Abstract
Initially known for their role in the rhizosphere in stimulating the seed germination of parasitic weeds such as the Striga and Orobanche species, and later as host recognition signals for arbuscular mycorrhizal fungi, strigolactones (SLs) were recently rediscovered as a new class of plant hormones involved in the control of shoot branching in plants. Herein, we report the synthesis of new SL analogs and, to our knowledge, the first study of SL structure-activity relationships for their hormonal activity in garden pea (Pisum sativum). Comparisons with their action for the germination of broomrape (Phelipanche ramosa) are also presented. The pea rms1 SL-deficient mutant was used in a SL bioassay based on axillary bud length after direct SL application on the bud. This assay was compared with an assay where SLs were fed via the roots using hydroponics and with a molecular assay in which transcript levels of BRANCHED1, the pea homolog of the maize TEOSINTE BRANCHED1 gene were quantified in axillary buds only 6 h after application of SLs. We have demonstrated that the presence of a Michael acceptor and a methylbutenolide or dimethylbutenolide motif in the same molecule is essential. It was established that the more active analog 23 with a dimethylbutenolide as the D-ring could be used to control the plant architecture without strongly favoring the germination of P. ramosa seeds. Bold numerals refer to numbers of compounds.
Strigolactones (SLs) represent the most recent class of hormones identified in plants for their role in repressing shoot branching. Their existence as a novel branching inhibitor signal was suggested through grafting experiments with high-branching mutants in pea (Pisum sativum; Beveridge et al., 1997; Beveridge, 2000) and in Arabidopsis (Arabidopsis thaliana; Turnbull et al., 2002; Booker et al., 2004). Analysis of the mutated genes indicated that some of them encode Carotenoid Cleavage Dioxygenase (CCD) enzymes, suggesting that the branching inhibitor was carotenoid derived (Sorefan et al., 2003; Booker et al., 2004; Matusova et al., 2005). When mutated in CCD7 or CCD8 genes, the plants were shown to be deficient in SLs. Treatment of these mutants by exogenous application of SLs inhibited the growth of lateral buds and restored the wild-type phenotype. Other branching mutants (max2/rms4) suspected to be affected in the hormone signaling pathway rather than in hormone synthesis were found to produce normal or higher amounts of SLs, and exogenous SL treatment did not suppress lateral bud growth. In the light of these data, some authors (Gomez-Roldan et al., 2008; Umehara et al., 2008; Domagalska and Leyser, 2011) concluded that SLs, or closely related molecules, were indeed the long-sought plant hormones that suppress lateral branch formation.
SLs have already been known as signaling molecules in the rhizosphere in both parasitic and symbiotic interactions. They were first identified in 1966 (Cook et al., 1966, 1972) as a seed germination stimulant of the parasitic weeds Orobanche and Striga, major agricultural pests around the Mediterranean Sea and in sub-Saharan Africa, respectively, where they constitute the major cause of crop damage. More recently, SLs have been shown to play a key role in the establishment of one of the most prevalent symbioses between the vast majority of land plants and arbuscular mycorrhizal (AM) fungi. SLs produced by plant roots stimulate spore germination and hyphal proliferation of AM fungi (Akiyama et al., 2005; Besserer et al., 2006). The hypothesis is that SLs boost their metabolism prior to root colonization (Besserer et al., 2006). The AM symbiosis, which involves an ancient phylum of fungi, the Glomeromycota, arose very early in land plant evolution and is thought to have been instrumental in allowing successful plant colonization of the terrestrial environment (Simon et al., 1993). It has been proposed that the primary role of SLs was to attract these highly beneficial fungal symbionts, because of the parasitic plants having exploited this system much later to detect their host (Bouwmeester et al., 2007). SLs have also been shown to be synthesized by a nonvascular plant, the moss Physcomitrella patens, and to play a role in both chemical communication between plants and plant extension control (Proust et al., 2011). All these data suggest that SLs are very ancient molecules. Their role as interkingdom signaling molecules and between plants, together with their effect on shoot branching, constitute an intriguing plant signaling story.
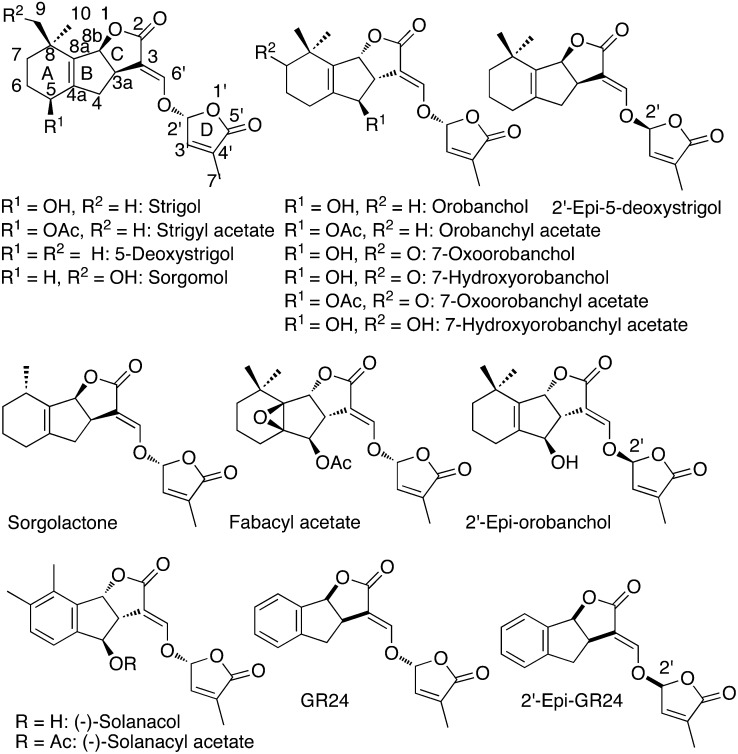
To date, at least 15 naturally occurring SLs have been identified in root exudates of monocotyledonous and dicotyledonous plant species (Xie and Yoneyama, 2010), and most of them have been structurally characterized, very often with difficulties. It is expected that many others will be identified later (Yoneyama et al., 2009; Zwanenburg et al., 2009). Predominant SLs differ in identity in different species, although relatives tend to have similar SLs. SLs present a common four-cycle skeleton (A, B, C, and D), with cycles A and B bearing various substituents and cycles C and D being lactone heterocycles connected by an ether enol bond (Fig. 1). The first analysis of the distribution of SLs in different species suggests that 5-deoxystrigol is generally shared by both monocots and dicots, while orobanchol and orobanchyl acetate, for which structural revisions were recently proposed (Ueno et al., 2011b; Vurro and Yoneyama, 2012), seem to be found in dicots but not in monocots. Solanacol (Xie et al., 2007; Chen et al., 2010), identified in tobacco (Nicotiana tabacum), is the only SL having an aromatic A-ring. The highly active synthetic analog of strigol, known as GR24 and commonly used in biological experiments, also has an aromatic A-ring (Fig. 1). All currently known natural SLs possess the same CD rings with a methyl at the C4′ position, suggesting the importance of this structure for bioactivity. The identification of SLs is difficult partly because they are produced by plants in extremely low quantities and are relatively unstable. The level of the endogenous SL 2′-epi-5-deoxystrigol in rice (Oryza sativa) roots is about 20 pg g−1 fresh weight (Umehara et al., 2008), but SL levels found in roots and in root exudates are strongly dependent on nutrient availability, notably phosphorus and nitrogen (Yoneyama et al., 2007, 2012). SLs are inherently unstable in water, particularly at pH > 7, as this nucleophilic agent cleaves the enol ether bond between the C- and D-rings (Akiyama and Hayashi, 2006) to furnish the hydroxymethylbutenolide 3 (Fig. 2) and the formyl tricyclic lactone 6 (Fig. 3). These compounds and GR24 analogs have also been found to be light sensitive (Johnson et al., 1981). Activities of SLs could be detected at concentrations as low as 10−13 m on AM fungi (Akiyama and Hayashi, 2006), 10−12 m on seeds of parasitic weeds (Xie and Yoneyama, 2010), and 10−8 m for GR24 on lateral buds (Gomez-Roldan et al., 2008).
Figure 1.
Natural SLs and analogs. Ac, Acetyl.
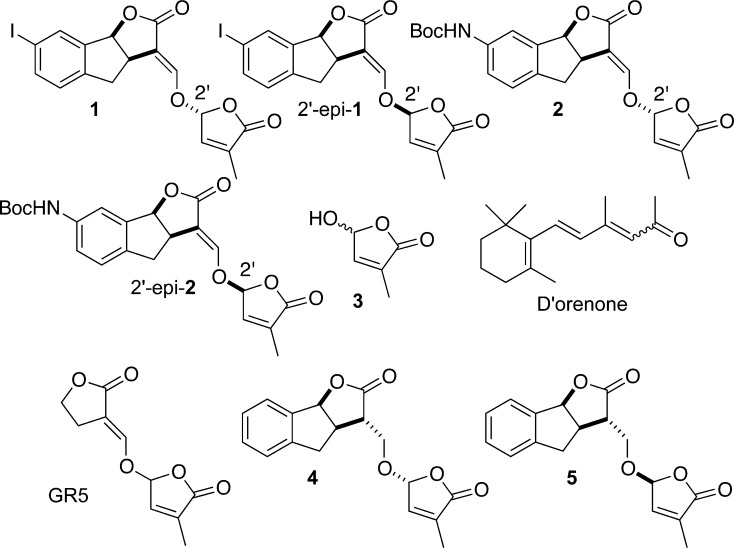
Figure 2.
Known SL analogs and d’orenone. Boc, t-Butoxycarbonyl.
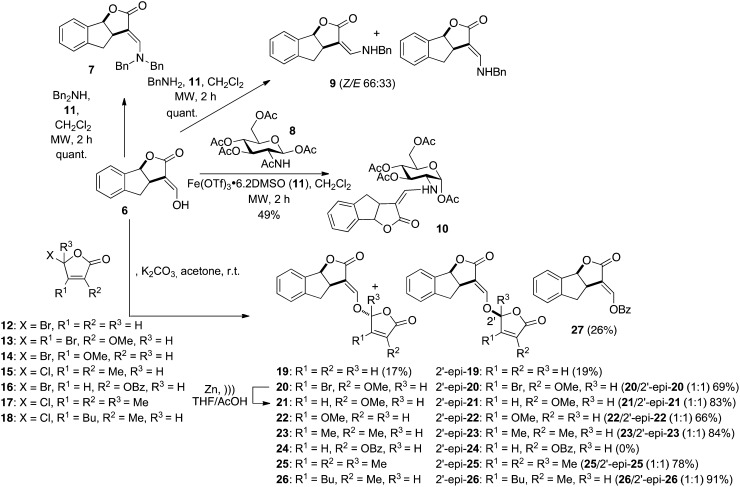
Figure 3.
Synthesis of D-modified GR24 analogs. Ac, Acetyl; Bn, benzyl; Bu, n-butyl; Bz, benzoyl; Me, methyl; MW, microwave irradiation; r.t., room temperature; Tf, trifluoromethanesulfonyl.
Most studies on the bioactivity of SLs investigated their action as a germination stimulant of parasitic seed weeds. The methyl substituent in the D-ring at C4′ is essential for bioactivity as a germination stimulant that underlines the importance of the D-ring (Zwanenburg et al., 2009). The presence of the 4-hydroxyl group seems to enhance the activity. It was recently reported that, generally, natural hydroxy-SLs are about 10- to 100-fold more active than their acetates on Orobanche minor and broomrape (Phelipanche ramosa) seed germination (Xie et al., 2008; Kim et al., 2010). The results are inverted for aromatic A-ring analogs of orobanchol for seeds of parasitic weeds (Malik et al., 2011). The C2′-(R) stereochemistry was also reported to be an important structural feature for potent germination stimulation activity for strigol, sorgolactone, orobanchol, orobanchyl acetate, and GR24 derivatives (Mangnus et al., 1992; Ueno et al., 2011a, 2011b). Recently, it was demonstrated that A-ring dimethyl substitution on GR24 lowers the germination activity (Malik et al., 2010). However, germination stimulation activity of SLs may be different from one parasitic plant species to another (Sato et al., 2005; Yoneyama et al., 2010; Ueno et al., 2011b). Structure-activity relationship (SAR) studies suggest that the CD ring moiety is the essential structure for stimulating seed germination (Zwanenburg et al., 2009). This CD structure unit was also proposed to be essential for AM fungi (Akiyama and Hayashi, 2006). Very recently, new data (Akiyama et al., 2010; Prandi et al., 2011) were made available concerning the hyphal branching activity. Structural requirements for this activity were reportedly very similar to those for germination stimulation of root parasites. However, the authors showed that the enol ether link between rings C and D can be replaced by an alkoxy or imino ether with little decrease in activity.
SL SAR studies are now required to define their role in repressing shoot branching. In this study, we used a branching bioassay developed with the pea SL-deficient mutant rms1 to analyze the hormonal activity of various natural SLs and synthetic analogs. This biological assay was completed by a molecular assay using the pea BRANCHED1 (PsBRC1) gene encoding a transcription factor of the plant-specific TCP family named from the first three identified members, the maize TEOSINTE BRANCHED1, CYCLOIDEA in Antirrhinum majus, and PCF-coding genes in rice. PsBRC1 was shown to be transcriptionally up-regulated by SLs in axillary buds of the SL-deficient rms1 mutant but not in the SL-response rms4 mutant, indicating that it is involved in the SL-signaling pathway to repress branching (Braun et al., 2012). This work represents, to our knowledge, the first SAR study of SLs for their hormonal activity in branching inhibition. SAR studies were performed in parallel for activity on the germination of P. ramosa. An SL analog with strong activity for branching inhibition in pea (Pisum sativum) together with low activity for broomrape seed germination is revealed.
RESULTS
Synthesis of SL Analogs
The SL SAR for the inhibition of bud outgrowth can be established as for seed germination of parasitic plants (Zwanenburg et al., 2009) by simplifying the SL skeleton. The relevant question is whether the bioactiphore is the same as that for seed germination of parasitic plants or for hyphal proliferation of AM fungi.
Several natural SLs were tested along with the well-known GR24, in which the A-ring was replaced by an aromatic ring. Several GR24 analogs (7, 9, 10, 27) lacking the D-ring were also synthesized (Fig. 3). Bold numerals refer to numbers of compounds. Reaction of tricycle 6 with benzylamine (dibenzylamine) furnished quantitatively under microwave irradiation the expected enamine 9 (7). The geometry of the enamine was determined by Nuclear Overhauser Enhancement Spectroscopy correlations observed between one benzylic methylene and H4 for 7 (only E geometry) and H6′ and H4 for the major Z isomer 9, respectively. Enamine 10 was prepared by the reaction of tricycle 6 with the commercially available GlcNAc acetate 8 in the presence of anhydrous ferric triflate under microwave irradiation (Stévenin et al., 2012). The exclusive formation of the Z enamine 10 could be rationalized by the possible presence of a hydrogen bond between the NH and the oxygen atom at C2 in this geometry. This geometry was largely studied as in the case of the protection of the amino group of amino sugars by the acylvinyl group (Gomez-Sanchez et al., 1984) or the primary amine-labile vinylogous amide type-protecting group. Variations of the D-ring substituents were performed using D-ring precursors 12 to 18 for the coupling with enol 6. In one case, the reaction with D-ring precursor 16 did not lead to the expected diastereoisomers 24/2′-epi-24 but to the transesterification product 27, even with the solid/liquid conditions (K2CO3/acetone) known to avoid this side reaction (Hoffmann et al., 1989).
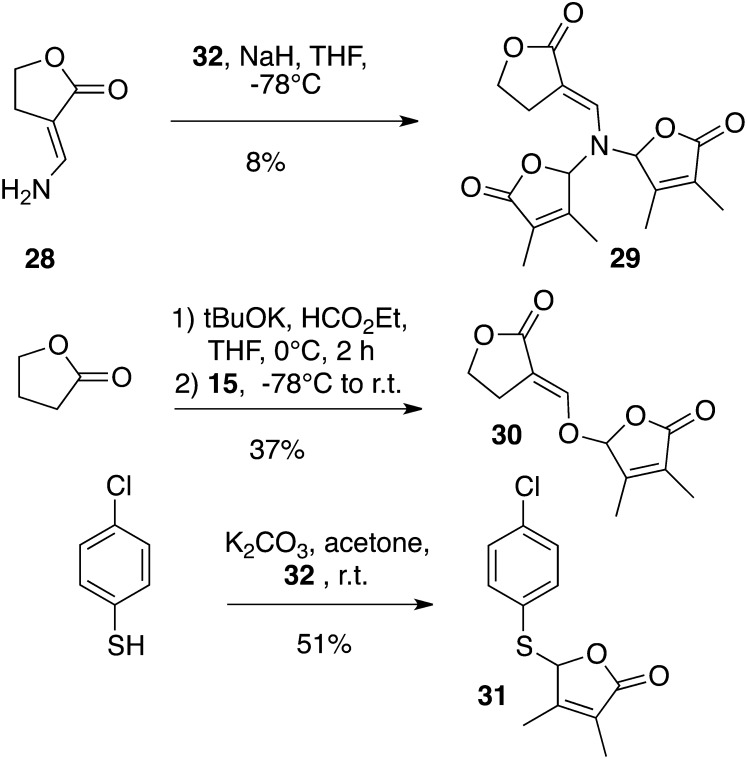
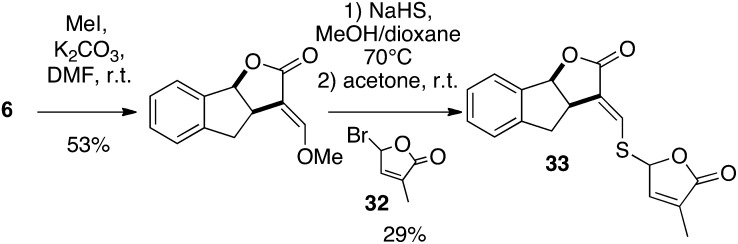
A racemic mixture of the AB-ring-truncated synthetic analog, GR5, was also prepared by a one-pot procedure involving the coupling of lactone with the suitable butenolide 32 (Macalpine et al., 1976) via an enol ether linkage (Fig. 4). The D analog of GR5 (30) was furnished from γ-butyrolactone, and the 3,4-dimethylbutenolide D-ring precursor 15 was easily obtained in bulk quantities (Canevet and Graff, 1978; Johnson et al., 1981). The influence of the replacement of the enol ether linkage by a thioenol ether and an enamine was also examined. Enamine 29 was obtained from the known 3-aminomethylenedihydrofuran-2-one 28 (Zanatta et al., 2003) by bis-alkylation with compound 15 in poor yield (8%). Preparations of 23/2′-epi-23 and SL mimic 31 were accomplished in the same way from enol 6 and commercially available 4-chlorobenzenethiol, respectively, in high (84% for 23/2′-epi-23; Fig. 3) to moderate (51% for 31; Fig. 4) yields. The bioisostere (33) of GR24 (Mangnus and Zwanenburg, 1992) was synthesized from enol 6 via treatment of the corresponding methoxymethylene derivative (Fig. 5) with sodium hydrosulfide in methanol (Just et al., 1976) to furnish the sodium thiolate directly alkylated with the 5-bromo-3-methyl-2(5H)-furanone (32). The analogs substituted at C2′ and C3′ GR24 analogs (25/2′-epi-25: R1 = R2 = R3 = Me) and (26/2′-epi-26: R1 = Bu, R2 = Me, R3 = H) were furnished by the coupling of enol 6 with the corresponding chloro D-ring precursor (17 or 18) with good yields (78% for 25/2′-epi-25, 91% for 26/2′-epi-26; Fig. 3).
Figure 4.
Synthesis of GR5 analogs and SL mimic 31. Et, Ethyl; r.t., room temperature; tBu, t-butyl.
Figure 5.
Synthesis of bioisostere 33 of GR24. DMF, Dimethylformamide; Me, methyl; r.t., room temperature.
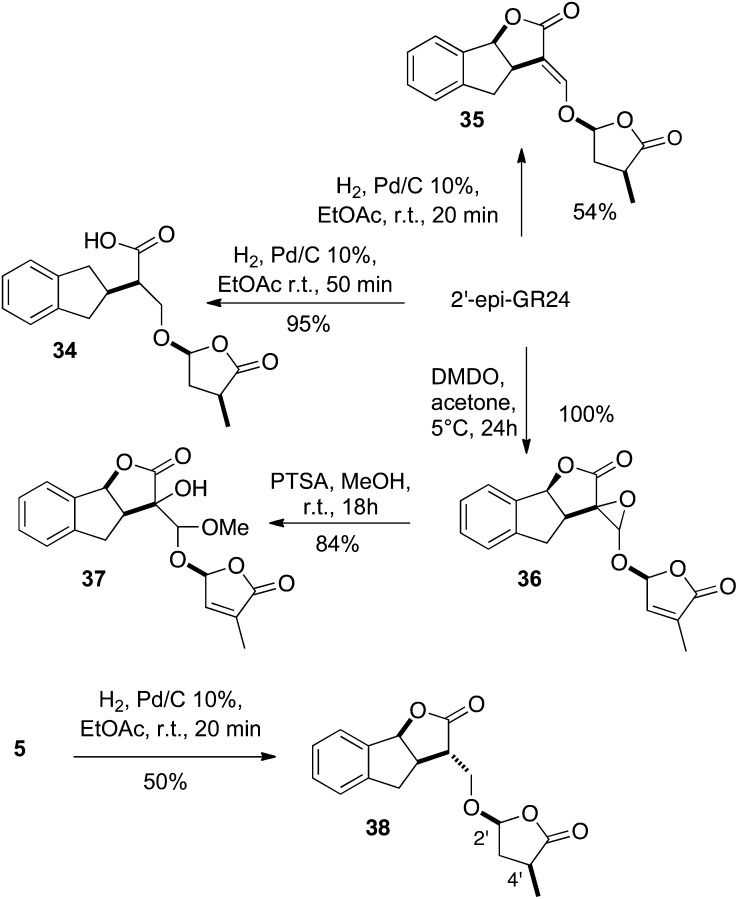
In order to examine the influence of the enol function at the C3C6′ position and that of the double bond of the D-ring on the biological activity, we synthesized derivatives 34 to 37 from 2′-epi-GR24 and derivative 38 from 5 (Fig. 6). Hydrogenation of 2′-epi-GR24 proceeded rapidly in ethyl acetate (EtOAc) in the presence of 10% palladium on carbon to give the completely reduced derivative 34 (two isomers), resulting from the concomitant hydrogenolysis of the benzylic carbon-oxygen bond. A time-controlled hydrogenation furnished the D-ring-reduced lactone 35 as a sole diastereoisomer in 54% yield. Similarly, hydrogenation of enone 5 led to bis-lactone 38 in 50% yield. The stereochemistry on the D-ring was proven by Nuclear Overhauser Enhancement Spectroscopy correlations observed between H4′ and H2′. Oxidation of 2′-epi-GR24 was performed regioselectively by the action of a diluted solution of dimethyl dioxirane to give a 7:3 mixture of epoxides 36 in high yield. Subsequent oxirane opening of 36 by methanol in an acidic medium led to alcohols 37 (7:3).
Figure 6.
Synthesis of reduced and oxidized GR24 analogs. DMDO, Dimethyl dioxirane; Me, methyl; PTSA, p-toluenesulfonic acid; r.t., room temperature.
Biological Activities of SLs and Analogs
A simple bioassay was developed using the high-branching SL-deficient rms1 mutant in garden pea to perform SAR studies on nine natural SLs and 33 analogs (structures are shown in Figs. 1–6) and to further clarify the structural requirements of this novel hormone in the control of shoot branching. Direct application of 10 µL of the solution to be tested on an axillary bud at a given node (generally node 3 or 4) was performed before its outgrowth, and the bud/branch length was measured 10 d later. The different compounds were first tested at a concentration of 1 µm and were considered inactive if no significant difference in the bud length was found compared with the mock control. For active compounds, lower concentrations were tested to analyze quantitative differences in bioactivity between molecules. Globally, when the treated bud was at node 3, its length was higher 10 d after treatment than when the treated bud was at node 4.
Apolar SLs Are More Active Than Hydroxy-SLs
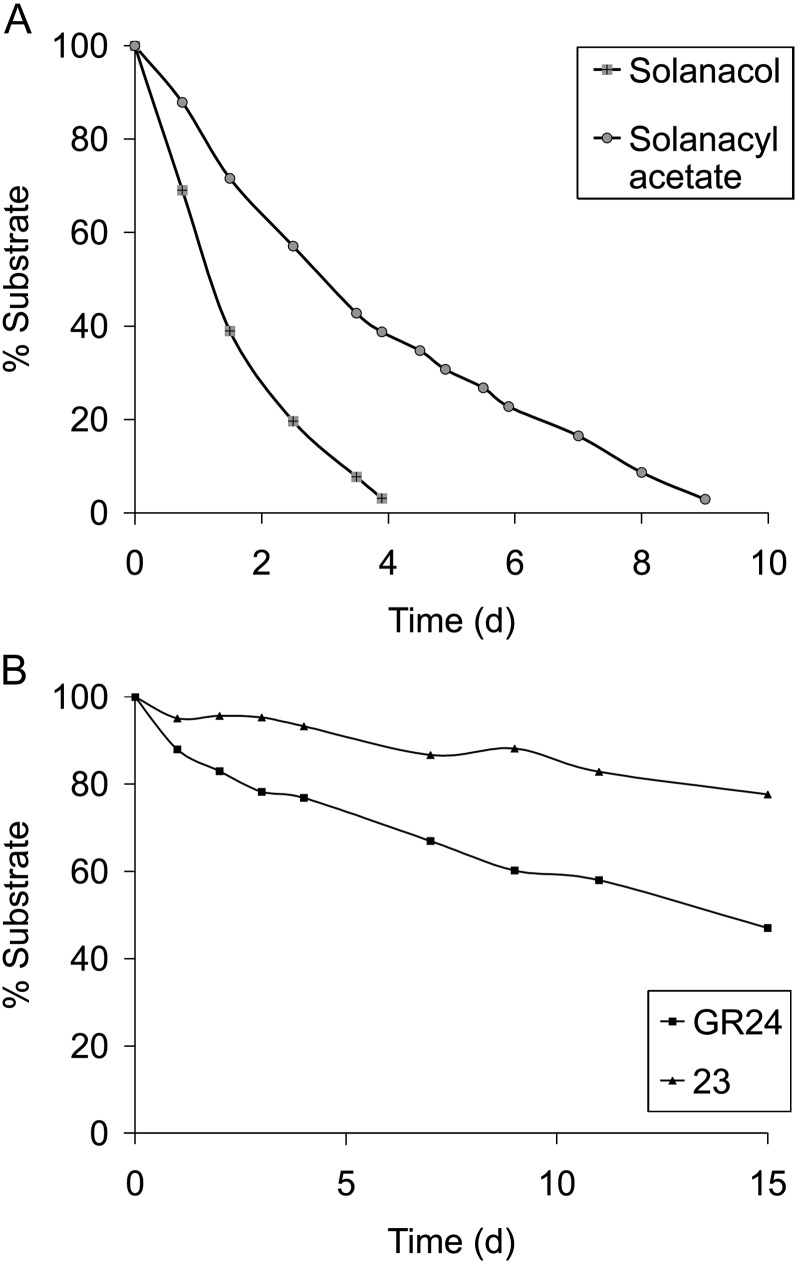
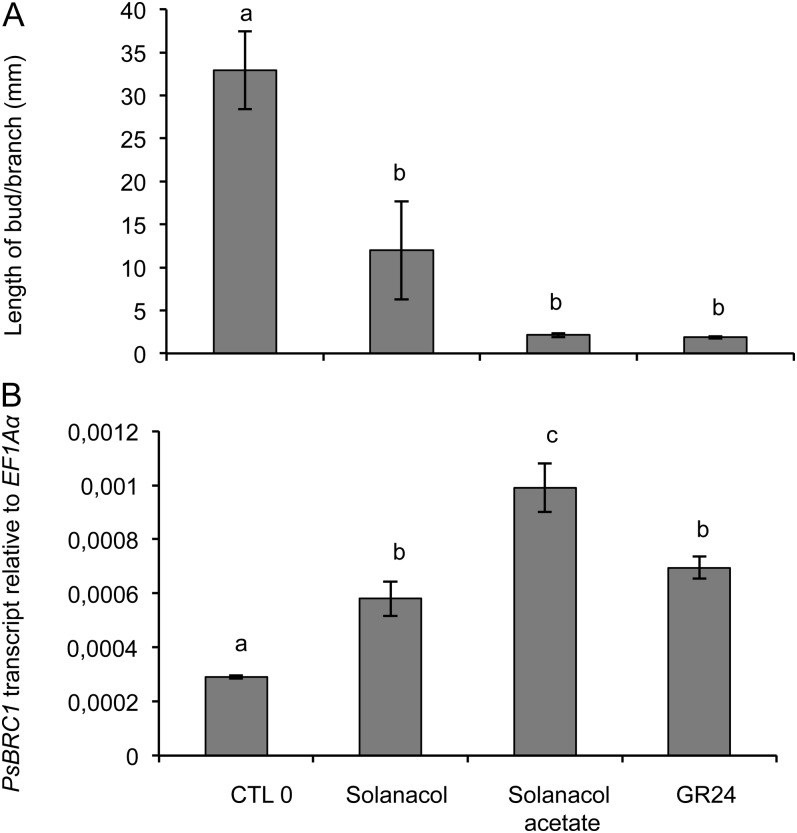
All tested natural SLs showed significant activities at a concentration of 1 µm (Table I). 5-Hydroxy-SL (strigol) and 4-hydroxy-SL (orobanchol; which was at the limit of significance in one experiment) showed less activity (Table I) than 5-acetate-SL (strigyl acetate) and 4-acetate-SL (orobanchyl acetate; e.g. at 500 nm, strigol was significantly less active than strigyl acetate [Kruskal-Wallis rank sum test, P = 0.00047]; Table I), as recently described for solanacol and solanacyl acetate (Chen et al., 2010). In all cases, acetate-SLs were always more active than their corresponding hydroxy-SLs, with the same dose-dependent activity for orobanchyl acetate, fabacyl acetate, and solanacyl acetate. 5-Deoxystrigol or sorgolactone without an oxygen function on the AB carbon bicycle presented high activity (Table I) better than the hydroxy-SLs (in Table I, compare 5-deoxystrigol and sorgolactone with strigol and orobanchol at 100 nm). In the case of fabacyl acetate, bud outgrowth inhibition activity was also found at the limit of significance at 10 nm (Table I). The best activity obtained with orobanchyl acetate and fabacyl acetate was in accordance with the fact that these SLs were identified in pea exudates (Gomez-Roldan et al., 2008; Xie et al., 2009). The activity differences between natural SLs observed in our bioassay may be attributed to the instability or low lipophilicity of the hydroxy-SLs, which may keep these molecules from reaching their putative receptors. We demonstrated that, in our conditions (ethanol/water), the acetate-SLs were more stable than the corresponding hydroxy-SLs (solanacol versus solanacyl acetate; Fig. 7A). D’orenone, mentioned as a putative intermediate in the SL biosynthesis pathway (Rani et al., 2008) until very recently (Alder et al., 2012), was also evaluated without success in bud outgrowth inhibition activity, even at 50 μm concentration (data not shown).
Table I. Bud outgrowth inhibition activity assay results for natural SLs and derivatives.
P shows comparison of the SL treatment with the control treatment (0 nm) using the Kruskal-Wallis rank sum test.
| Compound | Concentration | Length of Bud at Node 3 or 4 or 5 per Brancha | se | P |
|---|---|---|---|---|
| mm | ||||
| Strigol | 1 μm | 2.56b | 0.22 | 1.138e-06 |
| 500 nm | 12.49c | 2.22 | 0.04868 | |
| 500 nm | 8.47c | 2.81 | 0.8534 | |
| 100 nm | 12.33c | 2.52 | 0.09966 | |
| 100 nm | 13.99c | 3.39 | 0.6281 | |
| Strigyl acetate | 1 μm | 1.79c | 0.21 | 2.827e-05 |
| 500 nm | 1.82c | 0.21 | 0.0002111 | |
| 100 nm | 7.38c | 2.35 | 0.1297 | |
| 5-Deoxystrigol | 1 μm | 2.28b | 0.57 | 1.167e-07 |
| 100 nm | 3.99c | 1.44 | 0.01491 | |
| Orobanchol | 500 nm | 5.49c | 1.87 | 0.0005305 |
| 100 nm | 10.61c | 2.40 | 0.02416 | |
| Orobanchyl acetate | 500 nm | 1.71c | 0.16 | 0.001448 |
| 500 nm | 1.90c | 0.23 | 0.0003437 | |
| 100 nm | 2.92c | 0.50 | 0.0003486 | |
| Sorgolactone | 1 μm | 1.65b | 0.16 | 4.83e-08 |
| 100 nm | 2.32c | 0.36 | 0.0005833 | |
| 100 nm | 2.37d | 0.23 | 0.001486 | |
| Fabacyl acetate | 500 nm | 1.69c | 0.15 | 3.606e-05 |
| 100 nm | 2.52c | 0.28 | 0.05819 | |
| 100 nm | 1.62c | 0.14 | 0.002244 | |
| 10 nm | 2.08c | 0.21 | 0.08477 | |
| 1 nm | 2.41c | 0.81 | 0.9616 | |
| (±)-Solanacole | 1 μm | 6.87c | 1.74 | 0.0992 |
| 100 nm | 7.66c | 2.33 | 0.03308 | |
| (±)-2′-epi-Solanacole | 1 μm | 6.87c | 1.74 | 0.007101 |
| 100 nm | 14.53c | 2.96 | 0.7473 | |
| (–)-Solanacol | 1 μm | 5.34b | 1.20 | 1.64e-06 |
| 1 μm | 15.81b | 4.19 | 0.006184 | |
| 1 μm | 3.16c | 0.58 | 0.00733 | |
| (+)-Solanacol | 1 μm | 4.34b | 0.87 | 6.041e-06 |
| 1 μm | 9.58b | 3.16 | 0.000638 | |
| 1 μm | 4.68b | 1.44 | 0.0381 | |
| (±)-Solanacyl acetatee | 1 μm | 1.59c | 0.14 | 0.005552 |
| 1 μm | 1.59c | 0.14 | 2.654e-05 | |
| 100 nm | 3.87c | 0.89 | 0.01044 | |
| 100 nm | 3.13c | 0.57 | 0.03405 | |
| 10 nm | 6.26c | 1.66 | 0.1213 | |
| 10 nm | 2.40c | 0.50 | 0.002546 | |
| 1 nm | 12.13c | 1.49 | 0.1518 | |
| (+)-Solanacyl acetate (1 μm) | 1 μm | 2.70b | 0.39 | 3.19e-06 |
| 1 μm | 3.71c | 1.04 | 0.03391 | |
| (–)-Solanacyl acetate (1 μm) | 1 μm | 5.57b | 3.72 | 1.582e-05 |
| 1 μm | 1.90c | 0.14 | 0.00176 |
These data are means ± se (n = 24), obtained 10 d after treatment. bNode 3. cNode 4. dNode 5. eTested previously (Chen et al., 2010).
Figure 7.
A, Chemical stability of solanacol and solanacyl acetate in a solution of ethanol:water (1:4, v/v) at 21°C (pH 6.7). B, Chemical stability of GR24 and 23 in a solution of ethanol:water (1:4, v/v) at 21°C (pH 6.7).
The Stereochemistry at C2′ and the Enantiomeric Purity Have a Low Effect on Bioactivity
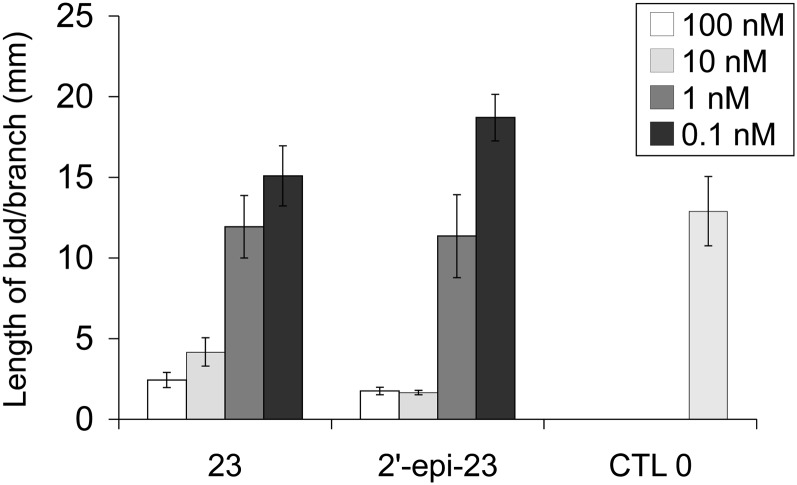
The synthesis of (+)- and (−)-solanacol and the corresponding (+)- and (−)-solanacyl acetate allowed us to compare their bioactivity and to conclude that no significant difference was observed concerning each pair of enantiomers (Fig. 8; for solanacol, P = 0.5601 in one experiment and P = 0.7028 in another one; for solanacyl acetate, P = 0.8, Kruskal-Wallis rank sum test). Similarly, the stereochemistry at C2′ had little influence on the bioactivity of the solanacol series (solanacol versus 2′-epi-solanacol; Table I; P = 0.148 for 1 µm, Kruskal-Wallis rank sum test). These results were also demonstrated for GR24 (Table II) and compound 23 possessing dimethyl substitution at C3′ and C4′ (Fig. 9; no significant difference except in one experiment at 10 nm; Kruskal-Wallis rank sum test, P = 2.358e-05) and 2 bearing protected t-butoxycarbonyl-amino substituents at the C7 position on the A aromatic ring (Table III; P = 0.3524 and P = 0.4518 in two different experiments, Kruskal-Wallis rank sum test). However, for the derivative bearing an iodo group at C7 on the A aromatic ring, isomer 1 was found to be more active than isomer 2′-epi-1 (Table III; P = 0.00183 and P = 0.01842 in two different experiments, Kruskal-Wallis rank sum test).
Figure 8.
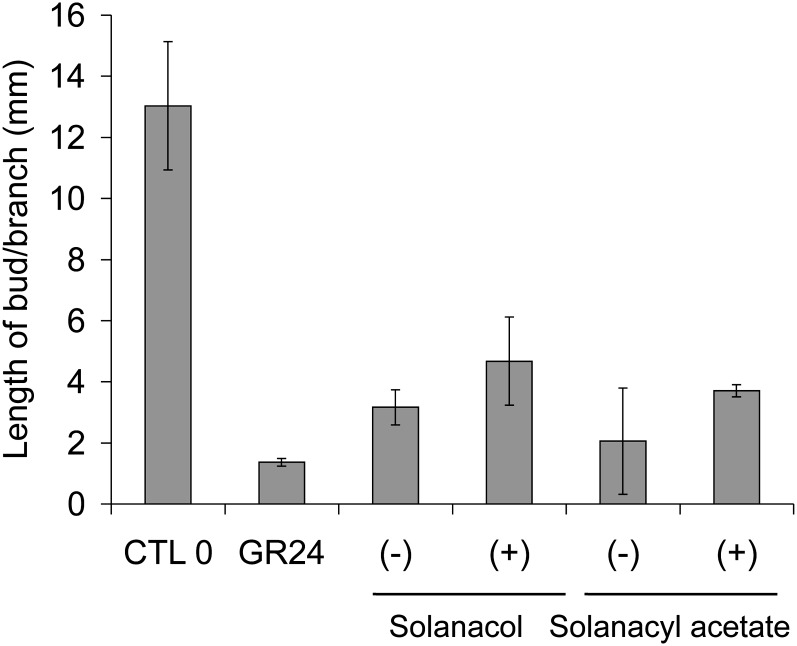
Activity of (+)- and (−)-solanacol and (+)- and (−)-solanacyl acetate. Lengths of axillary buds are shown at 10 d after direct application of a solution at 1 µm at node 4. Data are means ± se (n = 24). CTL 0, Control 0.
Table II. Bud outgrowth inhibition activity assay results for GR24 analogs: influence of the stereochemistry at C2′.
P shows comparison of the SL treatment with the control treatment (0 nm) using the Kruskal-Wallis rank sum test.
| Compound | Concentration | Length of Bud at Node 3 or 4 or 5 per Brancha | se | P |
|---|---|---|---|---|
| mm | ||||
| GR24/2′-epi-GR24 | (1:1) 1 μm | 1.13b | 0.06 | 0.0001434 |
| GR24 | 1 μm | 1.81c | 0.17 | 3.658e-08 |
| 1 μm | 2.32c | 0.21 | 7.703e-07 | |
| 1 μm | 3.16c | 1.03 | 3.123e-07 | |
| 1 μm | 5.02c | 1.08 | 0.0002323 | |
| 1 μm | 5.58c | 2.29 | 3.431e-07 | |
| 1 μm | 1.37b | 0.13 | 1.814e-05 | |
| 1 μm | 1.38b | 0.15 | 0.00296 | |
| 1 μm | 1.39b | 0.09 | 0.01278 | |
| 1 μm | 1.54b | 0.09 | 2.246e-05 | |
| 1 μm | 1.80b | 0.21 | 0.002495 | |
| 500 nm | 1.40b | 0.18 | 0.00419 | |
| 500 nm | 1.69b | 0.18 | 2.733e-05 | |
| 500 nm | 1.89b | 0.29 | 0.001434 | |
| 500 nm | 5.48b | 1.85 | 0.0006748 | |
| 100 nm | 1.87c | 0.13 | 5.42e-08 | |
| 100 nm | 4.39c | 0.38 | 1.598e-07 | |
| 100 nm | 7.26c | 1.75 | 0.001085 | |
| 100 nm | 16.78c | 3.76 | 0.001103 | |
| 100 nm | 1.44b | 0.06 | 6.515e-07 | |
| 100 nm | 1.57b | 0.13 | 0.001664 | |
| 100 nm | 1.66b | 0.13 | 7.789e-05 | |
| 100 nm | 3.15b | 1.10 | 0.006062 | |
| 100 nm | 3.65b | 1.88 | 0.0007414 | |
| 100 nm | 3.74b | 1.59 | 0.0001373 | |
| 100 nm | 3.74b | 0.82 | 0.001448 | |
| 100 nm | 4.83b | 1.50 | 0.0004835 | |
| 100 nm | 6.56b | 2.89 | 0.002068 | |
| 100 nm | 1.87d | 0.24 | 0.0007518 | |
| 10 nm | 14.05c | 1.79 | 0.1837 | |
| 10 nm | 14.99c | 2.39 | 0.2825 | |
| 10 nm | 28.74c | 3.46 | 0.1188 | |
| 10 nm | 1.76b | 0.12 | 2.011e-05 | |
| 10 nm | 1.90b | 0.24 | 0.03709 | |
| 10 nm | 3.93b | 1.53 | 0.003062 | |
| 10 nm | 11.85b | 2.24 | 0.942 | |
| 1 nm | 8.42c | 1.66 | 0.006272 | |
| 1 nm | 19.53c | 0.87 | 0.4435 | |
| 1 nm | 2.22b | 0.22 | 0.003631 | |
| 1 nm | 3.36b | 0.70 | 0.6577 | |
| 0.1 nm | 3.87b | 0.28 | 0.42 | |
| 0.01 nm | 16.32c | 1.28 | 0.1518 | |
| 2′-epi-GR24 | 1 μm | 1.23b | 0.08 | 0.0008346 |
| 23 | 1 μm | 1.80c | 0.11 | 7.656e-08 |
| 1 μm | 1.96c | 0.13 | 3.804e-08 | |
| 100 nm | 1.49c | 0.07 | 1.217e-07 | |
| 100 nm | 1.67c | 0.14 | 5.061e-08 | |
| 100 nm | 1.78c | 0.09 | 9.488e-09 | |
| 100 nm | 1.82c | 0.11 | 1.55e-05 | |
| 100 nm | 2.01c | 0.13 | 1.344e-08 | |
| 100 nm | 2.14c | 0.16 | 1.29e-06 | |
| 100 nm | 2.44c | 0.47 | 1.637e-05 | |
| 10 nm | 1.66c | 0.13 | 2.952e-06 | |
| 10 nm | 2.13c | 0.13 | 8.939e-09 | |
| 10 nm | 2.81c | 0.49 | 9.998e-06 | |
| 10 nm | 3.06c | 0.74 | 4.543e-05 | |
| 10 nm | 8.81c | 3.11 | 4.398e-05 | |
| 1 nm | 4.43c | 0.83 | 2.531e-07 | |
| 1 nm | 7.27c | 1.61 | 0.003078 | |
| 1 nm | 11.94c | 1.93 | 0.5845 | |
| 1 nm | 13.88c | 3.83 | 0.007361 | |
| 0.1 nm | 18.70c | 1.44 | 0.1024 | |
| 0.1 nm | 28.65c | 3.65 | 0.9561 | |
| 0.1 nm | 2.63b | 0.16 | 0.04531 | |
| 0.01 nm | 11.75c | 1.45 | 0.0814 | |
| 2′-epi-23 | 100 nm | 1.79c | 0.13 | 3.919e-05 |
| 100 nm | 1.76c | 0.23 | 7.853e-07 | |
| 10 nm | 1.84c | 0.11 | 1.931e-05 | |
| 10 nm | 1.66c | 0.13 | 2.952e-06 | |
| 1 nm | 11.36c | 2.57 | 0.386 | |
| 0.1 nm | 18.70c | 1.44 | 0.1024 |
These data are means ± se (n = 24), obtained 10 d after treatment. bNode 4. cNode 3. dNode 5.
Figure 9.
Comparative activity of SL analogs 23 and 2′-epi-23. Lengths of axillary buds are shown at 10 d after direct application at node 3. Data are means ± se (n = 24). CTL 0, Control 0.
Table III. Bud outgrowth inhibition activity assay results for GR24 analogs: influence of the substitution on the A-ring.
P shows comparison of the SL treatment with the control treatment (0 nm) using the Kruskal-Wallis rank sum test.
| Compound | Concentration | Length of Bud at Node 3 or 4 per Brancha | se | P |
|---|---|---|---|---|
| mm | ||||
| 1 | 1 μm | 2.55b | 0.20 | 5.113e-07 |
| 1 μm | 1.68c | 0.16 | 1.304e-05 | |
| 500 nm | 3.93b | 1.06 | 2.996e-06 | |
| 100 nm | 4.28c | 1.16 | 5.272e-05 | |
| 2′-epi-1 | 1 μm | 4.80b | 0.93 | 4.339e-06 |
| 1 μm | 7.24c | 2.46 | 0.02181 | |
| 2 | 1 μm | 4.09c | 1.74 | 0.1266 |
| 500 nm | 2.20c | 0.26 | 0.08286 | |
| 2′-epi-2 | 1 μm | 2.90c | 1.10 | 0.3367 |
| 500 nm | 4.90c | 2.80 | 0.1185 |
These data are means ± se (n = 24), obtained 10 d after treatment. bNode 3. cNode 4.
The ABC Part of the SL Backbone Can Be Removed without Affecting Bioactivity
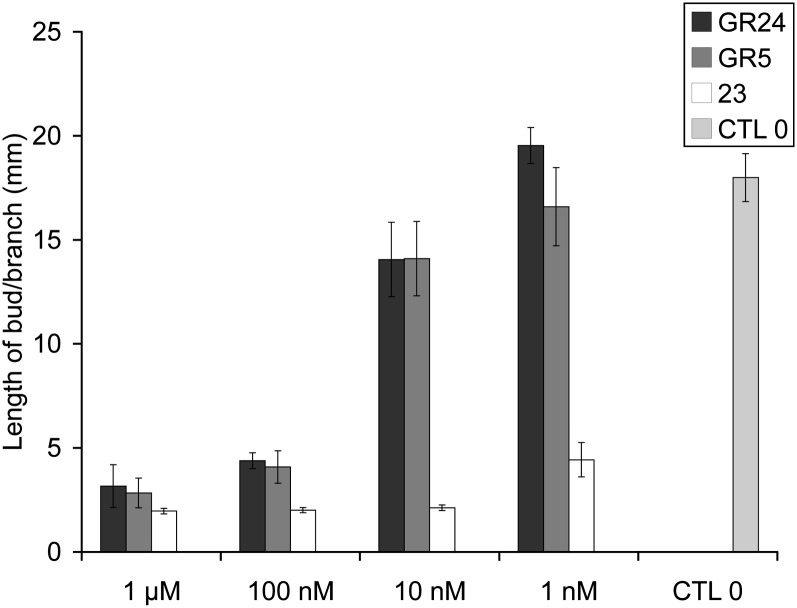
SAR studies on synthetic SL analogs revealed that the activity of GR24, the well-known synthetic SL with an aromatic A-ring, as well as the more simple SL analog GR5 lacking A- and B-rings was similar to the most active natural SLs orobanchyl acetate, sorgolactone, and fabacyl acetate in the same concentration range (Tables I, II, and VI). In some experiments, GR5 was even more active when compared with GR24 at 10 nm (P = 0.0236, Kruskal-Wallis rank sum test) and at some nodes in hydroponics (see below; Supplemental Figs. S1 and S2), as demonstrated recently in rice (Yamaguchi et al., 2010). The SL mimic 31, an analog of debranones (Fukui et al., 2011) with a 3,4-dimethylbutenolide D-ring and a 4-(chlorophenyl)thio group replacing the ABC part, also presented significant activity in branching repression at nanomolar concentrations (Table VI).
Table VI. Bud outgrowth inhibition activity assay results for GR5 analogs and SL mimic 31.
P shows comparison of the SL treatment with the control treatment (0 nm) using the Kruskal-Wallis rank sum test.
| Compound | Concentration | Length of Bud at Node 3 or 4 per Brancha | se | P |
|---|---|---|---|---|
| mm | ||||
| GR5 | 1 μm | 2.76b | 0.27 | 3.842e-06 |
| 1 μm | 2.84b | 0.71 | 6.845e-08 | |
| 1 μm | 3.45b | 0.31 | 2.456e-08 | |
| 100 nm | 2.29b | 0.26 | 3.78e-05 | |
| 100 nm | 4.08b | 0.77 | 1.373e-07 | |
| 100 nm | 6.46b | 1.25 | 0.0003009 | |
| 100 nm | 16.78b | 4.70 | 0.002689 | |
| 100 nm | 1.58c | 0.15 | 1.773e-05 | |
| 100 nm | 1.87c | 0.10 | 0.002514 | |
| 10 nm | 6.88b | 2.56 | 0.0006954 | |
| 10 nm | 13.70b | 2.05 | 0.08664 | |
| 10 nm | 14.10b | 1.79 | 0.1228 | |
| 10 nm | 28.35b | 4.40 | 0.3172 | |
| 10 nm | 5.15c | 1.85 | 0.03928 | |
| 1 nm | 16.59b | 1.88 | 0.7095 | |
| 1 nm | 8.16c | 2.12 | 0.5715 | |
| 29 | 1 μm | 24.17b | 4.19 | 0.06404 |
| 30 | 1 μm | 2.60b | 0.50 | 9.502e-08 |
| 100 nm | 6.15b | 1.29 | 5.678e-06 | |
| 10 nm | 11.24b | 1.64 | 0.001544 | |
| 1 nm | 17.30b | 1.48 | 0.6843 | |
| 31 | 1 μm | 1.81b | 0.11 | 8.726e-08 |
| 1 μm | 2.05b | 0.12 | 5.012e-07 | |
| 100 nm | 2.00b | 0.11 | 1.348e-07 | |
| 100 nm | 2.36b | 0.17 | 5.494e-06 | |
| 100 nm | 1.56c | 0.06 | 1.032e-07 | |
| 10 nm | 2.43c | 0.30 | 2.303e-06 | |
| 10 nm | 1.65c | 0.07 | 8.542e-07 | |
| 1 nm | 8.28b | 1.28 | 0.0003896 | |
| 1 nm | 1.91c | 0.21 | 0.0002094 | |
| 0.1 nm | 3.02c | 0.36 | 0.1417 | |
| 0.01 nm | 17.24b | 1.42 | 0.11 |
These data are means ± se (n = 24), obtained 10 d after treatment. bNode 3. cNode 4.
The D Part of the SL Backbone Attached to a Suitable Unsaturated System Is Essential for Bioactivity
The influence of the D-ring was established by the following results. The analogs of GR24, in which the enol ether group (>C = CH-O-) was replaced by an enamino group (>C = CH-N-) bearing either a benzyl (7, 9) or a glucosyl (10) group (Fig. 3), were inactive at 1 μm concentration (Table IV). The D-ring precursor 3 was also found to be inactive, as was the combination of 3 with the ABC tricyclic derivative 6 or 27 (Table IV). Modifications of the substitution of the D-ring led to a drastic effect on bioactivity. Substitutions by a methoxy group at the C3′ or C4′ position (20–22) or lack of a methyl group (19) at the same positions led to an activity loss (Table IV). However, both compounds 23 and 31 possessing dimethyl substitution at C3′ and C4′ were more active than GR24 and its GR5 analog 30 (Tables IV and VI; Fig. 10; 23/2′-epi-23 were significantly more active than GR5 at 10 nm; Kruskal-Wallis rank sum test, P = 0.01135). This higher activity could be correlated with the relative high stability of 23 in aqueous medium in comparison with GR24 (Fig. 7B). The substitution at C2′ by a methyl group (25) or at C3′ by a butyl group (26) instead of a methyl group (23) significantly decreased bioactivity (Table IV). To study the influence of the double bonds at C3C6′ and C3′C4′, reduced and oxidized analogs of GR24 were evaluated. Completely reduced derivative 34, 3,6′,3′,4′-tetrahydro-2′-epi-GR24 (38), and analogs with an oxidized enol ether group (36, 37) were either inactive or only slightly active (Fig. 6; Table V). The very low activity of epoxide 36 could be explained by the fast degradation (less than 1 h) of this compound under the test conditions (acetone/ethanol/water) via the opening of the oxirane ring and cleavage of the D-ring (data not shown). Analogs 35 with a saturated D-ring and 5, in which the carbon double bond in the CD-connecting enol ether was reduced to a single bond, presented significant activity (Table V) at micromolar concentrations but decreased rapidly as the concentration decreased. Finally, replacing the heteroatom attached at C6 by nitrogen or sulfur (29, 33; Figs. 4 and 5) showed no activity for bud outgrowth inhibition (Tables V and VI).
Table IV. Bud outgrowth inhibition activity assay results for GR24 analogs: influence of the D-ring.
P shows comparison of the SL treatment with the control treatment (0 nm) using the Kruskal-Wallis rank sum test.
| Compound | Concentration | Length of Bud at Node 3 or 4 per Brancha | se | P |
|---|---|---|---|---|
| mm | ||||
| 3 | 1 μm | 32.09b | 6.00 | 0.9167 |
| 6 | 500 nm | 21.32c | 2.67 | 0.7579 |
| 6/3 | (1:1) 500 nm | 19.55c | 3.68 | 0.9019 |
| 7 | 1 μm | 18.38b | 1.03 | 0.195 |
| 9 | 1 μm | 19.63b | 0.86 | 0.6184 |
| 10 | 1 μm | 17.89b | 1.53 | 0.4226 |
| 27/3 | (1:1) 1 μm | 29.81b | 3.69 | 0.2461 |
| 19 | 1 μm | 6.82c | 2.53 | 0.9097 |
| 20/2′-epi-20 | (1:1) 1 μm | 6.58c | 2.16 | 0.7445 |
| 21 | 1 μm | 18.09b | 0.11 | 0.2672 |
| 22/2′-epi-22 | (1:1) 1 μm | 17.56b | 1.52 | 0.1478 |
| 23/2′-epi-23 | (1:1) 1 μm | 2.67b | 0.19 | 1.895e-08 |
| (1:1) 1 μm | 2.55b | 0.28 | 4.191e-06 | |
| (1:1) 100 nm | 3.22b | 0.29 | 4.328e-08 | |
| 1:1) 100 nm | 1.81b | 0.12 | 5.225e-06 | |
| (1:1) 10 nm | 3.46b | 0.34 | 3.937e-08 | |
| 1:1) 10 nm | 1.89b | 0.14 | 1.233e-05 | |
| (1:1) 1 nm | 8.31b | 2.32 | 0.0247 | |
| 1:1) 1 nm | 4.37b | 1.19 | 0.0002118 | |
| (1:1) 0.1 nm | 8.07b | 1.50 | 0.03062 | |
| 25/2′-epi-25 | (1:1) 1 μm | 14.90b | 4.14 | 0.09312 |
| 26/2′-epi-26 | (1:1) 1 μm | 6.57b | 2.53 | 0.0002661 |
These data are means ± se (n = 24), obtained 10 d after treatment. bNode 3. cNode 4.
Figure 10.
Dose-dependent activity of the most active SL analogs GR24, GR5, and 23 for branching inhibition. Lengths of axillary buds are shown at 10 d after direct application at node 3. Data are means ± se (n = 24). CTL 0, Control 0.
Table V. Bud outgrowth inhibition activity assay results for GR24 analogs: influence of the double bonds at C3C6′ and C3′C4′ and influence of the nature of the heteroatom between C and D.
P shows comparison of the SL treatment with the control treatment (0 nm) using the Kruskal-Wallis rank sum test.
| Compound | Concentration | Length of Bud at Node 3 or 4 per Brancha | se | P |
|---|---|---|---|---|
| mm | ||||
| 34 | 1 μm | 15.64b | 1.49 | 0.02134 |
| 1 μm | 7.28c | 2.62 | 0.6719 | |
| 35 | 1 μm | 8.34b | 1.53 | 0.0001411 |
| 500 nm | 2.20c | 0.16 | 0.1321 | |
| 500 nm | 2.10c | 0.10 | 0.07155 | |
| 100 nm | 11.83c | 3.08 | 0.3319 | |
| 36 | 1 μm | 17.15c | 4.24 | 0.1831 |
| 1 μm | 10.22b | 1.84 | 0.0003181 | |
| 37 | 1 μm | 17.13b | 1.29 | 0.1482 |
| 1 μm | 5.53c | 2.71 | 0.1932 | |
| 4 | 1 μm | 4.15c | 0.83 | 0.08025 |
| 5 | 1 μm | 5.55b | 1.35 | 9.923e-06 |
| 1 μm | 11.38b | 2.60 | 0.01761 | |
| 1 μm | 1.87c | 0.15 | 0.0002597 | |
| 500 nm | 1.47c | 0.08 | 0.008959 | |
| 100 nm | 18.23b | 2.45 | 0.5782 | |
| 38 | 1 μm | 9.07b | 1.72 | 0.001774 |
| 33 | 1 μm | 25.20b | 4.74 | 0.162 |
These data are means ± se (n = 24), obtained 10 d after treatment. bNode 3. cNode 4.
Bioactivity of SLs When Applied via the Root System
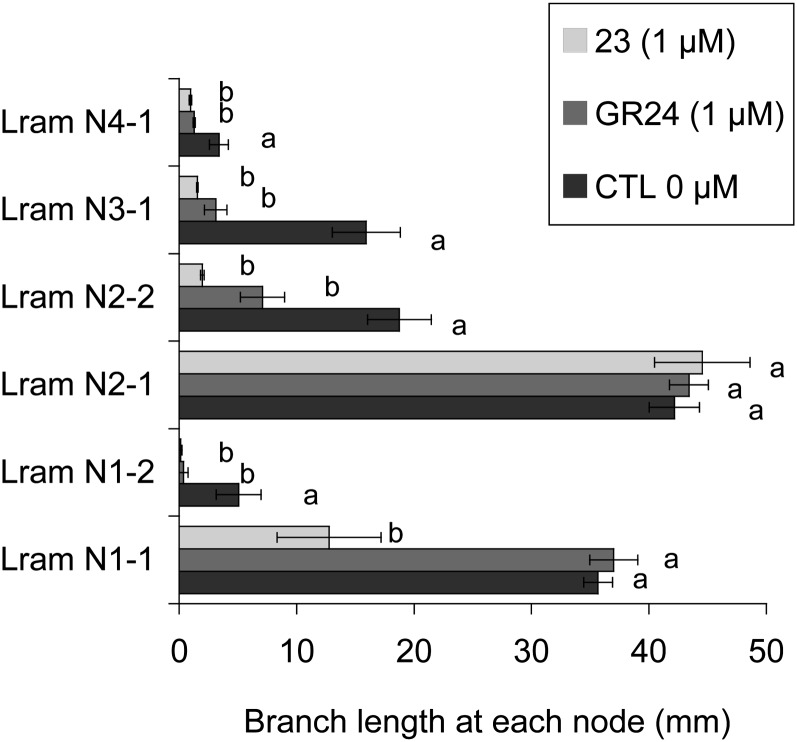
To investigate the effects of the most active SL analogs (GR24, GR5) and analog 23 on shoot branching when provided via the roots, we developed a hydroponic culture system in which pea seedlings were placed 6 d after sowing (before leaf expansion at node 3). SLs were added at this stage into the culture solution, and branching at each node was observed 2 weeks later. In a first experiment (Supplemental Fig. S1), GR24 and GR5 were added at a final concentration of 3 µm. Bud outgrowth was significantly repressed at the upper nodes (nodes 3 and 4) by treatment with either or both SLs. In pea, the number of axillary buds per axil differed along the stem and was highest at node 2, where four buds could be found, with one being larger than the remainder (the accessory buds; Gould et al., 1987). At the basal nodes (nodes 1 and 2), two branches per node were generally observed for our SL-deficient rms1 mutant line. The lengths of the different basal branches were measured and analyzed separately in the rms1 mutant grown on the hydroponic system. A significant reduction of the bud/branch length was observed only for the second branch at both nodes for GR5 and only at node 1 for GR24 (Supplemental Fig. S1). In a second experiment, GR24 and 23 were added at a final concentration of 1 µm. At the upper nodes, both SLs significantly repressed bud outgrowth. At the basal nodes, GR24 and 23 significantly reduced the branch length of the second one at both nodes (Fig. 11). Compound 23 also significantly reduced the growth of the main branch at node 1. To summarize, experiments with the hydroponic culture in which SLs were applied via the root system confirmed the results obtained with direct application onto the axillary bud, where GR5 and especially compound 23 showed higher bioactivity than did GR24 for branching inhibition.
Figure 11.
Bioassay on bud outgrowth using hydroponic culture. A comparison of the activity of GR24 and 23 at 1 µm is shown. Axillary bud lengths are shown at each node of rms1 plants 2 weeks after treatment. Lram Ni-j, Ramification length at node i. j = 1 means the main branch at this node, and j = 2 means the second branch at this node when visible. Data are means ± se (n = 12). Values that have the same letter (a or b) are not significantly different at P = 0.05. CTL 0, Control 0.
A Molecular Assay Using PsBRC1 Transcript Levels
The transcript level of PsBRC1 was reduced in the axillary buds of SL-deficient mutants in comparison with the wild type (Braun et al., 2012). This gene, mostly expressed in the axillary bud, is transcriptionally up-regulated by SLs without the requirement of protein synthesis, and its transcript level in the axillary bud correlates with the observed bud growth responses in the SL-deficient rms1 mutant (Braun et al., 2012; Dun et al., 2012). The activity of solanacol and solanacyl acetate, which showed a quantitative difference in bioactivity for bud outgrowth inhibition, was tested at the molecular level. Their effects on the PsBRC1 transcript level were quantified using real-time PCR in rms1 axillary buds 6 h after direct application of GR24, solanacol, and solanacyl acetate at a concentration of 1 µm. Bud length was measured 10 d after SL application to ensure their activity in this experiment (Fig. 12A). In comparison with the control without SL, at 1 µm, the three SLs induced a significant increase in PsBRC1 expression (Fig. 12B). At 500 nm, only GR24 and solanacyl acetate induced significant increases in PsBRC1 expression (Supplemental Fig. S3). At 1 µm, solanacyl acetate triggered a higher PsBRC1 expression than solanacol and GR24 (Fig. 12B). Consequently, phenotypic and molecular assays were in agreement for the branching control test of SLs, but differences were observed between assays. Solanacyl acetate showed higher activity than did GR24 according to the molecular assay, whereas both SLs showed the same activity in the branching assay.
Figure 12.
Effect of different SLs (1 µm) on PsBRC1 transcript levels in axillary buds at node 3 of rms1 plants. Plants were treated by direct application on the bud. A, Length of the axillary bud 10 d after treatment at node 3. Data are means ± se (n = 12). B, PsBRC1 transcript levels in the axillary bud 6 h after treatment. Data are means ± se (n = 3 pools of 30 plants). Values that have the same letter (a–c) are not significantly different at P = 0.05. CTL 0, Control 0.
Germination Stimulation Activity
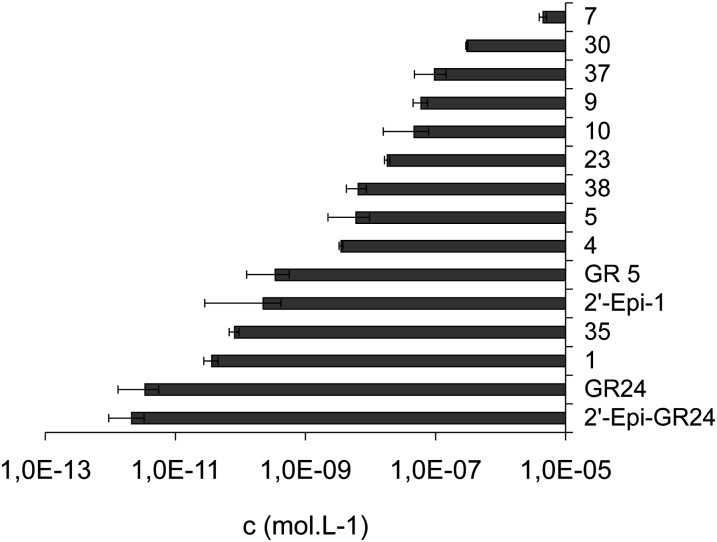
The germination stimulatory activity of natural SLs and analogs was extensively studied in the past with a range of one-half-maximal effective concentration (EC50) values (10−10 to 10−15 m) varying according to the root parasitic plants (Orobanche, Striga; Kim et al., 2010; Malik et al., 2011). In this study, the EC50 of each compound was determined with a range of concentrations tested between 10−16 and 10−4 m and the maximum percentage of germination. EC50 reflects the affinity of the compounds to the putative receptor over the effectiveness of the response induced. No significant difference was observed for the maximum germination percentage (from 94% ± 4% to 96% ± 3%) obtained between the different SLs (ANOVA, P < 0.05). Activities of SLs were then compared using their EC50. The most potent compounds for branching inhibition (GR24, GR5, 23) were examined for their activity on seed germination in comparison with other SL analogs. Seeds of P. ramosa were used, and in our hands, the best activity was observed for GR24 (EC50 ± se = 3.4 × 10−12 ± 2.1 × 10−12 mol L−1; Fig. 13). The stereochemistry at C2′ had no significant influence on the bioactivity in the GR24 series (GR24 versus 2′-epi-GR24, 3.4 × 10−12 ± 1.18 × 10−12 and 2.13 × 10−12 ± 1.18 × 10−12 mol L−1, respectively; Student’s t test, P < 0.05; Fig. 13). As already described (Johnson et al., 1981), GR5 analogs (Table VII) were less active than their corresponding GR24 analogs (about 10- to 1,000-fold). Interestingly, analog 23 presented moderate activity in comparison with GR5 or GR24 (about 100 and 10,000 times less active, respectively; Supplemental Fig. S4). The reduced analogs of GR24 at C3C6′ positions (4 and 5) and the reduced analog 35 at C3′C4′ presented, in our study, moderate and good activity for the germination of P. ramosa, respectively (Fig. 13). In contrast, these compounds showed no activity on Striga hermonthica and Orobanche crenata seed germination (Mangnus and Zwanenburg, 1992).
Figure 13.
EC50 (mol L−1) of various SL analogs toward P. ramosa seed germination.
Table VII. EC50 of SL analogs on P. ramosa.
| Compound | EC50 | se |
|---|---|---|
| m | ||
| GR24 | 2.13 × 10−12 | 1.18 × 10−12 |
| GR5 | 3.44 × 10−10 | 2.19 × 10−10 |
| 23 | 1.9 × 10−8 | 1.7 × 10−9 |
| 30 | 3.0 × 10−7 | 1.1 × 10−8 |
DISCUSSION
Pea Branching Assays
Few SLs were tested for shoot branching inhibition so far (Gomez-Roldan et al., 2008; Umehara et al., 2008; Chen et al., 2010), not only because the hormonal function of SLs was discovered a few years ago but also because of the difficulty of the treatment and the relatively large amount of samples required in some species such as rice, where a hydroponic culture has to be used (Umehara et al., 2008; Fukui et al., 2011). In this SAR study, in which 42 molecules were tested, often with different concentrations, pea was proven to be an excellent model. Its simple architecture is particularly suitable for exogenous hormone applications, and access to axillary buds is very easy. One major advantage of our bioassay with direct bud application is the small quantity (less than 1 mg) of molecule needed. Using this assay, the most active compounds showed a significant activity at a concentration of 10 nm. Notable activities at 1 nm were also observed for 23/2′-epi-23 and 31 in some experiments. These results indicate a highly sensitive perception system for SL and a particularly potent activity as demonstrated for AM fungi (Akiyama and Hayashi, 2006) and for seed germination (Xie and Yoneyama, 2010). Certain molecules may be applied through the roots for several days using a hydroponic system, and their effect on branching at several nodes can be analyzed. Using this system, response differences between buds depending on their positions on the stem or between buds at basal nodes were shown. The SLs tested with hydroponics (GR24, GR5, 23) were able to reduce branch lengths at all nodes except for those at basal nodes (nodes 1 and 2), where only the small branch was inhibited. At these particular nodes, corresponding to scale leaves, the SL-deficient rms1 mutant displayed two branches. Here, we found that even the most active SL was not able to reduce the main branch at node 2. At node 1, only 23 significantly reduced the growth of the main branch. In pea, wild-type lines often displayed one strong basal branching at nodes 1 and 2, suggesting that even endogenous SLs are not able to inhibit outgrowth of the main axillary bud at these nodes while axillary buds at upper nodes and accessory buds at basal nodes are inhibited. Consequently, the use of synthetic SL analogs to control shoot architecture may not be efficient to repress branching for all buds according to their positions along the stem. Two (nonexclusive) hypotheses are currently proposed for the action of SL to repress branching: (1) a direct and local action in the bud via PsBRC1 (Braun et al., 2012); (2) a systemic action by dampening auxin transport and enhancing competition between shoot branches (Crawford et al., 2010). The fact that at basal nodes, where more than two buds are present, SL treatment generally inhibits the small branch while the strongest one is not inhibited could suggest that SL treatment increases the competition between the two branches at these nodes. Nevertheless, here, we show that the most active SL, 23, is able to significantly inhibit the outgrowth/growth of both branches at node 1. This basal branching can also be inhibited by direct application of GR24 at high concentrations (e.g. 2 µm; Brewer et al., 2009), whereas only one application of GR24 at 100 nm is enough to inhibit axillary bud outgrowth at nodes 3 and 4. This is why we chose these nodes for our bioassay. Consequently, according to its position along the stem, the axillary bud will be more or less responsive to a local SL treatment or via the vascular stream. Because PsBRC1 integrates SL and cytokinin signals that act antagonistically to regulate bud outgrowth (Braun et al., 2012), it is also possible that the cytokinin response may explain the difference in bud outgrowth potential according to its position on the stem.
Analysis of transcript levels of PsBRC1 in a molecular assay was carried out to compare two SLs showing quantitatively different activities in our branching assay. Our results suggest that this molecular assay could be applied to test and compare the hormonal activity of SL analogs, particularly molecules with relatively low stability. This assay is based on transcript levels 6 h after SL application, whereas in the branching assay it is not clear how long the molecule needed to be active to repress bud outgrowth. This molecular assay could be used to investigate the lipophilicity of molecules and the penetration rate of the molecule in the tissue.
It is very likely that the activity for branching inhibition of the tested molecules is not toxicity. In our bioassay, when buds are “killed,” their color changes from green to white/yellow. To confirm the activity of the molecule in branching inhibition and to discriminate from a possible toxicity, the molecule could be tested on a SL response mutant (e.g. rms4). In the case of toxicity, branching inhibition should also be observed in this mutant, whereas if the molecule is active using the rms1 SL-deficient mutant, the same molecule should not inhibit bud outgrowth in rms4.
SL Structural Requirements for Repressing Branching
One major breakthrough in the discovery of SLs as a plant hormone can be seen in the study by Matusova et al. (2005) showing that SLs were derived from the carotenoid pathway. A SL biosynthesis scheme from β-carotene was proposed with olefin bond cleavage by CCD as a first step leading to the putative intermediate d’orenone, followed by several steps of reductions, allylic alcohol rearrangement, oxidation, allylic isomerizations, cyclizations, and coupling of the ABC intermediate with the D-ring precursor (Rani et al., 2008). From 5-deoxystrigol and 2′-epi-5-deoxystrigol, which may be common in many plants, further hydroxylations could lead to hydroxy-SLs and further acetylation to acetate-SLs. D’orenone was inactive in our conditions, demonstrating that this putative precursor could not be transformed to the active compound in the bud. Accordingly, a new biosynthesis scheme was proposed very recently (Alder et al., 2012) where trans-β-carotene is isomerized and then cleaved by CCD7 and CCD8 to give carlactone, a compound possessing A- and D-rings and the enol ether bridge, before being transformed in 5-deoxystrigol.
All nine natural SLs tested were active for branching inhibition, but with a quantitative difference in activity. Some synthetic SLs were as active as various natural SLs in the same range of concentrations (10−6 to 10−9 m), in which analog 23 was even more active. It appeared that lipophilicity is a major factor of the activity of natural SLs, since the hydrophobic natural SLs (5-deoxystrigol, sorgolactone, acetate-SLs) tested were more active in our bioassays than the hydroxy-SLs (strigol, orobanchol, solanacol).
All natural SLs identified so far from various plants bear invariant lactone heterocycles C and D, being connected by an ether enol bond. The SL structural requirements for shoot branching inhibition were demonstrated to be essential in this part of the molecule. Small modifications in this moiety of SLs led to drastic consequences on the bioactivity. If replacements of the carbon-carbon double bond at C3C6′or C3′C4′ and substitution at C2′ led to a significant reduction of the bioactivity, the addition of methyl at C3′ led to the very active compound 23 studied here. These results suggest that the D-ring is essential for bioactivity and that a small substitution at C3′ could modulate the interaction with the putative receptor and/or favor the formation of the active compound. The modifications on the AB part were not sufficient to substantially increase or decrease bioactivity. Similarly, the stereochemistry at C2′ or the absolute configuration had no effect on the bioactivity. The SAR of synthetic SLs showed that the ABC hydrophobic skeleton was not essential for the bud outgrowth inhibition activity and could easily be replaced by only the C-ring. These observations were also recently demonstrated in rice (Fukui et al., 2011), where debranones possessing only the D-ring connected to an aromatic cycle without the ABC part presented similar activity to GR24.
We could speculate that one of the functions of the ABC part is to provide a hydrophobic tail to facilitate the transport of SLs toward a putative receptor and influence the stability of the molecule. It is suggested that the SL instability is essential for their role in the rhizosphere as signaling the proximity of a host root to fungi and parasitic seeds (Parniske, 2008). Orobanche and Striga plants as well as AM fungi are obligate biotrophs and must colonize plant roots to obtain reduced carbon and to complete their life cycle. A steep SL concentration gradient may result from this instability, and spores and seeds will germinate only when located close to the host root. Whether this instability would also be essential for their hormonal role in the shoot is unlikely. The active branching inhibitor may not be one of the known SLs, and the SLs identified to date may only be the precursors. This hypothesis was proposed with the characterization of the dwarf and high-tillering rice d14 mutant and the cloning of the rice D14 gene encoding a protein of the α/β-hydrolase superfamily. D14 was suggested to act either at a later step of the active branching inhibitor biosynthesis or in its signaling pathway (Arite et al., 2009).
Other roles of SLs in regulating plant architectures were recently described, such as their involvement in root growth (Kapulnik et al., 2011; Ruyter-Spira et al., 2011), in the stimulation of cambium activity (Agusti et al., 2011), and in nodule formation in planta (Soto et al., 2010; Foo and Davies, 2011). Whether this SL SAR study for the control of branching applies for these novel hormonal effects will need further investigation.
Comparison with Structural Requirements of SLs for Hyphal Branching in AM Fungi and for Seed Germination of Parasitic Plants
The structural requirements of SLs for hyphal branching in AM fungi (Akiyama et al., 2010) and for seed germination of parasitic plants (Xie et al., 2008; Kim et al., 2010) have been extensively studied, revealing the importance of the stereochemistry at C2′ and the absolute configuration for both functions in contrast to SL requirements for branching inhibition. Various SL mimics were synthesized and tested (Zwanenburg et al., 2009; Zwanenburg and Mwakaboko, 2011) essentially for seed germination of the parasitic plant function, due to the relative easiness and well-known expertise to perform these bioassays.
One main common structural requirement between the three SL functions is the CD-ring moiety. The truncation of the AB-rings and even the ABC-rings (see compound 31) has no consequence on hormonal activity, whereas GR5 is inactive for hyphal branching and significantly less active for seed germination. The oxygenation of the SL backbone has a significant effect on the germination of parasitic weeds (Kim et al., 2010), contrary to the effect on AM fungi hyphal branching (Akiyama et al., 2010) or on pea branching inhibition.
Because in the putative receptor site, only nucleophilic functionalities would be available for initiating a reaction, two mechanisms involving in each case a Michael addition have been proposed for seed germination activity. Initially, Zwanenburg et al. (2009) suggested the initial binding of the SL followed by a nucleophilic attack in a Michael fashion at the enol ether bridge. Due to the activity in this recently found model of 2-aryloxyfuranone (Zwanenburg and Mwakaboko, 2011), Zwanenburg proposed an alternative mechanism. A nucleophile would add to the furanone also in a Michael fashion, followed by elimination of the group at C2 after an internal proton shift. Our results show that the enol function is important for bud outgrowth inhibition. Without this chemical function, the activity of the SL analogs decreases but always exists, as for hyphal branching in AM fungi (Akiyama et al., 2010) and for the parasitic plant model but differing according to the species (Mangnus and Zwanenburg, 1992; this study). The higher activity of 23 and 31 in comparison with other SLs in the pea branching test suggests that the mechanisms proposed by Zwanenburg and Mwakaboko (2011) for seed germination of parasitic plants may be different for branching inhibition: no internal proton shift could be involved with these last structures. Globally, important SL structure variations seem to affect bioactivity dramatically for AM fungi hyphal branching, moderately for parasitic seed germination stimulation, and weakly for the shoot branching inhibition. These observations would suggest that each system uses a distinct perception system. In accordance with our data, an alternative mechanism for the SL mode of action involving the hydrolysis of the butenolide D-ring was very recently proposed (Scaffidi et al., 2012).
This work confirmed the essential role of the D-ring for SL bioactivity for different biological functions. The γ-butyrolactones and specifically α,β-unsaturated furanones, including SLs with their D-ring, are important natural chemical mediators. Indeed, this class of compounds are found as pheromones (Tumlinson et al., 1977), allelochemicals, or N-acyl homo-Ser lactones, hormone-like signals by which bacteria can communicate with each other and coordinate their gene expression in a cell density-dependent manner, a process known as quorum sensing (Galloway et al., 2011). SLs represent a fascinating example of plant hormones with multiple signaling roles between different kingdoms, in parasitic interaction between plants, or between moss individuals to regulate their extension and the potential structure of moss communities (Proust et al., 2011). Whether these roles derived from their specific structures is an intriguing question (Tsuchiya and McCourt, 2012). The identification of SL receptors and signaling pathways in the different systems will help us better understand the evolution of functions of this remarkable and intriguing class of compounds.
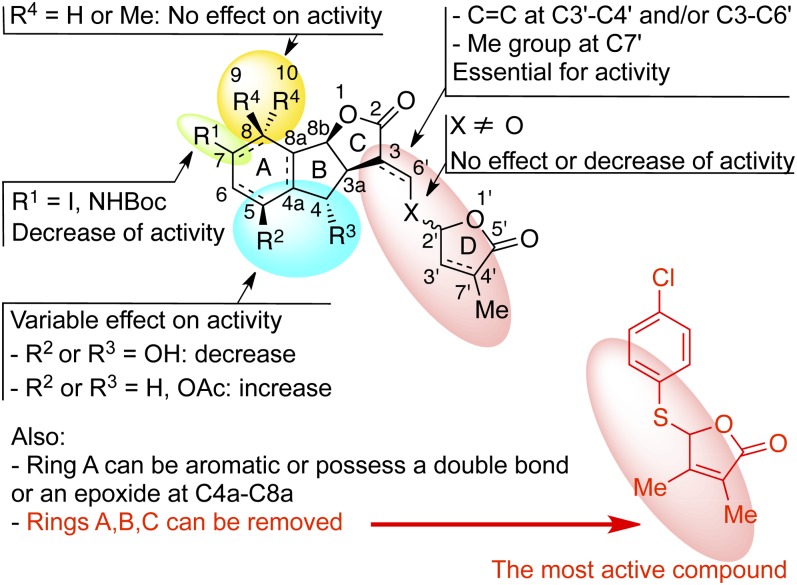
CONCLUSION
SL structural requirements for shoot branching inhibition were determined through the study of 42 various natural SLs and analogs and are summarized in Figure 14. The presence of the D-ring is essential for hormonal activity. The stereochemistry at C2′ is not an important structural feature for potent activity. The most active SL analogs for bud outgrowth repression were found to be GR5 and analogs 23 and 31 possessing a 3,4-dimethylbutenolide D-ring, and not GR24, which was previously found to be the most active SL analog for inducing arasitic weed germination (Zwanenburg et al., 2009) and for AM fungi hyphal branching (Besserer et al., 2006; Akiyama et al., 2010). These results suggest, to our knowledge for the first time, that branching signal recognition could present some distinctive features, compared with the perception of the signal for the different models. These results established that an SL analog, compound 23 or 31, could be used to control plant architecture without favoring the parasitic plant P. ramosa. We have demonstrated that the presence of both an α,β-unsaturated system and a methylbutenolide or dimethylbutenolide as the D-ring in the same molecule is essential. Our SAR study establishes that SLs show potent activity in a structure-dependent manner via a receptor-mediated mechanism but with low specificity concerning the stereochemistry or the substitution of SLs. Genetic approaches using branching mutants not responding to exogenous SL applications and analogy with other hormone signaling pathways have led to two candidate proteins for the SL receptor, the F-box protein encoded by the MAX2/RMS4/D3 gene and the D14 protein of the α/β-fold hydrolase superfamily (Arite et al., 2009; Beveridge and Kyozuka, 2010). Once identified, the SL receptor may be used for the development of other assays to investigate ligand specificity.
Figure 14.
Effects of SL structural modifications on bud outgrowth inhibition in the pea model. Ac, Acetyl; Boc, t-butoxycarbonyl; Me, methyl.
MATERIALS AND METHODS
General Remarks
Infrared (IR) spectra were recorded as neat. 1H- and 13C-NMR spectra were recorded as solutions in CDCl3, using residual protic solvent CHCl3 (δH = 7.24 ppm) or CDCl3 (δC = 77.23 ppm) as an internal reference. Mass spectra were determined by electrospray ionization (ESI). All reactions were monitored by thin-layer chromatography carried out on 0.2-mm aluminum silica gel precoated plates using UV light and a 5% ethanolic solution of phosphomolybdic acid and heat as staining agent. Flash chromatography was performed on 40- to 63-μm (400–230 mesh) silica gel 60 with EtOAc-heptane as eluents. Commercially available reagents and solvents were purified and dried when necessary by the usual methods. Tetrahydrofuran (THF) and diethyl ether were purified by distillation, under nitrogen, from sodium/benzophenone. N,N-Dimethylformamide and CH2Cl2 were dried by distillation from calcium hydride and acetone from anhydrous CaSO4. Unless otherwise mentioned, all other reagents were purchased from commercial sources and were used without further purification.
GR24, 2′-epi-GR24, and derivatives 1, 2′-epi-1, 2, 2′-epi-2, 4, and 5 (Fig. 2) were prepared according to the procedures described (Mangnus et al., 1992; Mangnus and Zwanenburg, 1992; Thuring et al., 1997; Reizelman et al., 2003); compound 3 was prepared according to the procedure described by Fell and Harbridge (1990) and d’orenone according to the procedure of Schachtschabel and Boland (2007). (+)- and (−)-Solanacol and solanacyl acetate were prepared by the procedure described previously in our laboratory (Chen et al., 2010). GR5 was synthesized by known procedures (Johnson et al., 1981), natural SLs (orobanchol, orobanchyl acetate, fabacyl acetate) were generously supplied by Prof. K. Yoneyama, and SLs (strigol, strigyl acetate, 5-deoxystrigol, sorgolactone) were derived by organic synthesis using known procedures or supplied by Bayer CropScience.
(3aR*,8bS*,E)-3-((Dibenzylamino)methylene)-3,3a,4,8b-tetrahydro-2H-indeno[1,2-b]furan-2-one 7
Tricycle 6 (Mangnus et al., 1992; 20 mg, 0.099 mmol, 1 eq.), dibenzylamine (20 mg, 0.099 mmol, 1 eq.), and Fe(OTf)3·6.2DMSO (11), where Tf = trifluoromethanesulfonyl and DMSO = dimethyl sulfoxide (Antoniotti and Duñach, 2008; 14 mg, 0.015 mmol, 0.15 eq.), were dissolved in anhydrous CH2Cl2 (1 mL). The reaction mixture was heated to 60°C under microwave irradiation (300 W) for 2 h. The reaction mixture was cooled and poured into a separation funnel containing CH2Cl2 (20 mL) and NaHCO3 (saturated aqueous 20 mL). The combined organic extracts were washed with water (10 mL), dried over Na2SO4, and evaporated under reduced pressure. The crude material was purified by chromatography on a silica gel (heptane:EtOAc 9:1 to 1:1) to give the desired product 7 (38 mg, quantitative) as a white solid. 1H-NMR (500 MHz, CDCl3) δ 7.64 (s, 1 H), 7.48 (d, J = 7.3 Hz, 1 H), 7.41 to 7.17 (m, 12 H), 7.15 (d, J = 7.6 Hz, 1 H), 5.72 (d, J = 8.2 Hz, 1 H), 4.54 (d, J = 15.6 Hz, 2 H), 4.32 (d, J = 15.6 Hz, 2 H), 3.87 to 3.80 (m, 1 H), 3.25 (dd, J = 16.2, 9.2 Hz, 1 H), 3.03 (dd, J = 16.2, 4.6 Hz, 1 H). 13C-NMR (75 MHz, CDCl3) δ 175.5 (C), 147.5 (CH), 142.8 (C), 139.9 (C), 136.5 (2 C), 129.9 (CH), 129.3 (4 CH), 128.3 (2 CH), 127.5 (5 CH), 126.7 (CH), 125.1 (CH), 95.1 (C), 84.6 (CH), 55.0 (br s, CH2), 53.4 (br s, CH2), 42.6 (CH2), 40.2 (CH). Mass spectrometry (MS) mass-to-charge ratio (m/z) 382 (MH+, 100%). High Resolution Mass Spectrometry (ESI) m/z calculated for C26H24NO2 [M + H+]: 382.1807; found: 382.1797.
(3aR*,8bS*,E and Z)-3-((Benzylamino)methylene)-3,3a,4,8b-tetrahydro-2H-indeno[1,2-b]furan-2-one 9
Prepared from tricycle 6 by the same procedure as for 7 to give 9 as a slightly yellow amorphous solid (58 mg, quantitative). Major isomer: 1H-NMR (300 MHz, acetone D6) δ 7.75 to 7.61 (br s, 1 H, NH), 7.32 to 7.07 (m, 9 H), 6.99 (d, J = 1.3 Hz, 1 H), 5.69 (d, J = 7.9 Hz, 1 H), 5.55 (d, AB, J = 6.2 Hz, 2 H), 3.74 (tt, J = 7.9, 3.2 Hz, 1 H), 3.23 (dd, J = 16.4, 8.1 Hz, 1 H), 3.23 (dd, J = 16.4, 2.1 Hz, 1 H). 13C-NMR (75 MHz, acetone D6) δ 174.0 (C), 149.4 (CH), 143.7 (C), 141.9 (C), 140.6 (C), 130.1 (CH), 129.5 (2 CH), 128.2 (CH), 128.1 (2 CH), 127.8 (CH), 126.8 (CH), 126.2 (CH), 94.4 (C), 86.0 (CH), 52.6 (CH2), 41.3 (CH2), 41.2 (CH). MS (ESI) m/z (%) 605 (50), 346 (25), 314 (100), 292 (30). HRMS (ESI) m/z calculated for C19H17NO2Na [M + Na+]: 314.1157; found: 314.1167.
(3aR*,8bS*,Z)-3-((1,3,4,6-Tetra-O-acetyl-2-amino-2-deoxy-α-d-glucopyranosyl)methylene)-3,3a,4,8b-tetrahydro-2H-indeno[1,2-b]furan-2-one 10
Tricycle 6 (20 mg, 0.099 mmol, 1 eq.), 2-acetamido-1,3,4,6-tri-O-acetyl-2-deoxy-β-d-glucopyranoside 8 (116 mg, 0.297 mmol, 3 eq.), and Fe(OTf)3·6.2DMSO (11; 43 mg, 0.045 mmol, 0.45 eq.) were dissolved in anhydrous CH2Cl2 (1 mL). The reaction mixture was heated to 60°C under microwave irradiation (300 W) for 2 h. The reaction mixture was cooled and poured into a separation funnel containing CH2Cl2 (20 mL) and NaHCO3 (sat. aq. 20 mL). The combined organic extracts were washed with water (10 mL), dried over Na2SO4, and evaporated under reduced pressure. The crude material was purified by chromatography on a silica gel (heptane:EtOAc 9:1 to 1:1) to give the desired mixture of inseparable diastereoisomers 10 (26 mg, 49%) as an off-white solid. 1H-NMR (500 MHz, CDCl3) δ 7.48 to 7.12 (m, 10H), 6.60 (d, J = 5.8 Hz, 1 H), 6.57 (d, J = 5.8 Hz, 1 H), 6.17 (d, J = 3.7 Hz, 1 H), 6.10 (d, J = 3.7 Hz, 1 H), 5.86 (d, J = 4.5 Hz, 1 H), 5.85 (d, J = 4.5 Hz, 1 H), 5.28 (t, J = 10.1 Hz, 1 H), 5.22 (t, J = 10.1 Hz, 1 H), 5.10 to 5.02 (m, 2 H), 4.31 (t, J = 3.7 Hz, 1 H), 4.28 (t, J = 3.7 Hz, 1 H), 4.06 to 3.98 (m, 4 H), 3.83 to 3.75 (m, 2 H), 3.49-3.42 (m, 2 H), 3.41-3.32 (m, 2 H), 2.85 (d, J = 16.5 Hz, 1 H), 2.77 (d, J = 16.5 Hz, 1 H), 2.20 (s, 3 H), 2.15 (s, 3 H), 2.05 (s, 6 H), 2.01 (s, 6 H), 1.99 (s, 3 H), 1.83 (s, 3 H). 13C-NMR (75 MHz, CDCl3) δ 173.8 (C), 173.7 (C), 170.7 (2 C), 170.0 (C), 169.9 (C), 169.8 (2 C), 169.1 (C), 169.0 (C), 145.6 (CH), 145.5 (CH), 142.3 (C), 142.0 (C), 140.1 (C), 140.0 (C), 129.9 (CH), 129.8 (CH), 127.7 (CH), 127.6 (CH), 126.7 (CH), 126.5 (CH), 125.5 (CH), 125.2 (CH), 97.6 (C), 97.3 (C), 91.1 (CH), 91.0 (CH), 86.2 (CH), 86.1 (CH), 71.7 (CH), 71.5 (CH), 70.0 (CH), 69.9 (CH), 68.1 (2 CH), 61.7 (2 CH2), 60.2 (CH), 60.0 (CH), 40.5 (2 CH2), 40.3 (CH), 40.2 (CH), 21.0 (CH3), 20.9 (3 CH3), 20.8 (3 CH3), 20.6 (CH3). MS m/z 554 (MNa+, 100%). HRMS (ESI) m/z calculated for C26H29NNaO11 [M + Na+]: 554.1639; found: 554.1633.
(3aR*,8bS*,E)-3-((((RS*)-5-Oxo-2,5-dihydrofuran-2-yl)oxy)methylene)-3,3a,4,8b-tetrahydro-2H-indeno[1,2-b]furan-2-one 19 and 2′-epi-19
Tricycle 6 (90 mg, 0.445 mmol, 1 eq.), 5-bromo-2(5H)-furanone 12 (Wolff and Hoffmann, 1988; 109 mg, 0.668 mmol, 1.5 eq.), and anhydrous K2CO3 (100 mg, 0.890 mmol, 2 eq.) were dissolved in anhydrous acetone (10.8 mL). The reaction mixture was stirred for 3 h and concentrated under reduced pressure. The residue was dissolved in EtOAc (10 mL), filtered, evaporated under reduced pressure, and purified by chromatography on a silica gel (EtOAc:heptane 2:3 to 4:1) to give the desired products 19 (22 mg, 17%) and 2′-epi-19 (24 mg, 19%) as white amorphous solids. 19: 1H-NMR (500 MHz, CDCl3) δ 7.50 to 7.48 (br s, 1 H), 7.46 (d, J = 2.4 Hz, 1 H), 7.37 (dd, J = 5.7, 1.3 Hz, 1 H), 7.35 to 7.20 (m, 3 H), 6.40 (dd, J = 5.7, 1.1 Hz, 1 H), 6.28 (t, J = 1.1 Hz, 1 H), 5.93 (d, J = 7.7 Hz, 1 H), 3.97 to 3.89 (m, 1 H), 3.41 (dd, J = 17.0 and 9.4 Hz, 1 H), 3.07 (dd, J = 17.0 and 3.0 Hz, 1 H). 13C-NMR (75 MHz, CDCl3) δ 171.4 (C), 169.0 (C), 151.2 (CH), 148.7 (CH), 142.8 (C), 139.1 (C), 130.4 (CH), 127.8 (CH), 126.8 (2 CH), 125.3 (CH), 114.0 (C), 102.2 (CH), 86.2 (CH), 39.1 (CH), 35.5 (CH2). IR νmax (film): 3,104, 2,925, 2,855, 1,793, 1,746, 1,680, 1,610 cm−1. HRMS (ESI) m/z calculated for C16H13O5 [M + H+]: 285.0763; found: 285.0760. 2′-Epi-19: 1H-NMR (500 MHz, CDCl3) δ 7.47 (d, J = 2.4 Hz, 1 H), 7.45 to 7.43 (br s, 1 H), 7.37 (dd, J = 5.7, 1.3 Hz, 1 H), 7.34 to 7.19 (m, 3 H), 6.39 (dd, J = 5.8, 1.3 Hz, 1 H), 6.29 (t, J = 1.1 Hz, 1 H), 5.93 (d, J = 7.9 Hz, 1 H), 3.95 to 3.87 (m, 1 H), 3.39 (dd, J = 17.0, 9.2 Hz, 1 H), 3.06 (dd, J = 17.0, 3.0 Hz, 1 H). 13C-NMR (75 MHz, CDCl3) δ 171.4 (C), 169.0 (C), 151.3 (CH), 148.8 (CH), 142.7 (C), 138.8 (C), 130.3 (CH), 127.7 (CH), 126.53 (CH), 126.50 (CH), 125.4 (CH), 113.9 (C), 102.3 (CH), 86.2 (CH), 39.0 (CH), 37.5 (CH2). IR νmax (film): 3,102, 2,931, 2,856, 1,793, 1,744, 1,679, 1,610 cm−1. HRMS (ESI) m/z calculated for C16H13O5 [M + H+]: 285.0763; found: 285.0769.
(3aR*,8bS*,E)-3-((((RS*)-3-Bromo-4-methoxy-5-oxo-2,5-dihydrofuran-2-yl)oxy)methylene)-3,3a,4,8b-tetrahydro-2H-indeno[1,2-b]furan-2-one 20 and 2′-epi-20
Obtained from tricycle 6 and 3,5-dibromo-2-methoxy-2(5H)-furanone 13 by the same procedure as for 19 to give the desired products 20/2′-epi-20 (1:1, unseparable mixture; 50 mg, 69%) as a colorless oil. 20/2′-epi-20 (1:1): 1H-NMR (300 MHz, CDCl3) δ 7.49 to 7.41 (m, 4 H), 7.35 to 7.21 (m, 6 H), 6.03 (d, J = 4 Hz, 2 H), 5.96 (d, J = 7.9 Hz, 2 H), 4.22 (s, 6 H), 4.00 to 3.94 (m, 2 H), 3.46 (dd, J = 17.0, 9.4 Hz, 2 H), 3.14 (ddd, J = 17.0, 11.9, 2.8 Hz, 2 H). 13C-NMR (75 MHz, CDCl3) δ 171.24 (C), 171.21 (C), 162.3 (2 C), 150.2 (CH), 149.6 (CH), 145.8 (2 C), 142.8 (C), 142.7 (C), 138.9 (C), 138.8 (C), 130.26 (CH), 130.24 (CH), 127.70 (CH), 127.68 (CH), 126.63 (CH), 126.59 (CH), 125.40 (CH), 125.35 (CH), 114.8 (C), 114.6 (C), 107.29 (C), 107.26 (C), 99.60 (CH), 99.57 (CH), 86.24 (CH), 86.19 (CH), 59.3 (2 CH3), 39.1 (CH), 39.0 (CH), 37.6 (CH2), 37.1 (CH2). IR νmax (film): 2,952, 2,856, 1,792, 1,744, 1,669, 1,608 cm−1. HRMS (ESI) m/z calculated for C17H13O6BrNa [M + Na+]: 414.9793; found: 414.9808.
(3aR*,8bS*,E)-3-((((RS*)-4-Methoxy-5-oxo-2,5-dihydrofuran-2-yl)oxy)methylene)-3,3a,4,8b-tetrahydro-2H-indeno[1,2-b]furan-2-one 21 and 2′-epi-21
A mixture of compound 20α/2′-epi-20 (1:1; 15 mg, 0.038 mmol, 1 eq.) and zinc (14 mg, 0.206 mmol, 5.4 eq.). AcOH (one drop) in THF (0.8 mL) was sonicated for 2 h under argon and filtered on a silica gel. The silica gel was washed with EtOAc (2 × 5 mL), and the filtrate was evaporated under reduced pressure. The residue was purified by preparative thin-layer chromatography (EtOAc:heptane 1:1) to afford the two diastereoisomers 21/2′-epi-21 (1:1; 10 mg, 83%) as a colorless oil. A sample of 21 (2.5 mg) was obtained by repeated PTLC (EtOAc:heptane 1:1). 21: 1H-NMR (500 MHz, CDCl3) δ 7.52 to 7.49 (m, 2 H), 7.35 (t, J = 7.6 Hz, 1 H), 7.30 (d, J = 7.6 Hz, 1 H), 7.25 (d, J = 7.6 Hz, 1 H), 6.25 to 6.24 (br s, 1 H), 5.99 (d, J = 1.6 Hz, 1 H), 5.97 (d, J = 7.9 Hz, 1 H) 3.98 to 3.91 (m, 1 H), 3.92 (s, 3 H), 3.04 (dd, J = 17.0, 9.5 Hz, 1 H), 3.12 (dd, J = 17.0, 2.8 Hz, 1 H). 13C-NMR (125 MHz, CDCl3) δ 171.4 (C), 164.0 (C), 151.3 (C), 150.8 (CH), 142.7 (C), 138.9 (C), 130.3 (CH), 127.7 (C), 126.6 (CH), 125.4 (CH), 113.8 (C), 109.3 (CH), 99.8 (CH), 86.1 (CH), 59.1 (CH3), 39.0 (CH), 37.6 (CH2). IR νmax (film): 2,937, 2,854, 1,799, 1,747, 1,681, 1,663 cm−1. HRMS (ESI) m/z calculated for C17H14O6Na [M + Na+]: 337.0688; found: 337.0704.
(3aR*,8bS*,E)-3-((((RS*)-3-Methoxy-5-oxo-2,5-dihydrofuran-2-yl)oxy)methylene)-3,3a,4,8b-tetrahydro-2H-indeno[1,2-b]furan-2-one 22 and 2′-epi-22
Prepared from tricycle 6 and 5-bromo-4-methoxy-2(5H)-furanone 14 (Wolff and Hoffmann, 1988) by the same procedure as for 19 to give the desired products 22/2′-epi-22 (1:1, unseparable mixture; 38 mg, 66%) as a colorless oil. 22/2′-epi-22 (1:1): 1H-NMR (300 MHz, CDCl3) δ 7.42 (d, J = 7.2 Hz, 2 H), 7.36 (t, J = 2.6 Hz, 2 H), 7.29 to 7.15 (m, 6 H), 5.94 (s, 2 H), 5.88 (d, J = 7.9 Hz, 2 H), 5.22 (d, J = 1.9 Hz, 2 H), 3.92 (s, 3 H), 3.90 (s, 3 H), 3.89 to 3.85 (m, 2 H), 3.38 (dd, J = 17.0, 9.4 Hz, 2 H), 3.05 (dd, J = 17.0, 3.0 Hz, 2 H). 13C-NMR (75 MHz, CDCl3) δ 175.25 (C), 175.23 (C), 171.34 (C), 171.31 (C), 168.46 (C), 168.42 (C), 150.6 (2 CH), 142.84 (C), 142.81 (C), 139.0 (C), 138.9 (C), 130.18 (CH), 130.17 (CH), 127.62 (CH), 127.59 (CH), 126.7 (CH), 126.5 (CH), 125.40 (CH), 125.3 (CH), 114.0 (C), 113.9 (C), 98.3 (CH), 98.1 (CH), 91.5 (CH), 91.4 (CH), 86.12 (CH), 86.10 (CH), 60.41 (CH3), 60.38 (CH3), 39.00 (CH), 38.96 (CH), 37.5 (CH2), 37.4 (CH2). IR νmax (film): 3,124, 3,029, 2,946, 2,858, 1,792, 1,745, 1,680, 1,646 cm−1. HRMS (ESI) m/z calculated for C17H14O6Na [M + Na+]: 337.0688; found: 337.0701.
(3aR*,8bS*,E)-3-((((RS*)-3,4-Dimethyl-5-oxo-2,5-dihydrofuran-2-yl)oxy)methylene)-3,3a,4,8b-tetrahydro-2H-indeno[1,2-b]furan-2-one 23 and 2′-epi-23
Prepared from tricycle 6 and 5-chloro-3,4-dimethyl-2(5H)-furanone 15 (Canevet and Graff, 1978) by the same procedure as for 19 to give the desired products 23/2′-epi-23 (1:1; 85 mg, 84%) as a colorless oil. The pure isomer 23 (colorless oil) could be obtained by separation by PTLC (EtOAc:heptane 3:7). 23: 1H-NMR (300 MHz, CDCl3) δ 7.49 (d, J = 7.2 Hz, 1 H), 7.44 (d, J = 2.6 Hz, 1 H), 7.35 to 7.20 (m, 3 H), 5.90 (s, 1 H), 5.89 (d, J = 7.9 Hz, 1 H), 3.93 to 3.85 (m, 1 H), 3.38 (dd, J = 17.0, 9.4 Hz, 1 H), 3.04 (dd, J = 17.0, 3.4 Hz, 1 H), 2.00 to 1.98 (m, 3 H), 1.86 to 1.84 (m, 3 H). 13C-NMR (75 MHz, CDCl3) δ 171.4 (C), 170.8 (C), 152.3 (C), 151.2 (CH), 142.7 (C), 139.1 (C), 130.2 (CH), 128.8 (C), 127.7 (CH), 126.7 (CH), 125.3 (CH), 113.1 (C), 102.5 (CH), 86.1 (CH), 39.1 (CH), 37.6 (CH2), 11.6 (CH3), 8.9 (CH3). IR νmax (film): 3,056, 2,927, 2,857, 1,775, 1,744, 1,677, 1,608 cm−1. HRMS (ESI) m/z calculated for C18H16O5Na [M + Na+]: 335.0895; found: 335.0899.
(3aR*,8bS*,E)-3-(((-(RS*)-2,3,4-Trimethyl-5-oxo-2,5-dihydrofuran-2-yl)oxy)methylene)-3,3a,4,8b-tetrahydro-2H-indeno[1,2-b]furan-2-one 25 and 2′-epi-25
Prepared from tricycle 6 and 5-chloro-2,3,4-trimethyl-2(5H)-furanone 17 by the same procedure as for 19 to give the desired products 25/2′-epi-25 (48.7 mg, 78%; mixture of diastereoisomers, 1:1) as a white amorphous solid. The furanone 17 was prepared from 5-hydroxy-2,3,4-dimethyl-2(5H)-furanone (Surmont et al., 2010) by the procedure of Canevet and Graff (1978). 25/2′-epi-25: 1H-NMR (300 MHz, CDCl3) δ 7.42 (d, J = 7.3 Hz, 2 H), 7.30 to 7.17 (m, 6 H), 7.09 (d, J = 2.6 Hz, 2 H), 5.88 (d, J = 7.9 Hz, 2 H), 3.90 to 3.83 (m, 2 H), 3.40 (dd, J = 17.0, 9.2 Hz, 2 H), 3.09 (dd, J = 17.0, 3.2 Hz, 2 H), 1.90 (d, J = 1.1 Hz, 6 H), 1.83 (d, J = 1.1 Hz, 6 H), 1.70 (s, 6 H). 13C-NMR (75 MHz, CDCl3) δ 171.6 (2 C), 170.9 (2 C), 155.7 (C), 155.6 (C), 147.6 (CH), 147.5 (CH), 142.8 (C), 142.7 (C), 139.07 (C), 138.98 (C), 130.1 (2 CH), 127.7 (CH), 127.6 (CH), 128.0 (C), 127.95 (C), 126.6 (CH), 126.5 (CH), 125.4 (CH), 125.2 (CH), 113.4 (C), 113.3 (C), 107.8 (C), 107.7 (C), 85.99 (CH), 85.97 (CH), 39.06 (CH), 38.98 (CH), 37.6 (CH2), 37.5 (CH2), 22.69 (CH3), 22.62 (CH3), 10.7 (2 CH3), 8.9 (2 CH3). IR νmax (film): 2,928, 1,777, 1,746, 1,673 cm−1. HRMS (ESI) m/z calculated for C19H19O5 [M + H+]: 327.1232; found: 327.1224.
(3aR*,8bS*,E)-3-(((-(RS*)-3-Butyl-4-methyl-5-oxo-2,5-dihydrofuran-2-yl)oxy)methylene)-3,3a,4,8b-tetrahydro-2H-indeno[1,2-b]furan-2-one 26 and 2′-epi-26
Prepared from tricycle 6 and 5-chloro-3-butyl-4-methyl-2(5H)-furanone 18 by the same procedure as for 19 to give the desired products 26/2′-epi-26 (80.2 mg, 91%; mixture of diastereoisomers, 1:1) as a white amorphous solid. The furanone 18 was prepared from 5-hydroxy-3-butyl-4-methyl-2(5H)-furanone (Schreiber and Wermuth, 1965) by the procedure of Canevet and Graff (1978). 1H-NMR (300 MHz, CDCl3) δ 7.48 to 7.43 (m, 4 H), 7.35 to 7.19 (m, 6 H), 6.03 (br s, 2 H), 5.93 (d, J = 7.7 Hz, 2 H), 3.96 to 3.87 (m, 2 H), 3.40 (dt, J = 17.3, 8.9 Hz, 2 H), 3.40 (dt, J = 17.3, 3.6 Hz, 2 H), 2.52 to 2.30 (m, 4 H), 1.89 (s, 6 H), 1.42 to 1.31 (m, 8 H), 0.94 (q, J = 7.2 Hz, 6 H). 13C-NMR (75 MHz, CDCl3) δ 171.41 (C), 171.37 (C), 171.02 (C), 170.98 (C), 156.43 (C), 156.28 (C), 151.4 (CH), 151.3 (CH), 142.7 (C), 142.6 (C), 139.0 (C), 138.9 (C), 130.2 (2 CH), 128.5 (C), 128.4 (C), 127.7 (CH), 127.6 (CH), 126.6 (CH), 126.5 (CH), 125.4 (CH), 125.2 (CH), 113.4 (C), 113.2 (C), 101.85 (CH), 101.82 (CH), 86.04 (CH), 86.02 (CH), 39.0 (2 CH), 37.63 (CH2), 37.56 (CH2), 29.46 (CH2), 29.43 (CH2), 26.03 (CH2), 25.98 (CH2), 22.89 (CH2), 22.81 (CH2), 13.9 (2 CH3), 8.9 (2 CH3). IR νmax (film): 2,930, 1,778, 1,749, 1,681 cm−1. HRMS (ESI) m/z calculated for C21H23O5 [M + H+]: 355.1545; found: 355.1535.
(E)-((3aR*,8bS*)-2-Oxo-3a,4-dihydro-2H-indeno[1,2-b]furan-3(8bH)-ylidene)methylbenzoate 27
Prepared from tricycle 6 and 3-bromo-2-benzoyloxy-2(5H)-furanone 16 by the same procedure as for 19 to give the compound 27 (20 mg, 26%) as a white solid. Furanone 16 was obtained by bromation of 2-benzoyloxy-2-buten-4-olide (Limberg and Thiem, 1995) by the same procedure as for 5-bromo-3-methyl-2(5H)-furanone (Macalpine et al., 1976). Melting point, 164.2°C to 165.3°C (heptane:EtOAc). 1H-NMR (300 MHz, CDCl3) δ 8.46 (d, J = 2.6 Hz, 1 H), 8.09 (d, J = 7.2 Hz, 1 H), 8.07 (d, J = 1.2 Hz, 1 H), 7.63 (dt, J = 7.3, 1.3 Hz, 1 H), 7.51 to 7.45 (m, 3 H), 7.31 to 7.17 (m, 3 H), 5.97 (d, J = 7.9 Hz, 1 H), 4.14 to 4.06 (m, 1 H), 3.59 (dd, J = 16.8, 9.4 Hz, 1 H), 3.21 (dd, J = 16.8, 3.4 Hz, 1 H). 13C-NMR (75 MHz, CDCl3) δ 171.0 (C), 162.1 (C), 143.9 (CH), 142.5 (C), 138.9 (C), 134.9 (CH), 130.5 (2 CH), 130.4 (CH), 129.2 (2 CH), 127.9 (CH), 127.8 (C), 126.7 (CH), 125.4 (CH), 116.7 (C), 86.1 (CH), 39.4 (CH), 37.8 (CH2). IR νmax (film): 3,076, 2,929, 2,855, 1,756, 1,684, 1,600 cm−1. HRMS (ESI) m/z calculated for C19H15O4 [M + H+]: 307.0970; found: 307.0971.
(E)-5,5′-(((2-Oxodihydrofuran-3(2H)-ylidene)methyl)azanediyl)bis(3,4-dimethylfuran-2(5H)-one) 29
To a −10°C solution of 3-aminomethylenedihydrofuran-2-one 28 (Zanatta et al., 2003; 100 mg, 0.876 mmol, 1 eq.) in THF (5 mL) was added NaH (67 mg, 60% weight in mineral oil, 1.665 mmol, 1.9 eq.), and the resulting solution was stirred under argon for 30 min at this temperature and then cooled to −78°C, and the chlorobutenolide 15 (321 mg, 2.191 mmol, 2.5 eq.) was added to the reaction mixture. The solution was warmed to room temperature, stirred for 2.5 h, quenched with AcOH (0.5 mL) followed by water, and extracted with EtOAc (3 × 10 mL). The combined organic layers were washed with brine (10 mL), dried (MgSO4), filtered, and evaporated under reduced pressure. The residue was purified by chromatography on a silica gel (EtOAc:heptane 1:1) to give 22 mg of the compound 29 (8%) as an amorphous solid. 1H-NMR (500 MHz, CDCl3) δ 7.87 (t, J = 2.4 Hz, 1 H), 5.83 (s, 1 H), 5.29 (s, 1 H), 4.43 to 4.25 (m, 2 H), 3.40 to 3.29 (m, 1 H), 3.40 to 3.29 (m, 1 H), 2.11 (s, 3 H), 1.89 (t, J = 1.0 Hz, 3 H), 1.78 (s, 6 H). 13C-NMR (125 MHz, CDCl3) δ 172.8 (C), 171.8 (C), 169.4 (C), 153.4 (C), 150. 8 (C), 131.8 (C), 128.0 (CH), 127.5 (C), 105.0 (C), 95.1 (CH), 86.7 (CH), 65.2 (CH2), 25.8 (CH2), 12.9 (CH3), 11.5 (CH3), 8.8 (CH3), 8.7 (CH3). IR νmax (film): 2,923, 1,748, 1,659, 1,589 cm−1. HRMS (ESI) m/z calculated for C17H20NO6 [M + H+]: 334.1278; found: 334.1291.
(E)-3,4-Dimethyl-5-((2-oxodihydrofuran-3(2H)-ylidene)methoxy)furan-2(5H)-one 30
Potassium tert-butoxide (469 mg, 0.418 mmol, 1.2 eq.) was added to a mixture of γ-butyrolactone (300 mg, 0.28 mL, 3.48 mmol, 1 eq.) and ethyl formate (0.34 mL, 4.18 mmol, 1.2 eq.) in THF (7.5 mL) at 0°C under argon and stirred for 2 h. It was then cooled to −78°C, and the chlorobutenolide 15 (562 mg, 3.83 mmol, 1.3 eq.) was gradually added. The mixture was then warmed to room temperature and stirred for 18 h. The reaction mixture was evaporated under reduced pressure, diluted with CH2Cl2 (10 mL), and the organic layer was washed with an aqueous solution of acetic acid (5%, 10 mL). The aqueous phase was extracted with CH2Cl2 (2 × 10 mL), and the combined organic layers were evaporated under reduced pressure. The crude product was purified by chromatography on a silica gel (heptane:EtOAc 3:2 to 1:1) to afford 30 (292 mg, 37%) as a white solid. Melting point, 93.4°C to 93.8°C (heptane:EtOAc). 1H-NMR (300 MHz, CDCl3) δ 7.42 (t, J = 2.8 Hz, 1 H), 5.93 (s, 1 H), 4.35 (t, J = 7.5 Hz, 2 H), 2.88 (td, J = 7.5, 2.8 Hz, 2 H), 1.99 (t, J = 1.0 Hz, 3 H), 1.87 (t, J = 1.0 Hz, 3 H). 13C-NMR (75 MHz, CDCl3) δ 171.9 (C), 170.9 (C), 152.5 (C), 150.0 (CH), 128.5 (C), 107.6 (CH), 102.3 (CH), 66.0 (CH2), 24.1 (CH2), 11.6 (CH2), 8.8 (CH2). IR νmax (film): 2,987, 2,923, 1,771, 1,748, 1,685 cm−1. HRMS (ESI) m/z calculated for C11H13O5 [M + H+]: 225.0763; found: 225.0755.
5-((4-Chlorophenyl)thio)-3,4-dimethylfuran-2(5H)-one 31
Prepared from 4-chlorobenzenethiol and 5-chloro-3,4-dimethyl-2(5H)-furanone 15 (Canevet and Graff, 1978) by the same procedure as for 19 to give the desired product 31 (361 mg, 51%) as a white solid. Melting point, 74.5°C to 76.9°C. 1H-NMR (300 MHz, CDCl3) δ 7.34 (dt, J = 8.7, 2.1 Hz, 2 H), 7.20 (dt, J = 8.7, 2.1 Hz, 2 H), 5.83 to 5.82 (m, 1 H), 1.96 (t, J = 1.0, 3 H), 1.64 (t, J = 1.0, 3 H). 13C-NMR (75 MHz, CDCl3) δ 172.6 (C), 155.2 (C), 135.4 (C), 135.2 (2 CH), 129.2 (2 CH), 128.2 (C), 126.2 (C), 88.1 (CH), 12.6 (CH3), 8.5 (CH3). IR νmax (film): 2,924, 1,758, 1,673, 1,476, 1,093, 985 cm−1. HRMS (ESI) m/z calculated for C12H12ClO2S [M + H]+: 255.0247; found: 255.0245.
(3aR*,8bS*,E)-3-(((R*S*)-4-Methyl-5-oxo-2,5-dihydrofuran-2-yl)thio)methylene)-3,3a,4,8b-tetrahydro-2H-indeno[1,2-b]furan-2-one 33
To a solution of tricycle 6 (100 mg, 0.495 mmol, 1 eq.) in dry N,N-dimethylformamide (2 mL) was added MeI (0.111 mL, 1.978 mmol, 4 eq.) and dry K2CO3 (89 mg, 0.643 mmol, 1.3 eq.). The reaction mixture was stirred for 3 h at room temperature and diluted with t-butylmethylether (20 mL), and the resulting mixture was washed with water (3 × 20 mL). The organic phase was separated, dried (MgSO4), filtered, and evaporated under reduced pressure. The residue was purified by chromatography on a silica gel (EtOAc:heptane 3:7) to give 68 mg of the ether intermediate (53%) as a white solid. To a solution of ether intermediate (19 mg, 0.087 mg, 1 eq.) in a mixture of methanol (0.4 mL) and dioxane (1 mL) was added at room temperature NaHS (10 mg, 0.87 mmol, 10 eq.). The reaction mixture was warmed to 70°C for 3 h, cooled to room temperature, and evaporated under reduced pressure. Acetone (2 mL) was added to the residue, followed by 5-bromo-3-methyl-2(5H)-furanone 32 (35 mg, 0.2 mmol, 2.3 eq.) and dry K2CO3 (26 mg, 0.19 mmol, 2.2 eq.). The resulting mixture was stirred for 3 h at room temperature and evaporated under reduced pressure. The residue was purified by chromatography on a silica gel (EtOAc:heptane 3:7 to 1:1) to give 8 mg of the thio compound 33 (29%) as a colorless oil (inseparable mixture of two diastereomers, 1:1). 33 (two diastereoisomers 1:1): 1H-NMR (500 MHz, CDCl3) δ 7.50 (d, J = 7.6 Hz, 2 H), 7.47 (d, J = 2.5 Hz, 1 H), 7.46 (d, J = 2.5 Hz, 1 H), 7.33 (t, J = 7.3 Hz, 2 H), 7.27 (t, J = 7.3 Hz, 2 H), 7.22 (t, J = 7.3 Hz, 2 H), 7.05 to 7.04 (m, 2 H), 6.19 (dt, J = 8.2, 1.6 Hz, 2 H), 5.96 (d, J = 7.9 Hz, 2 H), 3.84 to 3.79 (m, 2 H), 3.44 (dd, J = 16.7, 3.2 Hz, 1 H), 3.42 (dd, J = 16.7, 3.2 Hz, 1 H), 3.10 (dd, J = 16.7, 4.1 Hz, 1 H), 3.09 (dd, J = 16.7, 4.1 Hz, 1 H), 2.02 (s, 6 H). 13C-NMR (125 MHz, CDCl3) δ 171.82 (C), 171.79 (C), 168.5 (2 C), 143.6 (2 CH), 142.58 (C), 142.56 (C), 138.74 (C), 138.70 (C), 134.1 (2 C), 133.7 (CH), 133.4 (CH), 130.4 (2 CH), 129.9 (2 C), 127.9 (2 CH), 126.73 (CH), 126.70 (CH), 125.38 (CH), 125.36 (CH), 85.9 (2 CH), 83.3 (CH), 83.1 (CH), 40.9 (2 CH), 36.7 (2 CH2), 11.0 (2 CH3). IR νmax (film): 3,083, 3,030, 2,928, 2,853, 1,767, 1,740, 1,655, 1,621 cm−1. HRMS (ESI) m/z calculated for C17H15O4S [M + H+]: 315.0691; found: 315.0681.
(R*)-2-(2,3-Dihydro-1H-inden-2-yl)-3-((2S*,4S*)-4-methyl-5-oxotetrahydrofuran-2-yloxy)propanoic acid 34
To a solution of 2′-epi-GR24 (30 mg, 0.101 mmol, 1 eq.) in EtOAc (5 mL) was added the catalyst 10% palladium on carbon (16 mg). The flask was flushed with H2, and a positive pressure of H2 was maintained. The mixture was stirred at room temperature under 1 atmosphere of H2 for 2 h. Then the mixture was filtered through a layer of Celite, and the solvent was removed under reduced pressure. The residue was purified by chromatography on a silica gel (EtOAc) to give 29.3 mg of the lactone 34 (95%) as a colorless oil. 1H-NMR (300 MHz, acetone d6) δ 7.21 to 7.16 (m, 2 H), 7.13 to 7.08 (m, 2 H), 5.56 (dd, J = 5.5, 4.5 Hz, 1 H), 4.05 (dd, J = 9.6, 4.5 Hz, 1 H), 3.90 (dd, J = 9.6, 8.7 Hz, 1 H), 3.10 to 2.98 (m, 2 H), 2.86 to 2.61 (m, 6 H), 1.76 to 1.65 (m, 1 H), 1.24 (d, J = 7.2 Hz, 3 H). 13C-NMR (75 MHz, acetone d6) δ 178.6 (C), 174.8 (C), 143.43 (C), 143.40 (C), 127.18 (CH), 127.16 (CH), 125.1 (2 CH), 104.3 (CH), 71.2 (CH2), 40.5 (CH), 37.9 (CH2), 37.8 (CH2), 37.1 (CH2), 35.0 (CH), 16.8 (CH3). IR νmax (film): 3,200 to 2,480 (br), 2,938, 2,845, 1,775, 1,738, 1,706, 1,146 cm−1. MS (ESI) m/z (%) 327 (100). HRMS (ESI) m/z calculated for C17H20O5Na [M + Na+]: 327.1208; found: 327.1222.
(3aR*,8bS*,E)-3-(((2S*,4S*)-4-Methyl-5-oxotetrahydrofuran-2-yloxy)methylene)-3,3a,4,8b-tetrahydro-2H-indeno[1,2-b]furan-2-one 35
To a solution of 2′-epi-GR24 (25 mg, 0.084 mmol, 1 eq.) in EtOAc (12 mL) was added the catalyst 10% palladium on carbon (2.3 mg). The flask was flushed with H2, and a positive pressure of H2 was maintained. The mixture was stirred at room temperature under 1 atmosphere of H2 for 20 min. Then the mixture was filtered through a layer of Celite, and the solvent was removed under reduced pressure. The residue was purified by chromatography on silica gel (EtOAc:heptane 1:1) to give 13.6 mg of the lactone 35 (54%) as a colorless oil. 1H-NMR (500 MHz, CDCl3) δ 7.48 (d, J = 7.2 Hz, 1 H), 7.45 (d, J = 2.6 Hz, 1 H), 7.32 (td, J = 7.2, 2.5 Hz, 1 H), 7.27 (d, J = 7.2 Hz, 1 H), 7.23 (d, J = 7.2 Hz, 1 H), 5.93 (d, J = 7.9 Hz, 1 H), 5.84 (dd, J = 5.7, 3.0 Hz, 1 H), 3.95 to 3.88 (m, 1 H), 3.41 (dd, J = 19.0, 9.4 Hz, 1 H), 3.08 (dd, J = 19.0, 3.4 Hz, 1 H), 2.84 to 2.71 (m, 2 H), 2.08 (dd, J = 8.5, 3.2 Hz, 1 H), 1.43 (d, J = 7.0 Hz, 3 H). 13C-NMR (125 MHz, CDCl3) δ 177.4 (C), 171.6 (C), 152.2 (CH), 142.7 (C), 138.9 (C), 130.3 (CH), 127.7 (CH), 126.6 (CH), 125.5 (CH), 113.1 (C), 103.2 (CH), 86.1 (CH), 38.9 (CH), 37.7 (CH2), 36.0 (CH2), 33.8 (CH), 17.2 (CH3). IR νmax (film): 2,936, 2,878, 1,788, 1,746, 1,709, 1,680, 1,592 cm−1. MS (ESI) m/z (%) 623 (40), 355 (20), 323 (100). HRMS (ESI) m/z calculated for C17H16O5Na [M + Na+]: 323.0895; found: 323.0897.
(2′R*,3aS*,8bS*)-3′-((S*)-4-Methyl-5-oxo-2,5-dihydrofuran-2-yloxy)-3a,4-dihydrospiro[indeno[1,2-b]furan-3,2′-oxiran]-2(8bH)-one 36
To a solution of 2′-epi-GR24 (11 mg, 0.0369 mmol, 1 eq.) in CH2Cl2 (1 mL) was added in a solution of freshly distilled dimethyldioxirane (Adam et al., 1991) acetone (2.1 mL, approximately 0.1 m) and stirred at 5°C for 12 h. The solution was allowed to reach room temperature, and the volatiles were evaporated under reduced pressure to give 11.5 mg (quantitative) of the lactone 36 (two diastereoisomers 7:3) as a white solid. 1H-NMR (500 MHz, CDCl3) δ 7.51 (t, J = 7.0 Hz, 1 H), 7.40 to 7.27 (m, 3 H), 6.96 to 6.93 (m, 0.3 H), 6.90 to 6.87 (m, 0.7 H), 6.16 to 6.13 (m, 1 H), 6.09 (d, J = 6.6 Hz, 0.7 H), 6.05 (d, J = 7.5 Hz, 0.3 H), 5.39 (s, 0.7 H), 5.13 (s, 0.3 H), 3.47-3.18 (m, 3 H), 2.00 (s, 3 H). 13C-NMR (125 MHz, CDCl3) δ 171.7 (2 C), 170.8 (2 C), 143.6 (C), 143.2 (C), 142.2 (CH), 141.8 (CH), 138.2 (C), 137.4 (C), 135.8 (C), 135.4 (C), 131.0 (CH), 130.8 (CH), 127.9 (2 CH), 126.7 (CH), 126.6 (CH), 125.6 (CH), 125.3 (CH), 99.4 (CH), 99.1 (CH), 87.1 (CH), 86.5 (CH), 81.6 (CH), 79.9 (CH), 63.6 (C), 61.8 (C), 40.2 (CH), 35.6 (CH), 34.0 (CH2), 33.3 (CH2), 11.0 (CH3), 10.9 (CH3). IR νmax (film): 2,923, 2,854, 1,769, 1,665, 1,609 cm−1. MS (ESI) m/z (%) 378 (100), 337 (40). HRMS (ESI) m/z calculated for C17H14O6Na [M + Na+]: 337.0688; found: 337.0674.
(3R*,3aS*,8bS*)-3-Hydroxy-3-(methoxy((S*)-4-methyl-5-oxo-2,5-dihydrofuran-2-yloxy)methyl)-3,3a,4,8b-tetrahydro-2H-indeno[1,2-b]furan-2-one 37
To a solution of epoxide 36 (11 mg, 0.035 mmol, 1 eq.) in a mixture CH2Cl2:methanol (1 mL; 1:4) was added p-toluenesulfonic acid (1 mg). The mixture was stirred at room temperature for 4 h and overnight at −20°C. Then the solvents were removed under reduced pressure, and the residue was purified by chromatography on silica gel (EtOAc:heptane 1:1) to give 10.2 mg of the alcohol 37 (two isomers, 7:3; 84%) as a colorless oil. 1H-NMR (500 MHz, CDCl3) δ 7.54 to 7.21 (m, 4 H), 6.96 to 6.94 (m, 0.7 H), 6.93 to 6.91 (m, 0.3 H), 6.20 (t, J = 1.3 Hz, 0.7 H), 6.11 (t, J = 1.3 Hz, 0.3 H), 5.90 (d, J = 5.3 Hz, 0.7 H), 5.68 (d, J = 7.2 Hz, 0.3 H), 4.93 (s, 0.3 H), 4.89 (s, 0.7 H), 3.64 (s, 0.9 H), 3.57 (s, 2.1 H), 3.47 to 3.18 (m, 3 H), 1.97 (t, J = 1.3 Hz, 0.9 H), 1.95 (t, J = 2.1 Hz, 3 H). 13C-NMR (125 MHz, CDCl3) major isomer δ 175.0 (2 C), 144.2 (C), 143.4 (CH), 137.7 (C), 134.0 (C), 130.8 (CH), 127.7 (CH), 126.7 (CH), 125.2 (CH), 105.0 (CH), 101.1 (CH), 86.7 (CH), 79.9 (C), 57.1 (CH3), 48.9 (CH), 31.1 (CH2), 10.70 (CH3). MS (ESI) m/z (%) 378 (100), 337 (40). HRMS (ESI) m/z calculated for C18H18O7Na [M + Na+]: 369.0950; found: 369.0955.
(3aR*,8bS*)-3-((((2S*,4S*)-4-Methyl-5-oxotetrahydrofuran-2-yl)oxy)methyl)-3,3a,4,8b-tetrahydro-2H-indeno[1,2-b]furan-2-one 38
Prepared from compound 5 (Mangnus and Zwanenburg, 1992) by the same procedure as for 35 to give the desired product 38 (5 mg, 50%) as a colorless oil. 1H-NMR (500 MHz, CDCl3) δ 7.46 (d, J = 7.3 Hz, 1 H), 7.33 (t, J = 7.3 Hz, 1 H), 7.29 (d, J = 7.3, 1 H), 7.25 (d, J = 7.3 Hz, 1 H), 5.89 (d, J = 7.3 Hz, 1 H), 5.50 (t, J = 5.7 Hz, 1 H), 4.11 (dd, J = 9.5, 6.9 Hz, 1 H), 3.94 (dd, J = 9.5, 4.1 Hz, 1 H), 3.31 to 3.24 (m, 2 H), 3.12 (d, J = 14.2 Hz, 1 H), 2.68 to 2.56 (m, 3 H), 1.86 to 1.83 (m, 1 H), 1.35 (d, J = 6.9 Hz, 3 H). 13C-NMR (125 MHz, CDCl3) δ 178.5 (C), 176.2 (C), 141.8 (C), 139.0 (C), 130.1 (CH), 127.9 (CH), 126.4 (CH), 125.7 (CH), 103.1 (CH), 86.1 (CH), 69.1 (CH2), 47.6 (CH), 42.3 (CH2), 37.1 (CH), 36.6 (CH), 34.5 (CH2), 17.0 (CH3). IR νmax (film): 2,925, 2,853, 1,771, 1,608 cm−1. HRMS (ESI) m/z calculated for C17H18O5Na [M + Na+]: 325.1052; found: 325.1068.
Chemical Stability
Solanacol, solanacyl acetate, GR24, and 23 were tested for their chemical stability in an aqueous solution. Aqueous solutions of the compound to be tested (50 μg mL−1) were incubated at 21°C in the HPLC vials. The compounds were first dissolved in acetone (1 mL). Then, 50 μL of the previous solutions was diluted to the final concentration with ethanol (175 μL) and water (750 μL). Indanol (25 μL of a 1 mg mL−1 solution in acetone) as an internal standard was added to each solution. The time course of degradation was monitored by ultra-performance liquid chromatography analysis using an Acquity UPLC HSS C18 column (1.8 μm, 2.1 × 50 mm) eluted first by 5% acetonitrile in water containing 0.1% formic acid for 0.5 min, then by a gradient from 5% to 100% acetonitrile in water containing 0.1% formic acid within 6.5 min, and by 100% acetonitrile containing 0.1% formic acid for 3 min. The column was operated at 40°C with a flow rate of 0.6 mL min−1. Compounds eluted from the column were detected with a photodiode array detector. The relative quantity of remaining (no degraded) product was determined by integration comparison with the internal standard.
Plant Assays
Bud Outgrowth Inhibition Activity Assay
Pea (Pisum sativum) rms1 mutant plants (allele rms1-10 identified in the dwarf line Térèse; Beveridge et al., 1997; Rameau et al., 1997; Sorefan et al., 2003; Gomez-Roldan et al., 2008) deficient in SLs were used for the bioassay. The compound to be tested was applied directly to the bud with a micropipette as 10 µL of solution containing 0.1% acetone with 2% polyethylene glycol 1450, 50% ethanol, and 0.4% dimethyl sulfoxide. Twenty-four plants were sown per treatment in trays (two repetitions of 12 plants). The treatment was generally done 10 d after sowing on the axillary bud at node 4 (or 3). The branches at nodes 1 to 2 were removed to encourage the outgrowth of axillary buds at the nodes above. Nodes were numbered acropetally from the first scale leaf as node 1 and the cotyledonary node as node 0. Bud growth at node 4 (node 3 and node 5 in one bioassay) was measured 8 to 10 d after treatment with digital calipers. Generally, if poor activity or no activity was found at 1 μm concentration, no replication was done. Because the treatment in itself is stressful, we observed 10 d after the treatment some plants with dead buds (small yellow/white buds). Those plants for which the treatment had a toxic effect were eliminated from the analysis.
Pea Hydroponic Culture
The high-branching SL-deficient pea rms1 mutant plant rms1-10 was used. The nutrient solution (100%) was prepared by adding, in 1,000 L of water, the following macronutrients: HNO3 (0.28 L), (NH4)2HPO4 (120 g), Ca(NO3)2 (40 g), Mg(NO3)2 (140 g), KNO3 (550 g), (NH4)2MoO4 (0.05 g), H3BO3 (15 g), MnSO4·H2O (2 g), ZnSO4·0.7H2O (1 g), CuSO4·0.5H2O (0.25 g), and Sequestrene (10 g; Fe-EDTA solution). Pea seeds were germinated in wet sand for 6 d. Germinated seeds were placed in premade holes in the lid (35 holes per lid, 20 mm diameter) of a hydroponic polyvinyl chloride opaque pot containing the hydroponic culture solution (47 L, pH 5.8). Acetone or the compound to be tested (dissolved in acetone) was added to the hydroponic culture solution to give a final concentration of 0, 1, or 3 µm of the compound to be tested and 0.01% acetone. The hydroponic culture solution was continuously aerated by an aquarium pump and was replaced weekly. Eight days after the beginning of the treatment, branch/lateral bud lengths (nodes 1–6) were measured with an electronic caliper.
PsBRC1 Transcript Levels
The different compounds were applied directly to the bud as done in branching bioassays. The axillary buds at node 3 from 30 plants per treatment and biological repeat were dissected. Axillary buds were harvested, total RNA was isolated and quantified, complementary DNA was synthesized, and real-time PCR gene expression analyses were conducted as described (Braun et al., 2012). Quantitative real-time PCR analyses were performed using SYBR ROX RealMasterMix (5Prime). Cycling conditions for amplification were 95°C for 10 min, 50 cycles of 95°C for 5 s, 62°C for 5 s, and 72°C for 15 s, followed by 0.1°C s–1 ramping up to 95°C for fusion curve characterization. Three biological repeats were analyzed in duplicate. To calculate relative transcript levels, the comparative cycle method based on nonequal efficiencies was used (Pfaffl, 2001). Transcript levels for the different genes were expressed relative to expression of the EF1α gene (Johnson et al., 2006). Primer sequences were as follows: PsBRC1 forward (5′-TCGAAAGACGGAATCAAACA-3′) and PsBRC1 reverse (5′-TCCCTTGCTCTTTCTCTTGC-3′); EF1α forward (5′-TGTGCCAGTGGGACGTGTTG-3′) and EF1α reverse (5′-CTCGTGGTGCATCTCAACGG-3′).
Germination Stimulation Activity Assay
GR24 and SL analogs were resuspended in acetone at 10 mmol L−1 and then diluted with water at 1 mmol L−1 (water:acetone 99:1, v/v). Dilutions of 1 × 10−3 to 1 × 10−15 mol L−1 were then performed in water:acetone (99:1, v/v). Seeds of Phelipanche ramosa (from St. Martin de Fraigneau, France, 2005, on Brassica napus) were surface sterilized according to Dos Santos et al. (2003), resuspended in sterile water (10 g L−1), and distributed on 96-well plates (50 µL, approximately 100 seeds per well). After preconditioning (7 d and 21°C, on a dark, sealed plate), GR24 (10×) or other molecules were added and volumes were adjusted to 100 µL with water (water:acetone 999:1, v/v). Controls were made with water:acetone (999:1, v/v) and without seeds. Plates were incubated for germination (21°C, in the dark). After 4 d, the germinated seeds were counted with a stereomicroscope (SZX10; Olympus). Seeds were considered as germinated when the radicle protruded out of the seed coat. Each germination assay was repeated at least three times. For each compound tested, dose-response curves [g = f(c), where g = germination percentage, f = function, and c = concentration (mol L−1)] and EC50 were modeled with a four-parameter logistic curve computed with SigmaPlot 10.0.
Statistical Analyses
Because deviations from normality were observed for axillary bud length after SL treatment, the Kruskal-Wallis test was used to assess the significance of treatment in comparison with the control treatment (0 nm) using R Commander version 1.7-3 (Fox, 2005; Tables I–VI).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Bioassay on bud outgrowth using hydroponic culture: comparison of the activity of GR24 and GR5.
Supplemental Figure S2. Dose-dependent activity of the SL analogs GR24 and GR5.
Supplemental Figure S3. Effect of different SLs (500 nm) on PsBRC1 transcript levels in axillary buds at node 3 of rms1 plants.
Supplemental Figure S4. Stimulatory activity of GR24, GR5, 23, and 30 toward P. ramosa seed germination.
Supplementary Material
Acknowledgments
Bayer CropScience is acknowledged for the supply of strigol, sorgolactone, and 5-deoxystrigol and K. Yoneyama for the supply of fabacyl acetate, orobanchol, and orobanchyl acetate. We thank S. Bonhomme and R. Beau for their comments on the manuscript.
Glossary
- SL
strigolactone
- AM
arbuscular mycorrhizal
- SAR
structure-activity relationship
- EC50
half-maximal effective concentration
- ESI
electrospray ionization
- EtOAc
ethyl acetate
- THF
tetrahydrofuran
- MS
mass spectrometry
- m/z
mass-to-charge ratio
- IR
infrared
References
- Adam W, Bialas J, Hadjiarapoglou L. (1991) A convenient preparation of acetone solutions of dimethyldioxirane. Chem Ber 124: 2377 [Google Scholar]
- Agusti J, Herold S, Schwarz M, Sanchez P, Ljung K, Dun EA, Brewer PB, Beveridge CA, Sieberer T, Sehr EM, Greb T. (2011) Strigolactone signaling is required for auxin-dependent stimulation of secondary growth in plants. Proc Natl Acad Sci USA 108: 20242–20247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama K, Hayashi H. (2006) Strigolactones: chemical signals for fungal symbionts and parasitic weeds in plant roots. Ann Bot (Lond) 97: 925–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama K, Matsuzaki K, Hayashi H. (2005) Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435: 824–827 [DOI] [PubMed] [Google Scholar]
- Akiyama K, Ogasawara S, Ito S, Hayashi H. (2010) Structural requirements of strigolactones for hyphal branching in AM fungi. Plant Cell Physiol 51: 1104–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alder A, Jamil M, Marzorati M, Bruno M, Vermathen M, Bigler P, Ghisla S, Bouwmeester H, Beyer P, Al-Babili S. (2012) The path from β-carotene to carlactone, a strigolactone-like plant hormone. Science 335: 1348–1351 [DOI] [PubMed] [Google Scholar]
- Antoniotti S, Duñach E. (2008) Facile preparation of metallic triflates and triflimidates by oxidative dissolution of metal powders. Chem Commun (Camb) 44: 993–995 [DOI] [PubMed] [Google Scholar]
- Arite T, Umehara M, Ishikawa S, Hanada A, Maekawa M, Yamaguchi S, Kyozuka J. (2009) d14, a strigolactone-insensitive mutant of rice, shows an accelerated outgrowth of tillers. Plant Cell Physiol 50: 1416–1424 [DOI] [PubMed] [Google Scholar]
- Besserer A, Puech-Pagès V, Kiefer P, Gomez-Roldan V, Jauneau A, Roy S, Portais JC, Roux C, Bécard G, Séjalon-Delmas N. (2006) Strigolactones stimulate arbuscular mycorrhizal fungi by activating mitochondria. PLoS Biol 4: e226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge CA. (2000) Long-distance signalling and a mutational analysis of branching in pea. Plant Growth Regul 32: 193–203 [Google Scholar]
- Beveridge CA, Kyozuka J. (2010) New genes in the strigolactone-related shoot branching pathway. Curr Opin Plant Biol 13: 34–39 [DOI] [PubMed] [Google Scholar]
- Beveridge CA, Symons GM, Murfet IC, Ross JJ, Rameau C. (1997) The rms1 mutant of pea has elevated indole-3-acetic acid levels and reduced root-sap zeatin riboside content but increased branching controlled by graft-transmissible signal(s). Plant Physiol 115: 1251–1258 [Google Scholar]
- Booker J, Auldridge M, Wills S, McCarty D, Klee H, Leyser O. (2004) MAX3/CCD7 is a carotenoid cleavage dioxygenase required for the synthesis of a novel plant signaling molecule. Curr Biol 14: 1232–1238 [DOI] [PubMed] [Google Scholar]
- Bouwmeester HJ, Roux C, Lopez-Raez JA, Bécard G. (2007) Rhizosphere communication of plants, parasitic plants and AM fungi. Trends Plant Sci 12: 224–230 [DOI] [PubMed] [Google Scholar]
- Braun N, de Saint Germain A, Pillot JP, Boutet-Mercey S, Dalmais M, Antoniadi I, Li X, Maia-Grondard A, Le Signor C, Bouteiller N, et al. (2012) The pea TCP transcription factor PsBRC1 acts downstream of strigolactones to control shoot branching. Plant Physiol 158: 225–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer PB, Dun EA, Ferguson BJ, Rameau C, Beveridge CA. (2009) Strigolactone acts downstream of auxin to regulate bud outgrowth in pea and Arabidopsis. Plant Physiol 150: 482–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canevet JC, Graff Y. (1978) Friedel-Crafts reaction of aromatic derivatives with 1,4-dicarbonyl-2,3-ethylenic compounds. II. Alkylations by some 5-hydroxy or 5-chloro-2,5-dihydro-2-furannones: new method for synthesis of 1h-indene-1-carboxylic acids. Tetrahedron 34: 1935–1942 [Google Scholar]
- Chen VX, Boyer F-D, Rameau C, Retailleau P, Vors J-P, Beau J-M. (2010) Stereochemistry, total synthesis, and biological evaluation of the new plant hormone solanacol. Chemistry 16: 13941–13945 [DOI] [PubMed] [Google Scholar]
- Cook CE, Coggon P, McPhail AT, Wall ME, Whichard LP, Egley GH, Luhan PA. (1972) Germination stimulants. 2. Structure of strigol-potent seed germination stimulant for witchweed (Striga lutea Lour.). J Am Chem Soc 94: 6198–6199 [Google Scholar]
- Cook CE, Whichard LP, Turner B, Wall ME, Egley GH. (1966) Germination of witchweed (Striga lutea Lour.): isolation and properties of a potent stimulant. Science 154: 1189–1190 [DOI] [PubMed] [Google Scholar]
- Crawford S, Shinohara N, Sieberer T, Williamson L, George G, Hepworth J, Müller D, Domagalska MA, Leyser O. (2010) Strigolactones enhance competition between shoot branches by dampening auxin transport. Development 137: 2905–2913 [DOI] [PubMed] [Google Scholar]
- Domagalska MA, Leyser O. (2011) Signal integration in the control of shoot branching. Nat Rev Mol Cell Biol 12: 211–221 [DOI] [PubMed] [Google Scholar]
- Dos Santos CV, Letousey P, Delavault P, Thalouarn P. (2003) Defense gene expression analysis of Arabidopsis thaliana parasitized by Orobanche ramosa. Phytopathology 93: 451–457 [DOI] [PubMed] [Google Scholar]
- Dun EA, de Saint Germain A, Rameau C, Beveridge CA. (2012) Antagonistic action of strigolactone and cytokinin in bud outgrowth control. Plant Physiol 158: 487–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell SCM, Harbridge JB. (1990) 2,5-Dimethoxy-2,5-dihydrofuran: a convenient synthon for a novel mono-protected glyoxal. Synthesis of 4-hydroxybutenolides. Tetrahedron Lett 31: 4227–4228 [Google Scholar]
- Foo E, Davies NW. (2011) Strigolactones promote nodulation in pea. Planta 234: 1073–1081 [DOI] [PubMed] [Google Scholar]
- Fox J. (2005) The R commander: a basic-statistics graphical user interface to R. J Stat Softw 14: 1–41 [Google Scholar]
- Fukui K, Ito S, Ueno K, Yamaguchi S, Kyozuka J, Asami T. (2011) New branching inhibitors and their potential as strigolactone mimics in rice. Bioorg Med Chem Lett 21: 4905–4908 [DOI] [PubMed] [Google Scholar]
- Galloway WRJD, Hodgkinson JT, Bowden SD, Welch M, Spring DR. (2011) Quorum sensing in gram-negative bacteria: small-molecule modulation of AHL and AI-2 quorum sensing pathways. Chem Rev 111: 28–67 [DOI] [PubMed] [Google Scholar]
- Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pagès V, Dun EA, Pillot J-P, Letisse F, Matusova R, Danoun S, Portais J-C, et al. (2008) Strigolactone inhibition of shoot branching. Nature 455: 189–194 [DOI] [PubMed] [Google Scholar]
- Gomez-Sanchez A, Moya PB, Bellanato J. (1984) Protection of the amino group of amino-sugars by the acylvinyl group. Part I. Glycoside formation by the Fischer reaction. Carbohydr Res 135: 101–116 [Google Scholar]
- Gould KS, Cutter EG, Young JPW, Charlton WA. (1987) Positional differences in size, morphology, and in vitro performance of pea axillary buds. Can J Bot 65: 406–411 [Google Scholar]
- Hoffmann HMR, Schmidt B, Wolff S. (1989) Preparation of 5-bromotetronates [4-alkoxy-5-bromo-2(5h)-furanones] and a new concept for the synthesis of aflatoxins and related structure types: tributyltin hydride versus palladium-promoted intramolecular hydroarylation. Tetrahedron 45: 6113–6126 [Google Scholar]
- Johnson AW, Gowda G, Hassanali A, Knox J, Monaco S, Razavi Z, Rosebery G. (1981) The preparation of synthetic analogs of strigol. J Chem Soc Perkin Trans 1981: 1734–1743 [Google Scholar]
- Johnson X, Brcich T, Dun EA, Goussot M, Haurogné K, Beveridge CA, Rameau C. (2006) Branching genes are conserved across species: genes controlling a novel signal in pea are coregulated by other long-distance signals. Plant Physiol 142: 1014–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just G, Chung BY, Kim S, Rosebery G, Rossy P. (1976) Reactions of oxygen and sulfur anions with oxazolidine and thiazolidine derivatives of 2-mesyloxymethylglyceraldehyde acetonide. Can J Chem 54: 2089–2093 [Google Scholar]
- Kapulnik Y, Delaux P-M, Resnick N, Mayzlish-Gati E, Wininger S, Bhattacharya C, Séjalon-Delmas N, Combier J-P, Bécard G, Belausov E, et al. (2011) Strigolactones affect lateral root formation and root-hair elongation in Arabidopsis. Planta 233: 209–216 [DOI] [PubMed] [Google Scholar]
- Kim HI, Xie XN, Kim HS, Chun JC, Yoneyama K, Nomura T, Takeuchi Y. (2010) Structure-activity relationship of naturally occurring strigolactones in Orobanche minor seed germination stimulation. J Pestic Sci 35: 344–347 [Google Scholar]
- Limberg G, Thiem J. (1995) [beta]-Elimination of protected aldono-1,4-lactones as a general approach to the synthesis of 2-keto-3-deoxyaldonic acids containing four to six carbon atoms. Carbohydr Res 275: 107–115 [Google Scholar]
- Macalpine GA, Raphael RA, Shaw A, Taylor AW, Wild HJ. (1976) Synthesis of germination stimulant (+/−)-strigol. J Chem Soc Perkin Trans 1 410–416 [Google Scholar]
- Malik H, Kohlen W, Jamil M, Rutjes FPJT, Zwanenburg B. (2011) Aromatic A-ring analogues of orobanchol, new germination stimulants for seeds of parasitic weeds. Org Biomol Chem 9: 2286–2293 [DOI] [PubMed] [Google Scholar]
- Malik H, Rutjes F, Zwanenburg B. (2010) A new efficient synthesis of GR24 and dimethyl A-ring analogues, germinating agents for seeds of the parasitic weeds Striga and Orobanche spp. Tetrahedron 66: 7198–7203 [Google Scholar]
- Mangnus EM, Dommerholt FJ, Dejong RLP, Zwanenburg B. (1992) Improved synthesis of strigol analog Gr24 and evaluation of the biological activity of its diastereomers. J Agric Food Chem 40: 1230–1235 [Google Scholar]
- Mangnus EM, Zwanenburg B. (1992) Tentative molecular mechanism for germination stimulation of Striga and Orobanche seeds by strigol and its synthetic analogs. J Agric Food Chem 40: 1066–1070 [Google Scholar]
- Matusova R, Rani K, Verstappen FWA, Franssen MCR, Beale MH, Bouwmeester HJ. (2005) The strigolactone germination stimulants of the plant-parasitic Striga and Orobanche spp. are derived from the carotenoid pathway. Plant Physiol 139: 920–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parniske M. (2008) Arbuscular mycorrhiza: the mother of plant root endosymbioses. Nat Rev Microbiol 6: 763–775 [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prandi C, Occhiato EG, Tabasso S, Bonfante P, Novero M, Scarpi D, Bova ME, Miletto I. (2011) New potent fluorescent analogues of strigolactones: synthesis and biological activity in parasitic weed germination and fungal branching. Eur J Org Chem 2011: 3781–3793 [Google Scholar]
- Proust H, Hoffmann B, Xie X, Yoneyama K, Schaefer DG, Yoneyama K, Nogué F, Rameau C. (2011) Strigolactones regulate protonema branching and act as a quorum sensing-like signal in the moss Physcomitrella patens. Development 138: 1531–1539 [DOI] [PubMed] [Google Scholar]
- Rameau C, Bodelin C, Cadier D, Grandjean O, Miard F, Murfet IC. (1997) New ramosus mutants at loci Rms1, Rms3 and Rms4 resulting from the mutation breeding program at Versailles. Pisum Genet 29: 7–12 [Google Scholar]
- Rani K, Zwanenburg B, Sugimoto Y, Yoneyama K, Bouwmeester HJ. (2008) Biosynthetic considerations could assist the structure elucidation of host plant produced rhizosphere signalling compounds (strigolactones) for arbuscular mycorrhizal fungi and parasitic plants. Plant Physiol Biochem 46: 617–626 [DOI] [PubMed] [Google Scholar]
- Reizelman A, Wigchert SCM, del-Bianco C, Zwanenburg B. (2003) Synthesis and bioactivity of labelled germination stimulants for the isolation and identification of the strigolactone receptor. Org Biomol Chem 1: 950–959 [DOI] [PubMed] [Google Scholar]
- Ruyter-Spira C, Kohlen W, Charnikhova T, van Zeijl A, van Bezouwen L, de Ruijter N, Cardoso C, Lopez-Raez JA, Matusova R, Bours R, et al. (2011) Physiological effects of the synthetic strigolactone analog GR24 on root system architecture in Arabidopsis: another belowground role for strigolactones? Plant Physiol 155: 721–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato D, Awad AA, Takeuchi Y, Yoneyama K. (2005) Confirmation and quantification of strigolactones, germination stimulants for root parasitic plants Striga and Orobanche, produced by cotton. Biosci Biotechnol Biochem 69: 98–102 [DOI] [PubMed] [Google Scholar]
- Scaffidi A, Waters MT, Bond CS, Dixon KW, Smith SM, Ghisalberti EL, Flematti GR. (2012) Exploring the molecular mechanism of karrikins and strigolactones. Bioorg Med Chem Lett 22: 3743–3746 [DOI] [PubMed] [Google Scholar]
- Schachtschabel D, Boland W. (2007) Efficient generation of a trisporoid library by combination of synthesis and biotransformation. J Org Chem 72: 1366–1372 [DOI] [PubMed] [Google Scholar]
- Schreiber J, Wermuth CG. (1965) Etude de la condensation de l'acide pyruvique avec les aldéhydes aliphatiques non substitués en alpha. I. Produits de condensation dont la chaine carbonée est ramifiée-semialdéhydes méthyl-alcoyl maliques et semialdéhydes méthyl-alcoyl maléiques. Bull Soc Chim Fr 2242–2249 [Google Scholar]
- Simon L, Bousquet J, Levesque RC, Lalonde M. (1993) Origin and diversification of endomycorrhizal fungi and coincidence with vascular land plants. Nature 363: 67–69 [Google Scholar]
- Sorefan K, Booker J, Haurogné K, Goussot M, Bainbridge K, Foo E, Chatfield S, Ward S, Beveridge C, Rameau C, et al. (2003) MAX4 and RMS1 are orthologous dioxygenase-like genes that regulate shoot branching in Arabidopsis and pea. Genes Dev 17: 1469–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto MJ, Fernandez-Aparicio M, Castellanos-Morales V, Garcia-Garrido JM, Ocampo JA, Delgado MJ, Vierheilig H. (2010) First indications for the involvement of strigolactones on nodule formation in alfalfa (Medicago sativa). Soil Biol Biochem 42: 383–385 [Google Scholar]
- Stévenin A, Boyer F-D, Beau J-M. (2012) Highly selective formation of β-glycosides of N-acetylglucosamine using catalytic iron(III) triflate. Eur J Org Chem 2012: 1699–1702 [Google Scholar]
- Surmont R, Verniest G, De Kimpe N. (2010) Short synthesis of the seed germination inhibitor 3,4,5-trimethyl-2(5H)-furanone. J Org Chem 75: 5750–5753 [DOI] [PubMed] [Google Scholar]
- Thuring J, Keltjens R, Nefkens GHL, Zwanenburg B. (1997) Synthesis and biological evaluation of potential substrates for the isolation of the strigol receptor. J Chem Soc Perkin Trans 1 759–765 [Google Scholar]
- Tsuchiya Y, McCourt P. (2012) Strigolactones as small molecule communicators. Mol Biosyst 8: 464–469 [DOI] [PubMed] [Google Scholar]
- Tumlinson JH, Klein MG, Doolittle RE, Ladd TL, Proveaux AT. (1977) Identification of the female Japanese beetle sex pheromone: inhibition of male response by an enantiomer. Science 197: 789–792 [DOI] [PubMed] [Google Scholar]
- Turnbull CGN, Booker JP, Leyser HMO. (2002) Micrografting techniques for testing long-distance signalling in Arabidopsis. Plant J 32: 255–262 [DOI] [PubMed] [Google Scholar]
- Ueno K, Fujiwara M, Nomura S, Mizutani M, Sasaki M, Takikawa H, Sugimoto Y. (2011a) Structural requirements of strigolactones for germination induction of Striga gesnerioides seeds. J Agric Food Chem 59: 9226–9231 [DOI] [PubMed] [Google Scholar]
- Ueno K, Nomura S, Muranaka S, Mizutani M, Takikawa H, Sugimoto Y. (2011b) Ent-2′-epi-orobanchol and its acetate, as germination stimulants for Striga gesnerioides seeds isolated from cowpea and red clover. J Agric Food Chem 59: 10485–10490 [DOI] [PubMed] [Google Scholar]
- Umehara M, Hanada A, Yoshida S, Akiyama K, Arite T, Takeda-Kamiya N, Magome H, Kamiya Y, Shirasu K, Yoneyama K, et al. (2008) Inhibition of shoot branching by new terpenoid plant hormones. Nature 455: 195–200 [DOI] [PubMed] [Google Scholar]
- Vurro M, Yoneyama K. (2012) Strigolactones: intriguing biologically active compounds. Perspectives for deciphering their biological role and for proposing practical application. Pest Manag Sci 68: 664–668 [DOI] [PubMed] [Google Scholar]
- Wolff S, Hoffmann HMR. (1988) Aflatoxins revisited: convergent synthesis of the ABC moiety. Synthesis 760–763 [Google Scholar]
- Xie X, Kusumoto D, Takeuchi Y, Yoneyama K, Yamada Y, Yoneyama K. (2007) 2′-Epi-orobanchol and solanacol, two unique strigolactones, germination stimulants for root parasitic weeds, produced by tobacco. J Agric Food Chem 55: 8067–8072 [DOI] [PubMed] [Google Scholar]
- Xie X, Yoneyama K, Kusumoto D, Yamada Y, Yokota T, Takeuchi Y, Yoneyama K. (2008) Isolation and identification of alectrol as (+)-orobanchyl acetate, a germination stimulant for root parasitic plants. Phytochemistry 69: 427–431 [DOI] [PubMed] [Google Scholar]
- Xie XN, Yoneyama K. (2010) The strigolactone story. Annu Rev Phytopathol 48: 93–117 [DOI] [PubMed] [Google Scholar]
- Xie XN, Yoneyama K, Harada Y, Fusegi N, Yamada Y, Ito S, Yokota T, Takeuchi Y, Yoneyama K. (2009) Fabacyl acetate, a germination stimulant for root parasitic plants from Pisum sativum. Phytochemistry 70: 211–215 [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Umehara M, Akiyama K , inventors. December 2, 2010. Plant branching inhibitor, method for producing same, and plant branching inhibitory composition. Japan Patent Application No. WO/2010/137662 [Google Scholar]
- Yoneyama K, Awad AA, Xie XN, Yoneyama K, Takeuchi Y. (2010) Strigolactones as germination stimulants for root parasitic plants. Plant Cell Physiol 51: 1095–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama K, Xie X, Kim HI, Kisugi T, Nomura T, Sekimoto H, Yokota T, Yoneyama K. (2012) How do nitrogen and phosphorus deficiencies affect strigolactone production and exudation? Planta 235: 1197–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama K, Xie X, Yoneyama K, Takeuchi Y. (2009) Strigolactones: structures and biological activities. Pest Manag Sci 65: 467–470 [DOI] [PubMed] [Google Scholar]
- Yoneyama K, Xie XN, Kusumoto D, Sekimoto H, Sugimoto Y, Takeuchi Y, Yoneyama K. (2007) Nitrogen deficiency as well as phosphorus deficiency in sorghum promotes the production and exudation of 5-deoxystrigol, the host recognition signal for arbuscular mycorrhizal fungi and root parasites. Planta 227: 125–132 [DOI] [PubMed] [Google Scholar]
- Zanatta N, Barichello R, Pauletto MM, Bonacorso HG, Martins MAP. (2003) Convenient synthesis of 3-aminomethylenedihydrofuran-2-ones. Tetrahedron Lett 44: 961–964 [Google Scholar]
- Zwanenburg B, Mwakaboko AS. (2011) Strigolactone analogues and mimics derived from phthalimide, saccharine, p-tolylmalondialdehyde, benzoic and salicylic acid as scaffolds. Bioorg Med Chem 19: 7394–7400 [DOI] [PubMed] [Google Scholar]
- Zwanenburg B, Mwakaboko AS, Reizelman A, Anilkumar G, Sethumadhavan D. (2009) Structure and function of natural and synthetic signalling molecules in parasitic weed germination. Pest Manag Sci 65: 478–491 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.