Abstract
Protein kinase B (PKB, or Akt) plays a role in cell metabolism, growth, proliferation, and survival. Its activation is controlled by a multi-step process that involves phosphoinositide-3-kinase (PI3K).
Identification of the phosphoinositide-3-kinase–protein kinase B/Akt (PI3K-PKB/Akt) pathway and activating receptor tyrosine kinases (RTKs) began in earnest in the early 1980s through vigorous attempts to characterize insulin receptor signaling (for review, see Alessi 2001; Brazil and Hemmings 2001). These humble beginnings led to the identification of the components and mechanism of insulin receptor signaling via insulin receptor substrate (IRS) proteins to PI3K and consequent PKB/Akt-mediated activation by 3-phosphoinositide-dependent protein kinase 1 (PDK1). With the discovery of the potent contribution of PI3K and PKB/Akt activation to tumorigenesis, intense research into the regulation of this pathway led to the discovery of the negative regulators, the protein phosphatase 2 (PP2A), phosphatase and tensin homolog (PTEN), and the PH-domain leucine-rich-repeat-containing protein phosphatases (PHLPP1/2). More recently, the elusive PKB/Akt hydrophobic motif kinases—i.e., the mammalian target of rapamycin (mTOR), when associated with the mTOR complex 2 (mTORC2), and the DNA-dependent protein kinase (DNA-PK)—were identified, as was the ability of Ras to affect the PI3K-PKB/Akt pathway via PI3K, completing our current model of the PI3K-PKB/Akt pathway.
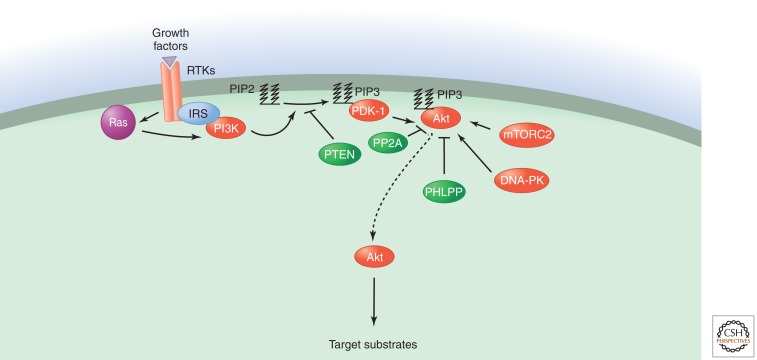
The PI3K-PKB/Akt pathway is highly conserved, and its activation is tightly controlled via a multistep process (as shown in Fig. 1) Activated receptors directly stimulate class 1A PI3Ks bound via their regulatory subunit or adapter molecules such as the insulin receptor substrate (IRS) proteins. This triggers activation of PI3K and conversion by its catalytic domain of phosphatidylinositol (3,4)-bisphosphate (PIP2) lipids to phosphatidylinositol (3,4,5)-trisphosphate (PIP3). PKB/Akt binds to PIP3 at the plasma membrane, allowing PDK1 to access and phosphorylate T308 in the “activation loop,” leading to partial PKB/Akt activation (Alessi et al. 1997). This PKB/Akt modification is sufficient to activate mTORC1 by directly phosphorylating and inactivating proline-rich Akt substrate of 40 kDa (PRAS40) and tuberous sclerosis protein 2 (TSC2) (Vander Haar et al. 2007). mTORC1 substrates include the eukaryotic translation initiation factor 4E binding protein 1 (4EBP1), and ribosomal protein S6 kinase, 70 kDa, polypeptide 1 (S6K1), which, in turn, phosphorylates the ribosomal protein S6 (S6/RPS6), promoting protein synthesis and cellular proliferation.
Figure 1.
PKB/Akt activation downstream of RTKs via the P13K pathway.
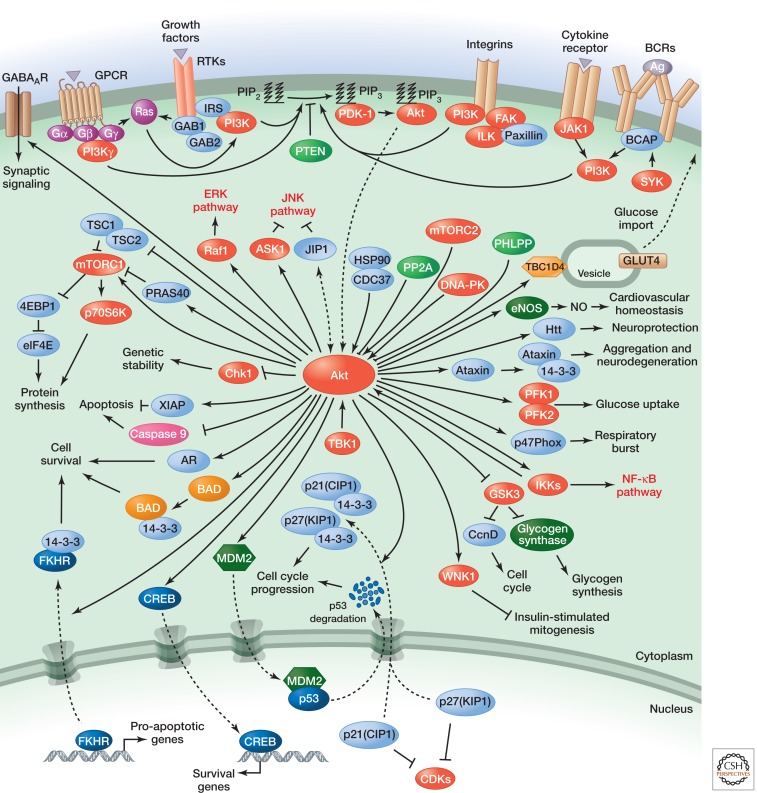
Phosphorylation of Akt at S473 in the carboxy-terminal hydrophobic motif, either by mTOR (Sarbassov et al. 2005) or by DNA-PK (Feng et al. 2004), stimulates full Akt activity. Full activation of Akt leads to additional substrate-specific phosphorylation events in both the cytoplasm and nucleus, including inhibitory phosphorylation of the pro-apoptotic FOXO proteins (Guertin et al. 2006). Fully active PKB/Akt mediates numerous cellular functions including angiogenesis, metabolism, growth, proliferation, survival, protein synthesis, transcription, and apoptosis (as shown in Fig. 2). Dephosphorylation of T308 by PP2A (Andjelković et al. 1996), and S473 by PHLPP1/2 (Brognard et al. 2007), and the conversion of PIP3 to PIP2 by PTEN (Stambolic et al. 1998) antagonize Akt signaling.
Figure 2.
Signalling events activating PKB/Akt and cellular functions regulated by PKB/Akt.
Acknowledgments
Figures adapted, with kind permission, from Cell Signaling Technology (http://www.cellsignal.com.)
Footnotes
Editors: Lewis Cantley, Tony Hunter, Richard Sever, and Jeremy Thorner
Additional Perspectives on Signal Transduction available at www.cshperspectives.org
REFERENCES
- Alessi DR 2001. Discovery of PDK1, one of the missing links in insulin signal transduction. Colworth Medal Lecture. Biochem Soc Trans 29: 1–14 [DOI] [PubMed] [Google Scholar]
- Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P 1997. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr Biol 7: 261–269 [DOI] [PubMed] [Google Scholar]
- Altomare DA, Testa JR 2005. Perturbations of the AKT signaling pathway in human cancer. Oncogene 24: 7455–7464 [DOI] [PubMed] [Google Scholar]
- Andjelković M, Jakubowicz T, Cron P, Ming XF, Han JW, Hemmings BA 1996. Activation and phosphorylation of a pleckstrin homology domain containing protein kinase (RAC-PK/PKB) promoted by serum and protein phosphatase inhibitors. Proc Natl Acad Sci 93: 5699–5704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozulic L, Hemmings BA 2009. PIKKing on PKB: Regulation of PKB activity by phosphorylation. Curr Opin Cell Biol 21: 256–261 [DOI] [PubMed] [Google Scholar]
- Brazil DP, Hemmings BA 2001. Ten years of protein kinase B signalling: A hard Akt to follow. Trends Biochem Sci 26: 657–664 [DOI] [PubMed] [Google Scholar]
- Brognard J, Sierecki E, Gao T, Newton AC 2007. PHLPP and a second isoform, PHLPP2, differentially attenuate the amplitude of Akt signaling by regulating distinct Akt isoforms. Mol Cell 25: 917–931 [DOI] [PubMed] [Google Scholar]
- Feng J, Park J, Cron P, Hess D, Hemmings BA 2004. Identification of a PKB/Akt hydrophobic motif Ser-473 kinase as DNA-dependent protein kinase. J Biol Chem 279: 41189–41196 [DOI] [PubMed] [Google Scholar]
- Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, Brown M, Fitzgerald KJ, Sabatini DM 2006. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCα, but not S6K1. Dev Cell 11: 859–871 [DOI] [PubMed] [Google Scholar]
- Manning BD, Cantley LC 2007. AKT/PKB signaling: Navigating downstream. Cell 129: 1261–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM 2005. Phosphorylation and regulation of Akt/PKB by the rictor–mTOR complex. Science 307: 1098–1101 [DOI] [PubMed] [Google Scholar]
- Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM, Siderovski DP, Mak TW 1998. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell 95: 29–39 [DOI] [PubMed] [Google Scholar]
- Vander Haar E, Lee SI, Bandhakavi S, Griffin TJ, Kim DH 2007. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol 9: 316–323 [DOI] [PubMed] [Google Scholar]