Abstract Abstract
The new spider genus and species Trogloraptor marchingtoni Griswold, Audisio & Ledford is described as the type of the new family Trogloraptoridae. The oblique membranous division of the basal segment of the anterior lateral spinnerets of Trogloraptor suggests that this haplogyne family is the sister group of the other Dysderoidea (Dysderidae, Oonopidae, Orsolobidae and Segestriidae). Trogloraptor is known only from caves and old growth forest understory in the Klamath-Siskiyou region of Oregon and California.
Keywords: Haplogynae, Caves, Pacific Northwest
Introduction
The spider fauna of North America is a rich one, with at least 68 families including 569 genera and comprising more than 3700 species (Ubick et al. 2005). In the last generation significant progress has been made at understanding this fauna. For example, continent-wide taxonomic treatments of major families have appeared, e.g., Araneidae (Levi 1968 to present), Gnaphosidae (Platnick 1975 and subsequent) and Theridiidae (Levi 1953 and subsequent), the largest genera of jumping spiders (Salticidae) have been revised, i.e., Habronattus (Griswold, 1987), Pelegrina (Maddison, 1996) and Phidippus (Edwards, 2004) and the Canadian government has produced a series of guides to Canadian spiders, e.g., Dondale and Redner (1982). Perhaps most important was Vince Roth’s key to families and genera (Roth 1982), which has been updated as the comprehensive and profusely illustrated Spiders of North America: an identification manual (Ubick et al. 2005). This makes it relatively easy to identify to family and even to genus any spider from America north of Mexico. Nevertheless, there are still surprises in store in this well known region, especially in remote or inaccessible places, such as caves. The troglofauna of the Pacific Northwest is poorly surveyed and several arachnid groups are known to have multiple undescribed species (Ledford et al. in prep.). In this paper we describe a remarkable haplogyne spider that fits into no known family, living or extinct (Jocqué and Dippenaar-Schoeman 2006; Penney and Selden 2011). Trogloraptor marchingtoni gen. et sp. n., type of the new family Trogloraptoridae, is described from caves in the Pacific Northwest, and a diagnostic table (Table 1) and character discussion are presented to distinguish it from all other spider families.
Table 1.
Diagnostic characters for Haplogynae families. Italics represents diagnosis from Trogloraptoridae.
| Family | AME | PME distance | Cheliceral bases | Cheliceral lamina | Posterior receptaculum | 3rd Entapophyses | Posterior spiracles | Posterior spiracle(s) | Emerit’s glands | Tarsal claw number | Serrula tooth rows | Labium-sternum junction | ALS basal segment | PLs AC gland spigots | CY gland spigots | Colulus |
| Caponiidae | present | separate | fused | present | absent | separate, short | two | advanced | absent | three | one | fused | entire | dispersed | absent | small |
| Diguetidae | absent | contiguous | fused | present | absent | fused | one | posterior | absent | three | one | fused | entire | dispersed | absent | small |
| Drymusidae | absent | contiguous | fused | present | absent | fused | one | posterior | absent | three | one | fused | entire | dispersed | absent | small |
| Dysderidae | absent | contiguous | free | absent | present | separate, short | two | advanced | absent | three | one | free | divided | dispersed | absent | small |
| Filistatidae | present | contiguous | fused | present | absent | separate, long | two | posterior | absent | three | one | fused | entire | dispersed | absent | absent |
| Leptonetidae | absent | contiguous | free | absent | absent | separate, long | one | posterior | present | three | one | fused | entire | in line | present | small |
| Ochyroceratidae | absent | contiguous | free | present | absent | separate, short | one | posterior | absent | three | one | fused | entire | in line | absent | large |
| Oonopidae | absent | contiguous | free | absent | present | separate, short | two | advanced | prs; abs | two | one | free | divided | dispersed | absent | small |
| Orsolobidae | absent | contiguous | free | absent | present | separate, short | two | advanced | absent | two | one | free | divided | dispersed | absent | small |
| Periegopidae | absent | contiguous | fused | present | absent | fused | one | posterior | absent | three | one | free | entire | dispersed | absent | small |
| Pholcidae | present | separate | fused | present | absent | absent | one | posterior | absent | three | one | fused | entire | dispersed | absent | small |
| Plectreuridae | present | separate | fused | present | absent | fused | one | posterior | absent | three | one | free | entire | dispersed | absent | small |
| Scytodidae | absent | contiguous | fused | present | absent | fused | one | posterior | absent | three | one | fused | entire | dispersed | absent | large |
| Segestriidae | absent | contiguous | free | absent | present | separate, short | two | advanced | absent | three | one | free | divided | dispersed | absent | small |
| Sicariidae | absent | contiguous | fused | present | absent | fused | one | posterior | absent | two | one; abs | fused | entire | dispersed | absent | large; abs |
| Telemidae | absent | contiguous | free | absent | absent | absent | two | posterior | present | three | one | fused | entire | in line | present | large |
| Tetrablemmidae | absent | contig./sep. | free | present | absent | fused | one | posterior | absent | three | one | free | entire | dispersed | absent | small |
| Trogloraptoridae | absent | separate | free | absent | absent | separate, long | one | posterior | absent | three | multiple | fused | divided | dispersed | absent | small |
Materials and methods
Species descriptions refer to a single adult individual for each sex, which is identified as a type or by the locality at which it was collected. All measurements are in millimeters and quantify the size of a structure at its widest or longest point. A section reporting the variation in the most conspicuous and variable features follows each description and represents multiple individuals (n), encompassing the full range in overall size.
Prior to examination with a Leo 1450VP Scanning Electron Microscope, all structures were cleaned with a fine brush and critical point dried. Spinneret preparations followed the methods of Griswold et al. (2005) consisting of a brief cleaning in an ultrasonicator and a gentle squeeze of the abdomen using forceps locked in place with a paperclip followed by overnight immersion in 100% ethanol in order to extend and separate the spinnerets. Large structures were examined using a Leica MZ 12.5 or MZ 16 stereomicroscope. Vulvae were carefully excised and placed in a pancreatin solution for 24–48 hours to digest extraneous tissue (Álvarez-Padilla and Hormiga 2007) then placed in water and manually cleaned. Light micrographs were prepared using a Nikon DXM1200 digital camera attached to a Leica MZ 16 stereomicroscope or a Leica DM 4000 compound microscope; multiple, stacked images were montaged using the program Helicon Focus®. The male pedipalp was checked for haematodochal expansion by placing it into boiling water for 2–3 minutes in a vial of hot 92% lactic acid solution (Sigma Aldrich, St Louis MO, USA), after which the pedipalp was transferred to distilled water (Ledford et al. 2011). The internal anatomy of the pedipalpal bulb and course of the reservoir were examined by immersing it in a solution of methyl salicylate (synthetic oil of wintergreen), from Mallinckrodt Baker Chemicals, Philipsburg NJ, USA (Miller et al. 2009). Abbreviations used in the text and figures are as follows: AC, aciniform gland spigots; ALE, anterior lateral eyes; ALS, anterior lateral spinnerets; AME, anterior median eyes; AT, atrium; BM, Brent McGregor; CG, Charles Griswold; ITC, inferior tarsal claw; JL, Joel Ledford; MAP, major ampullate gland spigot(s); mAP, minor ampullate gland spigot(s); Nu, nubbin (an aborted spigot); OAL, ocular area length; PER, posterior eye row; PI, piriform gland spigots; PLE, posterior lateral eyes; PLS, posterior lateral spinnerets; PME, posterior median eyes; PMS, posterior median spinnerets; RC, receptaculum; RD, Ron Davis; STC, superior tarsal claw(s). All specimens are deposited in the California Academy of Sciences (CAS).
Phylogenetic placement
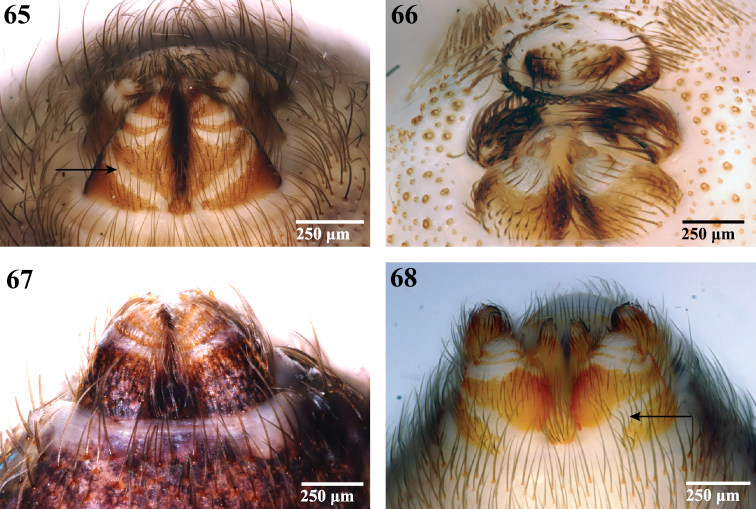
Trogloraptoridae have simple, haplogyne genitalia, with a single opening of the female vulva for fertilization and oviposition. This family differs from most other haplogyne clades. The Palpimanoidea (Archaeidae, Huttoniidae, Mecysmaucheniidae, Palpimanidae and Stenochilidae) have a foramen around the cheliceral bases, two protrusions posterior to the labral tongue, cheliceral peg teeth and a cheliceral gland mound (Wood et al. in press), all lacking in trogloraptorids. Austrochiloidea (Austrochilidae and Gradungulidae) have a clypeal hood, notched trichobothrial bases, and cylindrical gland spigots (Griswold et al. 2005), again all lacking in trogloraptorids. In spite of striking similarities in the raptorial and asymmetrical claws between gradungulids and trogloraptorids, these families are only distantly related. Trogloraptorids are not Entelegynae with secondarily simplified genitalia: the primitive tapetum in the indirect eyes (Figs 9, 11) and absence of tartipores from the spinnerets (Figs 69–74) rule this out (Griswold et al. 2005). The piriform pedipalpal bulb (Figs 9, 51–58) of the male, with tegulum and subtegulum fused, is a synapomorphy placing Trogloraptor in the clade Haplogynae. This clade contains, in addition to the new family Trogloraptoridae, 17 other families: Caponiidae, Diguetidae, Drymusidae, Dysderidae, Filistatidae, Leptonetidae, Ochyroceratidae, Oonopidae, Orsolobidae, Periegopidae, Pholcidae, Plectreuridae, Scytodidae, Segestriidae, Sicariidae, Telemidae and Tetrablemmidae (Platnick et al. 1991; Ramírez 2000). Haplogyne families are among the most clearly defined in the Araneae and well characterized by features of the chelicerae, spinning organs and posterior respiratory system. Trogloraptoridae are distinguished from Caponiidae, Diguetidae, Drymusidae, Filistatidae, Periegopidae, Pholcidae, Plectreuridae, Scytodidae and Sicariidae in having the chelicerae free at the base (Figs 9, 19, 23) (not fused at the base), from Dysderidae, Oonopidae, Orsolobidae, Segestriidae and Telemidae in having a single posterior spiracle (Figs 12, 60, 83) (not two posterior spiracles), from most Leptonetidae and Ochyroceratidae in having scattered AC gland spigots (Figs 73–80) (not AC gland spigots in rows on the PMS and/or PLS) and having the PME widely separated (Figs 9, 11) (not contiguous), from all Leptonetidae in having coxa-trochanter leg autospasy (not at the patella-tibia) and from Tetrablemmidae (as well as Diguetidae, Drymusidae, Periegopidae, Plectreuridae, Scytodidae and Sicariidae) in having long, separate 3rd abdominal entapophyses (Figs 60, 63, 64) (not fused entapophyses). For simplicity we depict 16 diagnostic characters across the 18 families of Haplogynae in Table 1. Another striking feature is the serrula with multiple tooth rows, otherwise found in Araneomorphae only in Hypochilidae. In many ways Trogloraptoridae are a collection of primitive character states. A clue to this family’s phylogenetic affinity comes from the basal segment of the ALS: the segment is crossed diagonally by a line of membranous cuticle, contrasting with the surrounding sclerotization (Fig. 68). This morphology was first reported by Simon (1893: 310) in Dysderidae, and is a probable synapomorphy for the Dysderoidea (Dysderidae, Oonopidae, Orsolobidae and Segestriidae) (Fig. 65). It is lacking in other Haplogynae, e.g., Figures 66, 67. This peculiar spinneret morphology in Trogloraptoridae suggests that this family is allied to the Dysderoidea. If so, the family Trogloraptoridae is a primitive member of Dysderoidea, lacking the paired, anteriorly advanced posterior tracheal spiracles and posterior receptaculum of the vulva that are synapomorphies uniting the four remaining families, Dysderidae, Oonopidae, Orsolobidae and Segestriidae.
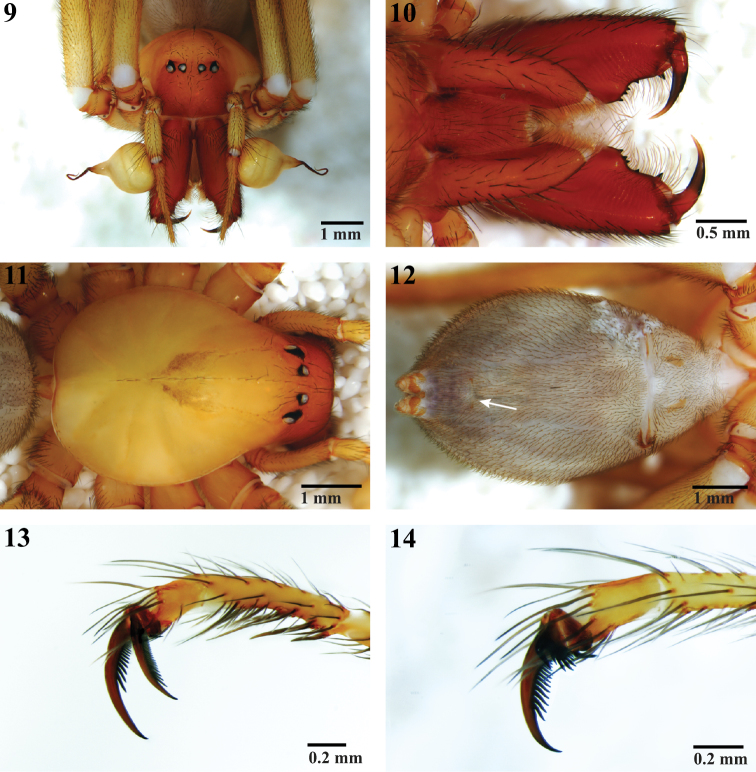
Figures 9–14.
Habitus and tarsi of male Trogloraptor marchingtoni (CASENT9040013) from M2 Cave. 9 front 10 mouthparts, ventral view 11 carapace, dorsal view 12 abdomen, ventral view, arrow to tracheal spiracle 13 tarsus I, prolateral; and 14 tarsus IV, prolateral.
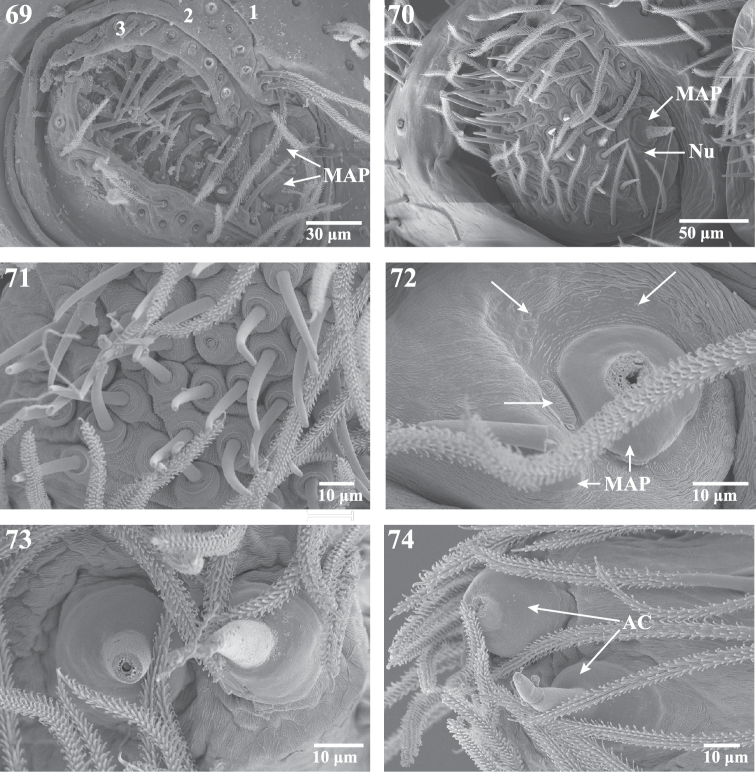
Figures 69–74.
Scanning electron micrographs of the ALS and PLS of Trogloraptor marchingtoni, female (CASENT9039440) and male (CASENT9040066) from No Name Cave and penultimate female from M2 Cave (CASENT9040012). 69 penultimatefemale, right ALS, numbers refer to the three ALS segments 70 male right ALS 71 female ALS piriform gland spigots (left image flipped to appear right) 72 female major ampullate gland spigots of ALS, arrows showing individual and grouped sensillae (right image flipped to appear left) 73 female PLS apex showing aciniform gland spigots (left image flipped to appear right); and 74 female right PLS apex. AC aciniform gland spigots MAP major ampullate gland spigot(s) Nu nubbin.
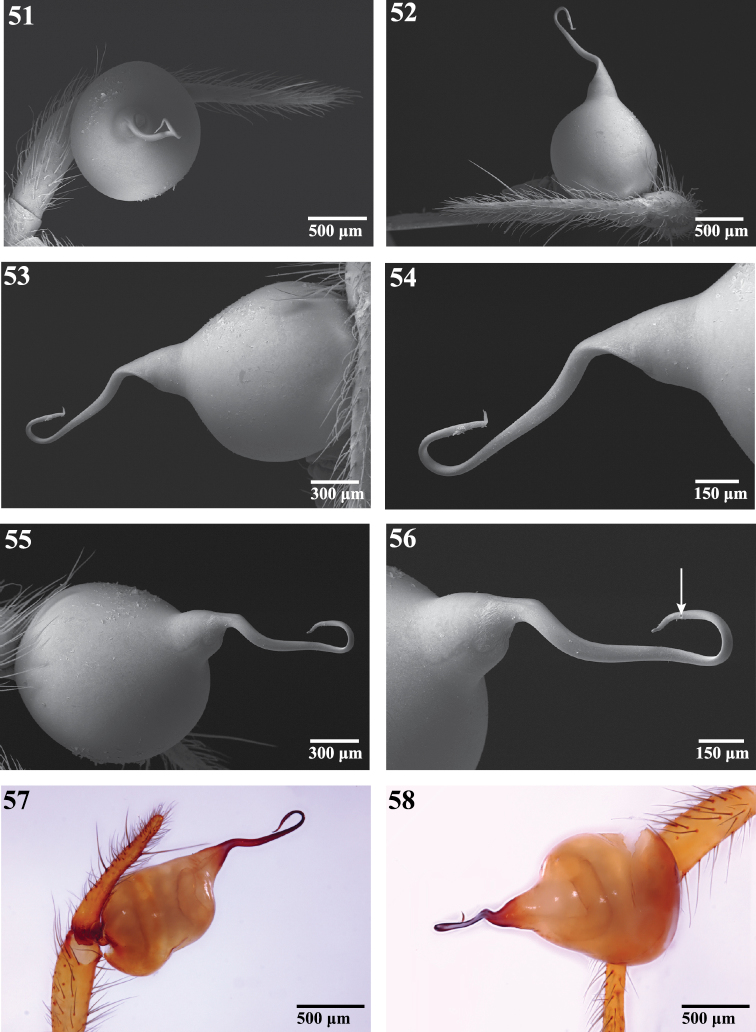
Figures 51–58.
Male pedipalp of Trogloraptor marchingtoni: 51–56 Scanning electron micrographs of right pedipalp 51 tarsus and bulb, apical view 52 tarsus and bulb, dorsal view 53 bulb, dorsal view 54 embolus, dorsal view 55 bulb, ventral view 56 embolus, ventral view, arrow to sperm pore 57, 58 Automontage of left pedipalp 57 prolateral view 58 retrolateral view. Figures 51–56 CASENT9040013 from M2 Cave 57, 58 CASENT9040066 from No Name Cave.
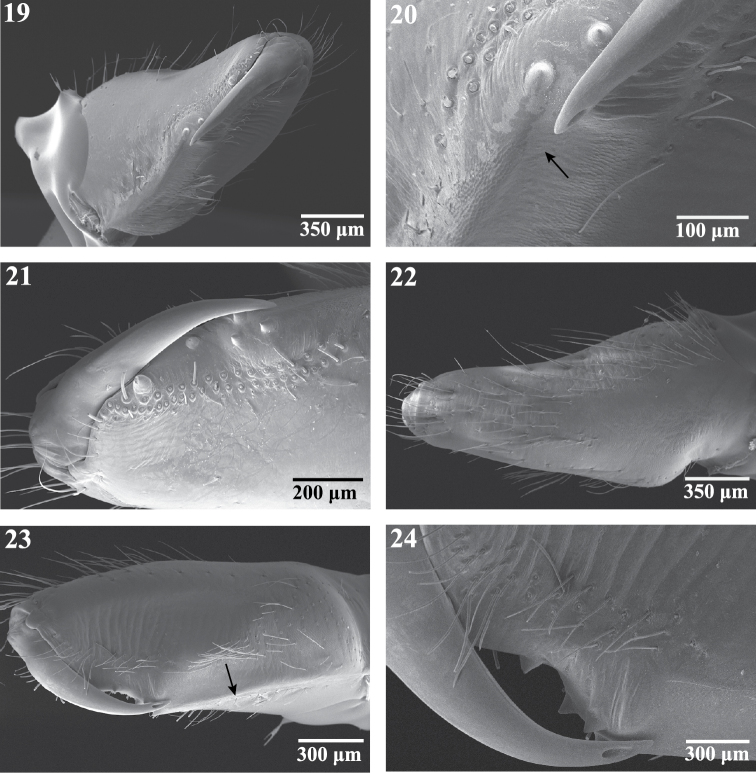
Figures 19–24.
Scanning electron micrographs of the right chelicera of female Trogloraptor marchingtoni (CASENT9040051) from M2 Cave. 19 mesal view 20 mesal view, arrow to cheliceral gland openings 21 retrolateral view 22 ectal view 23 prolateral view, arrow to weak laminar ridge; and 24 prolateral view, close up of fang and opening of poison gland.
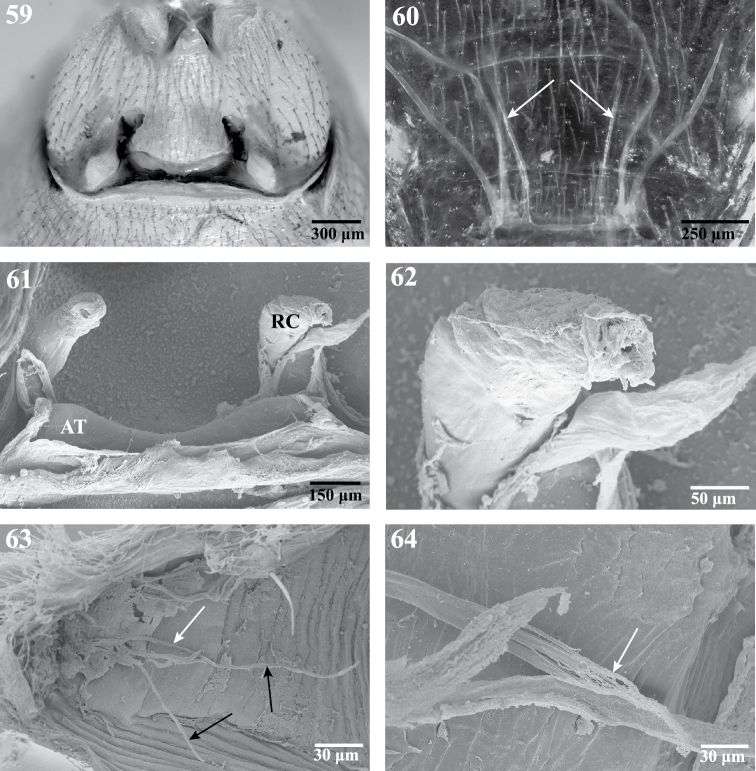
Figures 59–64.
Internal anatomy of Trogloraptor marchingtoni, female (CASENT9040051) from No Name Cave. 59 vulva, dorsal view 60 female posterior respiratory system, tracheae and apodemes, with arrows to median apodemes61–64 Scanning electron micrographs of the internal anatomy 61 vulva, dorsal view, AT, atrium, RC, receptaculum 62 apex of right receptaculum 63 posterior respiratory system, dorsal view, with white arrow to median apodeme and black arrows to lateral tracheal branches; and 64 apex of apodeme (white arrow), note frayed end typical of muscle attachment. Booklungs removed from preparation in 59, 61 and 62.
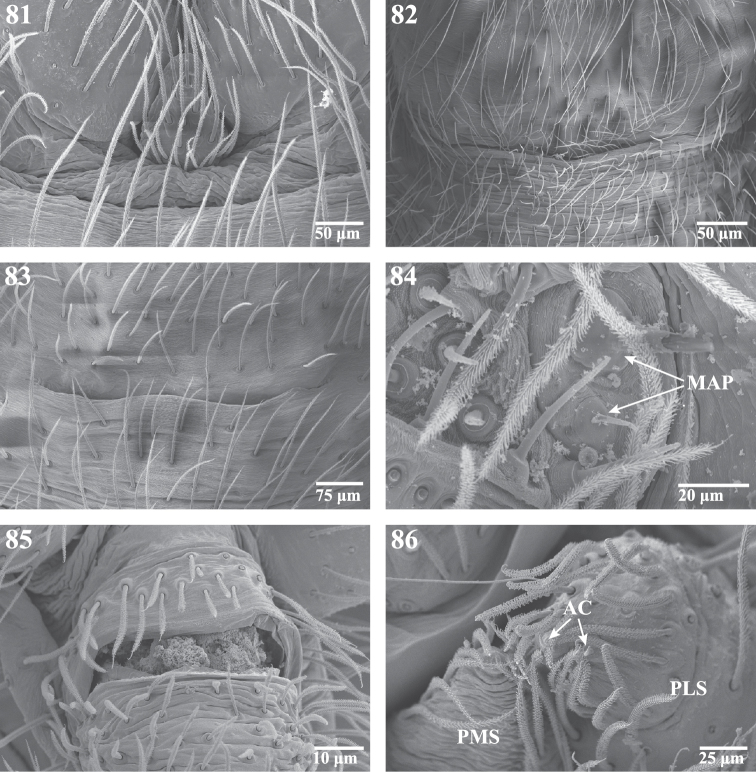
Figures 81–86.
Scanning electron micrographs of the spinnerets of Trogloraptor marchingtoni female (CASENT9039440) and male (CASENT9040066) from No Name Cave, and penultimate female from M2 Cave (CASENT9040012). 81 male colulus 82 male epiandrum 83 male posterior tracheal spiracle 84 penultimatefemale right ALS 85 female anal tubercle; and 86 male left PMS and PLS spinnerets, posterior, arrows to two aciniform gland spigots on PLS. AC aciniform gland spigots MAP major ampullate gland spigot(s) PMS posterior median spinnerets PLS posterior lateral spinnerets.
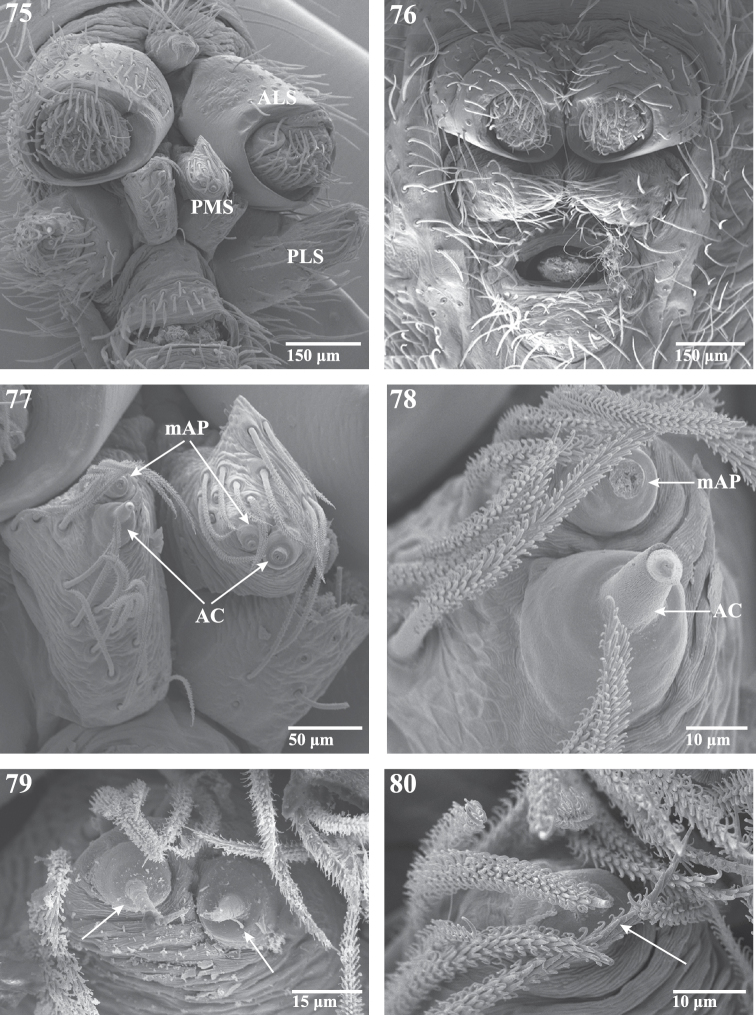
Figures 75–80.
Scanning electron micrographs of spinnerets of Trogloraptor marchingtoni from No Name Cave, female (CASENT9039440), male (CASENT9040066). 75 female spinneret overview (image flipped) 76 male spinneret overview 77 female PMS overview (image flipped) 78 female left PMS (image flipped) 79 female PMS close up showing aciniform gland spigots; and 80 male left PMS apex showing single aciniform gland spigot. AC aciniform gland spigots ALS anterior lateral spinneret mAP minor ampullate gland spigot(s) PMS posterior median spinnerets PLS posterior lateral spinnerets.
Figures 65–68.
Spinnerets, ventral view. 65 Segestriidae female, Ariadna sp. from Tinglewood, Western Australia (CASENT 9020550) 66 Pholcidae female, Artema atlanta from Hawaii, USA (CASENT 9047601) 67 Drymusidae female, Drymusa capensis from Table Mountain, South Africa (CASENT 9043173); and 68 Trogloraptoridae male, Trogloraptor marchingtoni (CASENT 9040065). Arrows point to membranous band on ALS base.
Taxonomy
Trogloraptoridae
Griswold, Audisio & Ledford fam. n.
urn:lsid:zoobank.org:act:0B46EFED-0BE4-4618-B14C-0EFFAA7CA4EA
Types.
Trogloraptor marchingtoni Griswold, Audisio and Ledford, here designated.
Diagnosis.
Ecribellate Haplogynae lacking AME, with ALE and PLE contiguous but PME separated (Figs 9, 11), chelicerae free at base and distally not forming a chela with fang (Figs 9, 10, 21, 24), Emerit’s glands absent from patellae and tibiae (Fig. 37), posterior respiratory system with broad spiracle closer to spinnerets than to epigastric furrow (Figs 12, 83), with paired, 2-branched lateral tracheal tubes and long, separate median entapophyses (Figs 60, 63), ALS basal article crossed by a diagonal membranous area (Fig. 68), and with all leg tarsi subsegmented and raptorial (Figs 13, 14, 29–32, 38, 44).
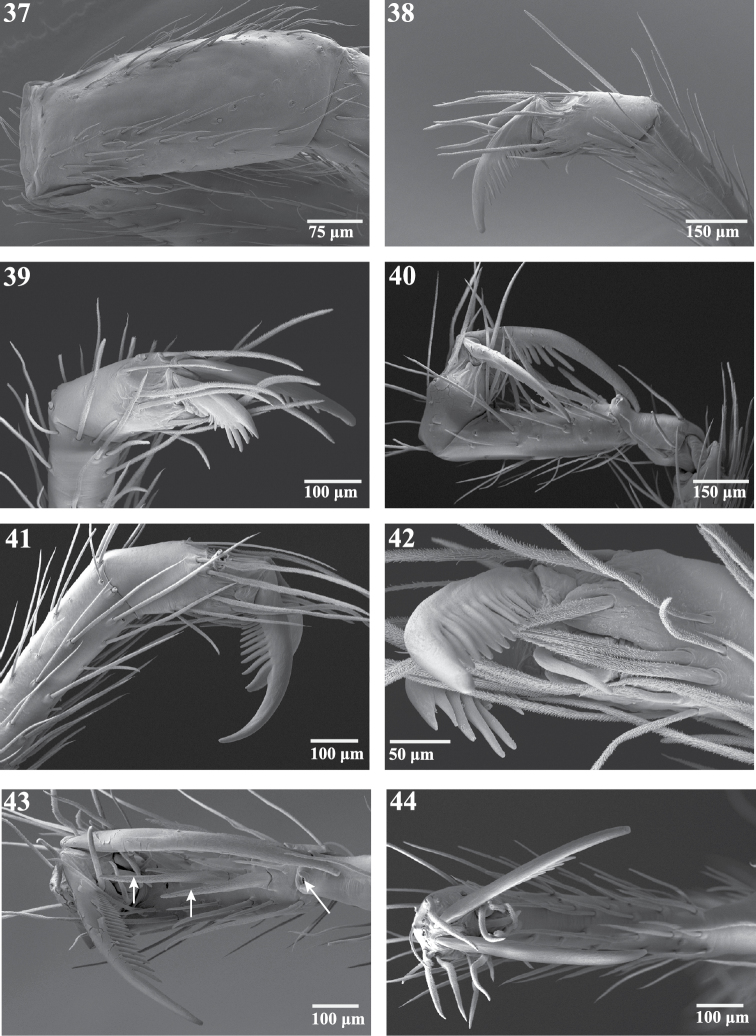
Figures 37–44.
Scanning electron micrographs of legs of Trogloraptor marchingtoni from No Name Cave. 37 left patella IV, dorsal view 38 left tarsus IV, retrolateral view 39 left tarsus IV, prolateral view 40 right tarsus III, retrolateral view 41 right tarsus IV, retrolateral view 42 left tarsus IV, retroventral view 43 right tarsus III, ventral view, arrows to spines 44 right tarsus IV, apical view. Figures 37–39, 42 (male, CASENT9040066) 40, 41, 43, 44 (female, CASENT9040051).
Figures 15–18.
Habitus of male Trogloraptor marchingtoni (CASENT9040013) from M2 Cave. 15, 16 dorsal views 17, 18 ventral views; note pedicel in Figure 16.
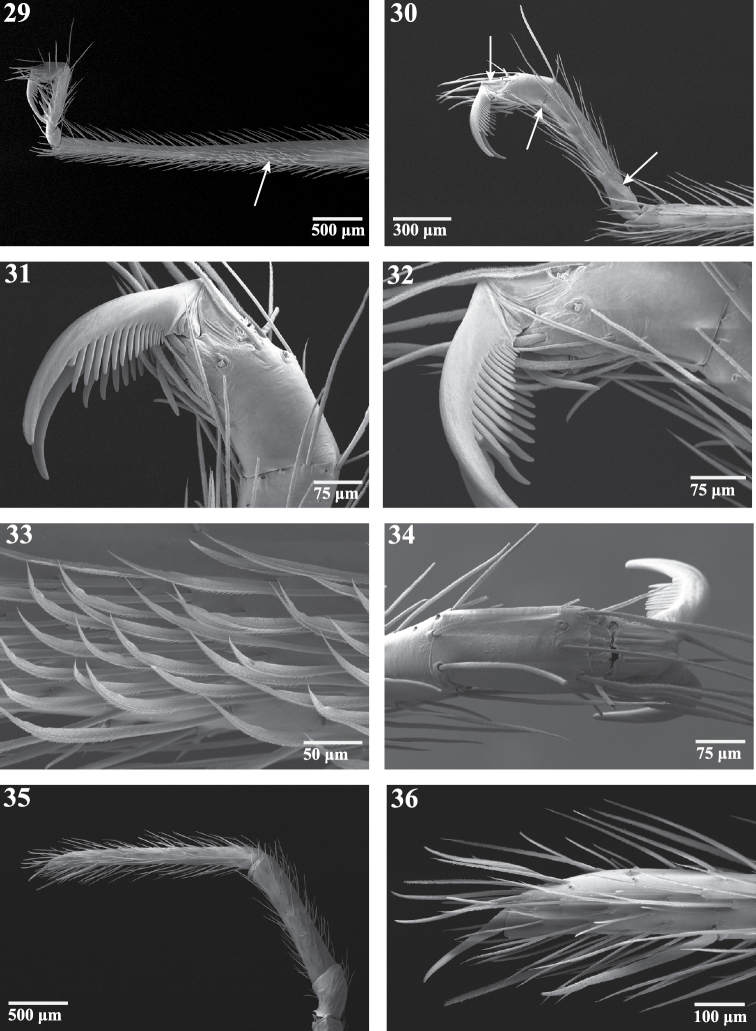
Figures 29–36.
Right appendages of female Trogloraptor marchingtoni (CASENT9040051) from No Name Cave. 29 metatarsus and tarsus of leg III, prolateral view, arrow to ventrolateral patch of curved, spinose setae 30 tarsus of leg IV, prolateral view, arrows to membranous regions 31, 32 tarsus of leg IV, prolateral view 33 curved setae onmetatarsus of leg III, prolateral view 34 tarsus of leg IV, dorsal view 35 pedipalp, prolateral view; and 36 tarsal claw of pedipalp.
Synapomorphies.
The extraordinary, subsegmented raptorial leg tarsi are unique among spiders and clearly autapomorphic for the family.
Trogloraptor
Griswold, Audisio & Ledford gen. n.
urn:lsid:zoobank.org:act:25F85266-612A-42BC-B2F9-72D3B5E0F7DC
http://species-id.net/wiki/Trogloraptor
Type species.
Trogloraptor marchingtoninew species, here designated.
Etymology.
The generic name refers to the cave habitat and raptorial tarsi.
Diagnosis.
By the characters of the family.
Synapomorphies.
As for the family.
Description.
Cephalothoraxwith carapace pear-shaped, narrowed anteriorly, pars cephalica faintly distinguished from pars thoracica, fovea indistinct (Figs 11, 16); six eyes, AME absent, ALE and PLE contiguous, PME separated from lateral eyes by their diameter, separated from each other by more than twice their diameter, shiny tapeta fill entire eyecup, of “primitive” type (Homann 1971) (Figs 9, 11); clypeus high, more than six times PME diameter, sloping anteriorly, ventral margin straight (Fig. 9); chelicerae free at base, without a boss (Figs 9, 22), with weak mesal lamellar ridge for basal 2/3 (Fig. 23), fang furrow with one large distal prolateral tooth and two promarginal and two retromarginal small proximal teeth; promargin with more than 30 elongate setae and setae on both margins at fang base (Figs 20, 21, 24), cheliceral gland opens as sparse pores near position of fang tip (Figs 20); fang without serrations along inner margin, apex longer than base, poison gland pore subapical, retrolateral (Figs 23, 24); no apparent chilum beneath clypeus but with small anterior sclerite between cheliceral bases, intercheliceral sclerite a narrow rectangle (Fig. 9); labrum elongate, with numerous plumose setae from base to middle, labral tongue free, longitudinally wrinkled apically, with minute, bristle-like setae distad of tongue apex (Fig. 27); pedipalpal coxa narrow, pointed apically, with membranous cuticle at apex and retroapical serrula (Fig. 10), serrula teeth in two rows (Figs 25, 26), with dorsal maxillary gland opening mesally near labrum (Figs 27, 28); labium narrow, sides converging, with weak basal notches, fused to sternum (Fig. 10); sternum heart-shaped, laterally undulate, anterior margin rounded on each side of labium, laterally with narrow lobes opposite coxae and rounded lobes between coxae, without free sclerites, posteriorly narrowly rounded between coxae IV (Fig. 18); coxae cylindrical, without retrocoxal hymen, trochanters shorter than coxae, apices straight, without notch (Fig. 18); narrow, slightly curved supracoxal sclerites above each leg coxa; leg formula 1243, legs elongate, femur I 1.69—2.30 times carapace length (Figs 1–8, 15, 17), sparsely covered with plumose setae, cuticle smooth or with fine fingerprint pattern (Figs 45–50), autospasy at coxa/trochanter joint, pairs of small sclerites visible in intersegmental membranes between coxae-trochanters and femora-patellae, metatarsi III and IV of female with ventrolateral patch of curved, spinose setae (Figs 29, 33), densest proventrally, Emerit’s glands absent from patellae (Fig. 37) and tibiae, legs with few spines except beneath tarsi I-III, pedipalp with dorsoapical spine on patella and median prolateral on tibia, female pedipalpal tarsus with three prolateral and one retrolateral spines; tarsal trichobothria absent, with only a single, subapical trichobothrium on metatarsi, 1–3 dorsal trichobothria on leg tibiae, more on pedipalpal tibia, bothrium with proximal hood (Fig. 50) or a smooth, entire ring, narrower apically (Fig. 49), trichome plumose, slightly narrowed basally (Fig. 49); tarsal organ near apex of pedipalp, at mid point of leg tarsi proximad of second membranous subsegmentation (Fig. 45), exposed, round, nearly flat, with central depressed circle or 1–2 raised sensillae (Figs 46–48); leg tarsi raptorial (Figs 5, 6, 13, 14, 29–32, 34), with flexible subsegmentations (Figs 13, 14, 30) near base and subapically in female tarsi I-IV, male I-III, subapical only in male tarsus IV, tarsi I-III with paired stout spines ventrally, one pair proximad of and three pair distad of basal subsegmentation (Figs 13, 40, 43, 44), tarsus IV lacks such spines (Figs 14, 30); leg tarsi with three claws, STC I-III slightly asymmetrical, proclaw longer (Figs 13, 43), STC teeth uniseriate, proclaws I-III with 8–9 teeth and fine basal comb, retroclaw with 15–25 fine teeth, STC IV sexually dimorphic, female claws equal (Fig. 44), male asymmetrical, retroclaw with 22 teeth, proclaw short, palmate, with one large and fan of 9 smaller teeth (Figs 14, 39), ITC long, curved, with distal and proximal teeth, tarsus without serrate accessory setae, claw tufts or scopulae (Figs 35, 36, 40–43); female pedipalp with long, smooth claw (Figs 35, 36). Abdomen oval, unsclerotized except at book lung openings, sparsely covered with setae (Figs 12, 15–18); pedicel with ventral sclerite contiguous to sternum, dorsum with lorum divided anteriorly (Fig. 16); male lacks epiandrous spigots (Fig. 82); anterior respiratory system booklungs, posterior respiratory system with broad spiracle closer to spinnerets than to epigastric furrow (Figs 12, 83), with paired, 2-branched lateral tracheal tubes and long, separate median entapophyses (Figs 60, 63), entapophyses tips frayed as muscle attachments (Fig. 64); colulus a large, oval sclerotized lobe, covered with hairs (Figs 75, 81); ALS with three segments (Fig. 69), basal segment divided obliquely by membranous cuticle (Fig. 68), with about 30 PI gland spigots, each with convex base and a narrow tapering shaft, shaft origin slightly sunken into base and encircled by a cuticular ridge (Fig. 71), female mesally with anterior large and posterior small MAP gland spigots (Figs 69, 72, 84); male resembles female except posterior MAP gland spigot replaced by a small nubbin (Fig. 70); PMS of female with two spigots with squat bases and narrow shafts (Figs 77, 78), male retains only the posterior (Fig. 80), suggesting that this is an AC gland spigot and the anterior is a mAP gland spigot, cuticle on mesal surface of PMS wrinkled (Fig. 77); PLS of female (Figs 73, 74) and male (Fig. 86) with two spigots with squat bases and narrow shafts, these probably AC gland spigots; female genitalia haplogyne, anterior edge of epigastric furrow sclerotized, vulva internally (Figs 59, 61) with median atrium and paired, lateral receptacula with sclerotized stalks and membranous apical bulbs (Fig. 62), apical bulbs may serve as muscle attachments; male pedipalp femur to tarsus lacking apophyses, cymbium narrow, without trichobothria or chemosensory scopulae, extending far beyond base of bulb (Figs 9, 15, 17, 51, 52, 57); male pedipalpal bulb piriform, swollen, lacking processes, embolus long, slender, recurved apically (Figs 51–58), spermpore subapical (Fig. 56), reservoir broad, making 1 ½ spiral within bulb (Figs 57, 58), basal haematodocha does not expand but bulb orientation twists slightly.
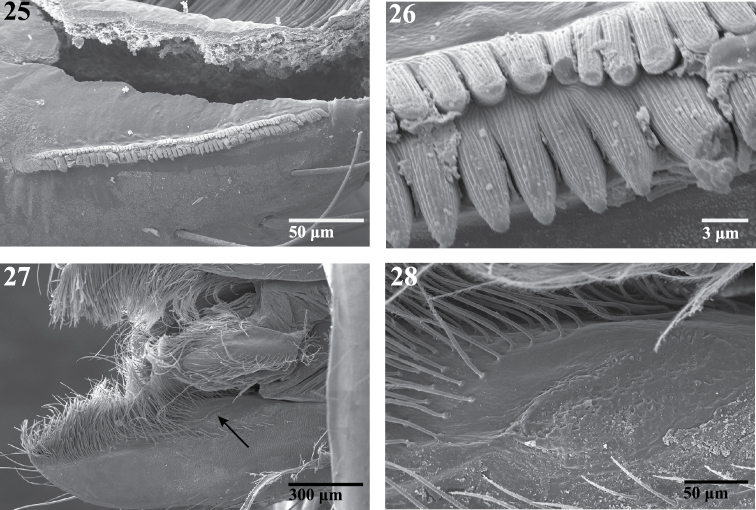
Figures 25–28.
Scanning electron micrographs of the endite of a female Trogloraptor marchingtoni (CASENT9040051) from M2 Cave. 25 serrula 26 serrula, close up, note multiple tooth rows 27 labrum and left endite, dorsal view, arrow to maxillary gland opening 28 maxillary gland opening, close up.
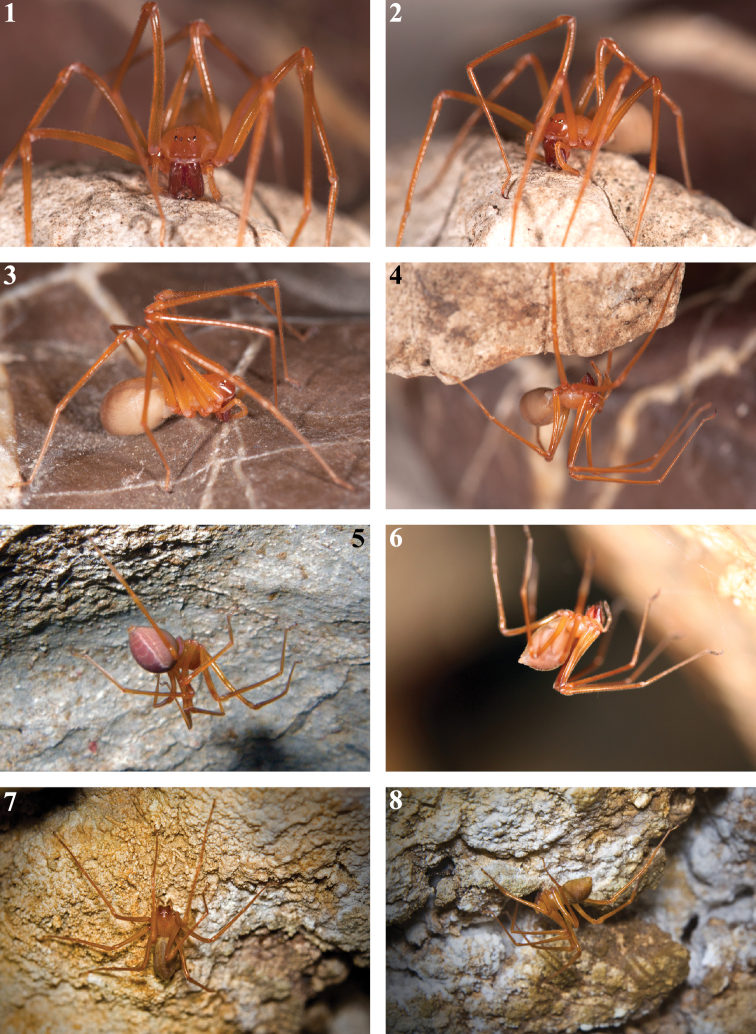
Figures 1–8.
Habitus of live Trogloraptor marchingtoni. 1–4 female in captivity (JL) 5 female in Lake Cave (CG) 6 female in M2 Cave (RD) 7, 8 female in No Name Cave (BM).
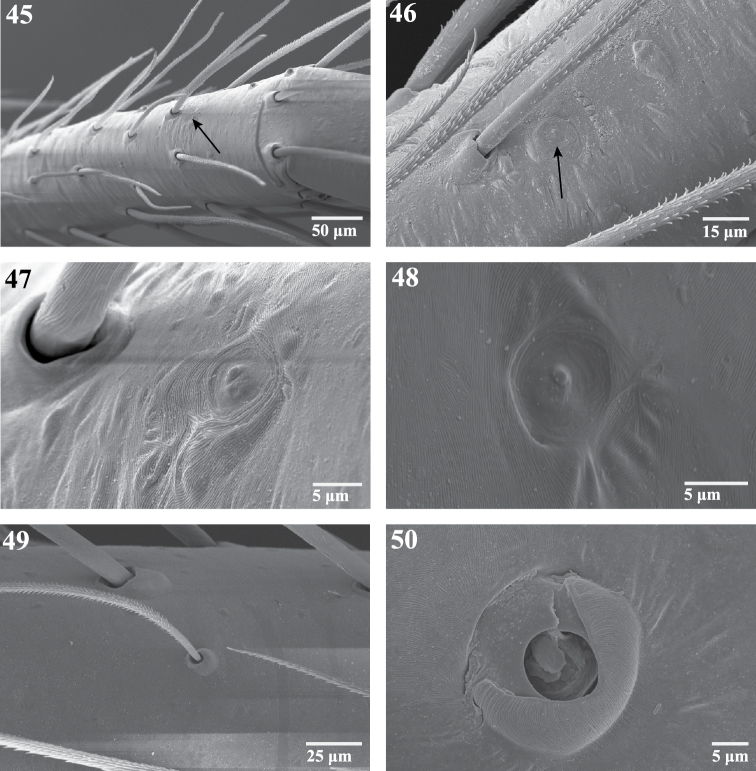
Figures 45–50.
Scanning electron micrographs of sensory organs of Trogloraptor marchingtoni. 45 right tarsus I, arrow to tarsal organ 46 tarsus IV, arrow to tarsal organ 47 right tarsus I, tarsal organ 48 tarsal organ on palp 49 trichobothrium on tibia of R leg III, prolateral view 50 trichobothrial base on metatarsus of right leg III. Figures 45, 47–49 (female, CASENT9040051) 46 (male,CASENT9040066) 50 (female, CASENT9040041).
Composition.
One species described, probably another known only from juveniles.
Distribution.
Known only from caves and old growth forest understory in the Klamath-Siskiyou region of Oregon and California.
Trogloraptor marchingtoni sp. n.
urn:lsid:zoobank.org:act:DD0946CA-9479-4DC5-BCA2-384B6B82599E
http://species-id.net/wiki/Trogloraptor_marchingtoni
Types.
Holotype male from M2 Cave, 15.7 km SSW Grants Pass, Josephine Co., Oregon, USA, collected 29 July 2010 by R. S. Davis and D. S. Snyder, CASENT9040013, and paratype female from No Name Cave, Josephine Co., Oregon, 17.8 km SSW Grants Pass, collected 16 Sept. 2010 by N. Marchington, CASENT9040065, deposited in CAS.
Etymology.
The specific name is a patronym in honor of Neil Marchington, cave biologist, Advisory Board member of the Western Cave Conservatory, Conservation Chair, Western Region, National Speleological Society and Deschutes County Deputy Sheriff, in gratitude for his help and kindness.
Diagnosis.
By the characters of the genus.
Male (Holotype). Total length 9.70. Markings as in Figs 9–12, 15–18, cephalothorax, legs and pedipalps yellow-brown, unmarked except for dark brown v-mark posteriorly on pars cephalica, clypeus and chelicerae orange brown, abdomen purple brown with faint light chevrons posteriorly on dorsum. Carapace 4.50 long, 3.10 wide; clypeus 1.33 high; ocular area 0.45 long, 1.30 wide; ratio of eyes ALE:PME:PLE, 1.08:1.00:1.08; diameter of PME 0.18; chelicerae 2.38 long; sternum 1.75 long, 1.88 wide; labium 1.08 long, 0.70 wide; pedipalpal coxa 1.50 long, 0.45 wide; leg measurements (Femur + Patella + Tibia + Metatarsus + Tarsus = [Total]): I: 8.25 + 1.40 + 9.25 + 9.00 + 1.45 = [29.35]; II: 7.75 + 1.35 + 8.05 + 8.00 + 1.40 = [26.55]; III: 6.40 + 1.40 + 6.25 + 5.70 + 1.60 = [21.35]; IV: 7.15 + 1.40 + 6.50 + 6.35 + 1.50 = [22.90]; pedipalp: 1.55 + 0.55 + 1.70 + 2.40 = [6.20]. Pedipalp as in Figs 9, 16, 51–58.
Variation (N=2): Total length 6.90—9.70; carapace length 1.19--1.45 times width, height 0.33—0.35 times width; PER width 2.89—3.00 times OAL; clypeal height 6.43—7.00 times PME diameter; clypeal height 1.74—2.11 times cheliceral length; sternum length 0.92—0.93 times width, labium length 1.54—1.55 times width, pedipalpal coxa length 2.81—3.33 times width; femur I length 1.83—2.30 times carapace length; metatarsus I length 2.03—2.06 times carapace length.
Female
(paratype): Total length 9.40. Markings as in male (Figs 1–8). Carapace 3.90 long, 2.90 wide; clypeus 0.93 high; ocular area 0.38 long, 1.28 wide; ratio of eyes ALE:PME:PLE, 1.00:1.00:1.07, diameter of PME 0.15; chelicerae 2.25 long; sternum 1.75 long, 1.88 wide; labium 0.95 long, 0.65 wide; pedipalpal coxa 1.38 long, 0.50 wide. Leg measurements (Femur + Patella + Tibia + Metatarsus + Tarsus = [Total]): I: 7.15 + 1.25 + 6.85 + 7.25 + 1.35 = [23.85]; II: 6.75 + 1.30 + 6.85 + 6.95 + 1.40 = [23.25]; III: 5.25 + 1.25 + 5.10 + 4.70 + 1.15 = [17.50]; IV: 5.85 + 1.25 + 5.60 + 5.15 + 1.20 = [19.05]; pedipalp: 1.50 + 0.50 + 1.05 + 2.05 = [5.10]. Genital region weakly sclerotized externally, vulva with median translucent atrium and sclerotized lateral receptaculae, receptacular apex membranous (Figs 59, 61, 62).
Variation (N=3): Total length 8.27—9.60; carapace length 1.30—1.40 times width, height 0.34—0.39 times width; PER width 2.71—3.40 times OAL; clypeal height 6.17—6.91 times PME diameter, 2.05—2.43 times cheliceral length; sternum length 0.93—0.96 times width; labium length 1.28—1.54 times width; pedipalpal coxa length 2.75—3.67 times width; femur I length 1.69—1.83 times carapace length; metatarsus I length 1.72—1.78 times carapace length.
Natural history.
This species has been collected in the dark zone of caves, hanging beneath a few strands of silk that are attached to the cave roof (Figs 5–8). Boulders and rotting logs were searched near the entrance to M2 Cave without finding any Trogloraptor. Nothing has been observed of its predatory or mating behavior. Living specimens were reared in climate controlled conditions and constructed a loose tangle of web from which they hung beneath. Multiple attempts to feed the specimens a variety of prey items failed, which may indicate a preference for specific prey.
Distribution.
Caves in southwestern Oregon.
Additional material examined
(all CAS). USA: OREGON: Josephine Co., No Name Cave, 17.8 km SSW Grants Pass, 16 Sept. 2010, N. Marchington, 1 ♀, CASENT9040051, 1 ♂, CASENT9040066, same data except 13 July 2011, 1♀, CASENT9039440; M2 Cave, 15.7 km SSW Grants Pass, 31 July 2010, Geo Graening, R. S. Davis and D. S. Snyder, 1 penultimate ♀, CASENT9040012, same data except 13 July 2011, N. Marchington, T. Audisio, C. Griswold, J. Ledford, D. Ubick, H. Wood and F. Álvarez-Padilla, 3 juveniles, CASENT9047599; Lake Cave near No Name Cave, 9.05 km S Wilderville, 13 July 2011, N. Marchington, T. Audisio, C. Griswold, J. Ledford, D. Ubick, H. Wood and F. Álvarez-Padilla, 1♂ (molted to maturity in captivity) CASENT9047600; Chapman Cave, 12 July 2011, N. Marchington, 2 juveniles, CASENT9039436.
Note.
A juvenile Trogloraptor specimen has been collected under debris in old growth redwood forest in far northwest California. The markings of this juvenile differ from the cave species, Trogloraptor marchingtoni. The northwest California specimen has dusky markings laterally on leg femora, a dusky Y marking extending back from the PLE to the posterior margin of the carapace and undulate dusky markings along the lateral carapace margin. These markings suggest that there may be at least one additional Trogloraptor species currently known only from the juvenile. Records for this specimen are as follows: CALIFORNIA: Del Norte Co., NE Crescent City, Jedediah Smith Redwood State Park, Ruth Perry Hatton Grove, US199 0.2 mi E junction with Walker Rd., elev. 60m, 10.29 km NNE Crescent City, old growth redwood, under woody debris, 31 March 2011, E. Garcia, C. Richart, A. Schoenhofer, D. Sitzmann, 1 juvenile, CASENT9040069 (CAS).
Conclusions
Western North America, especially the Klamath-Siskiyou region of northern California and southern Oregon is rich in biodiversity, particularly with respect to its endemic plants and invertebrates (Myers et al. 2000). This area is particularly notable for relicts, i.e., primitive relatives of otherwise widespread taxa. The coast redwood, Sequoia sempervirens, is a notable example. The coastal tailed frog (Ascapus truei Stejneger, 1899, Ascaphidae) and mountain beaver (Aplodontia rufa (Rafinesque, 1817)), Aplodontiidae) are considered the world’s most primitive living frog and rodent, respectively (Nielson et al. 2001; Adkins et al. 2001). Primitive arachnids and myriapods also occur in the Pacific Northwest. Shear and Leonard (2003) described the new millipede family Microlympiidae for a minute species from the Olympic Mountains of Washington. Several families of Travunioid harvestman are found here (Briggs 1971). The pseudoscorpion genera Pseudogarypus (Pseudogarypidae), Oreolpium (Garypinidae) and Pseudotyrannochthonius (Pseudotyrannochthoniidae) all have relict distributions in the Pacific Northwest. These are also among the most ancient of pseudoscorpion lineages (Harvey 1998; Harvey and Štáhlavský 2009). Atypoid mygalomorphs (Coyle 2005a, 2005b; Ramirez and Chi 2004), Hypochilidae (Hedin 2001; Paquin and Hedin 2005), the 8-eyed caponiid Calponia (Ubick, 2005) and the cribellate leptonetid genus Archoleptoneta (Ledford and Griswold 2010) comprise relatively ancient spider lineages characterized by the retention of ancestral character states. If Trogloraptoridae are the most primitive living members of the Dysderoidea we have another case of a notable relict from Western North America. If such a large and bizarre spider could have gone undetected for so long, who knows what else may lurk undiscovered in this remarkable part of the world.
Supplementary Material
Acknowledgements
Major funding for this project came from the Harriet Exline-Frizzell Fund with additional support coming from the Hagey Research Investment Fund of the California Academy of Sciences (CAS). Tracy and Charles acknowledge support from NSF BIR-9531307, “the CAS Summer Systematics Institute, an REU site,” R. Mooi, PI and from NSF DEB-0613775 “PBI: Collaborative research: the megadiverse, microdistributed spider family Oonopidae” C. Griswold, PI. Joel acknowledges support from the Schlinger Foundation Postdoctoral Fellowship at CAS. We thank Geo Graening, Neil Marchington, Ron Davis, Daniel Snyder and the Western Cave Conservancy for making the first known specimens available to us, Marshal Hedin and Axel Schoenhofer (San Diego State University) for making the redwood forest specimen available, and Neil Marchington, Darrell Ubick, Hannah Wood and Fernando Álvarez-Padilla for help with fieldwork. Vic Smith helped with imaging. Photographs of the living animals are by Ron Davis, Charles Griswold, Joel Ledford and Brent McGregor. The Bureau of Land Management (BLM), Grants Pass office, facilitated cave access. Darrell Ubick and Martín Ramírez provided valuable comments on the specimens, and Fernando Álvarez-Padilla, Anthea Carmichael, Ansie Dippenaar-Schoeman, Rosie Gillespie, Mark Harvey, Gustavo Hormiga, Rudy Jocqué, Leon Lotz, Norman Platnick, Martín Ramírez, Robert Raven and Tamás Szüts commented on presentations about this new spider family. Friendly reviews of drafts of the manuscript were provided by Facundo Labarque, Martín Ramírez, Norm Platnick, Bill Shear and Darrell Ubick. Michael Rix and an anonymous reviewer provided helpful comments. We especially thank Martín Ramírez for pointing out the potential phylogenetic significance of the diagonal band across the ALS base.
References
- Adkins RM, Gelke EL, Rowe D, Honeycutt RL. (2001) Molecular phylogeny and divergence time estimates for major rodent groups: Evidence from multiple genes. Molecular Biology and Evolution 18: 777-791. doi: 10.1093/oxfordjournals.molbev.a003860 [DOI] [PubMed] [Google Scholar]
- Álverez-Padilla F, Hormiga G. (2007) A protocol for digesting internal soft tissues and mounting spiders for scanning electron microscopy. Journal of Arachnology 35: 538-542. doi: 10.1636/Sh06-55.1 [DOI] [Google Scholar]
- Briggs T. (1971) Relict harvestmen from the Pacific Northwest. The Pan-Pacific Entomologist 47 (3): 165-178. [Google Scholar]
- Coyle FA. (2005a) Antrodiaetidae. In: Ubick D, Paquin P, Cushing PE, Roth V. (Eds). The Spider Genera of North America: An Identification Manual. The American Arachnological Society: 39-40.
- Coyle FA. (2005b) Mecicobothriidae. In: Ubick D, Paquin P, Cushing PE, Roth V. (Eds). The American Arachnological Society: 50-51.
- Dondale CD, Redner JH. (1982) The insects and arachnids of Canada, part 9. The sac spiders of Canada and Alaska, Araneae: Clubionidae and Anyphaenidae. Research Branch, Agriculture Canada, publ. 1724: 1-194. [Google Scholar]
- Edwards GB. (2004) Revision of the jumping spiders of the genus Phidippus (Araneae: Salticidae). Occasional Papers of the Florida State Collection of Arthropods 11: 1-156. [Google Scholar]
- Griswold CE. (1987) A revision of the jumping spider genus Habronattus F. O. P.-Cambridge (Araneae; Salticidae), with phenetic and cladistic analyses. University of California Publications in Entomology 107: 1-344. [Google Scholar]
- Griswold CE, Ramírez MJ, Coddington JA, Platnick NI. (2005) Atlas of Phylogenetic Data for Entelegyne Spiders (Araneae: Araneomorphae: Entelegynae) with Comments on Their Phylogeny. Proceedings of the California Academy of Sciences 56 (2): 1-324. [Google Scholar]
- Harvey MS. (1998) Pseudoscorpion groups with bipolar distributions: a new genus from Tasmania related to the holarctic Syarinus (Arachnida, Pseudoscorpiones, Syarinidae). Journal of Arachnology 26: 429-441. [Google Scholar]
- Harvey MS, Štáhlavský F. (2009) A review of the pseudoscorpion genus Oreolpium (Pseudoscorpiones: Garypinidae), with remarks on the composition of the Garypinidae and on pseudoscorpions with bipolar distributions. Journal of Arachnology 38: 294-308. doi: 10.1636/A09-53.1 [DOI] [Google Scholar]
- Hedin MC. (2001) Molecular insights into species phylogeny, biogeography, and morphological stasis in the ancient spider genus Hypochilus (Araneae: Hypochilidae). Molecular Phylogenetics and Evolution 18: 238-251. doi: 10.1006/mpev.2000.0882 [DOI] [PubMed] [Google Scholar]
- Homann H. (1971) Die Augen der Araneae: Anatomie, Ontogenese und Bedeutung für die Systematik (Chelicerata, Arachnida). Zeitschrift für Morphologie der Tiere 69: 201-272. doi: 10.1007/BF00277623 [DOI] [Google Scholar]
- Jocqué R, Dippenaar-Schoeman A. (2006) Spider families of the world. Royal Museum for Central Africa, 336 pp.
- Ledford JM, Griswold CE. (2010) A study of the subfamily Archoleptonetinae (Araneae, Leptonetidae) with a review of the morphology and relationships for the Leptonetidae. Zootaxa 2391: 1-32. [Google Scholar]
- Ledford JM, Paquin P, Cokendolpher J, Campbell J, Griswold C. (2011) Systematics of the Spider Genus Neoleptoneta Brignoli, 1972 (Araneae: Leptonetidae) with a Discussion of the Morphology and Relationships for the North American Leptonetidae. Invertebrate Systematics 25: 334-388. doi: 10.1071/IS11014 [DOI] [Google Scholar]
- Ledford JM, Audisio T, Griswold CE. (in prep.) Phylogeny of the cave spider genus Usofila (Araneae, Telemidae) in North America.
- Levi HW. (1953) Spiders of the genus Dipoena from America north of Mexico (Araneae, Theridiidae). American Museum Novitates 1647: 1-39. [Google Scholar]
- Levi HW. (1968) The spider genera Gea and Argiope in America (Araneae: Araneidae). Bulletin of the Museum of Comparative Zoology, Harvard 136: 319-352. [Google Scholar]
- Maddison WP. (1996) Pelegrina Franganillo and other jumping spiders formerly placed in the genus Metaphidippus (Araneae: Salticidae). Bulletin of the Museum of Comparative Zoology, Harvard 154: 215-368. [Google Scholar]
- Miller JA, Griswold CE, Yin CM. (2009) The symphytognathoid spiders of the Gaoligongshan, Yunnan, China (Araneae, Araneoidea): Systematics and diversity of micro-orbweavers. ZooKeys 11: 9-195. doi: 10.3897/zookeys.11.160 [DOI] [Google Scholar]
- Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J. (2000) Biodiversity hotspots for conservation priorities. Nature 403: 853-858. doi: 10.1038/35002501 [DOI] [PubMed] [Google Scholar]
- Nielson M, Lohman K, Sullivan J. (2001) Phylogeography of the tailed frog (Ascaphus truei): implications for the biogeography of the Pacific Northwest. Evolution 55: 147-160. [DOI] [PubMed] [Google Scholar]
- Paquin P, Hedin M. (2005) Hypochilidae. In: Ubick D, Paquin P, Cushing PE, Roth V. (Eds). The American Arachnological Society: 120-121.
- Penney D, Selden P. (2011) Fossil Spiders: the evolutionary history of a megadiverse order. Siri Scientific press, Monograph Series Volume 1, 128 pp.
- Platnick NI. (1975b) A revision of the Holarctic spider genus Callilepis (Araneae, Gnaphosidae). American Museum Novitates 2573: 1-32. [Google Scholar]
- Platnick NI, Coddington JA, Forster RR, Griswold CE. (1991) Spinneret Morphology and the Phylogeny of Haplogyne Spiders (Araneae, Araneomorphae). American Museum Novitates 3016, 1–73.
- Ramirez MG, Chi B. (2004) Cryptic speciation, genetic diversity and gene flow in the California turret spider Antrodiaetus riversi (Araneae: Antrodiaetidae). Biological Journal of the Linnean Society 82: 27-37. doi: 10.1111/j.1095-8312.2004.00312.x [DOI] [Google Scholar]
- Ramírez MJ. (2000) Respiratory system morphology and the phylogeny of haplogyne spiders (Araneae, Araneomorphae). Journal of Arachnology 28: 149-157. doi: 10.1636/0161-8202(2000)028[0149:RSMATP]2.0.CO;2 [DOI] [Google Scholar]
- Roth VD. (1982) Handbook for spider identification. Published by the author on behalf of the American Arachnological Society, Portal, Arizona, 62 pp. [Google Scholar]
- Shear WA, Leonard WP. (2003) Microlympiidae, a new millipede family from North America, and Microlympia echina, new genus and species. Zootaxa 243: 1-11. [Google Scholar]
- Simon E. (1893) Histoire naturelle des araignées. Paris, 1: 257-488. [Google Scholar]
- Ubick D. (2005) Caponiidae. In: Ubick D, Paquin P, Cushing PE, Roth V. (Eds). The American Arachnological Society: 75-76.
- Ubick D, Paquin P, Cushing PE, Roth V. (Eds) (2005) The Spider Genera of North America: An Identification Manual. The American Arachnological Society, 377 pages.
- Wood H, Griswold C, Gillespie R. (in press) Phylogenetic placement of pelican spiders (Archaeidae, Araneae), with insight into evolution of the “neck” and predatory behaviors of the superfamily Palpimanoidea. For Cladistics. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.