Abstract
OBJECTIVE:
To clarify whether persistent snoring in 2- to 3-year-olds is associated with behavioral and cognitive development, and to identify predictors of transient and persistent snoring.
METHODS:
Two hundred forty-nine mother/child pairs participated in a prospective birth cohort study. Based upon parental report of loud snoring ≥2 times weekly at 2 and 3 years of age, children were designated as nonsnorers, transient snorers (snored at 2 or 3 years of age, but not both), or persistent snorers (snored at both ages). We compared groups by using validated measures of behavioral and cognitive functioning. Potential predictors of snoring included child race and gender, socioeconomic status (parent education and income), birth weight, prenatal tobacco exposure (maternal serum cotinine), childhood tobacco exposure (serum cotinine), history and duration of breast milk feeding, and body mass relative to norms.
RESULTS:
In multivariable analyses, persistent snorers had significantly higher reported overall behavior problems, particularly hyperactivity, depression, and inattention. Nonsnorers had significantly stronger cognitive development than transient and persistent snorers in unadjusted analyses, but not after demographic adjustment. The strongest predictors of the presence and persistence of snoring were lower socioeconomic status and the absence or shorter duration of breast milk feeding. Secondary analyses suggested that race may modify the association of childhood tobacco smoke exposure and snoring.
CONCLUSIONS:
Persistent, loud snoring was associated with higher rates of problem behaviors. These results support routine screening and tracking of snoring, especially in children from low socioeconomic backgrounds; referral for follow-up care of persistent snoring in young children; and encouragement and facilitation of infant breastfeeding.
KEY WORDS: sleep-disordered breathing, behavior problems, preschool, environmental tobacco smoke, breastfeeding, overweight
What’s Known On This Subject:
Loud snoring, which spikes at ∼2 to 3 years of age, has been associated with behavior problems in school-aged children in cross-sectional studies, but no longitudinal studies have quantified predictors and the behavioral impact of persistent snoring in preschool-aged children.
What This Study Adds:
Persistent loud snoring, which occurs in 9% of children 2 to 3 years of age, is linked with behavior problems. Higher socioeconomic status and a history of breastfeeding were associated with lower rates of transient and persistent snoring in young children.
The American Academy of Pediatrics recommends that physicians routinely ask parents about snoring1 to screen for obstructive sleep apnea and milder forms of sleep-disordered breathing (SDB). Habitual snoring has been associated with cognitive and behavior problems in school-aged children, even when formal polysomnography results are ambiguous or negative.2–8 SDB treatment studies have reported encouraging short-term results in school-aged children,2,9 but there have been no published randomized clinical trials, and the few natural history studies have yielded mixed results on whether the resolution of snoring yields improved cognitive skills and/or behavior.10–12 Little is known about SDB in very young children, even though SDB symptoms spike at ∼2 to 3 years of age,13 and snoring-related arousals from sleep correlate with early mental development.14 Without intervention, many very young children continue to snore for years.13,15 The impact of persistent snoring on preschool-aged children is unknown; in older children, persistent snoring increases the chances for new or worsening behavior problems over time.10,16–18 Care decisions for preschool-aged children who snore are based on guidelines developed largely for older children and involve weighing the rare but real risks of interventions (eg, adenotonsillectomy)19,20 against suspected but unknown risks associated with persistent SDB.
Only limited longitudinal data are available to guide evidence-based prevention of persistent SDB. African American race, male gender, low birth weight, and low socioeconomic status have previously been shown to predict child SDB in cross-sectional studies,13,21–27 but these risks are difficult or impossible to modify. With respect to more modifiable risks, current SDB symptoms in infants and toddlers have been linked to exposure to environmental tobacco smoke (ETS) and a history of little to no breastfeeding during infancy.14,23,24,28 Similarly, cross-sectional studies have suggested that SDB symptoms later in childhood are associated with a history of short or absent breastfeeding, maternal smoking during pregnancy, ongoing ETS exposure, and current obesity.13,21,22,25,27,28 Notably, past studies have focused on predictors of SDB measured at a single time point, not its persistence over time, and ETS data have been gathered primarily from retrospective parent reports.
This study capitalizes on a large, prospective study of early childhood development to test 2 hypotheses. First, we hypothesized that children who persistently snore from 2 to 3 years of age will have more behavior problems and poorer cognitive development at 3 years of age than children who snore at neither or only 1 time point. Second, we hypothesized that demographic factors, infant feeding history, current body mass relative to norms, and prenatal and concurrent biomarkers for tobacco smoke exposure are associated with transient and persistent snoring.
Methods
Sample
The study cohort comprised mother/child pairs participating in the Health Outcomes and Measures of the Environment (HOME) Study, an ongoing prospective birth cohort in the Cincinnati, Ohio, metropolitan area.15,29 Beginning in March 2003, women were identified from 7 prenatal clinics associated with 3 hospitals. Eligible mothers were identified at ≤19 weeks of gestation, were ≥18 years of age, negative for HIV, and not taking medications for seizure or thyroid disorders. Of the 468 mothers who consented and were enrolled, 67 dropped out before delivery, 3 children were stillborn, and 9 sets of twins were excluded from current analyses. Of the 389 women with singleton live births, 280 and 258 completed sleep questionnaires at the 2-year and 3-year follow-up points, respectively, yielding a final sample of 249 (64%) with completed questionnaires at both time points.
Procedures
The Institutional Review Board of Cincinnati Children’s Hospital Medical Center provided approval and oversight. All mothers provided written informed consent before enrollment. Enrollees received phone calls every 3 months until the child turned 18 months, then every 6 months. Face-to-face interviews were conducted during yearly home and clinic visits. Unless otherwise indicated, all measures analyzed here were obtained at visits linked to each child’s second and third birthdays.
Assessment of Snoring and Snoring Group Classification
Snoring was assessed by using the validated Child Sleep Habits Questionnaire.15,30,31 Parents were asked to report how often their child “snored loudly” over the previous week: rarely (never or once/week), sometimes (2–4 times/week), or usually (5 or more times/week). Children were assigned to three groups:
Nonsnorers (n = 170; 68%) were reported to snore “rarely” at both time points.
Transient snorers (n = 57; 23%) snored ≥2 times/week at either age 2 or 3, but not both.
Persistent snorers (n = 22; 9%) snored ≥2 times/week at both age 2 and 3.
Assessment of Behavior
Parents completed the preschool form of the Behavior Assessment System for Children, an extensively validated behavior questionnaire.32 Age-normed T scores were generated for the Behavioral Symptoms Index composite and the 4 subscales that enter into it: hyperactivity, aggression, depression, and inattention. Higher scores reflect greater concerns.
Assessment of Cognitive and Motor Development
The second edition of the Bayley Scales of Infant Development was administered by a trained research associate according to standardized instructions.33 The Bayley Scales of Infant Development is widely accepted as one of the best overall assessment tools for measuring cognitive and motor development in young children.34 The second edition, the most updated version at the time of data collection, yields 2 composite age-normed scores: a Mental Development Index and a Psychomotor Development Index. Higher scores reflect better performance.
Assessment of Potential Predictors of Snoring Group
Caregivers were surveyed for collection of demographic characteristics including race, parental education, and household income during pregnancy. For analyses, race was coded as African American (n = 54; 22%) versus non-African American (n = 195; 78%); consistent with the local population, 94% of non-African American children in the sample were white (n = 183). Information about infant birth weight was collected via chart review. Prenatal exposure to tobacco was assessed through maternal serum cotinine measures collected at ∼16 and 26 weeks gestation as well as delivery, and through umbilical cord serum. Childhood tobacco exposure was assessed through serum collected annually from the children during a clinic visit. Serum cotinine analyses were completed at the Centers for Disease Control and Prevention Environmental Health Laboratories by using published methods.35 A detailed breast milk feeding survey was administered during each phone survey and face-to-face study visit until mothers reported they had discontinued breast milk feeding; duration of breast milk feeding was analyzed here. During annual visits, children were measured in triplicate for height and weight, which were used to generate BMI z scores (zBMI) by using age- and gender-based norms from the Centers for Disease Control and Prevention. Cotinine levels obtained at the 2- and 3-year measurement points were strongly associated, rs = 0.78, P < .0001, as were zBMI values, rp = 0.79, P < .0001. To minimize colinearity and simplify analyses, composite preschool cotinine and zBMI scores were calculated from the averages at the 2 time points.
Statistical Approach
Differences between the nonsnoring, transient snoring, and persistent snoring groups on demographic variables and potential snoring predictors were tested by using analysis of variance, Kruskal-Wallis H, and χ2. Group differences on behavioral and cognitive outcome variables were tested with analysis of variance and analysis of covariance, controlling for the demographic factors of child gender, race, and a composite socioeconomic status index (mean z score within the sample on maternal and paternal education and family income; results were unchanged in exploratory analyses that entered these variables separately).
Snoring predictors were tested by using ordinal logistic regression models that treated snoring as a continuum (nonsnoring, transient snoring, persistent snoring). Initially, we examined the bivariate associations of snoring group with 2 dichotomous variables (gender, race) and 6 continuous variables (socioeconomic status, birth weight, breast milk feeding, prenatal and preschool cotinine, preschool BMI z score). We then assessed these associations after adjusting for gender, race, and socioeconomic status (except when assessing associations for one of these demographic factors, at which time we adjusted for the other 2). Finally, we constructed a full model that entered all 8 of the predictors simultaneously.
Results
As shown in Table 1, the 3 groups did not significantly differ in gender distribution, birth weight or gestational age, or age at the time of assessment. In these unadjusted analyses, persistent snorers tended to have higher zBMI scores compared with nonsnorers and transient snorers. Persistent snorers had higher prenatal and childhood cotinine levels than transient snorers or nonsnorers. The snoring group was disproportionately African American and had lower parental education and family income, highlighting the importance of considering demographic factors in subsequent analyses.
TABLE 1.
Sample Characteristics
| Group | P | Group Differences | |||
|---|---|---|---|---|---|
| Nonsnorers | Transient Snorers | Persistent Snorers | |||
| Sample size, n | 170 | 57 | 22 | ||
| Boys, n, % | 78, 45.9 | 29, 50.9 | 9, 40.9 | .69 | |
| African American, n, % | 23, 13.5 | 19, 33.3 | 12, 54.6 | <.0001 | NS < TS, PS |
| Gestational age, wk | 39.2 ± 1.7 | 38.9 ± 1.3 | 39.1 ± 1.5 | .46 | |
| Birth weight, lbs | 7.6 ± 1.4 | 7.3 ± 1.4 | 7.7 ± 1.2 | .30 | |
| Age at 2-y evaluation, y | 2.08 ± 0.08 | 2.10 ± 0.09 | 2.09 ± 0.09 | .52 | |
| Age at 3-y evaluation, y | 3.10 ± 0.11 | 3.10 ± 0.12 | 3.11 ± 0.13 | .93 | |
| Family income, thousands of US dollars | 84.1 ± 40.6 | 62.5 ± 49.6 | 49.3 ± 45.6 | <.0001 | NS > TS, PS |
| Mother’s education, y | 16.2 ± 2.8 | 14.9 ± 3.1 | 13.4 ± 3.2 | <.0001 | NS > TS, PS |
| Father’s education, y | 16.0 ± 2.9 | 14.6 ± 2.9 | 13.5 ± 2.6 | <.0001 | NS > TS, PS |
| BMI z-score at 2-y evaluation, mean ± SD | −0.04 ± 1.03 | −0.21 ± 1.00 | 0.49 ± 1.27 | <.05 | PS > TS |
| BMI z score at 3-y evaluation, mean ± SD | 0.01 ± 1.09 | −0.10 ± 1.11 | 0.55 ± 1.37 | .07 | |
| Prenatal (maternal) cotinine, median (25th, 75th percentile) | 0.009 (0.002, 0.033) | 0.032 (0.007, 0.189) | 0.157 (0.035, 0.922) | <.0001 | NS < TS, PS |
| Serum cotinine at 2-y evaluation, median (25th, 75th percentile) | 0.036 (0.019, 0.076) | 0.153 (0.027, 0.461) | 1.230 (0.034, 2.07) | <.001 | NS < TS, PS |
| Serum cotinine at 3-y evaluation, median (25th, 75th percentile) | 0.022 (0.009, 0.047) | 0.063 (0.027, 0.474) | 0.710 (0.103, 2.14) | <.0001 | NS < TS, PS |
Unless otherwise indicated, group values are presented as mean ± SD. Statistical significance (P) values are based on χ2, analysis of variance, or Kruskal-Wallis H test. Follow-up tests of group differences were Tukey honestly significant difference for normally distributed variables and χ2 or Mann-Whitney U tests with Bonferroni corrections for categorical or nonnormal variables. NS, nonsnorers; TS, transient snorers; PS, persistent snorers.
As hypothesized, persistent snorers had significantly worse overall behavioral functioning (Table 2) than nonsnorers and transient snorers. This effect, which was particularly marked for hyperactivity, depression, and attention subscales, remained significant after adjusting for demographic covariates. Although transient and persistent snorers also had poorer cognitive scores than nonsnorers in unadjusted analyses, these differences were no longer significant after covarying for demographic factors. Motor development did not significantly differ across groups.
TABLE 2.
Group Differences in Behavior and Mental and Motor Development
| Group | Unadjusted | Adjusted | |||||
|---|---|---|---|---|---|---|---|
| Nonsnorers | Transient Snorers | Persistent Snorers | P | Group Differences | P | Group Differences | |
| BASC-2 | |||||||
| Overall BSI | 49.2 ± 7.7 | 50.9 ± 9.9 | 57.6 ± 8.8 | <.001 | PS > NS, TS | <.001 | PS > NS, TS |
| Hyperactivity | 49.7 ± 8.1 | 51.2 ± 9.3 | 58.5 ± 11.6 | <.001 | PS > NS, TS | <.001 | PS > NS, TS |
| Aggression | 47.5 ± 9.1 | 48.9 ± 11.1 | 53.2 ± 9.9 | <.05 | PS > NS | .09 | |
| Depression | 49.5 ± 9.2 | 51.5 ± 10.6 | 58.6 ± 10.7 | <.001 | PS > NS, TS | .001 | PS > NS, TS |
| Attention | 51.0 ± 7.2 | 50.6 ± 9.1 | 56.9 ± 8.1 | <.01 | PS > NS, TS | .01 | PS > NS, TS |
| BSID | |||||||
| Mental | 96.4 ± 12.8 | 89.7 ± 13.7 | 87.2 ± 13.9 | <.001 | NS > TS, PS | .67 | |
| Motor | 97.7 ± 14.7 | 95.1 ± 16.1 | 97.6 ± 10.7 | .53 | .69 | ||
Group values presented as unadjusted mean ± SD. BASC-2 scales are based on a normative mean = 50, standard deviation = 10, with higher scores indicating worse functioning. BSID scales are based on a normative mean = 100, standard deviation = 15, with higher scores indicating better functioning. Statistical significance (P values) and group differences are based on analysis of variance with Tukey HSD follow-up for unadjusted analyses, and analysis of covariance with Tukey HSD follow-up for analyses adjusted for child gender, race, and socioeconomic status. BASC-2, Behavior Assessment System for Children, second edition; BSID, Bayley Scales of Infant Development; BSI, Behavioral Symptoms Index; NS, nonsnorers; TS, transient snorers; PS, persistent snorers; HSD, honestly significant difference.
Table 3 summarizes the results of ordinal regression analyses. Child gender, birth weight, and preschool BMI z score were not significant predictors of transient or persistent snoring. In contrast, the presence and persistence of snoring was associated with African American race, lower socioeconomic status, and absent or shorter duration of breast milk feeding in unadjusted analyses and after adjustment for demographic variables. Higher prenatal cotinine levels were also associated with the presence and persistence of snoring in unadjusted analyses, but not after demographic adjustment. The full ordinal regression model that included all the predictors simultaneously found that transient and persistent snoring were associated with lower socioeconomic status and absent or shorter duration of breast milk feeding, as well as a trend toward association with higher preschool BMI z scores.
TABLE 3.
Results of Ordinal Regression Analyses Predicting Snoring Group
| Predictor | Unadjusted | Demographically Adjusted | Full Model | |||
|---|---|---|---|---|---|---|
| OR | P | OR | P | OR | P | |
| Gender | 1.05 (0.62, 1.78) | .85 | 1.21 (0.70, 2.12) | .49 | 0.93 (0.48, 1.81) | .83 |
| Race | 4.36 (2.38, 7.97) | <.001 | 2.20 (1.03, 4.71) | <.05 | 1.60 (0.61, 4.16) | .34 |
| Socioeconomic status | 0.44 (0.31, 0.61) | <.001 | 0.56 (0.37, 0.83) | <.01 | 0.52 (0.31, 0.89) | <.05 |
| Birth weight | 1.00 (1.00, 1.00) | .34 | 1.00 (1.00, 1.00) | .87 | 1.00 (1.00, 1.00) | .84 |
| Duration of breastfeeding | 0.70 (0.61, 0.81) | <.001 | 0.77 (0.67, 0.89) | <.001 | 0.73 (0.61, 0.87) | <.001 |
| Prenatal (maternal) cotinine | 1.01 (1.00, 1.02) | <.05 | 1.01 (1.00, 1.01) | .27 | 1.00 (0.99, 1.01) | .49 |
| Preschool cotinine | 1.26 (1.06, 1.50) | <.01 | 0.96 (0.79, 1.17) | .68 | 0.96 (0.76, 1.21) | .74 |
| Preschool BMI z score | 1.13 (0.87, 1.47) | .35 | 1.18 (0.90, 1.54) | .23 | 1.35 (0.96, 1.90) | .08 |
Parenthetical values reflect 95% confidence intervals. Unadjusted odds ratios and their associated significance (P) values reflect bivariate relationships between each predictor and group membership. Demographically adjusted odds ratios and P values reflect those relationships after covarying for gender, race, and socioeconomic status (unless the predictor is 1 of these 3 demographic factors, in which case the remaining 2 covariates were entered). Full-model odds ratios and P values reflect the predictor group associations after adjusting for all other variables in the table.
We then conducted a secondary analysis that examined whether race modified the cotinine effect in the ordinal regressions because there is evidence of racial differences in cotinine levels for individuals with comparable ETS exposure.36,37 The interaction term between race and cotinine was statistically significant for the unadjusted (P < .01) and demographically adjusted (P < .05) regression analyses, indicating a stronger relationship between higher cotinine levels and transient/persistent snoring behaviors for non-African Americans (unadjusted odds ratio [OR] = 2.50, demographically adjusted OR = 2.06) compared with African Americans (unadjusted OR = 0.93, demographically adjusted OR = 0.83). However, this interaction was not statistically significant (P = .19) when included in our full model.
Finally, exploratory analyses probed the regression coefficients associated with (1) other second-order interactions and (2) quadratic terms for continuous predictors. None of these reached a significance threshold of 0.01, which was considered liberal given the large number of exploratory analyses.
Discussion
Persistent Snoring and Daytime Behavioral and Cognitive Functioning
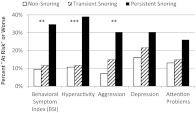
As predicted, children who were persistent snorers had significantly higher behavior problem scores at age 3 than those who never snored or who were transient snorers. To our knowledge, this was the first study to examine the relationship between the persistence of snoring and behavioral functioning in preschool-aged children.13,15 The results were consistent with reports on older children10,16–18 and remained significant after controlling for child gender, race, and socioeconomic status. As illustrated in Fig 1, “at risk” or worse overall behaviors (Behavioral Symptoms Index T ≥6032) were reported in 35% of persistent snorers, compared with 10% of nonsnorers and 12% of transient snorers. Preschool behavior and emotional problems of this magnitude were once dismissed as trivial, but are now recognized as significant sources of functional impairment at the population level.38–43
FIGURE 1.
Association between snoring group and behavioral and emotional functioning in the “at risk” range or worse (BASC-2 T score > 60). χ2 results: *P < .05, **P < .01, ***P = .001. BASC-2, Behavior Assessment System for Children, second edition.
In older children, SDB is most closely linked to their ability to regulate attention, emotions, and behaviors, and less strongly linked to cognitive/intellectual development.2 Our findings suggest that this also may be true for 2- to 3-year-olds. Although snoring was associated with cognitive development in unadjusted analyses, that association was no longer statistically significant after controlling for potential demographic confounds. No other study has examined the impact of persistent snoring in 2- to 3-year-old children; 1 cross-sectional study found associations between infants’ cognitive development and snore-related arousals but did not control for demographics.14 Both that study and the current work found no association between SDB and early motor development.
The findings from this study suggest that SDB in young children is associated with behavioral problems, potentially because of the cumulative effects of SDB on early childhood learning and on the development of neurologic systems that underlie attention and behavior regulation.2,3 Definitive causal conclusions cannot be drawn from these correlational findings. However, findings are consistent with animal and observational human studies that suggest that SDB-mediated sleep disruption and intermittent hypoxia can result in elevated oxidative stress, systemic inflammation, and changes in neural and neurobehavioral functioning.2 Behavioral improvements have been reported after surgical treatment of childhood SDB,2,9 but results are pending on a randomized trial to test the effect of such surgery on cognitive and behavioral functioning.44
Predictors of Persistent Snoring in 2- to 3-Year-Old Children
As has been reported in cross-sectional investigations,13,22–24,26 African American race and low socioeconomic status were predictors of transient and persistent snoring in 2- to 3-year-old children. Possible mechanisms include differences in craniofacial features, reduced access to health care, differences in health-related behaviors, and exposure to environmental toxicants.26,27,45 In our sample, low socioeconomic status was a more robust risk factor for transient and persistent snoring than was race.
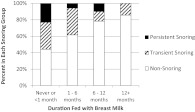
We found that children who were fed breast milk, especially across longer periods, were at markedly lower risk for persistent snoring, even after controlling for potential confounding variables. Whereas none of the children who were fed breast milk for >12 months developed persistent snoring, nearly one-fourth of those who were fed breast milk never or <1 month became persistent snorers (Fig 2). Two epidemiologic studies that coded breastfeeding dichotomously reported similar findings,13,25 and a study of habitually snoring children found that longer duration of breastfeeding between 2 and 5 months of age was associated with greater protection from obstructive sleep apnea.28 Authors of the latter study speculated that the act of breastfeeding promoted the development of a healthy upper airway structure and that breast milk provided immunologic protection against infections that promote SDB. Our data confirm that breast milk feeding may protect against persistent snoring during the early preschool years, and further suggest that extending breastfeeding beyond 5 months may result in incremental protection.
FIGURE 2.
Association between breast feeding duration and the presence and persistence of snoring at 2 to 3 years of age.
Hypotheses regarding tobacco smoke exposure were partially supported. In primary analyses, snoring was not associated with prenatal cotinine levels, and the bivariate association between snoring and preschool cotinine levels became nonsignificant after controlling for demographics. However, secondary analyses suggested more nuanced findings, in which higher preschool cotinine levels were associated with transient and persistent snoring among non-African American children (94% of whom were white), but not African American children. Of note, previous cross-sectional studies that have found associations between ETS exposure and childhood SDB have included few children of African descent,13,21,22,27 whereas no such associations were found in a sample that included a substantial number of African American children.24 Americans of European and African descent differ in nicotine metabolism and in the additives present in commonly smoked cigarettes.36,37 This could impact the putative mechanisms by which ETS exposure results in childhood SDB (smoke as an upper airway irritant, effect of nicotine and its withdrawal on neurologic control of sleep and respiration45). Although our race-specific findings warrant replication, present findings with use of an objective biomarker of nicotine exposure46 are consistent with previous findings based on less rigorous ETS measures; ongoing ETS exposure in at least a subset of preschool-aged children appears associated with transient and persistent snoring.
Birth weight, child gender, and age- and gender-corrected BMI were not consistently associated with transient or persistent snoring. Although children with low birth weight (<2500 g) have been shown to be at risk for later SDB,21,26 <5% of our sample fell in that range, and the overall sample was similar to Paavonen and colleagues’ normal birth weight (control) group.21 Previous findings on the associations of male gender and obesity with SDB have been mixed in children <6 years of age,13,25,27 with stronger findings in older children22,25 and adults.47 Although we cannot rule out an effect of extreme obesity, present data suggest little systematic associations of SDB with body weight or gender in the large majority of 2- to 3-year-old children.
Study Limitations
Our reliance on parent report for several measures is a limitation of this study. It might be useful to obtain an objective measure of SDB, although conventional polysomnographic indexes have tended to be weaker predictors of morbidity in older children than parent-reported loud snoring.2 Reassuringly, the observed association between persistent snoring and behavior problems seems unlikely to reflect reporter bias, which might have been plausible if behavior problems at age 3 had been associated with snoring at age 3 but not age 2; instead, the behavioral indexes of children who snored only at age 3 were nearly identical to those who never snored or snored only at age 2 (analyses not shown). There is a risk of statistical overcorrection when adjusting for parent education and income; SDB is partially heritable,48 and it is possible that a parent’s own SDB might influence his or her educational attainment or income. We did not assess parental SDB, so adjusted findings may have been biased toward nonsignificance. Finally, the demographic composition of our sample reflected the recruitment from metropolitan Cincinnati, Ohio, and sample attrition.15 Consequently, the generalizability of results to other populations cannot be guaranteed.
Conclusions
Our findings, which build upon previous work, highlight the importance of routine screening for snoring.1,49–52 It is important to ask specifically about snoring, because parents’ responses to more general sleep questions may not reflect this hallmark symptom of SDB.15 Screening is particularly important for children from lower socioeconomic backgrounds who are at higher risk for persistent SDB and its associated morbidity. It is also essential to document whether snoring persists. Owens and colleagues51 have developed a screening tool that has proven utility in the busy primary care setting. Failure to treat children with SBD is associated with an increased risk of behavioral morbidity, and previous research suggests that behavioral and medical morbidity may reverse with adequate SDB treatment.1,2,9 Finally, our findings support recommendations of the American Academy of Pediatrics regarding the prevention of tobacco smoke exposure53 and encouragement and facilitation of breastfeeding of infants.54
Glossary
- ETS
environmental tobacco smoke
- OR
odds ratio
- SDB
sleep-disordered breathing
- zBMI
age- and gender-adjusted BMI
Footnotes
This manuscript has not been published elsewhere, and all authors approve of its submission for review. Each author made a substantive intellectual contribution to the study. Dr Beebe participated in early conceptualization and design of sleep measurement methodology, conducted preliminary analyses, and collaborated with coauthors on final analyses and data interpretation; he took the lead role with drafting and revising the manuscript. Dr Rausch provided critical contributions to formulating the data analytic plan, conducted final data analyses, assisted with data interpretation, and reviewed and revised the manuscript for intellectual content. Dr Byars also participated in early conceptualization and design of sleep measurement methodology and collaborated with coauthors in interpretation of study findings and drafting and critically revising the manuscript. Dr Yolton was a primary investigator in the research laboratory that secured funding for this project. She had primary responsibility for the study design and oversight of its execution and participated in the conceptualization and design of the sleep measurement tools. Dr Yolton also played a significant role with respect to data collection and interpretation as well as critically reviewing and revising the manuscript. Dr Lanphear was the principal investigator who secured funding for this project; he also shared responsibility for the study design and oversight of its execution, data collection and interpretation, and critical review and revision of the manuscript.
None of the authors have any conflict of interest to declare with respect to this manuscript.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: This work was partially supported by grants from the National Institute of Environmental Health Sciences (R01 ES015517-01A1, P01 ES11261). Funded by the National Institutes of Health (NIH).
References
- 1.Section on Pediatric Pulmonology, Subcommittee on Obstructive Sleep Apnea Syndrome. American Academy of Pediatrics . Clinical practice guideline: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2002;109(4):704–712 [DOI] [PubMed] [Google Scholar]
- 2.Beebe DW. Neurobehavioral morbidity associated with disordered breathing during sleep in children: a comprehensive review. Sleep. 2006;29(9):1115–1134 [DOI] [PubMed] [Google Scholar]
- 3.Beebe DW. Cognitive, behavioral, and functional consequences of inadequate sleep in children and adolescents. Pediatr Clin North Am. 2011;58(3):649–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourke RS, Anderson V, Yang JS, et al. Neurobehavioral function is impaired in children with all severities of sleep disordered breathing. Sleep Med. 2011;12(3):222–229 [DOI] [PubMed] [Google Scholar]
- 5.Scullin MH, Ornelas C, Montgomery-Downs HE. Risk for sleep-disordered breathing and home and classroom behavior in Hispanic preschoolers. Behav Sleep Med. 2011;9(3):194–207 [DOI] [PubMed] [Google Scholar]
- 6.Blunden S, Lushington K, Kennedy D, Martin J, Dawson D. Behavior and neurocognitive performance in children aged 5-10 years who snore compared to controls. J Clin Exp Neuropsychol. 2000;22(5):554–568 [DOI] [PubMed] [Google Scholar]
- 7.O’Brien LM, Mervis CB, Holbrook CR, et al. Neurobehavioral implications of habitual snoring in children. Pediatrics. 2004;114(1):44–49 [DOI] [PubMed] [Google Scholar]
- 8.Aronen ET, Liukkonen K, Simola P, et al. Mood is associated with snoring in preschool-aged children. J Dev Behav Pediatr. 2009;30(2):107–114 [DOI] [PubMed] [Google Scholar]
- 9.Garetz SL. Behavior, cognition, and quality of life after adenotonsillectomy for pediatric sleep-disordered breathing: summary of the literature. Otolaryngol Head Neck Surg. 2008;138(suppl 1):S19–S26 [DOI] [PubMed] [Google Scholar]
- 10.Urschitz MS, Eitner S, Guenther A, et al. Habitual snoring, intermittent hypoxia, and impaired behavior in primary school children. Pediatrics. 2004;114(4):1041–1048 [DOI] [PubMed] [Google Scholar]
- 11.Chervin RD, Ruzicka DL, Archbold KH, Dillon JE. Snoring predicts hyperactivity four years later. Sleep. 2005;28(7):885–890 [DOI] [PubMed] [Google Scholar]
- 12.Gozal D, Pope DW. Long-term impact of snoring during early childhood on academic performance in middle school. Sleep. 2000;23:A29 [Google Scholar]
- 13.Bonuck KA, Chervin RD, Cole TJ, et al. Prevalence and persistence of sleep disordered breathing symptoms in young children: a 6-year population-based cohort study. Sleep. 2011;34(7):875–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montgomery-Downs HE, Gozal D. Snore-associated sleep fragmentation in infancy: mental development effects and contribution of secondhand cigarette smoke exposure. Pediatrics. 2006;117(3). Available at: www.pediatrics.org/cgi/content/full/117/3/e496 [DOI] [PubMed] [Google Scholar]
- 15.Byars KC, Yolton K, Rausch J, Lanphear B, Beebe DW. Prevalence, patterns, and persistence of sleep problems in the first 3 years of life. Pediatrics. 2012;129(2). Available at: www.pediatrics.org/cgi/content/full/129/2/e276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ali NJ, Stradling JR. Epidemiology and natural history of snoring and sleep-disordered breathing in children. In: Loughlin GM, Carroll JL, Marcus CL, eds. Sleep and Breathing in Children: A Developmental Approach. New York, NY: Marcel Dekker; 2000:555–574 [Google Scholar]
- 17.Ali NJ, Pitson DJ, Stradling JR. Natural history of snoring and related behaviour problems between the ages of 4 and 7 years. Arch Dis Child. 1994;71(1):74–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ali NJ, Pitson DJ, Stradling JR. Snoring, sleep disturbance, and behaviour in 4-5 year olds. Arch Dis Child. 1993;68(3):360–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Statham MM, Myer CM, III. Complications of adenotonsillectomy. Curr Opin Otolaryngol Head Neck Surg. 2010;18(6):539–543 [DOI] [PubMed] [Google Scholar]
- 20.Leong AC, Davis JP. Morbidity after adenotonsillectomy for paediatric obstructive sleep apnoea syndrome: waking up to a pragmatic approach. J Laryngol Otol. 2007;121(9):809–817 [DOI] [PubMed] [Google Scholar]
- 21.Paavonen EJ, Strang-Karlsson S, Räikkönen K, et al. Very low birth weight increases risk for sleep-disordered breathing in young adulthood: the Helsinki Study of Very Low Birth Weight Adults. Pediatrics. 2007;120(4):778–784 [DOI] [PubMed] [Google Scholar]
- 22.Urschitz MS, Guenther A, Eitner S, et al. Risk factors and natural history of habitual snoring. Chest. 2004;126(3):790–800 [DOI] [PubMed] [Google Scholar]
- 23.Montgomery-Downs HE, Gozal D. Sleep habits and risk factors for sleep-disordered breathing in infants and young toddlers in Louisville, Kentucky. Sleep Med. 2006;7(3):211–219 [DOI] [PubMed] [Google Scholar]
- 24.Kalra M, Lemasters G, Bernstein D, et al. Atopy as a risk factor for habitual snoring at age 1 year. Chest. 2006;129(4):942–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li AM, Au CT, So HK, Lau J, Ng PC, Wing YK. Prevalence and risk factors of habitual snoring in primary school children. Chest. 2010;138(3):519–527 [DOI] [PubMed] [Google Scholar]
- 26.Rosen CL, Larkin EK, Kirchner HL, et al. Prevalence and risk factors for sleep-disordered breathing in 8- to 11-year-old children: association with race and prematurity. J Pediatr. 2003;142(4):383–389 [DOI] [PubMed] [Google Scholar]
- 27.Kuehni CE, Strippoli MP, Chauliac ES, Silverman M. Snoring in preschool children: prevalence, severity and risk factors. Eur Respir J. 2008;31(2):326–333 [DOI] [PubMed] [Google Scholar]
- 28.Montgomery-Downs HE, Crabtree VM, Sans Capdevila O, Gozal D. Infant-feeding methods and childhood sleep-disordered breathing. Pediatrics. 2007;120(5):1030–1035 [DOI] [PubMed] [Google Scholar]
- 29.Geraghty SR, Khoury JC, Morrow AL, Lanphear BP. Reporting individual test results of environmental chemicals in breastmilk: potential for premature weaning. Breastfeed Med. 2008;3(4):207–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Owens JA, Spirito A, McGuinn M. The Children’s Sleep Habits Questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23(8):1043–1051 [PubMed] [Google Scholar]
- 31.Goodlin-Jones BL, Sitnick SL, Tang K, Liu J, Anders TF. The Children’s Sleep Habits Questionnaire in toddlers and preschool children. J Dev Behav Pediatr. 2008;29(2):82–88 [DOI] [PubMed] [Google Scholar]
- 32.Reynolds CR, Kamphaus RW. BASC–Behavioral Assessment System for Children Manual. Circle Pines, MN: American Guidance Service; 1992 [Google Scholar]
- 33.Bayley N. Bayley Scales of Infant Development Manual. 2nd ed. San Antonio, TX: Psychological Corporation; 1993 [Google Scholar]
- 34.Sattler JM. Assessment of Children: Cognitive Applications. 4th ed. San Diego, CA: Jerome M. Sattler; 2001 [Google Scholar]
- 35.Bernert JT, Jr, Turner WE, Pirkle JL, et al. Development and validation of sensitive method for determination of serum cotinine in smokers and nonsmokers by liquid chromatography/atmospheric pressure ionization tandem mass spectrometry. Clin Chem. 1997;43(12):2281–2291 [PubMed] [Google Scholar]
- 36.Caraballo RS, Giovino GA, Pechacek TF, et al. Racial and ethnic differences in serum cotinine levels of cigarette smokers: Third National Health and Nutrition Examination Survey, 1988-1991. JAMA. 1998;280(2):135–139 [DOI] [PubMed] [Google Scholar]
- 37.Wilson SE, Kahn RS, Khoury J, Lanphear BP. Racial differences in exposure to environmental tobacco smoke among children. Environ Health Perspect. 2005;113(3):362–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Egger HL, Angold A. Common emotional and behavioral disorders in preschool children: presentation, nosology, and epidemiology. J Child Psychol Psychiatry. 2006;47(3-4):313–337 [DOI] [PubMed] [Google Scholar]
- 39.Scheeringa MS, Haslett N. The reliability and criterion validity of the Diagnostic Infant and Preschool Assessment: a new diagnostic instrument for young children. Child Psychiatry Hum Dev. 2010;41(3):299–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keenan K, Boeldt D, Chen D, et al. Predictive validity of DSM-IV oppositional defiant and conduct disorders in clinically referred preschoolers. J Child Psychol Psychiatry. 2011;52(1):47–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keenan K, Wakschlag LS, Danis B, et al. Further evidence of the reliability and validity of DSM-IV ODD and CD in preschool children. J Am Acad Child Adolesc Psychiatry. 2007;46(4):457–468 [DOI] [PubMed] [Google Scholar]
- 42.Stalets MM, Luby JL. Preschool depression. Child Adolesc Psychiatr Clin N Am. 2006;15(4):899–917, viii–ix [DOI] [PubMed] [Google Scholar]
- 43.Lee SS, Lahey BB, Owens EB, Hinshaw SP. Few preschool boys and girls with ADHD are well-adjusted during adolescence. J Abnorm Child Psychol. 2008;36(3):373–383 [DOI] [PubMed] [Google Scholar]
- 44.Redline S, Amin R, Beebe D, et al. The Childhood Adenotonsillectomy Trial (CHAT): rationale, design, and challenges of a randomized controlled trial evaluating a standard surgical procedure in a pediatric population. Sleep. 2011;34(11):1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yolton K, Xu Y, Khoury J, et al. Associations between secondhand smoke exposure and sleep patterns in children. Pediatrics. 2010;125(2). Available at: www.pediatrics.org/cgi/content/full/125/2/e261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benowitz NL. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol Rev. 1996;18(2):188–204 [DOI] [PubMed] [Google Scholar]
- 47.Yaggi HK, Strohl KP. Adult obstructive sleep apnea/hypopnea syndrome: definitions, risk factors, and pathogenesis. Clin Chest Med. 2010;31(2):179–186 [DOI] [PubMed] [Google Scholar]
- 48.Varvarigou V, Dahabreh IJ, Malhotra A, Kales SN. A review of genetic association studies of obstructive sleep apnea: field synopsis and meta-analysis. Sleep. 2011;34(11):1461–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meltzer LJ, Johnson C, Crosette J, Ramos M, Mindell JA. Prevalence of diagnosed sleep disorders in pediatric primary care practices. Pediatrics. 2010;125(6). Available at: www.pediatrics.org/cgi/content/full/125/6/e1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Owens JA. The practice of pediatric sleep medicine: results of a community survey. Pediatrics. 2001;108(3). Available at: www.pediatrics.org/cgi/content/full/108/3/E51. [DOI] [PubMed] [Google Scholar]
- 51.Owens JA, Dalzell V. Use of the ‘BEARS’ sleep screening tool in a pediatric residents’ continuity clinic: a pilot study. Sleep Med. 2005;6(1):63–69 [DOI] [PubMed] [Google Scholar]
- 52.Meissner HH, Riemer A, Santiago SM, Stein M, Goldman MD, Williams AJ. Failure of physician documentation of sleep complaints in hospitalized patients. West J Med. 1998;169(3):146–149 [PMC free article] [PubMed] [Google Scholar]
- 53.Best, Committee on Environmental Health. Committee on Native American Child Health. Committee on Adolescence . From the American Academy of Pediatrics: Technical report—Secondhand and prenatal tobacco smoke exposure. Pediatrics. 2009;124(5). Available at: www.pediatrics.org/cgi/content/full/124/5/e1017 [DOI] [PubMed] [Google Scholar]
- 54.Gartner LM, Morton J, Lawrence RA, et al. American Academy of Pediatrics Section on Breastfeeding . Breastfeeding and the use of human milk. Pediatrics. 2005;115(2):496–506 [DOI] [PubMed] [Google Scholar]