated and cultured with CD4+CD25− Tresp (labeled with CFSE) at the indicated ratios in the presence of irradiated APC, and stimulated with anti-CD3 antibody and/or AChR-α peptides. After 4 days of in vitro culture, the proliferation of CFSE-labeled T cells was quantified, and expressed as a percentage of proliferating (CFSE low) cells. A). Relative ability of Tregs to suppress anti-CD3 induced T cell proliferation in the patient prior to treatment with GM-CSF (MG), after 5 days of treatment (MG + GM-CSF-1), 1 week after completion of treatment (MG + GM-CSF-2) and 4 weeks after completion of treatment (MG + GM-CSF-3) vs. control subjects: baseline proliferation - dark bars; proliferation after addition of Tregs - light bars. B). T cell proliferation/suppression studies stimulating with AChR-α 195–212 in the patient pre- and post-treatment with GM-CSF compared to control subjects. C). T cell proliferation/suppression studies stimulating with AChR-α 257–269 in the patient pre- and post-treatment compared to control subjects. For A, B, and C, control data is expressed as mean ± SEM of 14 subjects; percentage of proliferating cells were compared in Tresp alone and Tresp + Treg co-cultures, p <0.05.
DISCUSSION
We describe a patient with a prolonged myasthenic crisis whose disease was refractory initially to standard therapy. We administered GM-CSF partly because of the development of pancytopenia during his critical illness, but our published results in EAMG suggested that a beneficial therapeutic effect on his MG was a possibility.3,4 Admittedly, interpretation of the clinical efficacy of a single agent in this case is hampered by the multiple immunomodulatory treatments used and the patient’s complicated hospital course. Although a delayed response cannot be ruled out, this patient appeared not to respond to plasma exchange or IVIg. There are the occasional patients who do not respond to plasma exchange. It is unclear whether plasma exchange may not effectively clear the antibodies in someone with severe disease or if the resynthesis of antibodies outpaces the therapeutic exchange. The patient’s exam worsening during IVIG and continued to worsen 1 week after IVIG. GM-CSF may have tilted the balance in favor of tolerance thus limiting the ongoing production of antibodies and allowing plasma exchange and IVIG to be effective. Although the clinical efficacy of GM-CSF is difficult to assess in this case due to the confounding use of several other potent immunosuppressive agents, it is notable that the clinical course was inexorably progressive and unresponsive to therapy, and his clinical status only improved in temporal association with the initiation of GM-CSF treatment. Additionally, there was a measureable improvement in Treg function after the administration of GM-CSF which coincided with the improvement in disease. While it has been reported that other medications can expand Tregs, we are not aware that this has ever been demonstrated in a human patient, with clinical correlation, and on a longitudinal basis.
We demonstrated a significant down-regulation of Foxp3 expression by isolated Tregs (CD4+ CD25high CD127low cells) from the patient as compared to our control data. This reduced expression levels of Foxp3 in Tregs is in agreement with findings reported for other autoimmune diseases.7,8 A functional defect in these cells was also demonstrated with impaired in vitro suppression of both polyclonal and AChR-induced T cell proliferation. A quantitative or qualitative alteration in Tregs has also been noted in patients with a number of autoimmune diseases.9,10
Treatment with GM-CSF was associated with an increase in the number of circulating CD4+ cells expressing Foxp3 and an enhanced intensity of Foxp3 expression levels. An early improvement in Treg suppressive capacity for AChR-α induced T cell proliferation, and a subsequent enhancement in Treg suppression of polyclonal T cell proliferation was observed. These changes are consistent with the effects of GM-CSF in experimental MG.3,4The observed longitudinal changes of Treg function and expression of Foxp3 in direct response to therapeutic intervention is a novel observation.
In summary, we report the effects of GM-CSF treatment in a case of refractory myasthenic crisis. Clinical improvement in this case was associated with enhanced Treg function as demonstrated by serial in vitro T cell proliferation/suppression assays. While the use of multiple immunosuppressive drugs could have possibly contributed to these findings, the observed effects on Tregs are consistent with the reported immune modulatory effects of GM-CSF.1–4 Although limited to a single patient, these novel observations combined with our preclinical data3,4 argue that the effects of GM-CSF in MG should be studied in a clinical trial setting. Furthermore, our results may provide insight into the role of Tregs in MG, and may suggest the possibility of monitoring Treg function/phenotype as a biomarker of treatment response in MG.
ACKNOWLEDGEMENTS
This project was supported by the NIH (National Institute of Neurologic Disorders and Stroke, K08NS058800, MNM; and National Institute of Allergy and Infectious Diseases, RO1 AI 058190, BSP; and the Muscular Dystrophy Association (MDA 134545 to MNM, and MDA 157286 to JRS), and by the University of Illinois at Chicago (UIC) Center for Clinical and Translational Science (CCTS), Award Number UL1RR029879 from the National Center for Research Resources. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the UIC CCTS, MDA or the NIH.
The authors also wish to thank the following contributors: Margaret O’Connor RN and Jana Zawadzki Longtine for technical assistance and data collection.
Abbreviations
- ACHR
acetylcholine receptor
- APC
antigen presenting cell
- AU
arbitrary units
- BSA
bis(trimethylSilyl)Acetamide
- CA
California
- CFSE
carboxyfluorescein succinimidyl ester
- cc
milliliter
- DMSO
dimethyl sulfoxide
- EAMG
experimental autoimmune myasthenia gravis
- EDTA
ethylene-diamineteraacetic acid
- FACS
fluorescence activated cell sorter
- GM-CSF
granulocyte macrophage colony stimulating factor
- HBSS
Hanks balanced salt solution
- HEPES
N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid
- HPLC
high performance liquid chromatography
- IVIG
intravenous immunoglobulin
- IRB
institutional review board
- MMT
manual muscle testing
- MFI
mean fluorescent intensity
- MG
myasthenia gravis
- Mg
milligram
- µg
microgram
- Mmol/L
millimole/liter
- PBMC
peripheral blood mononuclear cells
- PBS
phosphate buffered saline
- PE
phycoerythrin
- PEX
plasmaexchange
- Thous/µl
thousands/microliter
- Treg
T regulatory cell
- WBC
white blood cell count
REFERENCES
- 1.Ganesh BB, Cheatem DM, Sheng JR, Vasu C, Prabhakar BS. GM-CSF-induced CD11c+CD8a− dendritic cells facilitate Foxp3+ and IL-10+ regulatory T cell expansion resulting in suppression of autoimmune thyroiditis. Int Immunol. 200;21:269–282. doi: 10.1093/intimm/dxn147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaudreau S, Guindi C, Menard M, Besin G, Dupuis G, Amrani A. Granulocyte-macrophage Colony stimulating factor prevents diabetes development in NOD mice by inducing tolerogenic dendritic cells that sustain the suppressive function of regulatory T cells. J Immunol. 2007;179:3638–3647. doi: 10.4049/jimmunol.179.6.3638. [DOI] [PubMed] [Google Scholar]
- 3.Sheng JR, Li LC, Ganesh BB, Vasu C, Prabhakar BS, Meriggioli MN. Suppression of experimental autoimmune myasthenia gravis (EAMG) by Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) is associated with an expansion of Foxp3+ regulatory T cells. J Immunol. 2006;177:5296–5306. doi: 10.4049/jimmunol.177.8.5296. [DOI] [PubMed] [Google Scholar]
- 4.Sheng JR, Li L, Ganesh BB, Prabhakar BS, Meriggioli MN. Regulatory T cells induced by GM-CSF suppress ongoing experimental myasthenia. Clin Immunol. 2008;128:172–180. doi: 10.1016/j.clim.2008.03.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanders DB, Tucker-Lipscomb B, Massey JM. A simple manual muscle test for myasthenia gravis: validation and comparison with QMG score. Ann NY Acad Sci. 2003;998:440–444. doi: 10.1196/annals.1254.057. [DOI] [PubMed] [Google Scholar]
- 6.Brocke S, Brautbar C, Steinman L, Abramsky O, Rothbard J, Neumann D, et al. In vitro proliferative responses and antibody titers specific to human acetylcholine receptor synthetic peptides in patients with myasthenia gravis and relation to HLA class II genes. J. Clin Invest. 1988;82:1894–1900. doi: 10.1172/JCI113807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Venken K, Hellings N, Thewissen M, Somers V, Hensen K, Rummens JL, et al. Compromised CD4+CD25high regulatory T-cell function in patients with relapsing-remitting multiple sclerosis is correlated with a reduced frequency of FOXP3-positive cells and reduced FOXP3 expression at the single-cell level. Immunology. 2008;123:79–89. doi: 10.1111/j.1365-2567.2007.02690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moes N, Rieux-Laucat F, Begue B, Verdier J, Neven B, Tapey N, et al. Reduced expression of FOXP3 and regulatory T-cell function in severe forms of early-onset autoimmune enteropathy. Gastroenterology. 2010;139:770–778. doi: 10.1053/j.gastro.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Torgerson TR. Regulatory T cells in human autoimmune diseases. Semin Immunopathol. 2006;28:63–76. doi: 10.1007/s00281-006-0041-4. [DOI] [PubMed] [Google Scholar]
- 10.Valencia X, Yarboro C, Illei G, Lipsky PE. Deficient CD4+CD25high T regulatory cell function in patients with active systemic lupus erythematosus. J Immunol. 2007;178:2579–2588. doi: 10.4049/jimmunol.178.4.2579. [DOI] [PubMed] [Google Scholar]