Abstract
Background
Adipose tissue expansion during obesity is associated with a state of low-grade inflammation and an increase in macrophage infiltration, which predisposes to insulin resistance and vascular malfunction. Growing evidence suggests that vitamin D3 has immunoregulatory effects and adipose tissue could be a target for vitamin D3 action. Preadipocytes, one of the major cell types in adipose tissue, are actively involved in inflammatory processes.
Objectives
This study investigated whether the active form of vitamin D3 (1,25(OH)2D3) affects the production of proinflammatory chemokines/cytokines and the monocyte recruitment by human preadipocytes.
Methods and Results
The secretion levels of MCP-1, IL-8 and IL-6 were significantly higher in preadipocytes than in differentiated adipocytes, suggesting that preadipocytes could be a major source of proinflammatory mediators. Cytokine profile analysis revealed that 1,25(OH)2D3 (10 nM) markedly reduced the release of MCP-1, IL-6 and IL-8 by preadipocytes. The involvement of NFκB signaling was shown by the upregulation of IκBα protein abundance by 1,25(OH)2D3 in preadipocytes. In addition, 1,25(OH)2D3 was able to decrease the migration of THP-1 monocytes. Treatment with proinflammatory stimuli, including macrophage conditioned (MC) medium, TNFα and IL-1β, led to a marked increase in protein release of MCP-1 and IL-6 by preadipocytes. Pretreatment with 1,25(OH)2D3 (10 nM and 100 nM) significantly decreased the stimulatory effects of MC medium, TNFα and IL-1β on MCP-1 expression and protein release, although the effect on stimulated release of IL-6 was less potent.
Conclusions
These results demonstrate that 1,25(OH)2D3 decreases the production of MCP-1 and other proinflammatory mediators by preadipocytes and reduces monocyte migration. Thus, vitamin D3 may protect against adipose tissue inflammation by disrupting the deleterious cycle of macrophage recruitment.
Keywords: 1,25-dihydroxyvitamin D3; preadipocytes; MCP-1; monocytes; inflammation; obesity
Introduction
White adipose tissue expansion during obesity is accompanied by increased infiltration of macrophages, and this is associated with a state of low-grade inflammation (1, 2). As an endocrine organ, adipose tissue secretes a number of protein factors which are directly involved in inflammation (3). The expression and release of some of these factors, including TNFα, IL-6, monocyte chemoattractant proteint-1 (MCP-1) and IL-8, have been shown to be elevated in obesity (4-6). Studies have suggested that the stromal-vascular (SV) fraction of adipose tissue is a major source of the production of proinflammatory factors in comparison with the mature adipocytes (7). Preadipocytes, a major component of the SV fraction, have been shown to function as macrophage-like cells and produce proinflammatory mediators (8, 9). Recent studies from our group and others have demonstrated that the release of MCP-1, IL-8 and IL-6 by human preadipocytes was substantially increased in response to the stimulation by macrophage-conditioned medium (9, 10). Therefore, preadipocytes could be a key player in adipose tissue inflammation in obesity.
The vitamin D system is increasingly recognised to have a range of physiological functions beyond calcium homeostasis and bone metabolism (11). The major circulating form of vitamin D is 25-hydroxycholecalciferol (25(OH)D3) which is converted to the biologically active factor, 1,25-dihydroxycholecalciferol (1,25(OH)2D3). The actions of 1,25(OH)2D3 are mediated through the vitamin D receptor (VDR) which modulates the transcription of a number of target genes (11). Growing evidence suggests that 1,25(OH)2D3 has immunoregulatory effects, such as modulating T-lymphocyte proliferation and function (12), and suppressing the production of inflammatory cytokines, chemokines and prostaglandins in cancer cells (13, 14). These actions of vitamin D may be through inhibiting the p38 kinase (15) and NF-κB signalling (16-18).
Clinical studies on vitamin D status in humans have suggested that there is a link between vitamin D deficiency and obesity (19, 20). Serum levels of 25(OH)D3 are inversely correlated with BMI and body fat mass in both children and adults (21, 22). There is also evidence from healthy subjects that lower levels of serum 25(OH)D3 are associated with an increase in systemic inflammation (23). The extent to which there is a role of vitamin D in adipose tissue function is not well understood. However, 1,25(OH)2D3 has been shown to inhibit the differentiation of 3T3-L1 cells and of porcine preadipocytes, and to repress the expression of adipogenic transcription factor genes (24, 25). A recent study has also shown that 1,25(OH)2D3 decreased the TNFα-stimulated expression and release of MCP-1 and adiponectin by differentiated human adipocytes (26). Although preadipocytes are important in adipose tissue inflammation, it is not known whether vitamin D modulates the inflammatory response in these precursor cells.
The present study has investigated the effect of 1,25(OH)2D3 on the production of proinflammatory chemokines/cytokines by human preadipocytes, with or without the stimulation of macrophage-derived factors. The study has also examined whether vitamin D3 acts on the NFκB signalling pathway in human preadipocytes. Finally, the effect of 1,25(OH)2D3 on monocyte migration has been explored.
Materials and methods
Culture of human preadipocytes
Human white preadipocytes derived from subcutaneous adipose tissue of a female Caucasian subject (BMI 21; age 44 years) were obtained from PromoCell (Heidelberg, Germany). Preadipocytes were cultured in preadipocyte growth medium supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml amphotericin B (Lonza, Tewkesbury, UK) at 37°C in a humidified atmosphere of 5% CO2/95% air. Preadipocytes were seeded onto 6-well or 24-well plates and grown until confluence. At confluence, cells were induced to differentiate (day 0) by incubation for 3 days in Dulbecco’s Modified Eagle’s Medium (DMEM) / Ham’s F12 (1:1) medium containing 32 μM biotin,1 μM dexamethasone, 200 μM 3-isobutyl-1-methyl-xanthine, 100 nM insulin, 11 nM L-Thyroxine (all from Sigma, Poole, Dorset, UK), 8 μM rosiglitazone (GlaxoSmithKline, Uxbridge, UK) and 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml amphotericin B. After induction, cells were further maintained in feeding medium containing 3% fetal calf serum (FCS; Sigma), 100 nM insulin, 32 μM biotin and 1 μM dexamethasone until full differentiation. Differentiation into adipocytes was confirmed by observing the accumulation of lipid droplets under the microscope.
Culture of THP-1 monocytes
The human THP-1 monocytic cell line was kindly provided by Professor Helen R Griffiths (Aston University, UK). Human THP-1 monocytes (1×106 cells/ml) were cultured in 24-well plates in Roswell Park Memorial Institute (RPMI-1640) medium with 10% FCS and 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml amphotericin B at 37°C. To prepare macrophage-conditioned (MC) medium, THP-1 monocytes were first differentiated by the addition of 100 nM phorbol 12-myristate 13-acetate (PMA; Sigma) for 48 h. The medium was then replaced with FCS-free RPMI 1640 medium (without PMA) for 24 h, and this conditioned (MC) medium was then harvested, filtered through a 0.22 μm filter, and stored at −80°C for later use.
Cell culture treatment
To assess the effect of vitamin D on cytokine secretion profile, preadipocytes were treated with 1,25(OH)2D3 (10 nM) (ENZO Life Sciences, Plymouth Meeting, PA, USA) for 24 h, and the control group of preadipocytes received no treatment. To further investigate whether vitamin D inhibits the effects of proinflammatory stimuli on cytokine/chemokine production, the cells were pre-incubated with vitamin D3 (10 nM and 100 nM) for 24 h and then treated with either MC medium, TNFα (5 ng/ml) or IL-1β (5 ng/ml) in the presence of vitamin D3 (10 and 100 nM) for a further 24 h.
To examine whether TNFα and IL-1β mediate the effects of MC medium on the expression and release of MCP-1 and IL-6, MC medium was pre-incubated with human TNFα or IL-1β neutralizing antibody (all from R&D Systems, Abingdon, UK) or mouse IgG (Sigma) as negative controls (1 μg/ml or 15 μg/ml) for 1 h. Preadipocytes were then treated with RPMI 1640 medium (control), MC medium, TNFα-neutralized MC medium, IL-1β-neutralized MC medium, or MC medium neutralised by both TNFα (7.5 μg/ml) and IL-1β (7.5 μg/ml) antibodies for 24 h.
To elucidate the potential signalling pathway, preadipocytes were preincubated with vitamin D3 (10 nM and 100 nM) for 24 h at 37°C.
At the end of each experiment, cells and the culture media were collected and stored at −80°C until analysis.
Measurement of MCP-1, IL-8 and IL-6 release
Protein release of MCP-1, IL-8, IL-6, adiponectin and leptin by preadipocytes and adipocytes was measured as the protein concentrations in cell culture medium, using human ELISA kits (R&D Systems, Abingdon, UK).
Cytokine protein array
Preadipocytes were pretreated with 10 nM vitamin D3 for 24 h, or without treatment, as controls. The cell culture medium (n=6 per group) was harvested 24 h later and pooled. The samples were incubated with a cocktail of biotinylated detection antibodies for 1 h at room temperature, and followed by the incubation with a human cytokine array (panel A) (R&D Systems) overnight at 4°C. The array was then incubated with streptavidin-horseradish peroxidase and the protein signals were detected by chemiluminescence (West Pico kit, Pierce, Loughborough, UK) and captured by Molecular Imager ChemiDoc XRS+ System (Bio-Rad, Hertfordshire, UK). The detected signals were then quantified as pixel density according to the manufacturer’s instructions.
Transmigration assay
THP-1 monocytes were suspended in RPMI 1640 (serum free) at 2×106/ml and added to the top chamber of HTS transwells (Fisher Scientific, Loughborough, UK). Preadipocytes were treated with or without vitamin D3 (10 and 100 nM) for 24 h; the medium was harvested and added to the lower chamber of transwells. In addition, the medium of untreated preadipocytes with freshly added vitamin D3 (10 nM) or preadipocyte growth medium (PGM) alone (without cells) was added to the lower chamber of transwells. After incubation for 4 h at 37°C, the number of monocytes that had migrated to the lower chamber was determined by the MTT assay using a cell density standard curve.
Western blotting
Total cellular protein was prepared by lysing cells in lysis buffer (50 mM Tris-HCl pH 6.7, 10% Glycerol, 4% SDS, 2% 2-mercaptoethanl) with freshly added protease inhibitor cocktail and phosphatase inhibitor cocktail (both from Sigma). Protein concentrations were determined by the BCA method. Protein samples (20 μg/lane) were resolved by 10% Tricine-SDS polyacrylamide slab gels (Mini Protean Tetra, Bio-Rad, Hemel Hempstead, UK), transferred onto a nitrocellulose membrane (Hybond™ C Extra, Amersham Bioscience, Little Chalfont, UK) by wet transfer (Trans Blot, Bio-Rad) at 100 V for 1 h. The transfer of proteins onto the membrane was assessed by Ponceau S staining.
For immunodetection, the membrane was blocked for 1 h at room temperature with Tris-buffered saline (TBS) containing 0.1% Tween 20 and 5% non-fat milk and incubated overnight at 4°C with an antibody for IκBα (New England BioLabs Ltd, Hitchin, UK) at a 1:1000 dilution in 5% non-fat milk TBS and 0.1% Tween-20 followed by an anti-mouse secondary antibody (Bio-Rad) at 1:2000 dilution. Signals were detected by chemiluminescence (West Pico kit, Pierce, Loughborough, UK) and scanned using Molecular Imager ChemiDoc XRS+ System (Bio-Rad). The size of the protein bands detected was estimated with PageRuler protein markers (Fermentas, York, UK). Subsequently, the membrane was further probed with the antibody detecting α-tubulin as a loading control.
Real-time PCR
Total RNA was extracted from cells using Trizol (Invitrogen, Paisley, UK) and the RNA concentration determined from the absorbance at 260 nm. First strand cDNA was reverse transcribed from 0.5 μg of total RNA using an iScript first strand synthesis kit (Bio-Rad) in a final volume of 10 μl. Real-time PCR amplification was performed in a final volume of 12.5 μl, containing cDNA (equivalent to 10 ng of RNA), optimized concentrations of primers, TaqMan probe (FAM-TAMRA) and a master mix made from qPCR core kit (Eurogentec, Seraing, Belgium) using a Stratagene Mx3005P instrument. The sequences of primers and probes for human β-actin, IL-6, MCP-1 and IL-8 were as described previously (27, 28). PCR amplification was performed in duplicate and the PCR cycling conditions were as follows: 95°C for 10 min followed by 40 cycles (95°C for 15 sec, 60°C for 1 min). Blank controls without cDNA were run in parallel. β-actin was used as a reference gene. All samples were normalised to the β-actin values and the results expressed as fold changes of Ct value relative to controls using the 2−ΔΔct formula (29).
Cell viability assay
To assess cell viability following various treatments, cells were incubated with 100 μl 3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) (Sigma) solution (5 mg/ ml in PBS) for 4 h at 37°C. Lysis buffer (100 μl; 20% SDS in 50% dimethyl formamide, pH 4.7) was added to each well and the cells were incubated for a further 16 h at 37°C. The absorbance was read at 570 nm using a spectrophotometer (Bio-Rad) at room temperature.
Statistical analysis
Data are expressed as means ± SEM. Differences between two groups were analyzed by Student’s unpaired t-test; ANOVA was employed for multi-group comparisons. Differences were considered as statistically significant when P<0.05.
Results
MCP-1, IL-8 and IL-6 are secreted mainly by human preadipocyte
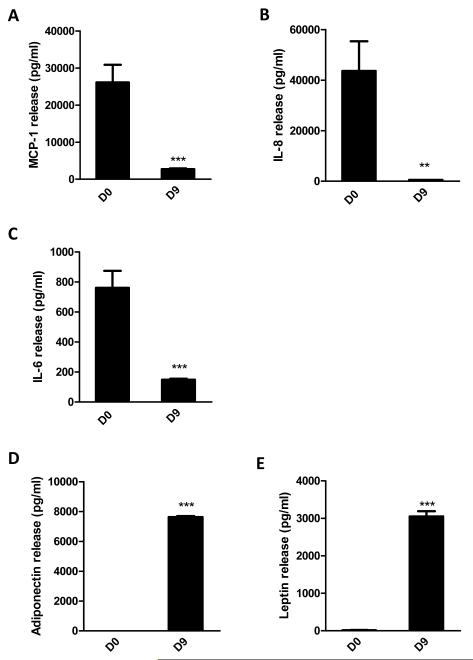
In initial experiments, the release of chemokines and cytokines by preadipocytes was examined. Measurements by ELISA indicate that preadipocytes are a more important source of the production of the chemokines MCP-1, IL-8 and of the cytokine IL-6 than adipocytes (Fig. 1A-C). The levels of the chemokines/cytokines were significantly higher in the medium from preadipocytes (D0) compared to differentiated adipocytes (D9); MCP-1 was increased 10-fold (P<0.001), IL-8 82-fold (P<0.001) and IL-6 5-fold (P<0.001) in the preadipocyte medium relative to the adipocyte medium. However, IL-1β was undetectable in cell culture medium from both cell types (data not shown). In contrast, the adipokines, adiponectin and leptin, were exclusively secreted by adipocytes (Fig. 1D-E).
Figure 1.
Secretion of MCP-1, IL-8 and IL-6 by human preadipocytes and differentiated adipocytes. Protein levels of MCP-1 (A), IL-8 (B), IL-6 (C), adiponectin (D) and leptin (E) in cell culture medium, accumulated over 72 h, of preadipocytes (D0) and adipocytes (D9) were measured by ELISAs. Similar numbers of preadipocytes and adipocytes were used. Results are expressed as means ± SEM for groups of 6 (where error bars are not visible, this reflects the low variation observed); **P<0.01, ***P<0.001 vs preadipocytes. The results were verified by three independent experiments.
Vitamin D3 decreases the protein release of MCP-1, IL-8 and IL-6 by human preadipocytes
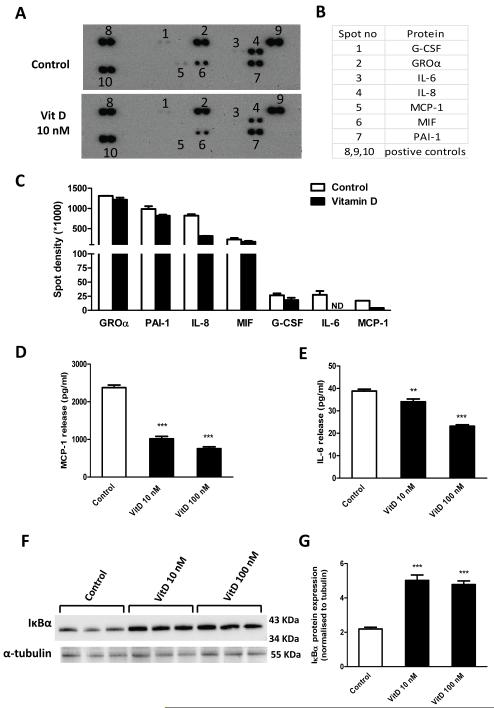
To explore the potential effect of vitamin D3 on the secretion profile of chemokines/cytokines by preadipocytes, a cytokine protein array was used. As shown in Fig. 2A-B, seven proteins (G-CSF, GROα, IL-6, IL-8, MCP-1, MIF and PAI-1) were detected in the medium from preadipocytes, both in the presence and absence of 1,25(OH)2D3. Quantification of the spots on the arrays by densitometry indicated that treatment with 1,25(OH)2D3 (10 nM) reduced the release of MCP-1, IL-8 and IL-6, compared to controls, and there was a slight reduction in G-CSF, MIF and PAI-1 release (Fig 2C). The reduction in MCP-1 and IL-6 release was confirmed by ELISA. Treatment with 1,25(OH)2D3 (10 and 100 nM) resulted in a dose-dependent decrease in MCP-1 (2-fold and 3-fold, both P<0.001) and IL-6 (10% and 40%, both P<0.01) in the medium compared to controls (Fig. 2D and 2E).
Figure 2.
Effects of vitamin D3 on cytokine secretion profile and NFκB activation. Preadipocytes were pretreated with or without vitamin D3 (10 nM) for 24 h. Cell culture medium was harvested 24 h later, mixed with a cocktail of biotinylated detection antibodies and then incubated with a human cytokine array (panel A, R&D). (A) Signals were detected by chemiluminescence. (B) The table shows the location of detected proteins on the membrane. (C) Detected signals were quantified as the pixel density. (D-E) levels of MCP-1 and IL-6 release into cell culture medium, measured by ELISA. Results are expressed as means ± SEM for groups of 6; the results were confirmed by three independent experiments. (F-G) preadipocytes were pre-incubated with vitamin D3 (10 and 100 nM) for 24 h; protein expression of IκBα in cell lysates was analyzed by western blotting (F) and the signals quantified by densitometry (G); the results were confirmed by two independent experiments. **P<0.01; ***P<0.001 vs controls.
Vitamin D inhibits activation of NF-κB signaling pathway
Since NF-κB activation is central in the signal transduction of proinflammatory cytokines, we next examined whether the effect of vitamin D3 on chemokine/cytokine production could be through inhibition of NF-κB in preadipocytes. As shown in Fig. 2F and 2G, 1,25(OH)2D3 (10 and 100 nM) significantly increased IκBα protein level (both 2-fold, P<0.001) in human preadipocytes.
Vitamin D3 reduces monocyte migration
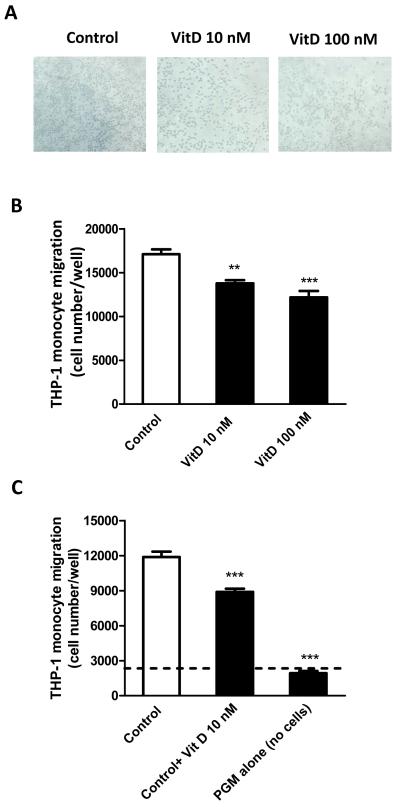
To examine whether vitamin D3 also affects immune cell recruitment by preadipocytes, THP-1 monocyte migration elicited by preadipocytes pretreated with vitamin D3 (10 and 100 nM) or without (control) for 24 h was determined. As shown in Fig. 3A-B, using the medium of preadipocytes pretreated with 1,25(OH)2D3 (10 and 100 nM) for 24 h, monocyte migration was significantly reduced (by 19% and 29%, P<0.01 and P<0.001). Further, addition of fresh 1,25(OH)2D3 (10 nM) to the control medium decreased monocyte migration (by 25%, P<0.001); PGM alone (without cells) caused the least migration of monocytes (Fig. 3C).
Figure 3.
Vitamin D3 reduces THP-1 monocyte transmigration. (A) Representative images of monocyte migration, photographed at 10x magnifications under a phase contrast microscope. (B) Preadipocytes growing in preadipocyte growth medium (PGM) were pretreated with vitamin D3 (10 and 100 nM) or without (control) for 24 h; the culture medium (150 μl) was harvested and added to the lower chamber of transwells. THP-1 monocytes (2×106/ml; 100 μl) were added to the upper chamber of the wells with a pore size of 5 μm. (C) The medium of preadipocytes without treatment (control) or the control medium with freshly added vitamin D3 (10 nM) (control+VitD), or PGM alone (without cells) was added to the lower chamber; THP-1 monocytes (2×106/ml; 100 μl) were placed in the upper chamber of the transwells. After incubation for 4 h at 37°C, the migration of monocytes was determined by the MTT assay. Results are expressed as means ± SEM for groups of 6; ***P<0.001 vs controls. The results were verified by four independent experiments.
TNFα and IL-1β neutralization inhibits the induction of MCP-1 and IL-6 in human preadipocytes by MC medium
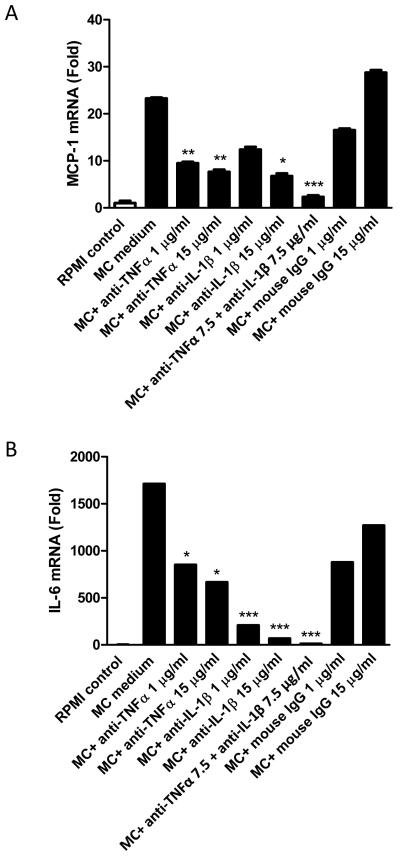
In the next experiments, whether TNFα and IL-1β play a key role in mediating the effects of MC medium on the expression of proinflammatory mediators by preadipocytes was examined. This was performed by assessing whether neutralising TNFα or IL-1β activity using specific antibodies antagonises the effect of MC medium on the expression of MCP-1 and IL-6. As shown in Fig. 4A and 4B), incubation of preadipocytes in the presence of MC medium led to a marked increase in mRNA levels for both MCP-1 (23-fold, P<0.001) and IL-6 (1710-fold, P<0.001), compared to controls maintained in unconditioned medium.
Figure 4.
Effects of TNFα and IL-1β neutralisation on MC medium-induced expression of MCP-1 and IL-6 by human preadipocytes. For blocking the activity of TNFα and IL-1β, MC medium was pre-incubated with human TNFα neutralizing antibody (1 and 15 μg/ml) or human IL-1β neutralizing antibody (1 and 15 μg/ml) or mouse IgG (as negative controls) for 1 h. Preadipocytes were then treated with either of RPMI 1640 medium (control), MC medium, TNFα neutralized MC medium, IL-1β neutralised MC medium, and MC medium neutralised by TNFα and IL-1β antibodies (7.5 μg/ml) for 24 h. Levels of MCP-1 (A) and IL-6 (B) mRNA in preadipocytes were measured by real-time PCR and normalised to β-actin. Data are means ± SEM for groups of 6. *P<0.05, **P<0.01, ***P<0.001 vs MC medium treatment.
Inhibition of TNFα or IL-1β significantly reduced, in a dose-dependent manner, the induction of MCP-1 and IL-6 gene expression in preadipocytes elicited by MC medium. Neutralisation of MC medium with an anti-TNFα antibody (1 or 15 μg/ml) reduced the MC-induced stimulation in MCP-1 (2-fold and 3-fold, both P<0.01) and IL-6 (2-fold and 2.5-fold, both P<0.05) mRNA levels. Similarly, neutralisation of MC medium with an anti-IL-1β antibody (1 or 15 μg/ml) led to marked reductions in MC medium-induced mRNA levels for MCP-1 (3-fold at high dose, P<0.05) and IL-6 (8-fold and 26-fold, both P<0.001). Furthermore, neutralisation of MC medium with both anti-TNFα (7.5 μg/ml) and anti-IL-1β antibodies (7.5 μg/ml) almost completely attenuated the induction of MCP-1 and IL-6 by MC medium (Fig. 4A and 4B).
The specificity of the TNFα and IL-1β neutralising antibodies was confirmed by there being no significant differences in expression levels of MCP-1 and IL-6 between preadipocytes treated with MC medium and MC medium pre-incubated with mouse IgG (1 or 15 μg/ml; Fig. 4A and 4B).
Vitamin D3 reduces the induction of chemokines/cytokines in preadipocytes by MC medium
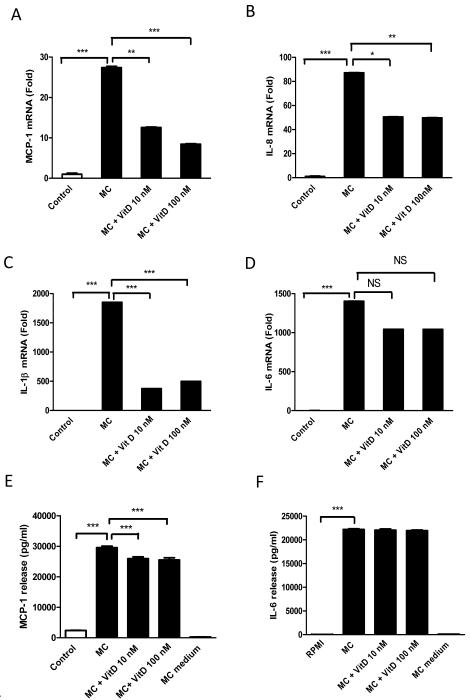
As macrophage-derived factors potently stimulate inflammatory responses in preadipocytes, the effects of vitamin D3 on MC medium-induced production of chemokines/cytokines by preadipocytes were examined. As shown in Fig. 5A-D, exposure to MC medium (100%) for 24 h dramatically increased mRNA levels of MCP-1 (27-fold, P<0.001), IL-8 (87-fold, P<0.001), IL-1β (1850-fold, P<0.001) and IL-6 (1403-fold, P<0.001) compared to controls. Consistent with the mRNA data, the MC medium substantially increased the release of MCP-1 (12-fold, P<0.001) and IL-6 (584-fold, P<0.001) compared to controls (Fig. 5F and 5G).
Figure 5.
Effects of vitamin D3 on MC medium-induced chemokine/cytokine production by human preadipocytes. Preadipocytes were pre-incubated with vitamin D3 (10 and 100 nM) for 24 h and treated with RPMI 1640 medium (control) or THP-1 MC medium for another 24 h. mRNA levels of MCP-1 (A), IL-8 (B), IL-1β (C) and IL-6 (D) were measured by real-time PCR and normalized to β-actin. Protein release of MCP-1 (E) and IL-6 (F) were determined by ELISA. Data are means ± SEM for groups of 6 (where error bars are not visible, this reflects the low variation observed); **P<0.01, ***P<0.001. The results were confirmed by three independent experiments.
Pre-incubation of preadipocytes with 1,25(OH)2D3 (10 and 100 nM) decreased the MC medium-induced gene expression of MCP-1 (2-fold and 3-fold, both P<0.01), IL-8 (both 1.7-fold, both P<0.05) and IL-1β (4.9-fold and 3.7-fold, both P<0.001) (Fig. 5A, 5B, and 5C). However, no significant (P>0.05) effect of 1,25(OH)2D3 was found on MC-induced IL-6 gene expression (Fig. 5D). At the level of protein secretion, 1,25(OH)2D3 (10 and 100 nM) led to a reduction in MC medium-stimulated MCP-1 release (12% and 13%, both P<0.01) (Fig. 5E), but had no effect on IL-6 release (Fig. 5F). The different scale of change in mRNA level and protein release for MCP-1 and IL-6 is likely to be due to differences in time-course; the mRNA level relates to the point at which the cells are taken while the protein release is the aggregate of the whole time-course of the experiment.
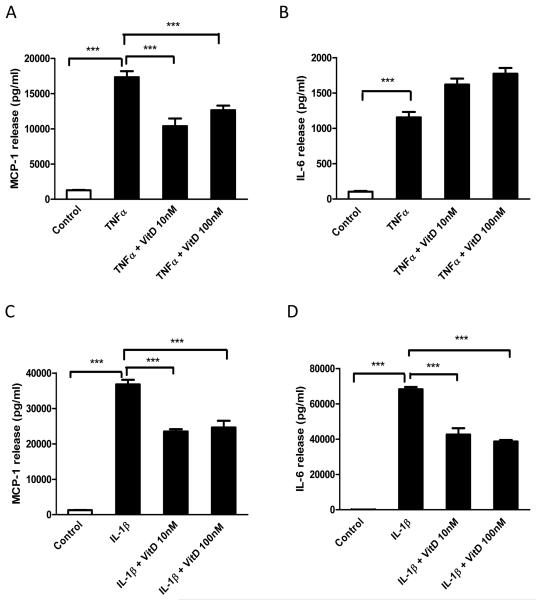
Vitamin D3 reduces TNFα and lL-1β-induced MCP-1 production by human preadipocytes
Since TNFα and IL-1β mediate the MC-medium induced release of MCP-1 and IL-6 by human preadipocytes, we further investigated whether vitamin D3 can antagonize the cytokine stimulated inflammatory response in preadipocytes. As shown in Fig. 6A and 6B, TNFα significantly increased the release of MCP-1 (13.5-fold, P<0.001) and IL-6 (11-fold, P<0.001) by preadipocytes. Pretreatment with 1,25(OH)2D3 (10 and 100 nM) significantly reduced MCP-1 release (40% and 27%, both P<0.001) induced by TNFα (Fig. 6A), but there was no inhibition of IL-6 release (Fig. 6B).
Figure 6.
Effects of vitamin D3 on TNFα- or IL-1β- induced MCP-1and IL-6 protein release by preadipocytes. Preadipocytes were pre-incubated with vitamin D3 (10 and 100 nM) for 24 h and then treated with TNFα (5 ng/ml) or IL-1β (5 ng/ml) for another 24 h in the presence of vitamin D3. Protein levels of MCP-1(A-C) and IL-6 (B-D) release were measured by ELISA. Data are means ± SEM for groups of 6; ***P<0.001. The results were verified by three independent experiments.
IL-1β substantially increased MCP-1 (28-fold, P<0.001) and IL-6 (656-fold, P<0.001) release from preadipocytes (Fig. 6C and 6D). Importantly, preincubation with 1,25(OH)2D3 (10 and 100 nM) significantly reduced MCP-1 (36% and 33%, both P<0.001) and IL-6 (38% and 43%, both P<0.001) release stimulated by IL-1β.
Vitamin D3 does not affect preadipocyte viability
Finally, the MTT assay for cell viability was conducted in parallel and showed that none of the treatments employed in this study affected cell viability (Fig. S1A-D).
Discussion
White adipose tissue as an active endocrine organ secretes a range of protein factors, including those involved in inflammation and inflammatory responses (3, 30). However, studies have suggested that the non-fat cells within adipose tissue are the major source of production of proinflammatory factors (7). In the present study, the basal release of proinflammatory chemokines/cytokines, such as MCP-1, IL-8 and IL-6 primarily occurred in preadipocytes compared with differentiated human adipocytes. We also demonstrate that under the stimulation of macrophage-derived factors, specifically TNFα and IL-1β, there was a considerable increase in the expression and release of these factors. Lipopolysaccharide (LPS) has also been shown to induce a major increase in the mRNA level of proinflammatory cytokines (IL-6, TNFα and IL-1β) and chemokines (MCP-1 and IL-8) in human preadipocytes, leading to a decrease in PPARγ activity and insulin-stimulated glucose uptake in adipocytes (31). Furthermore, the gene expression profile of preadipocytes has been shown to be closer to that of macrophages than to adipocytes, and preadipocytes can acquire high phagocytic activity when in contact with peritoneal macrophages (32). Collectively, it is suggested that preadipocytes are a major site of the production of proinflammatory chemokines/cytokines within adipose tissue, thereby playing a key role in adipose inflammation in obesity.
The emerging role of vitamin D3 in immune regulation suggests that this endocrine factor may modulate the inflammatory responses in adipose tissue. We demonstrate in this study that 1,25(OH)2D3 (10 nM) significantly reduced the basal release of MCP-1, IL-8 and IL-6 from preadipocytes. It should be noted that the doses of 1,25(OH)2D3 employed in the present work are similar to those used in several other studies (33), including a recent publication which showed that 1,25(OH)2D3 (100 nM) is effective in modulating MCP-1 and adiponectin production by human adipocytes (26). It should also be pointed out that since adipocytes store vitamin D, and adipocytes as well as monocytes/macrophages are able to locally convert 25(OH)D3 to 1,25(OH)2D3 (34, 35), the concentrations of vitamin D within adipose tissue could be higher than implied by the plasma levels. Further studies are required to determine the exact levels of vitamin D in obese adipose tissue.
The chemokine MCP-1 and its receptor CCR2 have been reported to be critical factors in promoting the recruitment of monocytes into adipose tissue (36). The release of MCP-1 by adipose tissue and the circulating levels of MCP-1 are elevated during the development of obesity (7, 37). Moreover, MCP-1 is also produced by macrophages (38) which increase further macrophage infiltration into adipose tissue, which may lead to a vicious cycle of monocyte recruitment. IL-8, a member of the CXC chemokine family, has been shown to be responsible for the recruitment of neutrophils into tumor tissue (39). Circulating levels of IL-8 are increased in obese subjects (40). It has been reported that in the early stage of high-fat diet feeding in mice, there is a transient increase in neutrophil infiltration in abdominal adipose tissue and this was partially attributed to IL-8 (41). The signalling pathways that are involved in the action of vitamin D3 in adipose tissue remain to be clarified. NF-κB activation is crucial in the signal transduction of proinflammatory cytokines and NF-κB knockdown reverses the upregulation of IL-6 gene expression in preadipocytes (42). The activation of NF-κB signalling involves the degradation of IκBα protein and translocation of p65 into the nucleus (43). In the present study, we show that 1,25(OH)2D3 increased IκBα protein expression in preadipocytes and this suggests that vitamin D3 may inhibit the activation of NF-κB signalling, probably by increasing the stability of IκBα protein. Similar to our results, previous studies have reported that in murine macrophages, human fibroblasts and peripheral blood mononuclear cells, vitamin D3 is able to stabilise IκBα protein, which inhibited p65 translocation to the nucleus and subsequently reduced NFκB activity (16-18, 44). Furthermore, in mesangial cells 1,25(OH)2D3 suppresses high glucose-induced MCP-1 expression and promoter activity by blunting NFκB activation (45).
In parallel with the effects of vitamin D3 on chemokine production, the present study demonstrates that vitamin D3 has an inhibitory effect on the chemoattractant properties of preadipocytes as pretreatment with 1,25(OH)2D3 (10 nM and 100 nM) reduced monocyte migration. Since 1,25(OH)2D3 has a short half-life of 15 h (46), the reduction in THP-1 cell migration induced by preadipocytes could be, at least in part, the result of the inhibitory effect of vitamin D3 on MCP-1 release. In addition, we show that 1,25(OH)2D3 alone also led to an inhibition on monocyte migration. These observations are novel, which suggests that vitamin D3 could be anti-inflammatory in human adipose tissue, reducing the basal release of MCP-1 and other proinflammatory factors by preadipocytes and limiting the infiltration of immune cells.
With the expansion of adipose tissue in obesity, there is an increased infiltration of immune cells, especially macrophages (47). There are two types of macrophages in adipose tissue: the classically activated M1 type and the alternatively activated M2 type (48) The newly recruited macrophages in adipose tissue in obesity are mainly derived from bone marrow and exhibit the M1 phenotype (48), which secrete a variety of cytokines such as TNFα and IL-1β (48). We show in this study that MC medium potently induces the upregulation of MCP-1 and IL-6 in preadipocytes, and that this is largely mediated by TNFα and IL-1β. Blocking TNFα and IL-1β with the neutralising antibody attenuated the elevation of MCP-1 and IL-6 mRNA elicited by MC medium (Fig.4). Therefore, TNFα and IL-1β are likely to be the primary mediators of the effects of macrophages on MCP-1 and IL-6 production in preadipocytes.
Although vitamin D3 has been shown to downregulate the expression of proinflammatory cytokines/chemokines (TNFα, IL-6, IL-1, and IL-8) in human monocytes stimulated with IFN-γ (49), little is known on whether vitamin D3 acts similarly in human adipose cells challenged by inflammatory stimuli. A key finding of the present study is the demonstration that 1,25(OH)2D3 was able to reduce the overexpression and release of MCP-1 induced by MC medium in preadipocytes (Fig. 5A and 5E). In addition, our data also show that 1,25(OH)2D3 effectively inhibited the increase in MCP-1 release induced by TNFα or IL-1β (Fig. 6A and 6C), suggesting that vitamin D3 has a protective role in restraining the inflammatory response in preadipocytes. Although there is a lack of data on the effects of vitamin D3 on preadipocytes, a recent study using mature human adipocytes has reported an inhibitory effect of 1,25(OH)2D3 on TNFα-elicited MCP-1 production (26). As shown by the present study, 1,25(OH)2D3 decreased the IL-1β-stimulated release of IL-6, but had no effect on the induction by MC medium and TNFα. This may suggest that vitamin D3 could be more effective in the reduction of chemokine production by preadipocytes under stimulated conditions.
In summary, this study demonstrates that human preadipocytes abundantly secrete proinflammatory proteins, and 1,25(OH)2D3 significantly decreased the release of MCP-1, IL-8 and IL-6 from preadipocytes. The effects of vitamin D3 may be through the inhibition of NFκB activation as 1,25(OH)2D3 upregulated IκBα protein abundance in preadipocytes. Furthermore, 1,25(OH)2D3 exhibits an inhibitory effect on THP-1 monocyte migration induced by preadipocytes. Importantly, pretreatment with 1,25(OH)2D3 ameliorated the stimulatory effect of macrophage-conditioned medium, TNFα and IL-1β on MCP-1 expression and protein release by preadipocytes. Our results suggest that vitamin D3 may protect against adipose tissue inflammation in obesity, at least in part, by lowering the release of MCP-1 and other proinflammatory mediators from preadipocytes and disrupting the vicious cycle of macrophage recruitment.
Supplementary Material
Figure S1. Cell viability of preadipocytes treated with vitamin D3 and MC, TNFα or IL-1β. Preadipocytes were pre-incubated with vitamin D3 (10 and 100 nM) for 24 h and then further incubated with RPMI 1640 medium (control) or THP-1 MC medium for another 24 h. Cell viability were determined by MTT assay. Data are means ± SEM for groups of 6. The results were verified by three independent experiments.
Acknowledgements
We thank Professor Helen R Griffiths (Aston University, UK) for providing the human THP-1 monocytes. We also thank Dr. Adrian O’Hara for IL-1β neutralizing antibody. This work was supported by the Medical Research Council (G0801226).
Funding resources: UK Medical Research Council (G0801226)
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Supplementary information is available at IJO’s website.
References
- 1.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92:347–355. doi: 10.1079/bjn20041213. [DOI] [PubMed] [Google Scholar]
- 4.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56:1010–1013. doi: 10.2337/db06-1656. [DOI] [PubMed] [Google Scholar]
- 6.Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab. 2007;92:1023–1033. doi: 10.1210/jc.2006-1055. [DOI] [PubMed] [Google Scholar]
- 7.Fain JN. Release of inflammatory mediators by human adipose tissue is enhanced in obesity and primarily by the nonfat cells: a review. Mediators Inflamm. 2010;2010:513948. doi: 10.1155/2010/513948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cousin B, Munoz O, Andre M, Fontanilles AM, Dani C, Cousin JL, et al. A role for preadipocytes as macrophage-like cells. FASEB J. 1999;13:305–312. doi: 10.1096/fasebj.13.2.305. [DOI] [PubMed] [Google Scholar]
- 9.Lacasa D, Taleb S, Keophiphath M, Miranville A, Clement K. Macrophage-secreted factors impair human adipogenesis: involvement of proinflammatory state in preadipocytes. Endocrinology. 2007;148:868–877. doi: 10.1210/en.2006-0687. [DOI] [PubMed] [Google Scholar]
- 10.Gao D, Bing C. Macrophage-induced expression and release of matrix metalloproteinase 1 and 3 by human preadipocytes is mediated by IL-1β via activation of MAPK signalling. J Cell Physiol. 2011 doi: 10.1002/jcp.22630. [DOI] [PubMed] [Google Scholar]
- 11.Verstuyf A, Carmeliet G, Bouillon R, Mathieu C. Vitamin D: a pleiotropic hormone. Kidney Int. 2010;78:140–145. doi: 10.1038/ki.2010.17. [DOI] [PubMed] [Google Scholar]
- 12.Hewison M. Vitamin D and immune function: an overview. Proc Nutr Soc. 2011:1–12. doi: 10.1017/S0029665111001650. [DOI] [PubMed] [Google Scholar]
- 13.Moreno J, Krishnan AV, Swami S, Nonn L, Peehl DM, Feldman D. Regulation of prostaglandin metabolism by calcitriol attenuates growth stimulation in prostate cancer cells. Cancer Res. 2005;65:7917–7925. doi: 10.1158/0008-5472.CAN-05-1435. [DOI] [PubMed] [Google Scholar]
- 14.Krishnan AV, Swami S, Feldman D. Vitamin D and breast cancer: inhibition of estrogen synthesis and signaling. J Steroid Biochem Mol Biol. 2010;121:343–348. doi: 10.1016/j.jsbmb.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 15.Nonn L, Peng L, Feldman D, Peehl DM. Inhibition of p38 by vitamin D reduces interleukin-6 production in normal prostate cells via mitogen-activated protein kinase phosphatase 5: implications for prostate cancer prevention by vitamin D. Cancer Res. 2006;66:4516–4524. doi: 10.1158/0008-5472.CAN-05-3796. [DOI] [PubMed] [Google Scholar]
- 16.Yu XP, Bellido T, Manolagas SC. Down-regulation of NF-kappa B protein levels in activated human lymphocytes by 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci U S A. 1995;92:10990–10994. doi: 10.1073/pnas.92.24.10990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harant H, Wolff B, Lindley IJ. 1Alpha,25-dihydroxyvitamin D3 decreases DNA binding of nuclear factor-kappaB in human fibroblasts. FEBS Lett. 1998;436:329–334. doi: 10.1016/s0014-5793(98)01153-3. [DOI] [PubMed] [Google Scholar]
- 18.Stio M, Martinesi M, Bruni S, Treves C, Mathieu C, Verstuyf A, et al. The Vitamin D analogue TX 527 blocks NF-kappaB activation in peripheral blood mononuclear cells of patients with Crohn’s disease. J Steroid Biochem Mol Biol. 2007;103:51–60. doi: 10.1016/j.jsbmb.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 19.Rajakumar K, Fernstrom JD, Holick MF, Janosky JE, Greenspan SL. Vitamin D status and response to Vitamin D(3) in obese vs. non-obese African American children. Obesity (Silver Spring) 2008;16:90–95. doi: 10.1038/oby.2007.23. [DOI] [PubMed] [Google Scholar]
- 20.Brock K, Huang WY, Fraser DR, Ke L, Tseng M, Stolzenberg-Solomon R, et al. Low vitamin D status is associated with physical inactivity, obesity and low vitamin D intake in a large US sample of healthy middle-aged men and women. J Steroid Biochem Mol Biol. 2010;121:462–466. doi: 10.1016/j.jsbmb.2010.03.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng S, Massaro JM, Fox CS, Larson MG, Keyes MJ, McCabe EL, et al. Adiposity, cardiometabolic risk, and vitamin D status: the Framingham Heart Study. Diabetes. 2010;59:242–248. doi: 10.2337/db09-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rajakumar K, de las Heras J, Chen TC, Lee S, Holick MF, Arslanian SA. Vitamin D status, adiposity, and lipids in black american and causasian children. J Clin Endocrinol Metab. 2011;96:1560–1567. doi: 10.1210/jc.2010-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peterson CA, Heffernan ME. Serum tumor necrosis factor-alpha concentrations are negatively correlated with serum 25(OH)D concentrations in healthy women. J Inflamm (Lond) 2008;5:10. doi: 10.1186/1476-9255-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kong J, Li YC. Molecular mechanism of 1,25-dihydroxyvitamin D3 inhibition of adipogenesis in 3T3-L1 cells. Am J Physiol Endocrinol Metab. 2006;290:E916–924. doi: 10.1152/ajpendo.00410.2005. [DOI] [PubMed] [Google Scholar]
- 25.Zhuang H, Lin Y, Yang G. Effects of 1,25-dihydroxyvitamin D3 on proliferation and differentiation of porcine preadipocyte in vitro. Chem Biol Interact. 2007;170:114–123. doi: 10.1016/j.cbi.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 26.Lorente-Cebrian S, Eriksson A, Dunlop T, Mejhert N, Dahlman I, Astrom G, et al. Differential effects of 1α,25-dihydroxycholecalciferol on MCP-1 and adiponectin production in human white adipocytes. Eur J Nutr. 2011 doi: 10.1007/s00394-011-0218-z. [DOI] [PubMed] [Google Scholar]
- 27.Bao Y, Bing C, Hunter L, Jenkins JR, Wabitsch M, Trayhurn P. Zinc-alpha2-glycoprotein, a lipid mobilizing factor, is expressed and secreted by human (SGBS) adipocytes. FEBS Lett. 2005;579:41–47. doi: 10.1016/j.febslet.2004.11.042. [DOI] [PubMed] [Google Scholar]
- 28.Wang B, Wood IS, Trayhurn P. Hypoxia induces leptin gene expression and secretion in human preadipocytes: differential effects of hypoxia on adipokine expression by preadipocytes. J Endocrinol. 2008;198:127–134. doi: 10.1677/JOE-08-0156. [DOI] [PubMed] [Google Scholar]
- 29.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 30.Pantanetti P, Garrapa GG, Mantero F, Boscaro M, Faloia E, Venarucci D. Adipose tissue as an endocrine organ? A review of recent data related to cardiovascular complications of endocrine dysfunctions. Clin Exp Hypertens. 2004;26:387–398. doi: 10.1081/ceh-120034142. [DOI] [PubMed] [Google Scholar]
- 31.Chung S, Lapoint K, Martinez K, Kennedy A, Boysen Sandberg M, McIntosh MK. Preadipocytes mediate lipopolysaccharide-induced inflammation and insulin resistance in primary cultures of newly differentiated human adipocytes. Endocrinology. 2006;147:5340–5351. doi: 10.1210/en.2006-0536. [DOI] [PubMed] [Google Scholar]
- 32.Charriere G, Cousin B, Arnaud E, Andre M, Bacou F, Penicaud L, et al. Preadipocyte conversion to macrophage. Evidence of plasticity. J Biol Chem. 2003;278:9850–9855. doi: 10.1074/jbc.M210811200. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Leung DY, Richers BN, Liu Y, Remigio LK, Riches DW, et al. Vitamin d inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol. 2012;188:2127–2135. doi: 10.4049/jimmunol.1102412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ching S, Kashinkunti S, Niehaus MD, Zinser GM. Mammary adipocytes bioactivate 25-hydroxyvitamin D and signal via vitamin D receptor, modulating mammary epithelial cell growth. J Cell Biochem. 2011;112:3393–3405. doi: 10.1002/jcb.23273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hewison M. Antibacterial effects of vitamin D. Nat Rev Endocrinol. 2011;7:337–345. doi: 10.1038/nrendo.2010.226. [DOI] [PubMed] [Google Scholar]
- 36.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sartipy P, Loskutoff DJ. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci U S A. 2003;100:7265–7270. doi: 10.1073/pnas.1133870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao D, Trayhurn P, Bing C. Macrophage-secreted factors inhibit ZAG expression and secretion by human adipocytes. Mol Cell Endocrinol. 325:135–142. doi: 10.1016/j.mce.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 39.Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14:6735–6741. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 40.Bruun JM, Lihn AS, Madan AK, Pedersen SB, Schiott KM, Fain JN, et al. Higher production of IL-8 in visceral vs. subcutaneous adipose tissue. Implication of nonadipose cells in adipose tissue. Am J Physiol Endocrinol Metab. 2004;286:E8–13. doi: 10.1152/ajpendo.00269.2003. [DOI] [PubMed] [Google Scholar]
- 41.Elgazar-Carmon V, Rudich A, Hadad N, Levy R. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J Lipid Res. 2008;49:1894–1903. doi: 10.1194/jlr.M800132-JLR200. [DOI] [PubMed] [Google Scholar]
- 42.Keophiphath M, Achard V, Henegar C, Rouault C, Clement K, Lacasa D. Macrophage-secreted factors promote a profibrotic phenotype in human preadipocytes. Mol Endocrinol. 2009;23:11–24. doi: 10.1210/me.2008-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–288. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 44.Cohen-Lahav M, Shany S, Tobvin D, Chaimovitz C, Douvdevani A. Vitamin D decreases NFkappaB activity by increasing IkappaBalpha levels. Nephrol Dial Transplant. 2006;21:889–897. doi: 10.1093/ndt/gfi254. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Z, Yuan W, Sun L, Szeto FL, Wong KE, Li X, et al. 1,25-Dihydroxyvitamin D3 targeting of NF-kappaB suppresses high glucose-induced MCP-1 expression in mesangial cells. Kidney Int. 2007;72:193–201. doi: 10.1038/sj.ki.5002296. [DOI] [PubMed] [Google Scholar]
- 46.Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr. 2008;88:582S–586S. doi: 10.1093/ajcn/88.2.582S. [DOI] [PubMed] [Google Scholar]
- 47.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 48.Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56:16–23. doi: 10.2337/db06-1076. [DOI] [PubMed] [Google Scholar]
- 49.Giulietti A, van Etten E, Overbergh L, Stoffels K, Bouillon R, Mathieu C. Monocytes from type 2 diabetic patients have a pro-inflammatory profile. 1,25-Dihydroxyvitamin D(3) works as anti-inflammatory. Diabetes Res Clin Pract. 2007;77:47–57. doi: 10.1016/j.diabres.2006.10.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Cell viability of preadipocytes treated with vitamin D3 and MC, TNFα or IL-1β. Preadipocytes were pre-incubated with vitamin D3 (10 and 100 nM) for 24 h and then further incubated with RPMI 1640 medium (control) or THP-1 MC medium for another 24 h. Cell viability were determined by MTT assay. Data are means ± SEM for groups of 6. The results were verified by three independent experiments.