SUMMARY
Introduction
Unlike other tapeworms, T. solium infections carry risk for neurocysticercosis. Differential diagnosis of human tapeworm infections relies on morphology of the scolex or proglottids, frequently unavailable. DNA-based assays are poorly available in endemic areas. Ziehl Neelsen staining has been suggested but not tested in controlled designs. We validated whether Ziehl Neelsen staining could differentiate T. solium and T. saginata eggs.
Methods
Tapeworm proglottids (33 specimens, 23 T. solium and 10 T. saginata) and eggs (31 specimens, 13 T. solium and 10 T. saginata) were stained. Four eggs from each sample were measured and average diameters were recorded.
Results
T. saginata eggs stained entirely magenta in seven of 13 cases. T. solium eggs stained entirely blue/purple in 4/18 cases and entirely magenta in one. Eggs of T. saginata were slightly larger and always ovoid, while T. solium eggs were smaller and were mostly spheric.
Conclusions
Ziehl Neelsen staining can occasionally distinguish fully mature T. solium from T. saginata eggs. This distinction is poorly sensitive and not completely specific. Differential staining suggest differences in embryophore components between species, evident along egg maturation. In this small series, egg morphology (shape, maximal diameter) provided appropriate differentiation between T. solium and T. saginata eggs.
Keywords: Taenia, Taenia solium, Taenia saginata, Ziehl Neelsen, cestodes, Perú
INTRODUCTION
Three big tapeworms lodge in the human intestine: Diphyllobothrium sp, Taenia solium, and Taenia saginata, with a fourth, Taenia asiatica, still in debate on whether it is a new species or a T. saginata subspecies.(Flisser et al., 2004; Garcia et al., 2007) From these, only Taenia solium can lead to severe disease because of its capacity to infect the human brain with its larval form causing neurocysticercosis, the major cause of acquired epilepsy in most of the world.(Garcia & Del Brutto, 2005) The differential diagnosis between these tapeworms is based on morphology of the adult tapeworm scolex or proglottids. In most cases, however, only tapeworm eggs are found in stool samples, and no parasite tissue is available. Although Diphyllobothrium eggs are easily distinguishable, eggs from T. solium and T. saginata can not be differentiated by microscopical examination. Only DNA-based probes have obtained specific identification between these eggs; this type of assays are hardly available in endemic areas.(Flisser et al., 2004; Garcia et al., 2007; Gonzalez et al., 2000; Mayta et al., 2000)
A few decades ago, Capron, A. and Rose, F. (1962) described the use of the acid-fast (Ziehl Neelsen) staining to distinguish T. solium from T. saginata.(Capron & Brygoo, 1959; Capron & Rose, 1962) While their work is occasionally quoted, it has neither been replicated nor refuted. We examined fresh and preserved material from both parasite species to validate whether this method can differentiate between these two tapeworm species.
MATERIAL AND METHODS
Species diagnosis of tapeworm material
Expelled parasite material from human origin was used for this study. Cases were defined as T. solium or T. saginata on the basis of carmine staining and counting of main uterine branches (Flisser et al., 2004; Garcia et al., 2007; Mayta et al., 2000) as well as PCR in tapeworm material.(Mayta et al., 2000) There were no discordances between results with either method.
Tapeworm proglottids
Pre-existing archive parasite material was used for this part of the study, comprising mature and gravid proglottids obtained from 33 patients (23 T. solium carriers, 10 T. saginata carriers) after anti-parasitic treatment.(Jeri et al., 2004) In some cases multiple gravid and pre-gravid proglottids were available (14 and 9 proglottids from two T. solium tapeworms, and 7 and 4 proglottids from two T. saginata tapeworms) and were processed to assess changes in staining according to proglottid maturation. As per our standard routine, proglottids were washed with distilled water, fixed in 10% formalin-phosphate buffered saline (PBS) and stored at room temperature to be later processed by histology.
Defined fresh and archive stool samples
Eggs from 8 pre- or post- treatment stool samples (2 T. saginata and 6 T. solium) were examined and stained in fresh, before any fixation. Also archive stool samples from 23 patients, preserved in 5% formalin-PBS, were used for staining (11 T. saginata, 12 T. solium). Stool samples were concentrated by tube sedimentation. Sediments were placed in microscopy slides with polilysine and left to dry for staining. Four eggs from each sample were measured and the average maximal and transversal diameters were recorded (Table 1).
Table 1.
Ziehl Neelsen staining and morphological characteristics of T. solium and T. saginata eggs in fresh and preserved stool samples.
| Fresh / | Ziehl Neelsen | Maximal | Transversal | ||
|---|---|---|---|---|---|
| 487 | Fresh | T. saginata | Magenta | 36.5 | 34 |
| 492 | Fresh | T. saginata | Magenta | 36 | 32.5 |
| 268 | Preserved | T. saginata | Magenta | 35 | 28 |
| 274 | Preserved | T. saginata | Magenta/blue | 35 | 28 |
| 279 | Preserved | T. saginata | Magenta | 38 | 31 |
| 301 | Preserved | T. saginata | Magenta/blue | 36 | 32 |
| 421 | Preserved | T. saginata | Magenta | 36 | 30 |
| 453 | Preserved | T. saginata | Magenta | 35 | 32.5 |
| 492 | Preserved | T. saginata | Magenta | 35 | 32.5 |
| 500 | Preserved | T. saginata | Magenta/blue | 35 | 30 |
| 508 | Preserved | T. saginata | Magenta/blue | 35 | 32.5 |
| 536 | Preserved | T. saginata | Magenta/blue | 35 | 32.5 |
| 543 | Preserved | T. saginata | Magenta/blue | 35 | 32.5 |
| 402 | Fresh | T. solium | Magenta/blue | 32.5 | 32.5 |
| 411 | Fresh | T. solium | Magenta/blue | 32.5 | 32.5 |
| 459 | Fresh | T. solium | Magenta/blue | 32.5 | 32.5 |
| 461 | Fresh | T. solium | Magenta/blue | 32 | 30 |
| 472 | Fresh | T. solium | Magenta/blue | 31 | 30 |
| 489 | Fresh | T. solium | Magenta/blue | 33.5 | 32.5 |
| 403 | Preserved | T. solium | Magenta | 32 | 32 |
| 405 | Preserved | T. solium | Blue | 29 | 29 |
| 408 | Preserved | T. solium | Magenta/blue | 32.5 | 32 |
| 409 | Preserved | T. solium | Magenta/blue | 33 | 32.5 |
| 410 | Preserved | T. solium | Blue | 32 | 32 |
| 411 | Preserved | T. solium | Magenta/blue | 33.7 | 32 |
| 413 | Preserved | T. solium | Magenta/blue | 32.5 | 32.5 |
| 428 | Preserved | T. solium | Magenta/blue | 32.5 | 31.5 |
| 457 | Preserved | T. solium | Magenta/blue | 32.5 | 30 |
| 458 | Preserved | T. solium | Blue | 33.3 | 30 |
| 472 | Preserved | T. solium | Blue | 32.5 | 31.5 |
| 474 | Preserved | T. solium | Magenta/blue | 28 | 28 |
Values correspond to the assessment of all examined egs (staining) or to the mean value obtained from measuring 4 eggs from the same sample (diameters).
Histology
Proglottids were washed to eliminate the excess of formalin and then passed through increasing ethanol concentrations (70°, 80°, 90°, and 100°) and then three times in xilol. Proglottid samples were then placed in paraffin blocks, sliced in 6 um sections, de-paraffined, and placed on microscopy slides with polilysine for staining (Luna, 1968).
Ziehl-Neelsen staining
Samples were stained with carbol-fuchsin 3% for 15′, washed with tap water, and then decolored with 70% ethanol 1% HCl for 2′. After a second washing the slide was contrasted with 3% methylene blue for 5′, washed again, and left to dry at room temperature.(Chapin & Lauderdale, 2007; Clavel et al., 1999).
RESULTS
Staining of Taenia eggs in proglottid material
Staining of more proximal and more distal gravid T. solium and T. saginata proglottids showed that the oncospheres always stain blue in both species, with magenta hooks. As the eggs mature, a blue oncospheral membrane is clearly defined, around which magenta blocks begin to form the embryophore. A substance apparently secreted from the oncosphere then begins to fill the space between blocks. This substance is initially blue in both species. As the embryophore matures and becomes thicker, coloration gets more intense, departing from blue to gradually acquire some mixed magenta tones in T. solium, and a more marked magenta color in material from T. saginata (Figure 1).
Figure 1.
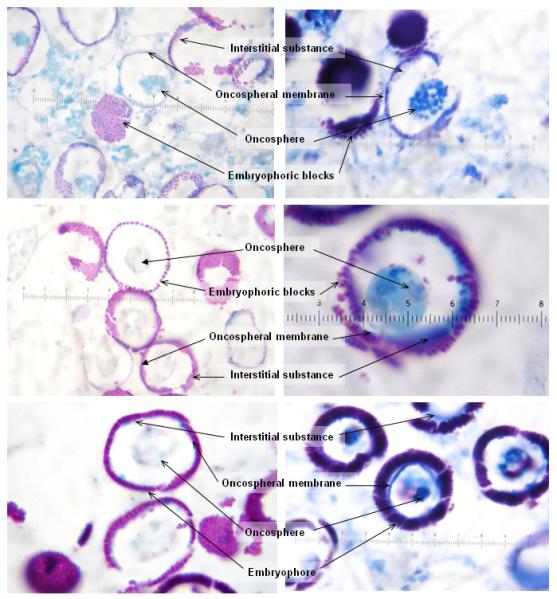
Histological sections showing stages of maturation of eggs in proglottids of Taenia saginata (left) and Taenia solium (right) showing oncospheres surrounded by an oncospheral membrane, small, magenta embryophoric blocks, and interstitial substance.
Staining of Taenia eggs from stool samples
There was no difference in staining of eggs from fresh versus preserved stool samples. In T. saginata eggs the external cover or embryophore was colored entirely magenta on Ziehl Neelsen in 7/13 cases (Figure 2a), and magenta with dark blue (some close to dark purple) areas in the remaining six. In T. solium eggs the embryophore stained usually in a mix of magenta and blue being entirely blue in four of the 18 cases (Figure 2b) and entirely magenta in one case (Table 1).
Figure 2.
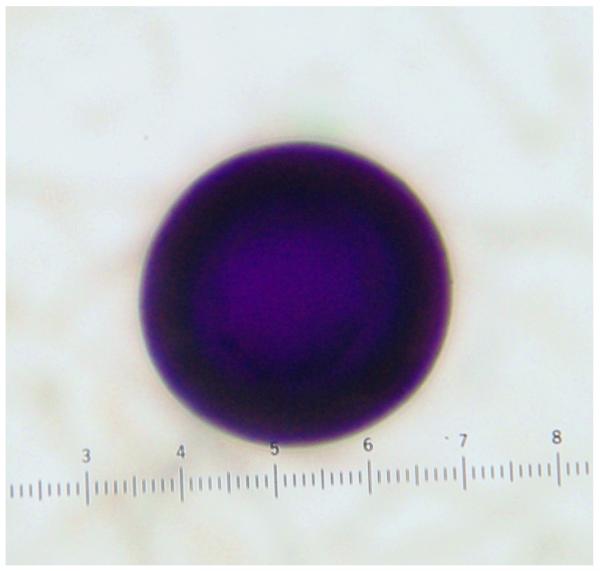
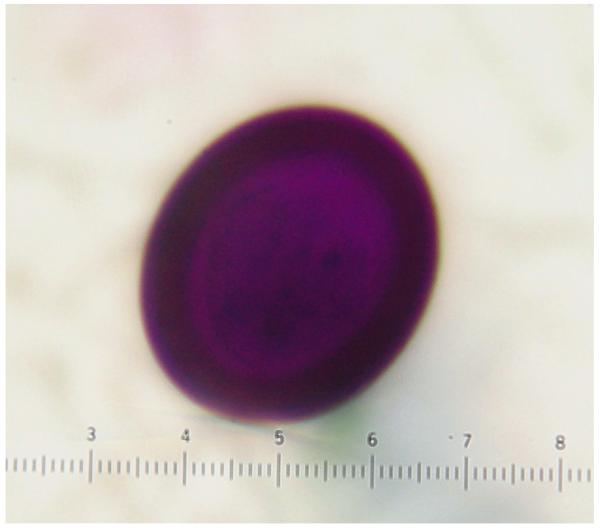
Mature eggs of Taenia solium (left) and Taenia saginata (right) as seen in stool samples, showing differences in staining tonalities.
Some apparent morphological differences could also be observed. The eggs of T. saginata were slightly larger, with a maximal diameter of 35.58 +/− 0.91 μm compared with 32.08 +/− 1.45 μm for T. solium eggs (n=13 for T. saginata, n=18 for T. solium; mean +/− SD; p<0.001, Mann Whitney test). T. saginata eggs were always ovoid (ratio between larger diameter and its transverse diameter was 1.14+/− 0.07), while most T. solium eggs were spheric (ratio was 1.03+/− 0.03; p<0.001 compared to T. saginata, Mann Whitney test). In 3 out of 18 cases, however, T. solium eggs looked ovoid in shape (ratios 1.11, 1.08, and 1.07) (Table 1).
In direct comparison of sensitivity and specificity with T. saginata and T. solium eggs, egg size and form were better predictors for species differentiation than Ziehl Neelsen staining (Table 2).
Table 2.
Sensitivity and specificity of Ziehl Neelsen staining and egg morphological to differentiate T. solium and T. saginata eggs.
| Criteria | Species | Sensitivity | Specificity | Youden’s J |
|---|---|---|---|---|
| Entirely magenta | T. saginata | 54% (7/13) | 96% (17/18) | 0.50 |
| Entirely blue/purple | T. solium | 50% (4/8) | 100% (13/13) | 0.50 |
| Ovoid | T. saginata | 100% (13/13) | 83% (15/18) | 0.83 |
| Spheric | T. solium | 83% (15/18) | 100% (13/13) | 0.83 |
| > 35 μm | T. saginata | 100% (13/13) | 100% (18/18) | 1.00 |
| < 35 μm | T. solium | 100% (18/18) | 100% (13/13) | 1.00 |
Values for spheric shape or maximal diameter < 35 μm as criteria to define T. solium are inverse to those of ovoid shape or larger eggs for T. saginata, due to direct group comparison
DISCUSSION
Taenia spp. eggs are covered by a thick embryophore composed by prismatic keratin blocks (which give it its typical radial appearance), kept together by a cement substance. By the time they reach the environment the embryophore is still surrounded by a colloid vitellum layer. It has been previously described that the vitelogen glands in the oncosphere produce a acid-fast resistant substance which takes the spaces between embryophoric blocks, likely responsible by the changes in coloration along egg maturation.(Capron & Brygoo, 1959; Capron & Rose, 1962)
This series showed that Ziehl Neelsen staining can occasionally distinguish fully mature T. solium from T. saginata eggs, in cases where the cover is entirely magenta (7/13 T. saginata), or entirely blue/purple (4/18 T. solium). Staining was mixed in 19/31 cases, and equivocal (magenta) in one case of T. solium. While this distinction provided a species diagnosis in 35% of cases, it is by no means absolute and seems poorly useful in practice. Its application assumes eggs are fully mature (which is not possible to determine in a proglottid), and carries some degree of subjectivity because color differences may be subtle, adding to the requirement of highly trained personal. In most centers, the numbers of specimens to be tested for species differentiation will be small, as will be the experience of the operators. Oncospheres stained blue in both species, not helping in differentiation. Morphological criteria (diameter and shape) seemed more consistent in this series. In this small series, an arbitrary cut off of 35 μm had 100% predictive value for differentiation. This had been reported before (Verster, 1969) but not really replicated. Morphological differences are however minor and would need to be replicated with T. saginata and T. solium material from other parts of the world.
Of interest, our data suggest differences in specific components of the embryophore between these two species, evident along egg maturation, compatible with previous data on different chemical composition of embryophoric blocks.(Morseth, 1966) Given that the ability of the oncosphere of T. solium to infect the human host and cause cysticercosis is not shared by T. saginata, further understanding and characterization of the enzymes and other active molecules present in one species but absent in the other may provide species-specific diagnostics and potential vaccine targets.
Acknowledgments
FINANCIAL SUPPORT
This work was partially supported by the Fogarty International Center, National Institutes of Health, Bethesda, USA (H.G., training grant number D43 TW001140); and the Bill and Melinda Gates Foundation (H.G., grants number 23981 and 33848).
REFERENCES
- Capron A, Brygoo ER. [Constitution of helminth eggs. I. Presence and formation of an acid-alcohol resistant substance in the shell] Bull Soc Pathol Exot Filiales. 1959;52:574–577. [PubMed] [Google Scholar]
- Capron A, Rose F. [On the composition of helminth eggs. II. Alcohol-acid-resistance in cestodes. Difference of Ziehl stainability of embryophores of Taenia saginata and Taenia solium] Bull Soc Pathol Exot Filiales. 1962;55:765–767. [PubMed] [Google Scholar]
- Chapin K, Lauderdale TL. Reagents, stains, and media: bacteriology. In: Murray PR, Baron EJ, Jorgensen JH, Landr ML, Pfaller MA, editors. Manual of Clinical Microbiology. 9th ed American Society for Microbiology; Washington, D.C: 2007. p. 344. [Google Scholar]
- Clavel A, Varea M, Doiz O, et al. Visualization of hydatid elements: comparison of several techniques. Journal of Clinical Microbiology. 1999;37:1561–1563. doi: 10.1128/jcm.37.5.1561-1563.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flisser A, Viniegra AE, Aguilar-Vega L, Garza-Rodriguez A, Maravilla P, Avila G. Portrait of human tapeworms. Journal of Parasitology. 2004;90:914–916. doi: 10.1645/GE-3354CC. [DOI] [PubMed] [Google Scholar]
- Garcia HH, Del Brutto OH. Neurocysticercosis: updated concepts about an old disease. Lancet Neurology. 2005;4:653–661. doi: 10.1016/S1474-4422(05)70194-0. [DOI] [PubMed] [Google Scholar]
- Garcia HH, Jimenez JA, Escalante H. Cestodes. In: Murray PR, Baron EJ, editors. Manual of Clinical Microbiology. ASM Press; Washington DC: 2007. pp. 2166–2174. [Google Scholar]
- Gonzalez LM, Montero E, Harrison LJ, Parkhouse RM, Garate T. Differential diagnosis of Taenia saginata and Taenia solium infection by PCR. Journal of Clinical Microbiology. 2000;38:737–744. doi: 10.1128/jcm.38.2.737-744.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeri C, Gilman RH, Lescano AG, et al. Species identification after treatment for human taeniasis. Lancet. 2004;363:949–950. doi: 10.1016/S0140-6736(04)15791-7. [DOI] [PubMed] [Google Scholar]
- Luna LG, editor. Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology. 3rd Ed McGraw-Hill; New York: 1968. p. 28. [Google Scholar]
- Mayta H, Talley A, Gilman RH, et al. Differentiating Taenia solium and Taenia saginata infections by simple hematoxylin-eosin staining and PCR-restriction enzyme analysis. Journal of Clinical Microbiology. 2000;38:133–137. doi: 10.1128/jcm.38.1.133-137.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morseth DJ. Chemical composition of embryophoric blocks of Taenia hydatigena, Taenia ovis, and Taenia pisiformis eggs. Experimental Parasitology. 1966;18:347–354. doi: 10.1016/0014-4894(66)90037-3. [DOI] [PubMed] [Google Scholar]
- Verster A. A taxonomic revision of the genus Taenia Linnaeus, 1758 s. str. Onderstepoort Journal of Veterinary Research. 1969;36:3–58. [PubMed] [Google Scholar]


