Abstract
Background
Increasing evidence links altered intestinal flora in infancy to eczema and asthma. No studies have investigated the influence of maternal intestinal flora on wheezing and eczema in early childhood.
Objective
To investigate the link between maternal intestinal flora during pregnancy and development of wheeze and eczema in infancy.
Methods
Sixty pregnant women from the Boston area gave stool samples during the third trimester of their pregnancy and answered questions during pregnancy about their own health, and about their children’s health when the child was 2 and 6 months of age. Quantitative culture was performed on stool samples and measured in log10colony-forming units(CFU)/gram stool. Primary outcomes included infant wheeze and eczema in the first 6 months of life. Atopic wheeze, defined as wheeze and eczema, was analyzed as a secondary outcome.
Results
In multivariate models adjusted for breastfeeding, daycare attendance and maternal atopy, higher counts of maternal total aerobes (TA) and enterococci (E) were associated with increased risk of infant wheeze (TA: OR 2.32 for 1 log increase in CFU/g stool [95% CI 1.22, 4.42]; E: OR 1.57 [95% CI 1.06, 2.31]). No organisms were associated with either eczema or atopic wheeze.
Conclusions & Clinical Relevance
In our cohort, higher maternal total aerobes and enterococci were related to increased risk of infant wheeze. Maternal intestinal flora may be an important environmental exposure in early immune system development.
Keywords: infant wheeze, eczema, asthma, microbiota, intestinal flora, maternal flora
Introduction
Prenatal and early life microbial exposures affect immune system development and tolerance. The human intestinal microbiota, which consists of a complex community of hundreds of bacterial species,[1, 2] represents a vital environmental influence on the developing immune system. Increasing evidence suggests that the composition of the intestinal flora in early life may influence the development of an allergic phenotype[3-5] and immune response to infection.[6]
Several studies have investigated the associations between infant intestinal flora and allergy and asthma-related outcomes. Observational studies have shown decreases in the prevalence of Lactobacillus, Bacteroides and Bifidobacteria species, and higher burdens of S. aureus, E. coli or C. difficile in allergic as compared to non-allergic children.[7-10] Microbial community structure and overall diversity may also be important influences on child outcomes, with development of a diverse gut microbiota early in life being related to decreased risk of allergy in some studies.[11-13] Early infant wheeze is common [14] and is often due to viral respiratory infection. Though infrequent wheeze may be transient, recurrent wheezing in early life is associated with an increased risk of child asthma.[15-17] Some trials of probiotics or prebiotics aimed at altering infant intestinal flora have shown beneficial effects on both allergy-related outcomes such as eczema[18, 19] as well as respiratory infections[20, 21].
Maternal intestinal flora influences the composition of infant flora;[22] however no studies to date have examined its relationship with disease outcomes in infants. Our objective was to investigate the association between maternal intestinal flora and wheeze and eczema in infancy.
Materials and Methods
Study cohort
Pregnant women were recruited at their 24-week prenatal visit from three outpatient facilities affiliated with Brigham and Women’s Hospital in Boston between July 2003 and November 2005 as previously described.[13, 23] Inclusion criteria were maternal age between 18 years and 44 years, plans to deliver at Brigham and Women’s Hospital, and maternal ability to speak English or Spanish. Subjects were not recruited based on any specific disease such as allergy or asthma. Informed consent was obtained from participating mothers. The study protocol was approved by the Institutional Review Board of the Brigham and Women’s Hospital and conforms to the principles expressed in the Declaration of Helsinki.
Questionnaire and review of medical records
A questionnaire was administered to each participating woman between her 24-week prenatal visit and delivery to obtain information on demographics, general health, and history of allergic diseases and/or symptoms in her and in the father of her child. Information on labor and delivery was obtained from review of medical records. When the child was 2 and 6 months of age, a telephone questionnaire (modified from the American Thoracic Society-Division of Lung Diseases questionnaire[24]) was administered by trained research assistants to the child’s primary caretaker.
Stool collection and culture
A stool sample was collected from participating women between their 24-week prenatal visit and delivery. At least one gram of stool was collected into a sterile specimen container and frozen for transport to the laboratory. Samples were weighed, serially diluted with sterile phosphate-buffered-saline (PBS) in an anaerobic chamber, and plated onto enriched or selective agar media. Media for the recovery of obligate anaerobes included pre-reduced Brucella base agar containing 5% sheep blood, enriched with hemin, and vitamin K (BMB), BMB containing 5% laked sheep blood, 100 μg/ml of kanamycin, and 7.5 μg/ml vancomycin (BKV) for Bacteroides and Prevotella species, and Rogosa selective agar for lactobacilli and bifidobacteria (ROG). Facultative organisms were recovered using tryptic soy base agar with 5% sheep blood agar (TSA), bile esculin azide agar (BEA) for enterococci, and MacConkey agar (MAC) for Enterobacteriaceae. All media with the exception of ROG, which was prepared in-house according to manufacturer’s directions (Difco), was obtained from PML Microbiologicals, Tuluatin, OR. Agar plates inoculated for the recovery of obligate anaerobes (BMB, BKV, and ROG) were incubated in an anaerobic chamber for a minimum of 96 hours before enumeration. Media used for the isolation of facultative species (TSA, BEA, and MAC) were removed from the chamber and incubated for 48 hours in air. Following incubation, colonies were enumerated on the various selective and nonselective media, and Gram’s stains were performed. All counts were recorded as log10colony-forming units (CFU)/gram dry weight of stool so that all counts were based on a consistent denominator unaffected by the amount of liquid in the stool. The lower limit of detection of the various organisms cultured was 1.5 log10CFU/gram.
Counts were analyzed relative to the dry input mass of stool: (1) total anaerobes, (2) total aerobes, (3) total Gram-negative anaerobes, (4) lactobacilli and bifidobacteria (combined), (5) Enterobacteriaceae, and (6) enterococci.
Outcomes
Primary outcomes were wheeze and eczema. Wheeze was defined as an answer of “yes” to “Has your child had any wheezing or whistling in the chest?” at either the 2 month or 6 month follow-up interview. Eczema was defined as parental report of doctor-diagnosed eczema in the first 6 months of life. Secondarily we also looked at atopic wheeze, defined as both doctor-diagnosed eczema and wheezing in the first 6 months of life.
Covariates
Variables considered for inclusion in the multivariate analysis included maternal and paternal asthma, maternal and paternal atopy (defined as report of doctor-diagnosed asthma, allergy or eczema), smoking during or just before pregnancy, maternal age, birthweight, maternal antibiotic use, gestational age, mode of delivery, child gender, child race, breastfeeding, presence of children under age 14 at home, daycare attendance, presence of children under age 2 at daycare, and infant antibiotic use.
Statistical Analysis
Stepwise regression was used to build multivariate models. Variables associated at p<.20 and potential confounders were included in initial models. Final models included variables associated with the outcome at p<.05 and/or those that caused a change of greater than 10% in the effect estimate for the microbial exposure of interest. Final models were adjusted for breastfeeding daycare attendance and maternal atopy. All analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC, USA).
Results
Table 1 shows characteristics of the 60 sets of parents and 61 infants (one set of twins) included in the study. Mean age of mothers was 34 (SD 6.4). Sixty percent of mothers and 18% of fathers had atopy. Few mothers smoked just before or during pregnancy. The majority of children were white. Rates of caesarean section were slightly higher than national rates, though consistent for the mean maternal age in our cohort;[25] approximately 50% of these were elective. Only 2 (3%) of the infants were pre-term (33 weeks). There were high rates of both wheezing and eczema in the infants.
Table 1.
Characteristics of parents and children from the AMIGA study
| Parents | Mean (SD) or N (%) |
|---|---|
| Age | 34 (6.4) |
| Smoked during pregnancy | 2 (3%) |
| Maternal history of asthma | 9 (15%) |
| Maternal history of atopy1 | 36 (60%) |
| Paternal history of asthma | 6 (10%) |
| Paternal history of atopy1 | 11 (18%) |
| Ceasarean-section | 22 (36%) |
|
| |
| Infants | |
|
| |
| Sex (N, %male) | 37 (61%) |
| Race (N, % white) | 42 (70%) |
| Birthweight (gm) | 3327 (542) |
| Gestational age (wks) | 39 (1.7) |
| In daycare | 16 (26%) |
| ≥1 other child <14 years in home | 33 (54%) |
| Breast feeding2 | 30 (49%) |
| Antibiotic use in first 6 months | 6 (10%) |
| Wheezing (N, %) | 17 (28%) |
| Eczema | 13 (21%) |
| Atopic Wheeze3 | 5 (8%) |
doctor-diagnosed asthma, eczema or allergies
breast-fed at least daily in first 6 months of life
doctor-diagnosed eczema and wheezing
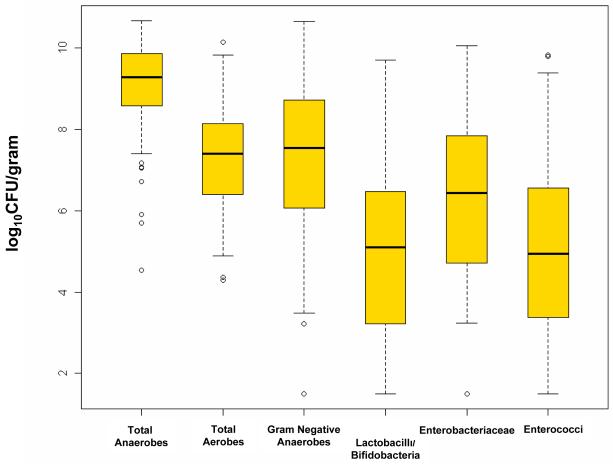
The distribution of types of microorganisms identified in the stool samples from mothers is shown in Figure 1. The highest median counts (in log10CFU/g stool) were of total anaerobes. Counts of lactobacilli and bifidobacteria (combined) and of enterococci were an average of 2 logs lower per gram of fecal material cultured than Gram-negative anaerobes but had a wide range of variation.
Figure 1. Distribution of Cultured Bacteria in Maternal Flora.
Median values and interquartile range for bacteria cultured from maternal stool samples.
Maternal flora and infant outcomes
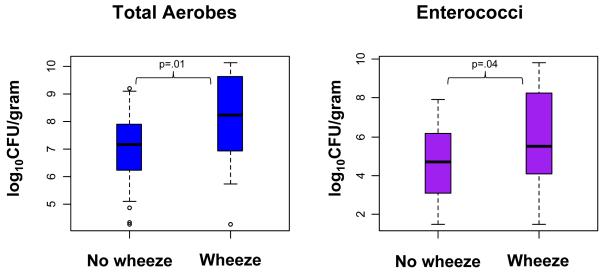
We first examined the bivariate relationships between maternal stool flora and infant outcomes. There was a significant association between higher counts of total aerobes and of enterococci in maternal stool with development of wheezing in infants. There was no significant association with eczema alone nor with atopic wheeze (Table 2). Figure 2 shows the differences in distribution of total aerobes and enterococci in mothers of children with and without wheezing. There were no significant associations between maternal total anaerobes, total Gram-negative anaerobes, the combination of lactobacilli and bifidobacteria or Enterobacteriaceae with any of the outcomes (data not shown).
Table 2.
Unadjusted and multivariate models for the association between maternal stool organisms and infant wheeze, eczema and atopic wheeze
| Model* | Wheeze | Eczema | Atopic Wheeze | ||||
|---|---|---|---|---|---|---|---|
| OR [95% CI] | p value | OR [95% CI] | p value | OR [95% CI] | p value | ||
| Total Aerobes | 1 | 1.88 [1.14, 3.09] | 0.01 | 1.02 [0.66, 1.58] | 0.9 | 1.44 [0.73, 2.85] | 0.3 |
| 2 | 2.32 [1.22, 4.42] | 0.01 | 1.12 [0.70, 1.75] | 0.7 | 1.64 [0.82,3.27] | 0.16 | |
|
| |||||||
| Enterococci | 1 | 1.35 [1.02, 1.79] | 0.035 | 1.00 [0.76, 1.33] | 0.99 | 1.10 [0.73, 1.64] | 0.66 |
| 2 | 1.57 [1.06, 2.31] | 0.02 | 1.05 [0.77, 1.45] | 0.75 | 1.53 [0.83, 2.87] | 0.18 | |
Model 1=unadjusted;
Model 2=adjusted for breastfeeding, daycare attendance, maternal atopy
Figure 2. Maternal total aerobes and enterococci in relation to wheezing status in children.
Median and interquartile range of maternal bacteria by child wheezing status up to age 6 months (unadjusted).
Adjusting for covariates (breastfeeding,daycare attendance and maternal atopy) in the multivariate model strengthened both the effect size and significance of the bivariate associations. A one unit increase in log10CFU of total aerobes per gram of stool was associated with a greater than 2-fold increase in the odds of wheezing in infants (p=.01). A one unit increase in log10CFU of enterococci per gram of stool was associated with a 1.6-fold higher odds of wheezing (p=.02) (Table 2). There were no significant associations with eczema alone nor with atopic wheeze.
Discussion
We examined associations between the specific bacterial components of the intestinal flora of pregnant women during their 3rd trimester in relation to the development of wheeze and eczema in their children. The infants of pregnant women with higher total aerobes and enterococci in their 3rd trimester stool samples had higher rates of wheeze when evaluated up to age 6 months. Adjusting for breastfeeding and daycare attendance strengthened this association. Although prior studies have examined the effect of maternal flora on infant flora or blood cytokines, to our knowledge this is the first study to examine the relationship between maternal intestinal flora and infant disease outcomes such as wheeze and eczema.
Normal immune maturation depends upon interactions between the host immune system and the intestinal microbiota.[26-29] Possible influences affecting early infant bowel flora include mode of delivery,[23, 30, 31] early infant diet,[10, 32-35] and composition of the maternal flora.[22, 36] Maternal factors contributing to the development of the microbiota in neonates and infants include the composition of the mother’s intestinal[22] and vaginal[37, 38] flora as well as breast-milk[39] flora. One study from Japan suggests that maternal intestinal flora may be more important than vaginal flora in shaping infant intestinal flora.[22] The potential influence of maternal flora on infant outcomes is indirectly exemplified by trials of probiotics. Maternal consumption of Lactobacillus species has been shown to influence infant flora[40] and interventional studies of probiotics to prevent atopic disease have had more success with combined maternal prenatal supplementation in addition to infant supplementation,[18, 19, 41, 42] rather than solely the latter.[43] Given that maternal probiotic ingestion may influence infant flora and outcomes, it is not surprising that maternal flora may also affect the development of disease outcomes or symptoms in infants.
One large study of over 3000 children has investigated the influence of maternal vaginal flora on child asthma-related outcomes, specifically asthma hospitalization and use of medications for asthma. They found higher risk of hospitalizations for wheezing in children of mothers colonized with Ureaplasma urealyticum, and higher risk of asthma in those whose mothers were colonized with staphylococci (a facultative anaerobe).[38] Our group has found previously that components of maternal stool influence cord blood immune response.[23] However no previous study has examined maternal intestinal flora in relation to child wheeze or allergy outcomes.
At birth an infant’s gastrointestinal tract is sterile. Early gut colonization occurs through encounters with microbial species in the environment, with exposure to the mother’s microbiota being a primary influence. Facultative species colonize intestinal ecosystems first, followed by increasing numbers of obligate anaerobes until a predominance of obligate anaerobes is present by the end of the first year of life, at which time a stable microbial community resembling that of an adult is established.[44, 45] Over the last several decades, detailed analyses of intestinal colonization of infants have shown that concomitant with the rise in prevalence of allergic diseases, the composition of early infant intestinal flora has changed, with a greater early predominance of facultative anaerobes, lower anaerobe to facultative anaerobe ratio, and later colonization with obligate anaerobes such as Bacteroides and bifidobacteria.[46, 47] Together with the rising prevalence of allergic disease,[48-50] this may represent ecological evidence that early colonization with obligate anaerobes is protective. Furthermore, animal models suggest that the obligate anaerobe Bacteroides fragilis may play a key role in the development of immune tolerance.[51, 52] Our prior findings that anaerobes in maternal stool were positively correlated with the anti-inflammatory cytokine IL-10 in cord blood[23] together with our current finding that higher maternal total aerobes and enterococci (a facultative anaerobe) were associated with higher risk of wheezing is consistent with the suggested importance of anaerobes, though this is indirect evidence. Analyses in larger cohorts are needed to confirm these findings.
Early childhood wheezing is often due to viral respiratory infection and may or may not be related to allergy or subsequent development of asthma. Regardless of whether the children go on to develop asthma, early childhood wheezing is an important cause of morbidity and health care utilization in young children.[49, 53] It may be that our findings suggest that infant immune response to respiratory infection could be affected by altered microbial exposure, which has been suggested by prior studies.[6, 20, 21] Specific types of intestinal bacteria may stimulate antibody production, enhance cytokine release, or augment phagocytosis, all of which could potentially reduce the severity of infection.[54, 55] Prior trials of probiotic supplementation have shown reduced severity or incidence of respiratory tract infections [20, 21, 56, 57]. Even in the absence of an effect on atopic asthma, a protective effect of specific microbes against wheezing in response to viral respiratory infection is interesting and could have significant implications on respiratory health in childhood.
If confirmed in future studies, our findings have several implications. Maternal intestinal flora may need to be considered an important modifiable environmental exposure in the development of child immunity and tolerance. Further investigations and elucidation of specific species that confer either increased or decreased risk will be valuable in planning prevention studies in which pre- or probiotics are used to manipulate maternal intestinal flora during pregnancy for the prevention of child allergy.
These data must be interpreted in the context of the study design. First, the small number of subjects may have limited our power to detect associations with other genera, such as lactobacilli and bifidobacteria, which have been found to be important in studies of infant flora and allergy; our findings will need to be confirmed in larger cohorts. Second, outcomes were assessed at a very early age. Our questionnaires did not distinguish between wheezing due to infection, often the cause of wheeze in early childhood, and wheezing related to allergy. Though a multitude of studies relate wheezing in response to viral infection in early life as a risk factor for subsequent asthma,[15-17, 58-60], infection-related wheezing can also be transient. Prior data shows that children with recurrent wheeze in the first year of life had a threefold increase in the odds of asthma at age 7 years.[61] In our study because we did not examine recurrent wheeze, we likely captured both transient and recurrent wheezers in our analysis. As stated above, however, if our findings do not relate to allergy or asthma but instead represent a modifiable risk factor for severe viral infections, these results remain important in understanding immune system development and have implications for potential future studies and interventions. Third, we did not find any association with eczema, one of the early steps in the “atopic march” and there may have been too few subjects with atopic wheeze to find an effect. An association in the same direction was suggested with atopic wheeze though did not reach statistical significance. Fourth, although the cohort was not recruited for any history of allergy, asthma or other specific disease, the high rate of atopy among mothers suggests the possibility of selection bias and may limit the generalizability of our finding. However, rates of atopy in the general population have been reported to be as high as 40-54%[62-64], and enrichment of our sample for atopic mothers may have increased the incidence of our primary outcomes and thus would increase our power to detect differences.
In summary, we found that bacteria in maternal stool during pregnancy identified by quantitative culture were associated with early infant wheeze. Specifically, higher total aerobes and counts of enterococci were associated with increased risk of wheezing in the first 6 months of life. Though our findings need to be confirmed in larger studies, this is the first evidence to our knowledge that bacterial members of the larger microbial community present in maternal stool may influence child immune-related outcomes. Future studies will clarify which types of bacteria may reduce risk, and these data will inform interventional studies using prebiotic foods or probiotic supplementation during pregnancy to modulate the immune response potentially decreasing risk of wheezing or infection in children.
Acknowledgments
We would like to thank all of the study participants and staff of the AMIGA study.
Funding and Conflicts of Interest: This work was supported by a grant from the Harvard National Institute of Environmental Health Sciences Center to J. C. Celedón. N.E.Lange is supported by NIH grant HL07427.
Abbreviations
- CFU
colony-forming units
- g
gram
- OR
odds ratio
- PBS
phosphate-buffered saline
- BMB
Brucella-base agar with 5% sheep blood enriched with hemin and vitamin K
- BKV
Brucella-base agar with 5% laked sheep blood with kanamycin and vancomycin
- ROG
Rogosa selective agar
- TSA
Tryptic soy agar with 5% sheep blood
- BEA
ile esculin azide agar
- MAC
MacConkey agar
Footnotes
None of the authors have any conflicts of interest to report.
References
- 1.Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292:1115–1118. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- 2.Frank DN, Pace NR. Gastrointestinal microbiology enters the metagenomics era. Curr Opin Gastroenterol. 2008;24:4–10. doi: 10.1097/MOG.0b013e3282f2b0e8. [DOI] [PubMed] [Google Scholar]
- 3.Ly NP, Litonjua A, Gold DR, Celedon JC. Gut microbiota, probiotics, and vitamin D: Interrelated exposures influencing allergy, asthma, and obesity? J Allergy Clin Immunol. 2011 doi: 10.1016/j.jaci.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McLoughlin RM, Mills KH. Influence of gastrointestinal commensal bacteria on the immune responses that mediate allergy and asthma. J Allergy Clin Immunol. 2011 doi: 10.1016/j.jaci.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 5.Weiss ST. Bacterial components plus vitamin D: The ultimate solution to the asthma (autoimmune disease) epidemic? J Allergy Clin Immunol. 2011 doi: 10.1016/j.jaci.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hatakka K, Savilahti E, Ponka A, Meurman JH, Poussa T, Nase L, Saxelin M, Korpela R. Effect of long term consumption of probiotic milk on infections in children attending day care centres: double blind, randomised trial. BMJ. 2001;322:1327. doi: 10.1136/bmj.322.7298.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bjorksten B, Naaber P, Sepp E, Mikelsaar M. The intestinal microflora in allergic Estonian and Swedish 2-year-old children. Clin Exp Allergy. 1999;29:342–346. doi: 10.1046/j.1365-2222.1999.00560.x. [DOI] [PubMed] [Google Scholar]
- 8.Bjorksten B, Sepp E, Julge K, Voor T, Mikelsaar M. Allergy development and the intestinal microflora during the first year of life. J Allergy Clin Immunol. 2001;108:516–520. doi: 10.1067/mai.2001.118130. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe S, Narisawa Y, Arase S, Okamatsu H, Ikenaga T, Tajiri Y, Kumemura M. Differences in fecal microflora between patients with atopic dermatitis and healthy control subjects. J Allergy Clin Immunol. 2003;111:587–591. doi: 10.1067/mai.2003.105. [DOI] [PubMed] [Google Scholar]
- 10.Penders J, Thijs C, van den Brandt PA, Kummeling I, Snijders B, Stelma F, Adams H, van Ree R, Stobberingh EE. Gut microbiota composition and development of atopic manifestations in infancy: the KOALA Birth Cohort Study. Gut. 2007;56:661–667. doi: 10.1136/gut.2006.100164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sjogren YM, Jenmalm MC, Bottcher MF, Bjorksten B, Sverremark-Ekstrom E. Altered early infant gut microbiota in children developing allergy up to 5 years of age. Clin Exp Allergy. 2009;39:518–526. doi: 10.1111/j.1365-2222.2008.03156.x. [DOI] [PubMed] [Google Scholar]
- 12.Wang M, Karlsson C, Olsson C, Adlerberth I, Wold AE, Strachan DP, Martricardi PM, Aberg N, Perkin MR, Tripodi S, Coates AR, Hesselmar B, Saalman R, Molin G, Ahrne S. Reduced diversity in the early fecal microbiota of infants with atopic eczema. J Allergy Clin Immunol. 2008;121:129–134. doi: 10.1016/j.jaci.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Forno E, Onderdonk AB, McCracken J, Litonjua AA, Laskey D, Delaney ML, Dubois AM, Gold DR, Ryan LM, Weiss ST, Celedon JC. Diversity of the gut microbiota and eczema in early life. Clin Mol Allergy. 2008;6:11. doi: 10.1186/1476-7961-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mallol J, Garcia-Marcos L, Sole D, Brand P. International prevalence of recurrent wheezing during the first year of life: variability, treatment patterns and use of health resources. Thorax. 2010;65:1004–1009. doi: 10.1136/thx.2009.115188. [DOI] [PubMed] [Google Scholar]
- 15.Ly NP, Gold DR, Weiss ST, Celedon JC. Recurrent wheeze in early childhood and asthma among children at risk for atopy. Pediatrics. 2006;117:e1132–1138. doi: 10.1542/peds.2005-2271. [DOI] [PubMed] [Google Scholar]
- 16.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332:133–138. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 17.Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, Printz MC, Lee WM, Shult PA, Reisdorf E, Carlson-Dakes KT, Salazar LP, DaSilva DF, Tisler CJ, Gern JE, Lemanske RF., Jr. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178:667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalliomaki M, Salminen S, Poussa T, Arvilommi H, Isolauri E. Probiotics and prevention of atopic disease: 4-year follow-up of a randomised placebo-controlled trial. Lancet. 2003;361:1869–1871. doi: 10.1016/S0140-6736(03)13490-3. [DOI] [PubMed] [Google Scholar]
- 19.Kalliomaki M, Salminen S, Poussa T, Isolauri E. Probiotics during the first 7 years of life: a cumulative risk reduction of eczema in a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2007;119:1019–1021. doi: 10.1016/j.jaci.2006.12.608. [DOI] [PubMed] [Google Scholar]
- 20.Arslanoglu S, Moro GE, Boehm G. Early supplementation of prebiotic oligosaccharides protects formula-fed infants against infections during the first 6 months of life. J Nutr. 2007;137:2420–2424. doi: 10.1093/jn/137.11.2420. [DOI] [PubMed] [Google Scholar]
- 21.Arslanoglu S, Moro GE, Schmitt J, Tandoi L, Rizzardi S, Boehm G. Early dietary intervention with a mixture of prebiotic oligosaccharides reduces the incidence of allergic manifestations and infections during the first two years of life. J Nutr. 2008;138:1091–1095. doi: 10.1093/jn/138.6.1091. [DOI] [PubMed] [Google Scholar]
- 22.Mikami K, Takahashi H, Kimura M, Isozaki M, Izuchi K, Shibata R, Sudo N, Matsumoto H, Koga Y. Influence of maternal bifidobacteria on the establishment of bifidobacteria colonizing the gut in infants. Pediatr Res. 2009;65:669–674. doi: 10.1203/PDR.0b013e31819ed7a8. [DOI] [PubMed] [Google Scholar]
- 23.Ly NP, Ruiz-Perez B, Onderdonk AB, Tzianabos AO, Litonjua AA, Liang C, Laskey D, Delaney ML, DuBois AM, Levy H, Gold DR, Ryan LM, Weiss ST, Celedon JC. Mode of delivery and cord blood cytokines: a birth cohort study. Clin Mol Allergy. 2006;4:13. doi: 10.1186/1476-7961-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferris BG. Epidemiology Standardization Project (American Thoracic Society) Am Rev Respir Dis. 1978;118:1–120. [PubMed] [Google Scholar]
- 25.CDC National Vital Statistics Reports. 2005.
- 26.Noverr MC, Huffnagle GB. The ‘microflora hypothesis’ of allergic diseases. Clin Exp Allergy. 2005;35:1511–1520. doi: 10.1111/j.1365-2222.2005.02379.x. [DOI] [PubMed] [Google Scholar]
- 27.Smith K, McCoy KD, Macpherson AJ. Use of axenic animals in studying the adaptation of mammals to their commensal intestinal microbiota. Semin Immunol. 2007;19:59–69. doi: 10.1016/j.smim.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Sudo N, Sawamura S, Tanaka K, Aiba Y, Kubo C, Koga Y. The requirement of intestinal bacterial flora for the development of an IgE production system fully susceptible to oral tolerance induction. J Immunol. 1997;159:1739–1745. [PubMed] [Google Scholar]
- 29.Round JL, O’Connell RM, Mazmanian SK. Coordination of tolerogenic immune responses by the commensal microbiota. J Autoimmun. 2010;34:J220–225. doi: 10.1016/j.jaut.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gronlund MM, Lehtonen OP, Eerola E, Kero P. Fecal microflora in healthy infants born by different methods of delivery: permanent changes in intestinal flora after cesarean delivery. J Pediatr Gastroenterol Nutr. 1999;28:19–25. doi: 10.1097/00005176-199901000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Morelli L. Postnatal development of intestinal microflora as influenced by infant nutrition. J Nutr. 2008;138:1791S–1795S. doi: 10.1093/jn/138.9.1791S. [DOI] [PubMed] [Google Scholar]
- 32.Duramad P, Harley K, Lipsett M, Bradman A, Eskenazi B, Holland NT, Tager IB. Early environmental exposures and intracellular Th1/Th2 cytokine profiles in 24-month-old children living in an agricultural area. Environ Health Perspect. 2006;114:1916–1922. doi: 10.1289/ehp.9306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fanaro S, Chierici R, Guerrini P, Vigi V. Intestinal microflora in early infancy: composition and development. Acta Paediatr Suppl. 2003;91:48–55. doi: 10.1111/j.1651-2227.2003.tb00646.x. [DOI] [PubMed] [Google Scholar]
- 34.Klaassens ES, Boesten RJ, Haarman M, Knol J, Schuren FH, Vaughan EE, de Vos WM. Mixed-species genomic microarray analysis of fecal samples reveals differential transcriptional responses of bifidobacteria in breast- and formula-fed infants. Appl Environ Microbiol. 2009;75:2668–2676. doi: 10.1128/AEM.02492-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, van den Brandt PA, Stobberingh EE. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118:511–521. doi: 10.1542/peds.2005-2824. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi H, Mikami K, Nishino R, Matsuoka T, Kimura M, Koga Y. Comparative analysis of the properties of bifidobacterial isolates from fecal samples of mother-infant pairs. J Pediatr Gastroenterol Nutr. 2010;51:653–660. doi: 10.1097/MPG.0b013e3181f0e032. [DOI] [PubMed] [Google Scholar]
- 37.Stencel-Gabriel K, Gabriel I, Wiczkowski A, Paul M, Olejek A. Prenatal priming of cord blood T lymphocytes by microbiota in the maternal vagina. Am J Reprod Immunol. 2009;61:246–252. doi: 10.1111/j.1600-0897.2009.00687.x. [DOI] [PubMed] [Google Scholar]
- 38.Benn CS, Thorsen P, Jensen JS, Kjaer BB, Bisgaard H, Andersen M, Rostgaard K, Bjorksten B, Melbye M. Maternal vaginal microflora during pregnancy and the risk of asthma hospitalization and use of antiasthma medication in early childhood. J Allergy Clin Immunol. 2002;110:72–77. doi: 10.1067/mai.2002.125833. [DOI] [PubMed] [Google Scholar]
- 39.Gronlund MM, Gueimonde M, Laitinen K, Kociubinski G, Gronroos T, Salminen S, Isolauri E. Maternal breast-milk and intestinal bifidobacteria guide the compositional development of the Bifidobacterium microbiota in infants at risk of allergic disease. Clin Exp Allergy. 2007;37:1764–1772. doi: 10.1111/j.1365-2222.2007.02849.x. [DOI] [PubMed] [Google Scholar]
- 40.Gueimonde M, Sakata S, Kalliomaki M, Isolauri E, Benno Y, Salminen S. Effect of maternal consumption of lactobacillus GG on transfer and establishment of fecal bifidobacterial microbiota in neonates. J Pediatr Gastroenterol Nutr. 2006;42:166–170. doi: 10.1097/01.mpg.0000189346.25172.fd. [DOI] [PubMed] [Google Scholar]
- 41.Abrahamsson TR, Jakobsson T, Bottcher MF, Fredrikson M, Jenmalm MC, Bjorksten B, Oldaeus G. Probiotics in prevention of IgE-associated eczema: a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2007;119:1174–1180. doi: 10.1016/j.jaci.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 42.Kukkonen K, Savilahti E, Haahtela T, Juntunen-Backman K, Korpela R, Poussa T, Tuure T, Kuitunen M. Probiotics and prebiotic galacto-oligosaccharides in the prevention of allergic diseases: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2007;119:192–198. doi: 10.1016/j.jaci.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 43.Taylor AL, Dunstan JA, Prescott SL. Probiotic supplementation for the first 6 months of life fails to reduce the risk of atopic dermatitis and increases the risk of allergen sensitization in high-risk children: a randomized controlled trial. J Allergy Clin Immunol. 2007;119:184–191. doi: 10.1016/j.jaci.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 44.Adlerberth I, Wold AE. Establishment of the gut microbiota in Western infants. Acta Paediatr. 2009;98:229–238. doi: 10.1111/j.1651-2227.2008.01060.x. [DOI] [PubMed] [Google Scholar]
- 45.Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5:e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adlerberth I, Lindberg E, Aberg N, Hesselmar B, Saalman R, Strannegard IL, Wold AE. Reduced enterobacterial and increased staphylococcal colonization of the infantile bowel: an effect of hygienic lifestyle? Pediatr Res. 2006;59:96–101. doi: 10.1203/01.pdr.0000191137.12774.b2. [DOI] [PubMed] [Google Scholar]
- 47.Adlerberth I, Strachan DP, Matricardi PM, Ahrne S, Orfei L, Aberg N, Perkin MR, Tripodi S, Hesselmar B, Saalman R, Coates AR, Bonanno CL, Panetta V, Wold AE. Gut microbiota and development of atopic eczema in 3 European birth cohorts. J Allergy Clin Immunol. 2007;120:343–350. doi: 10.1016/j.jaci.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 48.Asthma and Allergy Foundation of America: Allergy Facts and Figures.
- 49.Akinbami LJ. The State of Childhood Asthma, United States, 1980-2005Advance Data from Vital Health and Statistics: CDC. 2006 [PubMed] [Google Scholar]
- 50.Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004;59:469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 51.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 52.Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci U S A. 2010;107:12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wright AL. Epidemiology of asthma and recurrent wheeze in childhood. Clin Rev Allergy Immunol. 2002;22:33–44. doi: 10.1007/s12016-002-0004-z. [DOI] [PubMed] [Google Scholar]
- 54.Erickson KL, Hubbard NE. Probiotic immunomodulation in health and disease. J Nutr. 2000;130:403S–409S. doi: 10.1093/jn/130.2.403S. [DOI] [PubMed] [Google Scholar]
- 55.Corthesy B, Gaskins HR, Mercenier A. Cross-talk between probiotic bacteria and the host immune system. J Nutr. 2007;137:781S–790S. doi: 10.1093/jn/137.3.781S. [DOI] [PubMed] [Google Scholar]
- 56.Hojsak I, Abdovic S, Szajewska H, Milosevic M, Krznaric Z, Kolacek S. Lactobacillus GG in the prevention of nosocomial gastrointestinal and respiratory tract infections. Pediatrics. 125:e1171–1177. doi: 10.1542/peds.2009-2568. [DOI] [PubMed] [Google Scholar]
- 57.Hojsak I, Snovak N, Abdovic S, Szajewska H, Misak Z, Kolacek S. Lactobacillus GG in the prevention of gastrointestinal and respiratory tract infections in children who attend day care centers: a randomized, double-blind, placebo-controlled trial. Clin Nutr. 29:312–316. doi: 10.1016/j.clnu.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 58.Carroll KN, Hartert TV. The impact of respiratory viral infection on wheezing illnesses and asthma exacerbations. Immunol Allergy Clin North Am. 2008;28:539–561. viii. doi: 10.1016/j.iac.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med. 2000;161:1501–1507. doi: 10.1164/ajrccm.161.5.9906076. [DOI] [PubMed] [Google Scholar]
- 60.Kusel MM, de Klerk NH, Kebadze T, Vohma V, Holt PG, Johnston SL, Sly PD. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J Allergy Clin Immunol. 2007;119:1105–1110. doi: 10.1016/j.jaci.2006.12.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ly NP, Gold DR, Weiss ST, Celedon JC. Recurrent wheeze in early childhood and asthma at age 7 years among children at risk for atopy. Proc Am Thoracic Soc. 2005;2:A700. doi: 10.1542/peds.2005-2271. [DOI] [PubMed] [Google Scholar]
- 62.Arbes SJ, Jr., Gergen PJ, Elliott L, Zeldin DC. Prevalences of positive skin test responses to 10 common allergens in the US population: results from the third National Health and Nutrition Examination Survey. J Allergy Clin Immunol. 2005;116:377–383. doi: 10.1016/j.jaci.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 63.Britton J, Pavord I, Richards K, Knox A, Wisniewski A, Wahedna I, Kinnear W, Tattersfield A, Weiss S. Factors influencing the occurrence of airway hyperreactivity in the general population: the importance of atopy and airway calibre. Eur Respir J. 1994;7:881–887. [PubMed] [Google Scholar]
- 64.Gergen PJ, Arbes SJ, Jr., Calatroni A, Mitchell HE, Zeldin DC. Total IgE levels and asthma prevalence in the US population: results from the National Health and Nutrition Examination Survey 2005-2006. J Allergy Clin Immunol. 2009;124:447–453. doi: 10.1016/j.jaci.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]