Abstract
OBJECTIVE:
Cholestasis predisposes to fat-soluble vitamin (FSV) deficiencies. A liquid multiple FSV preparation made with tocopheryl polyethylene glycol-1000 succinate (TPGS) is frequently used in infants with biliary atresia (BA) because of ease of administration and presumed efficacy. In this prospective multicenter study, we assessed the prevalence of FSV deficiency in infants with BA who received this FSV/TPGS preparation.
METHODS:
Infants received FSV/TPGS coadministered with additional vitamin K as routine clinical care in a randomized double-blinded, placebo-controlled trial of corticosteroid therapy after hepatoportoenterostomy (HPE) for BA (identifier NCT 00294684). Levels of FSV, retinol binding protein, total serum lipids, and total bilirubin (TB) were measured 1, 3, and 6 months after HPE.
RESULTS:
Ninety-two infants with BA were enrolled in this study. Biochemical evidence of FSV insufficiency was common at all time points for vitamin A (29%–36% of patients), vitamin D (21%–37%), vitamin K (10%–22%), and vitamin E (16%–18%). Vitamin levels were inversely correlated with serum TB levels. Biochemical FSV insufficiency was much more common (15%–100% for the different vitamins) in infants whose TB was ≥2 mg/dL. At 3 and 6 months post HPE, only 3 of 24 and 0 of 23 infants, respectively, with TB >2 mg/dL were sufficient in all FSV.
CONCLUSIONS:
Biochemical FSV insufficiency is commonly observed in infants with BA and persistent cholestasis despite administration of a TPGS containing liquid multiple FSV preparation. Individual vitamin supplementation and careful monitoring are warranted in infants with BA, especially those with TB >2 mg/dL.
KEY WORDS: cholestasis, nutrition, liver, vitamin deficiency
What’s Known on This Subject:
Cholestasis predisposes to the development of fat-soluble vitamin (FSV) deficiency. D-α tocopheryl polyethylene glycol-1000 succinate and coadministered FSVs are absorbed in spite of cholestasis.
What This Study Adds:
Infants with biliary atresia with total bilirubin >2 mg/dL are at risk for fat-soluble vitamin (FSV) deficiency. A multivitamin preparation containing d-α tocopheryl polyethylene glycol-1000 succinate alone is not effective in treating biochemical FSV insufficiency in cholestatic infants.
Biliary atresia (BA) leads to obstruction of the extrahepatic biliary tree. Treatment by surgical hepatoportoenterostomy (HPE) restores bile flow in ∼50% of infants.1 Many infants do not have optimal bile flow even after timely HPE with the resulting chronic cholestasis leading to malabsorption and nutritional deficiencies, cirrhosis, and end-stage liver disease. Early after HPE, a total serum bilirubin >2 mg/dL, a marker of poor bile flow, has been found to be associated with poorer short-term outcome.2,3 Infants with BA are at risk for malabsorption of dietary lipid and fat-soluble vitamins (FSVs) due to insufficient intraluminal bile acid concentrations.4 Thus, routine FSV supplementation after HPE has been advocated to prevent biochemical and clinical deficiency states of vitamins A, D, E, and K.5 To provide these vitamins, 2 approaches are typically employed: (1) supplementation with large doses of each individual vitamin (either orally or intramuscularly) or (2) use of multiple vitamin preparations that contain increased levels of FSV. Most of the high-dose multiple vitamin preparations were developed for supplementation in infants and children with other diseases characterized by fat malabsorption, such as cystic fibrosis,6 and not designed specifically for those with cholestasis. A liquid multiple FSV preparation made with d-α tocopheryl polyethylene glycol-1000 succinate (TPGS; a micelle forming water-soluble form of vitamin E), FSV/TPGS, is frequently used in the management of infants with BA because of ease of administration and presumed efficacy due to the inclusion of TPGS, which enhances absorption of FSV independent of luminal bile acids.7 The efficacy of these preparations in preventing vitamin deficiencies in cholestasis in general, and BA in particular, has not been evaluated, despite their widespread clinical use.
The Biliary Atresia Research Consortium, a multiinstitutional clinical research consortium funded by the National Institutes of Health now called the Childhood Liver Disease Research and Education Network, has been conducting a randomized, placebo-controlled, double blinded trial of corticosteroid therapy after HPE for BA since 2004. In this study all enrolled infants are treated with a standardized dose of a liquid multiple FSV/TPGS preparation with additional supplementation of individual vitamins as needed (Table 1). Serum levels of FSV are monitored routinely during this study, allowing for a unique means of evaluating the efficacy of the vitamin supplementation in preventing FSV deficiencies in BA. The objective of this substudy was to evaluate FSV status longitudinally in patients enrolled in this study from 2005 to 2009 and to determine serum markers predictive of biochemical vitamin insufficiency.
TABLE 1.
Target FSV Levels and Replacement Regimens
| Vitamin | Target Range (Serum Level) | Supplementation Strategy |
|---|---|---|
| A (retinol) | 19–77 μg/dL retinol:RBP molar ratio >0.8 | Increments of 5000 IU (up to 25–50 000 IU/d) orally or monthly intramuscular administration of 50 000 IU |
| D (25-hydroxy vitamin D) | 15–45 ng/mL | Increments of 1200 to 8000 IU orally daily of cholecalciferol or ergocalciferol; alternatively calcitriol at 0.05 to 0.20 μg/kg per d |
| E (α tocopherol) | 3.8–20.3 μg/mL vitamin E:total serum lipids ratio >0.6 mg/g | Increments of 25 IU/kg of TPGS orally daily (to 100 IU/kg per d) |
| K (phytonadione) | INR ≤ 1.2 | 1.2 < INR ≤ 1.5 2.5 mg vitamin K orally daily, 1.5 < INR ≤ 1.8 2.0–5.0 mg vitamin K intramuscular and 2.5 mg vitamin K orally daily, INR > 1.8 2.0–5.0 mg vitamin K intramuscular and 5.0 mg vitamin K orally daily |
Methods
Study Participants
Infants enrolled in a randomized double-blinded, placebo-controlled trial of corticosteroid therapy after HPE for BA were the subjects of this investigation (identifier NCT 00294684). Infants were enrolled in this study within 72 hours of their HPE. Independent of their randomization status (placebo or corticosteroids), all infants in this trial received standardized FSV/TPGS supplementation and monitoring as described below. Informed consent was obtained from the parent or guardian for all study participants, and institutional review board approval was obtained at each local center.
Vitamin Administration
Postoperatively, as soon as enteral intake was tolerated, study participants received 2 mL per os daily of a commercially available FSV/TPGS (ADEKs or AquADEKs, trademarks owned by or under license by Axcan Pharma US, Inc, Birmingham, AL). The type of multivitamin preparation was changed from ADEKs to AquADEKs ∼32 months after the initiation of the study because the manufacturer changed the composition of this product to the latter formulation. Detailed compositional comparison of ADEKs and AquADEKs is provided in Supplemental Table 4. The TPGS (an amphipathic molecule) potentially serves to enhance absorption of FSV that are comixed in these preparations.7 The vitamin composition of the 2 preparations differed in that AquADEKs contained more vitamin A as β carotene, 25% more vitamin E, and 4 times more vitamin K than ADEKs. Additional vitamin K (phytonadione) was coadministered with the FSV/TPGS 3 times each week at a dose of 2.5 mg. Vitamin K was crushed and added to the FSV/TPGS. If vitamin insufficiency was documented by persistently low serum levels or ratios, additional vitamin supplements were added at the clinical discretion of the medical team, based on recommended dosing regimens available as part of the study guidelines (Table 1). FSV/TPGS was provided to study participants at no cost as part of a cooperative research and development agreement between Axcan Pharma US, Inc and the National Institute of Diabetes and Digestive and Kidney Diseases.
Monitoring
Blood testing for prothrombin time/international normalized ratio (INR), total bilirubin (TB), total lipids, retinol binding protein (RBP), retinol, α tocopherol, and 25-hydroxyvitamin D was performed 1, 3, and 6 months after HPE. TB was equated with the sum of conjugated and unconjugated bilirubin in circumstances where TB was not measured directly. In some cases, adequate blood samples could not be obtained for testing of all vitamin levels at all time points.
Vitamin Assays
Vitamin, RBP, and total lipids were performed in the Clinical Translational Research Center Core Laboratory at Children’s Hospital Colorado (Aurora, CO) by the same methods that were used to establish normal ranges in the literature. Retinol and tocopherol levels were assayed from deproteinized serum or plasma by high performance liquid chromatography.8 RBP level was assayed by immunonephelometry by using the BNII Analyzer9 and according to the manufacturer (Siemens, Deerfield, IL). Total serum lipids were measured spectrophotometrically when reacted with vanillin as described.10 Twenty-five-hydroxy vitamin D was assayed by radioimmunoassay according to the manufacturer (Diasorin, Stillwater, MN). The range of detectable levels and the performance parameters of the assays are listed in Supplemental Table 5. Pediatric reference ranges used for target vitamin levels in this study (Table 1) were followed from published criteria.9–11 Vitamin insufficiency was defined biochemically as vitamin measurements (serum 25-hydroxyvitamin D for vitamin D, retinol:RBP ratio for vitamin A and serum α tocopherol:total serum lipids ratio for vitamin E) below the lower limit of the target range or elevated INR as a surrogate marker for vitamin K deficiency (Table 1). Serum levels of the protein-induced by vitamin K antagonist II were not available for routine clinical use in this investigation. The authors of previous studies have validated that low serum 25-hydroxyvitamin D and low ratios of retinol:RBP and α tocopherol:total serum lipids are the preferred indices of vitamin status and are associated with clinical deficiency states in cholestatic children.12–17 INR, which is most often used in clinical practice, was used to estimate vitamin K deficiency, although other indices may be more specific.18
Statistical Analyses
Vitamin levels and TB were available at 1, 3, and 6 months post-HPE on varying number of subjects. Data were analyzed separately at each time point. Analysis of FSV sufficiency was undertaken in all infants including those who received supplements in addition to FSV/TPGS. Descriptive statistics (Ns, means, and SDs) were reported on the vitamin levels. Vitamin levels were classified as low, sufficient, or high, and were also classified as sufficient or insufficient by using the cutoffs in Table 1. The parameters for deficiency of vitamin D and indicators for supplementation of vitamin D have evolved since this study was initiated. The current analysis utilizes a cutoff of 15 ng/mL, although the implications of a higher cutoff of 20 ng/mL as recently recommended by the Institute of Medicine are depicted in relevant graphic depictions of the data.19 Percentages of subjects who are vitamin insufficient are reported. To assess the relationship between TB and vitamin insufficiency, patients were stratified based on TB level (elevated [>2 mg/dL] versus not elevated). The level of 2 mg/dL was chosen on the basis of its utility as a biomarker of bile flow and as a predictor of short-term outcome in infants with BA.2,3 The ensuing 2 × 2 tables were analyzed by using Fisher’s exact test, and the 2-sided P value for H0: no association between TB level and vitamin insufficiency was reported. All analyses were performed by using SAS/STAT (SAS Institute, Inc, Cary, NC).
Results
Enrollment and Demographics
Between September 21, 2005, and October 31, 2008, 92 infants with BA were enrolled in this study (final enrollment planned to be 144 infants for the corticosteroid trial; identifier NCT 00294684). Data were collected up to July 1, 2009. Information was available at entry 1, 3, and 6 months post HPE in 92, 88, 86, and 77 infants, respectively. The numbers of subjects who died or underwent liver transplantation were 0 at 1 month, 3 at 3 months, and 10 at 6 months. Forty-seven percent of the subjects were boys, 23% Hispanic, 57% white, 13% African American, and 30% other races (Asian, Native American, and unknown).
Vitamin Levels During Supplementation
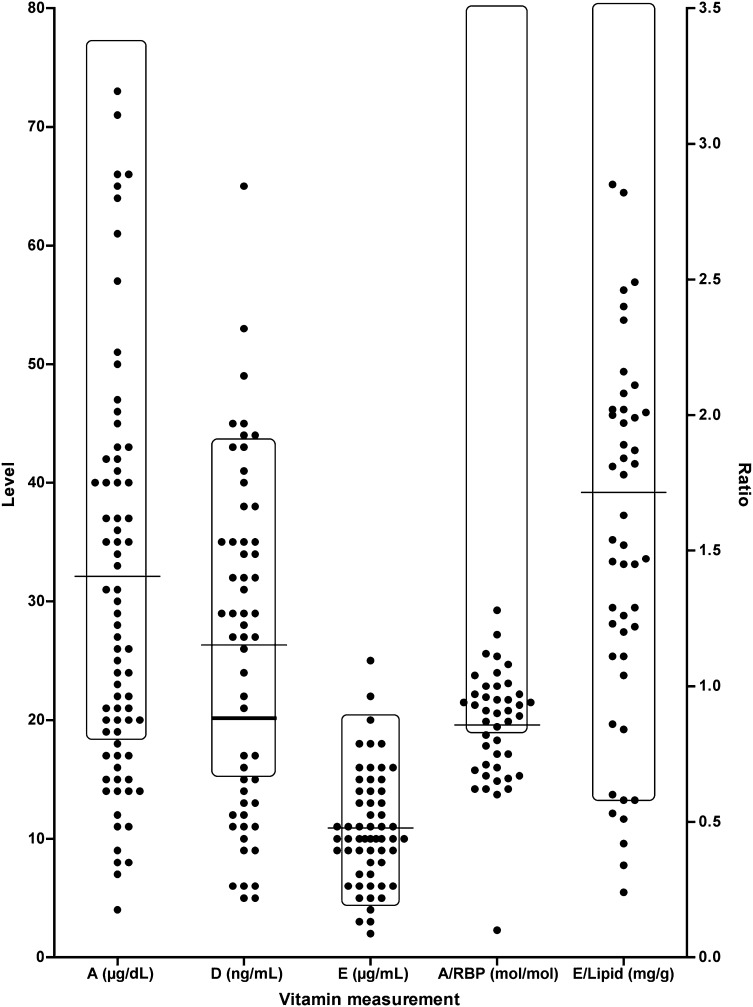
Serum FSV levels at different time intervals after HPE are shown in Table 2. Serum vitamin levels in patients with BA were grouped as low, sufficient, or high relative to reference ranges (Table 1) and are shown in Supplemental Fig 4 according to time after HPE. A representative scatterplot of specific levels at 6 months after HPE is seen in Fig 1. Biochemical evidence of insufficiency of at least 1 vitamin was noted in 58%, 53%, and 57% of the infants at 1, 3, and 6 months after HPE, respectively. High FSV levels were generally uncommon and seen more so at the earlier time points (Supplemental Fig 4). FSV levels differed somewhat between the ADEKs and AquADEKs supplementation eras (Supplemental Table 6). The limited number of subjects only receiving ADEKs (n = 46) or AquADEKs (n = 41) did not permit a detailed analysis of the prevalence of insufficiency at each specific time point in ADEKs versus AquADEKs supplemented subjects.
TABLE 2.
Serum Vitamin Levels Relative to Time After HPE
| Time After HPE | |||
|---|---|---|---|
| 1 mo (n = 88)a | 3 mo (n = 86) | 6 mo (n = 77) | |
| Retinol (μg/dL) | 50.8 ± 34.7 (67)b | 34.1 ± 21.0 (64) | 30.3 ± 19.1 (57) |
| Retinol/RBPc | 0.9 ± 0.2 (55) | 0.9 ± 0.2 (51) | 0.9 ± 0.2 (44) |
| 25-hydroxyvitamin D (ng/mL) | 20.2 ± 11.0 (63) | 28.1 ± 20.2 (62) | 26.7 ± 14.2 (57) |
| α tocopherol (E) (μg/mL) | 11.4 ± 6.1 (68) | 12.1 ± 6.7 (65) | 10.8 ± 4.8 (59) |
| E/lipids (mg/g)d | 1.6 ± 1.0 (66) | 2.2 ± 3.7 (64) | 1.7 ± 1.0 (51) |
| INR | 1.0 ± 0.2 (69) | 1.1 ± 0.2 (69) | 1.1 ± 0.2 (62) |
n refers to the number of subjects remaining in the study at the time point indicated.
Mean ± SD (number of measurements of a specific vitamin for subjects at that time point).
Molar ratio of retinol to RBP.
Ratio of vitamin E (α tocopherol) to total lipids.
FIGURE 1.
Scatterplot of serum vitamin levels 6 months after HPE. Individual values measured from each subject are shown. The rectangle represents the target range for a specific measurement. The horizontal line represents the mean for each measurement. An additional thick horizontal line indicates the revised level for vitamin D of 20 ng/mL. Levels for vitamins A, D, and E are indicated on the left vertical axis in μg/dL, ng/mL, and μg/mL, respectively. Ratios for A/RBP (molar) and E/lipid (mg/g) are indicated on the right vertical axis.
Vitamin Insufficiency and TB >2 mg/dL
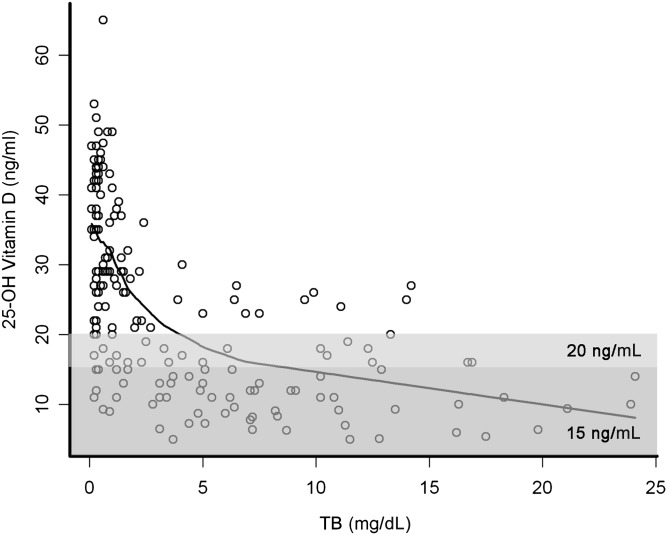
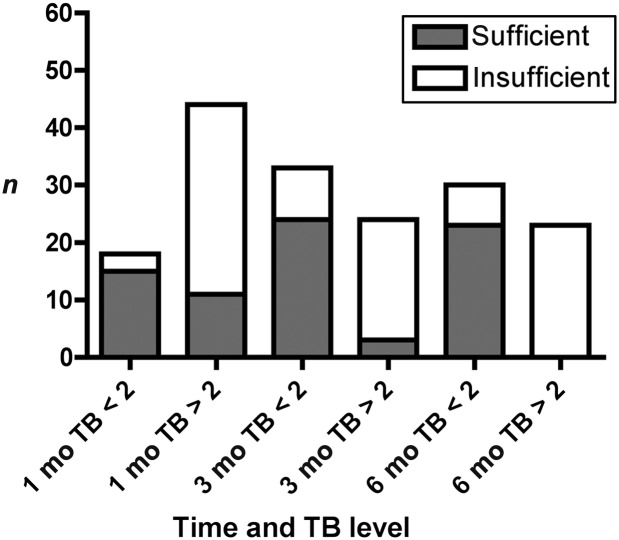
Vitamin levels, in general, were negatively correlated with TB (Fig 2). A TB of <2 mg/dL has often been used as a marker of evidence of good bile flow after HPE, with the corollary being that a TB >2 mg/dL indicates impaired bile flow. In light of this clinical observation, a detailed examination was performed of the prevalence of vitamin sufficiency relative to a TB of <2 mg/dL. Vitamin levels were generally sufficient in infants whose TB was <2 mg/dL, whereas there was a relatively high level of insufficiency in those with TB >2 mg/dL (Table 3 and Fig 3). In fact, at 3 and 6 months post HPE, only 3 of 24 and 0 of 23 infants, respectively, with TB >2 mg/dL were sufficient in all FSV. Not all vitamin levels were drawn at all time points. The ability to obtain adequate blood specimens coupled with the potential perceived necessity for the sample influenced the availability of serum for the vitamin level. At 1, 3, and 6 months after HPE, the percentage of subjects who did not have a vitamin level obtained was similar between those with a TB <2 mg/dL versus those with a TB >2 mg/dL (data not shown). There was a trend toward fewer measurements in those with TB as <2 mg/dL.
FIGURE 2.
Scatterplot of serum 25-hydroxyvitamin D levels plotted against TB. All measured 25-hydroxyvitamin D levels from all time points after HPE are plotted relative to total serum bilirubin. Spline correlation is depicted by the solid line. The darker shaded box reflects measurements that would be categorized as insufficient with a cutoff of 15 ng/mL, whereas the lighter shaded box reflects the revised cutoff of 20 ng/mL.
TABLE 3.
Vitamin Deficiency Relative to TB Level
| TB <2 mg/dL | TB ≥2 mg/dL | P | |||
|---|---|---|---|---|---|
| Sufficient | Deficient | Sufficient | Deficient | ||
| Retinol (<20 μg/dL)a | |||||
| 1 mo | 22b | 0 | 34 | 11 | .0118 |
| 3 mo | 34 | 6 | 11 | 13 | .0016 |
| 6 mo | 31 | 4 | 5 | 14 | <.0001 |
| Retinol/RBP (molar ratio <0.8) | |||||
| 1 mo | 17 | 3 | 22 | 13 | .1238 |
| 3 mo | 30 | 9 | 3 | 9 | .0018 |
| 6 mo | 25 | 6 | 0 | 10 | <.0001 |
| α tocopherol (E) (<3.8 μg/mL) | |||||
| 1 mo | 21 | 1 | 39 | 7 | .2602 |
| 3 mo | 40 | 0 | 21 | 4 | .0187 |
| 6 mo | 36 | 0 | 17 | 3 | .0411 |
| E/lipids (<0.6 mg/g) | |||||
| 1 mo | 21 | 0 | 33 | 12 | .0068 |
| 3 mo | 40 | 0 | 13 | 11 | <.0001 |
| 6 mo | 32 | 0 | 8 | 8 | <.0001 |
| 25-hydroxyvitamin D (<15 ng/mL) | |||||
| 1 mo | 19 | 2 | 21 | 21 | .0019 |
| 3 mo | 37 | 3 | 12 | 10 | .0008 |
| 6 mo | 34 | 1 | 4 | 15 | <.0001 |
| INR (K) (>1.2) | |||||
| 1 mo | 23 | 0 | 39 | 7 | .0864 |
| 3 mo | 39 | 1 | 15 | 14 | <.0001 |
| 6 mo | 33 | 2 | 13 | 11 | .0004 |
Vitamin levels are measured in serum.
The number of subjects with this finding.
FIGURE 3.
Prevalence of fat-soluble vitamin sufficiency relative to TB level ≥ or <2 mg/dL. The presence (unfilled bar) or absence (shaded bar) of any FSV insufficiency (A, D, E, or K as defined in Table 1) at a given time point post HPE is depicted in the bar graph. The prevalence is reported for those infants with a TB <2 mg/dL relative to those with a TB ≥2 mg/dL. The y axis represents absolute numbers of subjects reported.
Discussion
FSV deficiencies are among the most common nutritional deficiencies in infants and children with cholestatic liver diseases, capable of causing rickets and bone fractures, coagulopathy with hemorrhage, cerebellar ataxia, and impaired vision.12 In cholestasis, low serum 25 hydroxyvitamin D levels,13 low ratio of retinol:RBP,15 and low ratio of α tocopherol:total serum lipids17 have been shown to correlate with clinically important deficiencies of the respective vitamins. In this study, we used a conservative cutoff for biochemical deficiency of vitamin D (25-hydroxyvitamin D level <15 ng/mL). If a higher cutoff were to be used, as has been suggested by others,14 then a higher frequency of vitamin D deficiency would have been identified (Figs 1 and 2). Cross-sectional studies of cholestatic children have revealed that >50% will have biochemical evidence of insufficiency of vitamins A, D, and E.4,15,20–23
In the current study, the effectiveness of a standardized regimen of vitamin supplementation in achieving normal serum levels of FSV was assessed in infants with BA after HPE. Supplementation with the FSV/TPGS preparation plus additional vitamin K failed to prevent FSV insufficiency in up to 58% of infants. The 2 vitamin preparations (ADEKs and AquADEKs) resulted in similar aggregated serum levels of FSV. This investigation was neither powered nor designed to directly compare the adequacy of ADEKs versus AquADEKs as a FSV supplement. Despite receiving increasing doses of individual FSV in an attempt to achieve vitamin sufficiency, many infants in this study continued to display persistent insufficiency. Analysis of the requirements of FSV in infants with BA and persistent cholestasis are on-going. However, from this study, it can be concluded that infants with BA who maintain a serum TB at or above 2 mg/dL remain at high risk for FSV insufficiency and that the FSV/TPGS supplements used in this study plus additional vitamin K were ineffective in preventing all FSV deficiencies in a significant percentage of infants with BA.
There are a number of potential strategies taken to prevent the development of FSV deficiency in infants with cholestasis. Oral supplementation of individual vitamin preparations at doses in significant excess of the Dietary Reference Intake is 1 recommended approach.5 This requires prescription of several vitamin formulations and necessitates close monitoring of individual serum vitamin levels and INR. In the setting of significant cholestasis, oral administration of even large doses of standard vitamin formulations may be inadequate.24 Alternatively, periodic intramuscular administration of vitamins can circumvent poor intestinal absorption; however, it is generally not favored by patients nor their families. In the United States, preparations are available for intramuscular administration of vitamins A, D, and K, but not for vitamin E. Another approach is the chemical modification of a vitamin to promote greater water solubility. The conjugation of α tocopheryl succinate with polyethylene glycol-1000 created an amphipathic molecule (TPGS) that undergoes self-micellization at concentrations as low as 0.02 mM, which precludes the necessity for bile acid micellar solubilization.25 This formulation of tocopherol has excellent intestinal absorption even in the setting of severe cholestasis.24 When TPGS is coadministered with other FSV, enhanced absorption of the unmodified vitamins has been observed in the setting of significant cholestasis.7 This property formed the basis for the commercially available liquid preparations of multiple FSV (with larger doses of FSV than standard multivitamin preparations) suspended in TPGS that were used in this study. Although initially formulated for use in cystic fibrosis, these preparations had the potential for relatively simplified administration of all FSV to infants with cholestasis and were thus chosen as the standard form of vitamin supplementation for infants with BA in this prospective study.
Unfortunately, these 2 TPGS-based liquid multivitamin preparations were ineffective in correcting or preventing the development of FSV deficiency in the majority of infants with BA, especially those with persistent cholestasis. Deficiencies in vitamins A, D, and E were quite common, being significantly more common in infants whose TB was >2 mg/dL. Elevated INR as an estimate of vitamin K deficiency was prevalent but less so than deficiencies of vitamins A and D. Low absolute serum vitamin E levels were less common, although when normalized for total serum lipids, which is the accurate marker for vitamin E deficiency in cholestasis, insufficiency was similar to that observed for vitamin K. Effects of individual vitamin supplementation were not analyzed due to limits in sample size. The importance of these findings lies in the common clinical practice of prescribing the FSV/TPGS preparations used in this study as the only vitamin supplement for infants with BA, with the assumption that absorption was adequate and that monitoring was unnecessary. This is clearly not the case in those infants who remain cholestatic, necessitating other approaches for vitamin supplementation and attention to frequent monitoring.
Finally, it is recognized that the participants evaluated in this study were randomized to a 14-week course of corticosteroids or placebo during the course of this study (identifier NCT 00294684) and that the blind for this ongoing trial will not be broken until 2013. The finding of a large proportion of infants with FSV deficiency justified early publication of these findings. Despite the probability that half of the patients reported received corticosteroids, there is no evidence that corticosteroid therapy alters absorption or metabolism of any of the FSV and thus would not affect serum vitamin levels. If the corticosteroid therapy had a beneficial effect on bile flow, which could improve FSV absorption, this would be reflected by lower serum TB and would not alter the conclusions of this study. Thus, we believe that the conclusions reached in regard to the effectiveness of the FSV/TPGS supplements are valid despite the patients participating in this ongoing clinical trial regardless of the final results of that study. One of the potential benefits of corticosteroid therapy could be improved FSV status if corticosteroids are found to improve bile flow after HPE.
Conclusions
It is clear that infants with BA who have evidence of persistent cholestasis, as manifest by serum TB in excess of 2 mg/dL, remain at high risk for FSV insufficiency despite supplementation with an available FSV/TPGS supplement plus additional vitamin K that is commonly used in clinical practice. Six months after HPE, the prevalence of biochemical evidence of FSV insufficiency in infants with persistent cholestasis (elevated serum TB) is 100%, 79%, 50%, and 46%, respectively, for vitamins A, D, E, and K (Table 3). Thus, careful monitoring for vitamin insufficiency is warranted in infants with BA particularly those whose TB is >2 mg/dL. Current strategies that can successfully correct and prevent FSV deficiency include adding large supplements of individual vitamins, switching to more water-soluble forms (eg, TPGS or 1,25-dihydroxyvitamin D), or administration of intramuscular injections. Development of more effective multiple vitamin supplements for childhood cholestasis will be a challenge because of the varying percentage of patients with persistent deficiency of each of the FSV and thus a regimen of individual vitamin supplementation coupled with timely monitoring of serum levels appears to be necessary.
Supplementary Material
Acknowledgments
We thank the outstanding study coordinators and staff who helped conduct these investigations, the Children’s Hospital Colorado core facility that performed the vitamin measurements, Robert Abel for his expert assistance with the data analysis, and the generous families and children who participated in this research.
Glossary
- BA
biliary atresia
- FSV
fat-soluble vitamin
- HPE
hepatoportoenterostomy
- INR
international normalized ratio
- RBP
retinol binding protein
- TB
total bilirubin
- TPGS
D-α tocopheryl polyethylene glycol-1000 succinate
Footnotes
This trial has been registered at www.clinicaltrials.gov (identifier NCT00294684).
FINANCIAL DISCLOSURE: Dr Sokol is a consultant for and on the scientific advisory board of Yasoo Health, Inc, which is the distributor for AquADEKs and ADEKs. The company had no involvement in designing the research, interpreting the data, or writing the article; the other authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: This work was supported by the National Center for Research Resources (5M01 RR00069 [Dr Sokol], UL1RR025780 [Colorado], UL1RR024153[Pittsburgh], UL1RR024134 [Philadelphia], UL1RR024131 [San Francisco], UL1RR025005 [Baltimore], UL1RR025741 [Chicago], UL1RR029877 [New York], UL1RR026314 [Cincinnati], UL1RR024992 [St. Louis]) and U01 grants from the National Institute of Diabetes, Digestive and Kidney Diseases (DK 62453 [Dr Sokol], DK 62497 [Dr Bezerra], DK 62481 [Dr Haber], DK 62470 [Dr Karpen], DK 62500 [Dr Robuck], DK 62530 [Dr Schwarz], DK 62445 [Drs Suchy and Kerkar], DK 62466 [Dr Shneider], DK 62456 [Dr Magee], DK 62452 [Dr Turmelle], and DK 62436 [Dr Whitington]). Funded by the National Institutes of Health (NIH).
References
- 1.Sokol RJ, Shepherd RW, Superina R, Bezerra JA, Robuck P, Hoofnagle JH. Screening and outcomes in biliary atresia: summary of a National Institutes of Health workshop. Hepatology. 2007;46(2):566–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Superina R, Magee JC, Brandt ML, et al. Childhood Liver Disease Research and Education Network . The anatomic pattern of biliary atresia identified at time of Kasai hepatoportoenterostomy and early postoperative clearance of jaundice are significant predictors of transplant-free survival. Ann Surg. 2011;254(4):577–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shneider BL, Brown MB, Haber B, et al. Biliary Atresia Research Consortium . A multicenter study of the outcome of biliary atresia in the United States, 1997 to 2000. J Pediatr. 2006;148(4):467–474 [DOI] [PubMed] [Google Scholar]
- 4.Sokol RJ, Heubi JE, Iannaccone S, Bove KE, Balistreri WF. Mechanism causing vitamin E deficiency during chronic childhood cholestasis. Gastroenterology. 1983;85(5):1172–1182 [PubMed] [Google Scholar]
- 5.Feranchak AP, Sokol RJ. Medical and nutritional management of cholestasis in infants and children. In: Suchy F, Balistreri W, Sokol R, eds. Liver Disease in Children. New York, NY: Cambridge University Press; 2007:190–231 [Google Scholar]
- 6.Wilson DC, Rashid M, Durie PR, et al. Treatment of vitamin K deficiency in cystic fibrosis: Effectiveness of a daily fat-soluble vitamin combination. J Pediatr. 2001;138(6):851–855 [DOI] [PubMed] [Google Scholar]
- 7.Argao EA, Heubi JE, Hollis BW, Tsang RC. d-Alpha-tocopheryl polyethylene glycol-1000 succinate enhances the absorption of vitamin D in chronic cholestatic liver disease of infancy and childhood. Pediatr Res. 1992;31(2):146–150 [DOI] [PubMed] [Google Scholar]
- 8.Kaplan LA, Miller JA, Stein EA. Simultaneous measurement of serum retinol, tocopherols, carotene, and carotenoids by high performance liquid chromatography. Journal of Laboratory and Clinical Analysis. 1987;1:147–152 [Google Scholar]
- 9.Lockitch G, Halstead AC, Quigley G, MacCallum C. Age- and sex-specific pediatric reference intervals: study design and methods illustrated by measurement of serum proteins with the Behring LN Nephelometer. Clin Chem. 1988;34(8):1618–1621 [PubMed] [Google Scholar]
- 10.Heubi JE, Sokol RJ, McGraw CA. Comparison of total serum lipids measured by two methods. J Pediatr Gastroenterol Nutr. 1990;10(4):468–472 [DOI] [PubMed] [Google Scholar]
- 11.Feranchak AP, Sontag MK, Wagener JS, Hammond KB, Accurso FJ, Sokol RJ. Prospective, long-term study of fat-soluble vitamin status in children with cystic fibrosis identified by newborn screen. J Pediatr. 1999;135(5):601–610 [DOI] [PubMed] [Google Scholar]
- 12.Sokol RJ. Fat-soluble vitamins and their importance in patients with cholestatic liver diseases. Gastroenterol Clin North Am. 1994;23(4):673–705 [PubMed] [Google Scholar]
- 13.Chongsrisawat V, Ruttanamongkol P, Chaiwatanarat T, Chandrakamol B, Poovorawan Y. Bone density and 25-hydroxyvitamin D level in extrahepatic biliary atresia. Pediatr Surg Int. 2001;17(8):604–608 [DOI] [PubMed] [Google Scholar]
- 14.Wagner CL, Greer FR, American Academy of Pediatrics Section on Breastfeeding. American Academy of Pediatrics Committee on Nutrition . Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122(5):1142–1152 [DOI] [PubMed] [Google Scholar]
- 15.Feranchak AP, Gralla J, King R, et al. Comparison of indices of vitamin A status in children with chronic liver disease. Hepatology. 2005;42(4):782–792 [DOI] [PubMed] [Google Scholar]
- 16.Farrell PM, Levine SL, Murphy MD, Adams AJ. Plasma tocopherol levels and tocopherol-lipid relationships in a normal population of children as compared to healthy adults. Am J Clin Nutr. 1978;31(10):1720–1726 [DOI] [PubMed] [Google Scholar]
- 17.Sokol RJ, Heubi JE, Iannaccone ST, Bove KE, Balistreri WF. Vitamin E deficiency with normal serum vitamin E concentrations in children with chronic cholestasis. N Engl J Med. 1984;310(19):1209–1212 [DOI] [PubMed] [Google Scholar]
- 18.Strople J, Lovell G, Heubi J. Prevalence of subclinical vitamin K deficiency in cholestatic liver disease. J Pediatr Gastroenterol Nutr. 2009;49(1):78–84 [DOI] [PubMed] [Google Scholar]
- 19.Ross CA, Taylor CL, Yaktine AL, Del Valle HB. Dietary Reference Intakes for Calcium and Vitamin D. In: Io M, ed. Washington, DV. Washington, DC: National Academies Press; 2011 [PubMed] [Google Scholar]
- 20.Sokol RJ, Guggenheim MA, Heubi JE, et al. Frequency and clinical progression of the vitamin E deficiency neurologic disorder in children with prolonged neonatal cholestasis. Am J Dis Child. 1985;139(12):1211–1215 [DOI] [PubMed] [Google Scholar]
- 21.Tazawa Y, Nakagawa M, Yamada M, et al. Serum vitamin E levels in children with corrected biliary atresia. Am J Clin Nutr. 1984;40(2):246–250 [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi A, Kawai S, Ohkubo M, Ohbe Y. Serum 25-hydroxy-vitamin D in hepatobiliary disease in infancy. Arch Dis Child. 1979;54(5):367–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heubi JE, Hollis BW, Tsang RC. Bone disease in chronic childhood cholestasis. II. Better absorption of 25-OH vitamin D than vitamin D in extrahepatic biliary atresia. Pediatr Res. 1990;27(1):26–31 [DOI] [PubMed] [Google Scholar]
- 24.Sokol RJ, Butler-Simon N, Conner C, et al. Multicenter trial of d-alpha-tocopheryl polyethylene glycol 1000 succinate for treatment of vitamin E deficiency in children with chronic cholestasis. Gastroenterology. 1993;104(6):1727–1735 [DOI] [PubMed] [Google Scholar]
- 25.Traber MG, Kayden HJ, Green JB, Green MH. Absorption of water-miscible forms of vitamin E in a patient with cholestasis and in thoracic duct-cannulated rats. Am J Clin Nutr. 1986;44(6):914–923 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.