Abstract
Expression of chemokine receptors on T helper 2 cells and eosinophils has been postulated to be the mechanism by which these cells are selectively recruited to the lung during allergic inflammatory reactions. Mouse models have provided evidence to show that blocking the ligands for these receptors is successful in abrogating the pathophysiological effects of allergen challenge. However, recent studies describing the effect of genetic deletions of these chemokine receptors have not confirmed the results obtained with ligand knockouts or neutralising antibodies. Coupled with the realisation that, because of a lack of species cross-reactivity, it is not possible to test small molecule antagonists against human receptors in the original in vivo animal models, the future of chemokine receptor therapeutics is in question. However, recent advances have been made regarding the therapeutic potential of blocking the chemokine receptors CCR3, CCR4 and CCR8 in allergic airway disease.
Introduction
Allergic inflammation, such as asthma, is a T helper (Th)2-driven response associated with the selective recruitment of allergen-specific Th2 cells to sites of inflammation. These Th2 cells influence the inflammatory response through generation of specific cytokines, including interleukin (IL)-4, IL-13 and IL-5. One important consequence of Th2 cell involvement is the associated influx of large numbers of eosinophils, which are thought to contribute to the pathogenesis of the disease. Allergic inflammation in the lung is characterised by airway hyperresponsiveness (AHR). Eosinophils, Th2 cells and mast cells can all contribute to AHR, although controversy remains over which cell type is the predominant effector of this response. In developing therapies for asthma, the goal is to inhibit AHR and not just leukocyte recruitment.
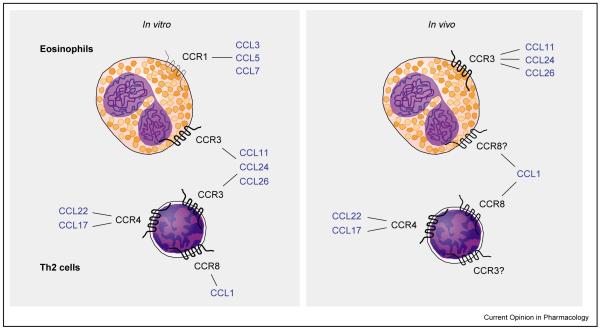
Chemokines are a group of structurally related chemotactic cytokines that signal through 7-transmembrane G-protein-coupled receptors expressed by leukocytes. The discovery that certain chemokine receptors are differentially expressed on the surface of effector T cells has suggested that this might be the mechanism by which Th2 cells are selectively recruited to the lung. In vitro analysis has determined that not only do effector T cells express a restricted repertoire of receptors but also that they preferentially migrate to the chemokines that bind these receptors [1,2]. Thus, it has been shown that CCL11 (eotaxin), CCL22 (monocyte-derived chemokine), CCL17 (thymus and activation-regulated chemokine) and CCL1 (I-309 [human] or TCA-3 [mouse]) are chemokines which induce the selective migration of Th2 cells but not Th1 cells. CCL11 binds exclusively to the chemokine receptor (CCR)3, whereas CCL22 and CCL17 both interact with CCR4. CCL1 is the only known ligand for CCR8. Interestingly, CCR3 is also expressed by eosinophils, for which CCL11 is a potent chemoattractant. The only other chemokine receptor expressed by eosinophils is CCR1, but this is generally expressed at very low levels. Interest in CCR3, CCR4 and CCR8 as potential therapeutic targets in asthma developed when it was discovered that these receptors exhibited restricted expression profiles on cells believed to be involved in the asthmatic response. CCR3 is reported to be expressed by eosinophils, Th2 cells, mast cells and basophils, whereas CCR4 and CCR8 are expressed by Th2 cells (Figure 1). Thus, these chemokine receptors are potential targets for the treatment of allergic inflammation, as they have the advantage of being expressed by selective leukocyte populations.
Figure 1.
Potential chemokine receptor–ligand interactions on human eosinophils and Th2 cells. Recent studies have highlighted differences between in vivo and in vitro receptor expression.
In this review, we describe recent findings from in vivo studies of allergic inflammation regarding the function of chemokines and their receptors. These studies include those using both ligand blockade and receptor knockout (KO) strategies. We discuss how recent advances in the fields of chemokine biology and allergic airway inflammation allow us to better interpret some of the conflicting results. Finally we consider how the results of all of these studies impact on the search to find chemokine receptor antagonists for anti-asthma therapeutics.
CCL11 and CCR3
Multiple studies have shown that neutralisation of CCL11 results in a decrease in both airway inflammation and AHR [3–5]. More specifically, it has been shown that CCL11 blockade reduces trafficking of Th2 cells and eosinophils [6]. In contrast, the CCL11 KO mouse showed only partial protection against development of allergic airway inflammation, reinforcing the idea that there is a certain amount of redundancy in the chemokine network [7,8]. Three members of the CCL11 family have been identified in humans (CCL11, CCL24 [eotaxin-2] and CCL26 [eotaxin-3]) [9–11], whereas only two forms are expressed in the mouse [12,13]. These eotaxins could have distinct, as well as overlapping, functions. Although they are all upregulated during allergen challenge [14,15], recent studies have determined that, in response to the Th2 cytokine IL-4, they are generated by distinct cell types [16,17]. These findings suggest that CCL11, CCL24 and CCL26 may be regulated both temporally and spatially during disease progression.
Antagonism of the CCL11 receptor (CCR3) was predicted to be a valid therapeutic strategy for the treatment of asthma. However, the recent development of a CCR3 KO mouse has added further complexity to our understanding of how CCR3 regulates leukocyte trafficking to the lung during allergic inflammation [18••]. Although eosinophil recruitment to the lung after allergen challenge was significantly reduced in CCR3 KO mice, AHR was unexpectedly increased [18••]. It was proposed that this was a result of increased accumulation of mast cells in the trachea following allergen challenge. Related studies showed that CCR3-deficient mice were completely protected from allergen-induced AHR when the model system was changed so that mast cells were not recruited to the trachea [19••]. These studies indicate that, in addition to Th2 cells and eosinophils, mast cells may also be critical for the development of AHR.
CCL22, CCL17 and CCR4
Neutralising either CCL22 or CCL17 has been shown to abrogate lung eosinophilia and AHR [20,21]. It was proposed that this was caused by inhibition of Th2 cell trafficking. Further studies utilised a model based on the adoptive transfer of allergen-specific effector T cells to track Th2 cell migration to the lung following allergen challenge in mice receiving anti-CCL22 antibodies [6]. These experiments established that CCL22 and CCR4 contribute to the recruitment of Th2 cells to the lung, demonstrating for the first time an in vivo relevance for the expression of this receptor on T cells. In contrast, CCR4 deficiency afforded no protection against the development of allergic inflammation or AHR [22]. This might be explained by the intrinsic differences either in blocking a ligand rather than a receptor, or in using a genetically deficient mouse rather than neutralising antibodies. As antibodies against CCR4 are not available, this theory cannot be tested directly. An alternative explanation for these results is that there are other, as yet undiscovered, receptors for CCL22 and CCL17. In a separate study, the effect of CCR4 KO was examined in a model of chronic allergic airways disease; a significant reduction in both eosinophilia and AHR was reported [23•]. A potential caveat of these findings is that the allergen used was Aspergillus fumigatus, and it was noted that fungal spores were more rapidly cleared in the CCR4-deficient mice. Thus, it is not known whether the observed reduction in allergic inflammatory response was a direct effect, caused by a reduction in Th2 cell trafficking, or an indirect effect, caused by enhanced elimination of the fungal spores by a distinct CCR4-dependent mechanism. Indeed, protection from lipopolysaccharide-induced lung inflammation was initially reported in CCR4-deficient mice [22]. Taken together, these studies indicate that CCR4 might be important in modulating both the innate and acquired immune responses.
CCL1 and CCR8
The in vivo role of the CCL1/CCR8 chemokine axis in Th2-mediated inflammation is more controversial. Recent studies have shown that, although both CCL1 protein and CCR8 mRNA are upregulated in the murine lung following allergen sensitisation and challenge, neutralisation of the ligand has no effect on recruitment of Th2 cells to the lungs [24••,25••]. Interestingly, one of these studies reported no effect of CCL1 blockade on eosinophil recruitment [25••], whereas the other reported a modest reduction in eosinophil recruitment [24••]. Moreover, in the latter study, it was shown that direct instillation of CCL1 into the lungs of allergen-sensitised challenged mice induced the recruitment of eosinophils but not Th2 cells. Taken together, these data indicate that CCL1 is unlikely to be involved in recruitment of Th2 cells to the lung after allergen challenge, but could possibly play a role in eosinophil trafficking. Interestingly, both studies failed to show an effect on AHR.
Intriguingly, three CCR8 KO studies have been reported that give very different insights into the functional role of CCR8 in allergic reactions in vivo. The first study demonstrated that CCR8 KO mice had diminished Th2 responses and impaired eosinophil recruitment in a variety of Th2-driven airway inflammation models [26••]. In contrast, the two other studies found no effect of CCR8 deficiency on the development of allergen-driven airway inflammation [25••,27••]. A CCR8 blocking antibody was also used, but did not affect development of inflammation [27••]. Differences in either the model protocol or the genetic background of the CCR8KO mice might explain the variation seen in the pathology of these mice. These data, taken together with findings from the CCL1 blocking studies, imply that the CCL1/CCR8 axis might be involved in recruitment of eosinophils in some models. However, it seems unlikely that CCR8 is important for specific recruitment of Th2 cells to the lung in vivo.
CXCL12 and CXCR4
CXCR4 is expressed constitutively on all T cells and on a wide range of other cells, including CD34+ stem cells, B cells and monocytes. It is not, however, expressed by eosinophils. As deficiency of either CXCR4 or CXCL12 (SDF-1α) is lethal, it was originally hypothesised that CXCR4 played an important role in the homeostatic homing mechanism of multiple leukocyte populations. Therefore, it was surprising that blockade of this chemokine receptor with a blocking monoclonal antibody caused a significant reduction in both airway inflammation and AHR [28]. These results were interpreted as evidence that CXCR4 plays a role in Th2 cell trafficking during allergic inflammation. Interestingly, although CXCR4 is an unlikely therapeutic target because of its widespread expression, a recent paper by Lukacs, Berlin, Schols, Skerlj and Bridger [29•] showed that CXCR4 blockade with the specific CXCR4 antagonist AMD3100 effectively abrogates both leukocyte recruitment and AHR in a murine model of allergic inflammation. This therefore represents the first publication showing that a small molecular weight antagonist directed against a specific chemokine receptor can modify disease progression.
Chemokine receptor expression after allergen challenge of atopic asthmatics
To establish a role for specific chemokines and their receptors in allergic airway inflammation in humans, several studies have examined chemokine receptor expression on T cells within the lungs of asthmatic patients following allergen challenge. In one such study, significantly greater numbers of CCR4+ T cells were found in bronchial biopsies after segmental allergen challenge than in pre-challenge biopsies, biopsies from non-atopic patients or biopsies from patients with ‘Th1-type’ lung diseases such as sarcoidosis or chronic obstructive pulmonary disease [30••]. Over 90% of T cells were CCR4+ in allergen-challenged atopic asthmatics; the authors concluded that these were probably Th2 cells, as they co-stained for IL-4. 28% of these CCR4+ T cells were also found to be CCR8+. Surprisingly, in this study, the authors were unable to detect any T cells expressing CCR3 in the airways, with expression being confined to eosinophils. Expression of CCL22 and CCL17 was increased in airway epithelium following segmental allergen challenge. Interestingly, although expression of CCL1 was not detected, the number of CCR8+ cells infiltrating the airway mucosa was found to correlate with the degree of airflow limitation during the late-phase reaction. Another study has also found that allergen challenge promotes recruitment of CCR4+ T cells to either the lung or skin after allergen challenge of atopic asthmatics [31]. Together, these data support the idea that Th2 lymphocytes are recruited to the airways by interaction both of CCL22/CCL17 with CCR4 and, perhaps, CCL1 with CCR8.
Chemokine receptor expression is dynamic in vivo
There are various aspects of chemokine receptor biology that influence the conclusions drawn from individual studies in vitro and in vivo. The reported selectivity of chemokine receptor expression is appealing in terms of anti-inflammatory therapy, as it is possible to target selected leukocyte populations. However, our current knowledge of chemokine receptor expression has been gained largely from studies of isolated blood leukocytes and, in the case of T cells, on artificially polarised T cell lines or clones. There is now evidence from both in vitro and in vivo studies to show that chemokine receptor expression can be modulated either temporally or by the local tissue environment. Certainly, chemokine expression on T cells can be altered after activation, as well as during differentiation [32,33]. Eosinophils in bronchoalveolar lavage fluid from patients with eosinophilic lung disease express low levels of CCR3 and increased levels of CXCR4 compared with blood eosinophils, implying that eosinophils in different tissue compartments express a different receptor repertoire [34]. Moreover, injection of either CCL11 or CCL24 into atopic or non-atopic skin induces recruitment of neutrophils, as well as eosinophils and basophils [35•]. Although the authors found low levels of CCR3 on blood neutrophils, eotaxin did not elicit migration of neutrophils in vitro. Similarly, in vivo delivery of CCL1 to allergen-sensitised mice resulted in recruitment of eosinophils rather than Th2 cells [24••]. This effect of CCL1 on eosinophil recruitment might relate to the recent observation that allergen-sensitised eosinophils exhibit an altered repertoire of chemokine receptors [36]. Thus, in contrast to eosinophils isolated from the blood of wild-type mice or IL-5 transgenic mice, eosinophils from the bronchoalveolar lavage fluid of allergen-sensitised mice express CCR8 and migrate to CCL1. All of these studies imply that cell surface expression of chemokine receptors is a dynamic process in vivo, which might not necessarily be mimicked by experiments on isolated cells in vitro.
Relevance of mouse models to human disease
A comparison of the methodologies used in the animal studies cited in this review reveals some important differences. Protocols differ with respect to the allergen used, the mode of sensitisation and the number and timings of airway challenges. These differences are sometimes reflected by changes in the extent and duration of inflammation (compare [24••] with [25••]) [37], but can also result in mechanistic differences in the development of the disease (e.g. compare [18••] with [19••]) (Table 1). Therefore, results of individual studies should be interpreted with this in mind. Differences between model protocols might also contribute to the inconsistencies in data obtained by various groups. In addition, human asthma is a chronic inflammatory condition, whereas mouse models of asthma are generally acute and short-term. It could be argued, therefore, that models with evidence of chronic inflammation might better reflect human disease, and are more relevant when trying to determine the therapeutic potential of drugs for the treatment of asthma in humans.
Table 1.
Contrasting effects of blocking chemokine receptors or their ligands during allergic airway inflammation.
| Receptor | Knockout | Ligand | Blocking antibodies |
|---|---|---|---|
| CCR3 | Decreased eosinophils, increased AHR [18••] | CCL11 | Decreased eosinophils [4,7,8] |
| Reduced Th2 recruitment [6] | |||
| CCR4 | No protection [22] | CCL22 | Decreased eosinophils and AHR [20] |
| Reduced AHR [23•] | CCL17 | Reduced Th2 recruitment [6] | |
| Decreased eosinophils and AHR [21] | |||
| CCR8 | Decreased eosinophils [25••] | CCL1 | Decreased eosinophils [24••] |
| No protection [24••,26••] | No effect [24••] |
Prospects for therapeutic intervention
Animal data for blocking chemokines are compelling, and the prospect of targeting chemokine receptors on leukocytes is attractive because G-protein-coupled receptors are historically good targets. However, the perception that CCR3, CCR4 and CCR8 play a critical role in the selective recruitment of Th2 cells to sites of allergic inflammation has not been confirmed by in vivo studies with chemokine receptor KO mice. Further studies are necessary to determine whether these results are caused by the model system studied (i.e. the use of KOs versus antibodies) or the particular allergen used. When trying to understand and manipulate recruitment of Th2 cells to tissue sites, further complexity arises from the realisation that T regulatory cells, which can attenuate the allergic response, express a similar chemokine receptor repertoire to Th2 cells. Thus, CD4+/CD25+ regulatory T cells isolated from blood express CCR4 and CCR8, and differentially migrate to their ligands [38]. Future work will be necessary to determine whether regulatory T cells from lungs express similar patterns of receptors, and whether it is possible to influence recruitment of allergen-specific Th2 cells by promoting the development and movement to the lung of regulatory T cells.
Another challenge facing the pharmaceutical industry is to develop small molecule inhibitors of chemokines, given that species cross-reactivity for most chemokine receptors is lost as the affinity for the human receptor increases [39,40••]. Thus, in vivo validation in animal models as ‘proof-of-concept’ is impossible, as has been the case for BX471, a small molecule antagonist of CCR1 [41]. When looking for chemokine receptor antagonists for asthma, it is possible (although expensive) to use large animals, as there are allergy models using non-human primates. However, despite these potential problems, the number of small molecule receptor antagonists is growing rapidly, and programmes for CXCR2, CXCR4, CCR1 and CCR5 have entered Phase I clinical trials [40••,42].
Conclusions
Mouse models have generated vast amounts of data regarding the role of specific chemokines in recruiting leukocytes to the lung after allergen challenge. However, the validity of these data depends on the ability of the model to represent the human disease. Further work is needed to determine how CCR3, CCR4 and CCR8 interact to mediate the recruitment of Th2 cells and eosinophils to the allergic lung, and to confirm these results in human studies. Although some studies appear contradictory, further analyses of the methods employed and the wider use of reagents in several models are likely to answer these apparent contradictions. With small molecule antagonists for several chemokine receptors now in development, it seems likely that a new generation of therapeutics for asthma will ultimately be available.
Acknowledgements
The authors are supported by the Wellcome Trust (Reference 057704 to Clare Lloyd and 058190 to Sara Rankin).
Abbreviations
- AHR
airway hyperresponsiveness
- CCR/CXCR
chemokine receptor
- IL
interleukin
- KO
knockout
- Th
T helper
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Sallusto F, Mackay CR, Lanzavecchia A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science. 1997;277:2005–2007. doi: 10.1126/science.277.5334.2005. [DOI] [PubMed] [Google Scholar]
- 2.Bonecchi R, Bianchi G, Bordignon PP, D’Ambrosio D, Lang R, Borsatti A, Sozzani S, Allavena P, Gray PA, Mantovani A, Sinigaglia F. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998;187:129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonzalo J-A, Lloyd CM, Kremer L, Finger E, Martinez-A. C, Siegelman MH, Cybulsky MI, Gutierrez-Ramos J-C. Eosinophil recruitment to the lung in a murine model of allergic inflammation. The role of T cells, chemokines and adhesion receptors. J Clin Invest. 1996;98:2332–2345. doi: 10.1172/JCI119045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonzalo J-A, Lloyd CM, Wen D, Albar JP, Wells TNC, Proudfoot A, Martinez-A C, Dorf M, Bjerke T, Coyle AJ, Gutierrez-Ramos J-C. The coordinated action of CC chemokines in the lung orchestrates allergic inflammation and airways hyperresponsiveness. J Exp Med. 1998;188:157–167. doi: 10.1084/jem.188.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell EM, Kunkel SL, Strieter RM, Lukacs NW. Temporal role of chemokines in a murine model of cockroach allergen-induced airway hyperreactivity and eosinophilia. J Immunol. 1998;161:7047–7053. [PubMed] [Google Scholar]
- 6.Lloyd CM, Delaney T, Nguyen T, Tian J, Martinez CA, Coyle AJ, Gutierrez-Ramos JC. CC Chemokine receptor (CCR)3/eotaxin is followed by CCR4/monocyte-derived chemokine in mediating pulmonary T helper lymphocyte type 2 recruitment after serial antigen challenge in vivo. J Exp Med. 2000;191:265–273. doi: 10.1084/jem.191.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rothenberg ME, MacLean JA, Pearlman E, Luster AD, Leder P. Targeted disruption of the chemokine eotaxin partially reduces antigen-induced tissue eosinophilia. J Exp Med. 1997;185:785–790. doi: 10.1084/jem.185.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Y, Loy J, Ryseck RP, Carrasco D, Bravo R. Antigen-induced eosinophilic lung inflammation develops in mice deficient in chemokine eotaxin. Blood. 1998;92:3912–3923. [PubMed] [Google Scholar]
- 9.Ponath PD, Qin S, Ringler DJ, Clark-Lewis I, Wang J, Kassam N, Smith H, Shi X, Gonzalo JA, Newman W, et al. Cloning of the human eosinophil chemoattractant, eotaxin. Expression, receptor binding and functional properties suggest a mechanism for the selective recruitment of eosinophils. J Clin Invest. 1996;97:604–612. doi: 10.1172/JCI118456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White JR, Imburgia C, Dul E, Appelbaum E, O’Donnell K, O’Shannessy DJ, Brawner M, Fornwald J, Adamou J, Elshourbagy NA, et al. Cloning and functional characterization of a novel human CC chemokine that binds to the CCR3 receptor and activates human eosinophils. J Leukoc Biol. 1997;62:667–675. doi: 10.1002/jlb.62.5.667. [DOI] [PubMed] [Google Scholar]
- 11.Kitaura M, Suzuki N, Imai T, Takagi S, Suzuki R, Nakajima T, Hirai K, Nomiyama H, Yoshie O. Molecular cloning of a novel human CC chemokine (eotaxin-3) that is a functional ligand of CC chemokine receptor 3. J Biol Chem. 1999;274:27975–27980. doi: 10.1074/jbc.274.39.27975. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalo J-A, Jia G-Q, Aguirre V, Friend D, Coyle AJ, Jenkins NA, Lin GS, Katz H, Lichtman A, Copeland N, et al. Mouse eotaxin expression parallels eosinophil accumulation during lung allergic inflammation but it is not restricted to a Th2-type response. Immunity. 1996;4:1–14. doi: 10.1016/s1074-7613(00)80293-9. [DOI] [PubMed] [Google Scholar]
- 13.Zimmermann N, Hogan SP, Mishra A, Brandt EB, Bodette TR, Pope SM, Finkelman FD, Rothenberg ME. Murine eotaxin-2: a constitutive eosinophil chemokine induced by allergen challenge and IL-4 overexpression. J Immunol. 2000;165:5839–5846. doi: 10.4049/jimmunol.165.10.5839. [DOI] [PubMed] [Google Scholar]
- 14.Menzies-Gow A, Robinson DS. Eosinophil chemokines and chemokine receptors: their role in eosinophil accumulation and activation in asthma and potential as therapeutic targets. J Asthma. 2001;38:605–613. doi: 10.1081/jas-100107538. [DOI] [PubMed] [Google Scholar]
- 15.Romagnani S. Cytokines and chemoattractants in allergic inflammation. Mol Immunol. 2002;38:881–885. doi: 10.1016/s0161-5890(02)00013-5. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe K, Jose PJ, Rankin SM. Eotaxin-2 generation is differentially regulated by lipopolysaccharide and IL-4 in monocytes and macrophages. J Immunol. 2002;168:1911–1918. doi: 10.4049/jimmunol.168.4.1911. [DOI] [PubMed] [Google Scholar]
- 17.Cuvelier SL, Patel KD. Shear-dependent eosinophil transmigration on interleukin 4-stimulated endothelial cells: a role for endothelium-associated eotaxin-3. J Exp Med. 2001;194:1699–1709. doi: 10.1084/jem.194.12.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18••.Humbles AA, Lu B, Friend DS, Okinaga S, Lora J, Al-Garawi A, Martin TR, Gerard NP, Gerard C. The murine CCR3 receptor regulates both the role of eosinophils and mast cells in allergen-induced airway inflammation and hyperresponsiveness. Proc Natl Acad Sci USA. 2002;99:1479–1484. doi: 10.1073/pnas.261462598. [This paper describes the generation of CCR3 KO mice and the effects on the development of airway inflammation and AHR. Although eosinophils were reduced following allergen challenge of CCR3 KO mice, this was associated with an increase in AHR. The authors determined that this was due to abnormal trafficking of mast cells in the CCR3 KO mice, and postulate a role for CCR3 in mast cell homing.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19••.Ma W, Bryce PJ, Humbles AA, Laouini D, Yalcindag A, Alenius H, Friend DS, Oettgen HC, Gerard C, Geha RS. CCR3 is essential for skin eosinophilia and airway hyperresponsiveness in a murinemodel of allergic skin inflammation. J Clin Invest. 2002;109:621–628. doi: 10.1172/JCI14097. [This study reveals that CCR3 KO mice sensitised epicutaneously do not develop AHR following allergen challenge, and highlights the importance of the protocol used for interpreting individual mouse model studies (compare with [18••]).] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalo JA, Pan Y, Lloyd CM, Jia GQ, Yu G, Dussault B, Powers C, Proudfoot AE, Coyle AJ, Gearing D, Gutierrez-Ramos JC. Mouse monocyte-derived chemokine is involved in airway hyperreactivity and lung inflammation. J Immunol. 1999;163:403–411. [PubMed] [Google Scholar]
- 21.Kawasaki S, Takizawa H, Yoneyama H, Nakayama T, Fujisawa R, Izumizaki M, Imai T, Yoshie O, Homma I, Yamamoto K, Matsushima K. Intervention of thymus and activation-regulated chemokine attenuates the development of allergic airway inflammation and hyperresponsiveness in mice. J Immunol. 2001;166:2055–2062. doi: 10.4049/jimmunol.166.3.2055. [DOI] [PubMed] [Google Scholar]
- 22.Chvatchko Y, Hoogewerf AJ, Meyer A, Alouani S, Juillard P, Buser R, Conquet F, Proudfoot AE, Wells TN, Power CA. A key role for CC chemokine receptor 4 in lipopolysaccharide-induced endotoxic shock. J Exp Med. 2000;191:1755–1764. doi: 10.1084/jem.191.10.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23•.Schuh JM, Power CA, Proudfoot AE, Kunkel SL, Lukacs NW, Hogaboam CM. Airway hyperresponsiveness, but not airway remodeling, is attenuated during chronic pulmonary allergic responses to Aspergillus in CCR4–/– mice. FASEB J. 2002;16:1313–1315. doi: 10.1096/fj.02-0193fje. [This paper was the first to show that lack of CCR4 results in decreased AHR in an in vivo allergen challenge model. See also annotation [27••].] [DOI] [PubMed] [Google Scholar]
- 24••.Bishop B, Lloyd CM. CCL1 promotes recruitment of eosinophils but not Th2 cells during the development of allergic airways disease. J Immunol. 2003;170:4810–4817. doi: 10.4049/jimmunol.170.9.4810. [See annotation [27••].] [DOI] [PubMed] [Google Scholar]
- 25••.Chung CD, Kuo F, Kumer J, Motani AS, Lawrence CE, Henderson WRJ, Venkataraman C. CCR8 is not essential for the development of inflammation in a mouse model of allergic airway disease. J Immunol. 2003;170:581–587. doi: 10.4049/jimmunol.170.1.581. [See annotation [27••].] [DOI] [PubMed] [Google Scholar]
- 26••.Chensue SW, Lukacs NW, Yang TY, Shang X, Frait KA, Kunkel SL, Kung T, Wiekowski MT, Hedrick JA, Cook DN, et al. Aberrant in vivo T helper type 2 cell response and impaired eosinophil recruitment in CC chemokine receptor 8 knockout mice. J Exp Med. 2001;193:573–584. doi: 10.1084/jem.193.5.573. [See annotation [27••].] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27••.Goya I, Villares R, Zaballos A, Gutierrez J, Kremer L, Gonzalo JA, Varona R, Carramolino L, Serrano A, Pallares P, et al. Absence of CCR8 does not impair the response to ovalbumin-induced allergic airway disease. J Immunol. 2003;170:2138–2146. doi: 10.4049/jimmunol.170.4.2138. [These papers ([23•, 24••–27••]) highlight apparent contradictions regarding the in vivo role of the CCR8/CCL1 axis in mediating Th2 recruitment.] [DOI] [PubMed] [Google Scholar]
- 28.Gonzalo JA, Lloyd CM, Peled A, Delaney T, Coyle AJ, Gutierrez-Ramos JC. Critical involvement of the chemotactic axis CXCR4/stromal cell-derived factor-1 alpha in the inflammatory component of allergic airway disease. J Immunol. 2000;165:499–508. doi: 10.4049/jimmunol.165.1.499. [DOI] [PubMed] [Google Scholar]
- 29•.Lukacs NW, Berlin A, Schols D, Skerlj RT, Bridger GJ. AMD3100, a CxCR4 antagonist, attenuates allergic lung inflammation and airway hyperreactivity. Am J Pathol. 2002;160:1353–1360. doi: 10.1016/S0002-9440(10)62562-X. [This study is the first to use a small molecule antagonist to manipulate progression of allergic pulmonary inflammation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30••.Panina-Bordignon P, Papi A, Mariani M, Di Lucia P, Casoni G, Bellettato C, Buonsanti C, Miotto D, Mapp C, Villa A, et al. The C-C chemokine receptors CCR4 and CCR8 identify airway T cells of allergen-challenged atopic asthmatics. J Clin Invest. 2001;107:1357–1364. doi: 10.1172/JCI12655. [This study was the first to determine that CCR4 and CCR8 are present on Th2 cells in the lung after allergen challenge of atopic asthmatics.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nouri-Aria KT, Wilson D, Francis JN, Jopling LA, Jacobson MR, Hodge MR, Andrew DP, Till SJ, Varga EM, Williams TJ, et al. CCR4 in human allergen-induced late responses in the skin and lung. Eur J Immunol. 2002;32:1933–1938. doi: 10.1002/1521-4141(200207)32:7<1933::AID-IMMU1933>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 32.D’Ambrosio D, Iellem A, Bonecchi R, Mazzeo D, Sozzani S, Mantovani A, Sinigaglia F. Selective up-regulation of chemokine receptors CCR4 and CCR8 upon activation of polarized human type 2 Th cells. J Immunol. 1998;161:5111–5115. [PubMed] [Google Scholar]
- 33.Colantonio L, Recalde H, Sinigaglia F, D’Ambrosio D. Modulation of chemokine receptor expression and chemotactic responsiveness during differentiation of human naive T cells into Th1 or Th2 cells. Eur J Immunol. 2002;32:1264–1273. doi: 10.1002/1521-4141(200205)32:5<1264::AID-IMMU1264>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 34.Nagase H, Kudo K, Izumi S, Ohta K, Kobayashi N, Yamaguchi M, Matsushima K, Morita Y, Yamamoto K, Hirai K. Chemokine receptor expression profile of eosinophils at inflamed tissue sites: decreased CCR3 and increased CXCR4 expression by lung eosinophils. J Allergy Clin Immunol. 2001;108:563–569. doi: 10.1067/mai.2001.118292. [DOI] [PubMed] [Google Scholar]
- 35•.Menzies-Gow A, Ying S, Sabroe I, Stubbs VEL, Soler D, Williams TJ, Kay AB. Eotaxin (CCL11) and eotaxin-2 (CCL24) induce recruitment of eosinophils, basophils, neutrophils and macrophages as well as features of early- and late-phase allergic reactions following cutaneous injection in human atopic and nonatopic volunteers. J Immunol. 2002;169:2712–2718. doi: 10.4049/jimmunol.169.5.2712. [This study is the first to describe the in vivo effects of chemokine delivery into humans. Cutaneous injection of either eotaxin-1 or eotaxin-2 was seen to induce recruitment of neutrophils and macrophages, as well as eosinophils and basophils.] [DOI] [PubMed] [Google Scholar]
- 36.Oliveira SH, Lira S, Martinez A, Wiekowski M, Sullivan L, Lukacs NW. Increased responsiveness of murine eosinophils to MIP-1beta (CCL4) and TCA-3 (CCL1) is mediated by their specific receptors, CCR5 and CCR8. J Leukoc Biol. 2002;71:1019–1025. [PubMed] [Google Scholar]
- 37.Lloyd CM, Gonzalo JA, Nguyen T, Delaney T, Tian J, Oettgen H, Coyle AJ, Gutierrez-Ramos JC. Resolution of bronchial hyperresponsiveness and pulmonary inflammation is associated with IL-3 and tissue leukocyte apoptosis. J Immunol. 2001;166:2033–2040. doi: 10.4049/jimmunol.166.3.2033. [DOI] [PubMed] [Google Scholar]
- 38.Iellem A, Mariani M, Lang R, Recalde H, Panina-Bordignon P, Sinigaglia F, D’Ambrosio D. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2001;194:847–853. doi: 10.1084/jem.194.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Owen C. Chemokine receptors in airway disease: which receptors to target? Pulm Pharmacol Ther. 2001;14:193–202. doi: 10.1006/pupt.2001.0281. [DOI] [PubMed] [Google Scholar]
- 40••.Proudfoot AE. Chemokine receptors: multifaceted therapeutic targets. Nat Rev Immunol. 2002;2:106–115. doi: 10.1038/nri722. [This review provides an excellent overview of the chemokine family as potential therapeutic targets for multiple inflammatory conditions.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liang M, Rosser M, Ng HP, May K, Bauman JG, Islam I, Ghannam A, Kretschmer PJ, Pu H, Dunning L, et al. Species selectivity of a small molecule antagonist for the CCR1 chemokine receptor. Eur J Pharmacol. 2000;389:41–49. doi: 10.1016/s0014-2999(99)00863-8. [DOI] [PubMed] [Google Scholar]
- 42.D’Ambrosio D, Mariani M, Panina-Bordignon P, Sinigaglia F. Chemokines and their receptors guiding T lymphocyte recruitment in lung inflammation. Am J Respir Crit Care Med. 2001;164:1266–1275. doi: 10.1164/ajrccm.164.7.2103011. [DOI] [PubMed] [Google Scholar]